Abstract
Background:
In addition to mandatory haematological monitoring, treatment guidelines recommend routine monitoring of adverse effects and physical health in patients prescribed clozapine.
Methods:
NHS trusts/healthcare organisations participated in a clinical audit in the context of a UK quality improvement programme addressing clozapine-prescribing practice.
Results:
Data relating to 6948 adult patients prescribed clozapine were submitted by 63 NHS trusts/healthcare organisations. Of 481 patients treated with clozapine for up to 18 weeks, there was documented pretreatment screening of blood pressure, heart rate and ECG in at least 90%, and body weight, plasma lipids, plasma glucose/glycated haemoglobin (HbA1c) and physical examination in approximately 80%. During the first 2 weeks of clozapine treatment there was documented daily measurement of both heart rate and blood pressure in 82% and body temperature in 77%. In a subsample of 411 patients, of the 72% who had weekly side-effect assessments documented in the first month of treatment, a structured assessment tool had been used in 29%. Treatment monitoring up to 18 weeks included an ECG in 90%, C-reactive protein (CRP) or creatine kinase in 42%, and troponin or B-type natriuretic peptide (BNP) in 29%. In the 5908 patients prescribed clozapine for at least 1 year, blood pressure and body weight/body mass index were documented in at least 80%, plasma lipids in 78% and plasma glucose in 73%, with an ECG in 55%. Two-thirds were prescribed medication to manage side effects of clozapine and one third of those with a diagnosis of schizophrenia were prescribed a second antipsychotic medication.
Conclusion:
The findings suggest that for most patients treated with clozapine in UK mental health services, physical health screening and side-effect monitoring follow recommended practice, but there was limited use of structured side-effect assessment tools. Monitoring for clozapine-induced myocarditis during the early risk period using markers of inflammation such as CRP, and cardiac damage such as troponin and BNP, was less consistent. This may partly reflect the variation in guideline recommendations for monitoring for myocarditis and partly the selected use of such tests when prompted by cardiac symptoms. The relatively common coprescription of medications for the majority of people on longer-term clozapine treatment may well further increase side-effect burden and physical health risks, reinforcing the need for continuing systematic monitoring.
Keywords: antipsychotic medication, clozapine, physical health monitoring, quality improvement, schizophrenia, side effects
Introduction
The antipsychotic medication, clozapine, has the most convincing evidence for benefit in treatment-resistant schizophrenia and there are indications that it can reduce aggression, suicidality and mortality in schizophrenia.1–3 Weighing up the risks and benefits of clozapine treatment in any individual involves the assessment of adverse effects. These include several uncommon but serious problems, such as neutropenia/agranulocytosis, myocarditis/cardiomyopathy,4,5 pneumonia6 and seizures. In addition, there are side effects that may become serious if overlooked, such as constipation,7–9 weight gain and metabolic syndrome,10,11 and others that are potentially discomfiting or distressing such as hypersalivation, sedation, orthostatic hypotension and nocturnal enuresis.12 Clozapine is associated with high rates of cardio-metabolic risk factors which may be both under-detected and under-treated.13 The prevalence of specific side effects changes during the course of treatment, and effective monitoring of clozapine side effects should take this into account.
Aside from the mandatory haematological monitoring, guideline recommendations for clinicians for the physical health and side-effect monitoring of patients prescribed clozapine treatment are not entirely consistent.14 While the elements of screening and on-treatment monitoring relating to body weight, plasma lipids, glycaemic control, blood pressure and heart rate are included in most relevant protocols, monitoring for clozapine-induced cardiotoxicity using measures of C-reactive protein (CRP), creatine kinase (CK) or troponin is not universally accepted as part of a routine protocol, and while electrocardiography is commonly recommended, echocardiography rarely is.14–16
The Prescribing Observatory for Mental Health (POMH-UK) coordinates audit-driven quality improvement (QI) programmes addressing aspects of prescribing practice in adult mental health services. In 2019, the baseline audit was conducted for a QI programme on the use of clozapine, one aim of which was to determine compliance with evidence-based practice standards for monitoring of physical health, aside from the obligatory haematological monitoring for neutropenia and agranulocytosis.
Methods
All 64 POMH-UK member trusts and healthcare services were invited to participate in the baseline clinical audit of the QI programme. The data collection tool was designed to capture information related to performance against practice standards derived from the National Institute for Health and Care Excellence (NICE) Clinical Guideline CG178, ‘Psychosis and schizophrenia in adults: prevention and management’17 and ‘The Maudsley prescribing guidelines in psychiatry’, 13th edition.18 Four of these standards referred to monitoring of physical health and adverse effects: pretreatment screening should include physical examination with assessment of the cardiovascular system; monitoring in the first 2 weeks of treatment should include at least daily assessment of temperature, blood pressure and heart rate; in the first month of treatment there should be at least weekly assessment for common side effects, such as cardiac symptoms, hypotension, constipation and weight gain; patients established on clozapine treatment for more than 1 year should have an annual medication review, taking account of therapeutic response and recognised side effects. In addition, the data collected from clinical records included psychiatric diagnoses, other psychotropic medication prescribed, the reasons for the initiation of clozapine treatment, whether clozapine was prescribed outside of its licensed indication (‘off-label’) and clozapine treatment history.
In each participating trust and healthcare service, the data were collected by clinicians and clinical audit staff on a sample of eligible patients (prescribed clozapine and under the care of adult mental health services) and submitted pseudonymously online to POMH-UK using Formic software.19 Ethical approval was not required for such audit-based QI initiatives.20
The data were analysed using SPSS.21
Results
A total of 872 clinical teams in 63 NHS trusts/healthcare organisations in the UK participated in the baseline audit, submitting pseudonymous data relating to the treatment of 6948 patients, all of whom were prescribed clozapine under the care of adult mental health services. Table 1 shows the demographic and clinical characteristics of these patients; 481 had been receiving clozapine for 18 weeks or less (usually subject to weekly haematological monitoring), 559 had been receiving clozapine for longer than 18 weeks but less than 1 year (usually fortnightly haematological monitoring) and 5908 had been prescribed clozapine for longer than 1 year (usually monthly haematological monitoring).
Table 1.
Demographic and clinical characteristics of the total national sample (n = 6948) at the baseline audit.
| Demographic and clinical characteristics | 6948 adult patients n (%) |
||
|---|---|---|---|
| Gender | Male | 4857 (70) | |
| Female | 2091 (30) | ||
| Age | Median age in years | 44 | |
| Range in years | 16–90 | ||
| Ethnicity | White/White British | 5477 (79) | |
| Asian/Asian British | 491 (7) | ||
| Black/Black British | 405 (6) | ||
| Mixed or other | 372 (5) | ||
| Not collected/stated/refused | 203 (3) | ||
| Clinical diagnosis: ICD-10 category (for at least 1% of the sample) | Schizophrenia spectrum disorder (F20-29) | 6571 (95) | |
| Disorder due to psychoactive substance use (F10-19) | 363 (5) | ||
| Borderline/emotionally unstable personality disorder (F60.3) | 312 (5) | ||
| Personality disorder other than borderline/emotionally unstable personality disorder (F60-69) | 173 (3) | ||
| Learning disability (F70-79) | 169 (2) | ||
| Neurotic, stress-related and somatoform disorders (F40-48) | 149 (2) | ||
| Affective disorder, other than bipolar disorder (F30-39) | 128 (2) | ||
| Disorder of psychological development (F80-89) | 94 (1) | ||
| Bipolar disorder (F31) | 67 (1) | ||
| Patient status | Inpatients | Detained under the Mental Health Act | 1459 (21) |
| Informal status | 160 (2) | ||
| Outpatients/community patients | Community Treatment Order | 287 (4) | |
| No Community Treatment Order | 5042 (73) | ||
| Clinical service (for at least 1% of the sample) | Community mental health team | 5152 (74) | |
| Forensic inpatient team | 823 (12) | ||
| Inpatient rehabilitation service | 415 (6) | ||
| Acute adult inpatient team | 311 (4) | ||
| Forensic community team | 111 (2) | ||
| Early intervention service | 89 (1) | ||
| PICU inpatient team | 42 (1) | ||
| Home treatment/crisis team | 38 (1) | ||
ICD, international classification of diseases; PICU, psychiatric intensive care unit.
Pretreatment screening
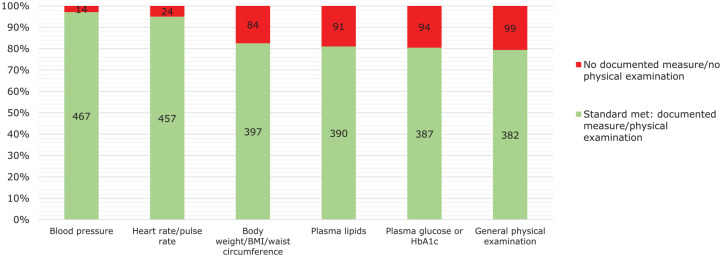
Pretreatment measures of blood pressure, heart rate, body weight/body mass index (BMI), plasma lipid levels, plasma glucose or glycated haemoglobin (HbA1c) and a general physical examination were reported for the subsample of 481 patients treated with clozapine for fewer than 18 weeks. Figure 1 shows that baseline measures for blood pressure and heart rate were documented in almost all of these patients (97% and 95%, respectively), body weight had been recorded in 83%, plasma lipid levels in 81%, plasma glucose or HbA1c in 80%, and a general physical examination was documented as having been conducted in 79%. A baseline electrocardiogram (ECG) had been conducted in 90% of cases.
Figure 1.
Documented pretreatment measures of blood pressure, pulse rate, body weight, plasma lipids, plasma glucose and a general physical examination in 481 patients who had been treated with clozapine for fewer than 18 weeks.
BMI, body mass index; HbA1c, haemoglobin A1c.
Monitoring in the first 2 weeks of clozapine treatment
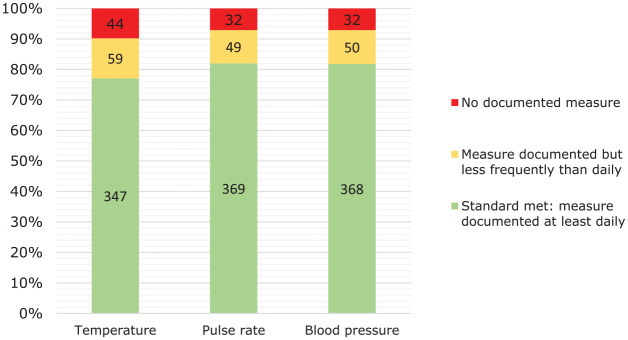
Of the subsample of 481 patients who were undergoing weekly haematological monitoring, 31 had been receiving treatment for fewer than 2 weeks and were therefore excluded from the analysis of data relating to monitoring in the first 2 weeks of clozapine treatment. For the 450 eligible patients, Figure 2 shows that during the first 2 weeks of clozapine treatment there was documented daily measurement of body temperature in 77% and measurement less frequently than daily in an additional 13%. For both heart rate and blood pressure, the respective figures were 82% and 11%.
Figure 2.
Documented daily measurements of temperature, pulse rate and blood pressure in the first 2 weeks of clozapine treatment in 450 patients who had been treated with clozapine for between 2 weeks and 18 weeks.
Monitoring of side effects in the first 18 weeks of treatment
Several tests relevant to cardiovascular side effects were documented in the clinical records of the subsample of 481 patients who had been treated with clozapine for up to 18 weeks: 431 (90%) had an ECG, 204 (42%) had measures of the amount of CRP or CK in the blood, and 138 (29%) had been tested for troponin or B-type natriuretic peptide (BNP) blood levels.
To determine the proportion of patients who had been assessed weekly for side effects within the first month of treatment, 70 patients who had been receiving clozapine for fewer than 4 weeks were excluded. Of the remaining 411 patients treated with clozapine for between 4 weeks and 18 weeks, 55 (13%) had no documented assessment of side effects in the first month of treatment while 88 (21%) had documented weekly assessments using a structured assessment tool and 210 (51%) had documented weekly assessments but without the use of a structured assessment tool. For 58 (14%) patients, assessment of side effects was documented as having occurred less frequently than weekly, with a structured assessment tool being used in 17 (29%).
Longer-term medication review
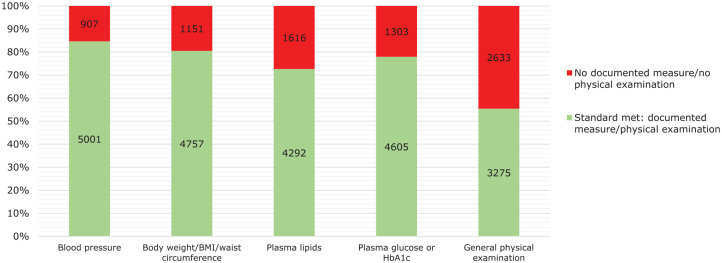
From the clinical records of the 5908 patients treated with clozapine for more than 1 year, data were collected on the measurement of blood pressure, pulse rate, body weight, plasma lipids and plasma glucose, as well as a general physical examination. As can be seen from Figure 3, there were documented measures of blood pressure for 5001 (85%) patients and body weight/BMI for 4757 (81%) within the past year, while for plasma glucose and plasma lipids, the proportions were slightly lower at 4605 (78%) and 4292 (73%), respectively. Physical examination was documented for 3275 (55%) patients. In 422 (7%) of these patients, none of these physical health checks were documented in the clinical records in the previous year.
Figure 3.
Documented measurements in the previous year of blood pressure, pulse rate, body weight, plasma lipids, plasma glucose and a general physical examination in 5908 patients treated with clozapine for more than 1 year.
BMI, body mass index; HbA1c, haemoglobin A1c.
There was documented evidence that an ECG had been conducted in the last year for 3072 (52%) patients in this subsample receiving continuing clozapine treatment for at least 1 year. CRP or CK had been measured in 945 (16%) patients and troponin or BNP had been measured in 295 (5%), while an echocardiogram had been conducted for 59 (1%).
In the patients prescribed clozapine for more than 1 year, information was collected on medications prescribed to manage side effects associated with clozapine treatment. Medication was prescribed to treat hypersalivation in 1701 (29%) patients, oesophageal reflux/nausea in 1178 (20%), dyslipidaemia 1138 (19%), type 2 diabetes in 796 (13%), hypertension in 644 (11%), tachycardia in 477 (8%) and nocturnal enuresis in 104 (2%), and to treat or prevent constipation in 1642 (28%), seizures/fits in 287 (5%) and weight gain in 125 (2%).
A total of 5633 (95%) patients had a diagnosis of schizophrenia spectrum disorder, of whom 1803 (32%) were prescribed a second antipsychotic in combination with clozapine. The two antipsychotic medications most commonly coprescribed were amisulpride (n = 683, 38%) and aripiprazole (n = 613, 34%). In addition, other psychotropic medications were commonly coprescribed: an antidepressant medication for 2096 (37%) patients, valproate for 1000 (18%), a benzodiazepine for 840 (15%), an anticholinergic agent for 726 (13%) and lamotrigine for 293 (5%).
Discussion
Pretreatment screening
Clozapine treatment is associated with the emergence of cardio-metabolic risk factors such as weight gain, an adverse lipid profile and the development of diabetes. Before treatment is started, relevant baseline measures, specifically heart rate, blood pressure, body weight/BMI, plasma lipids, plasma glucose or HbA1c and a general physical examination, are recommended across relevant, evidence-based treatment guidelines such those by the Scottish Intercollegiate Guidelines Network,22 NICE,17 the Maudsley prescribing guidelines18 and the Royal Australian and New Zealand College of Psychiatrists.23 These baseline measures were not fully documented for about one in five patients in our audit subsample, for whom the ability to assess the nature and severity of the emergent side-effect burden of clozapine treatment would therefore be limited. However, most patients had a baseline ECG in their clinical records. Although treatment guidelines do not consistently recommend conducting a baseline ECG prior to starting treatment with clozapine, it may be considered good practice to do so as it allows certain contraindications to clozapine treatment to be identified and provides a valuable reference should cardiac side effects emerge subsequently.
Early on-treatment monitoring
The first few weeks of clozapine treatment, which are almost always a period of dosage titration, are commonly associated with hypotension and tachycardia, as well as side effects such as fever, hypersalivation and nocturnal enuresis.12,24 While the Maudsley prescribing guidelines18 recommend daily measurement of heart rate, blood pressure and temperature during dosage titration, other guidelines refer to blood pressure measurement after 1 month of treatment17,22 or more generally to the need for blood pressure monitoring.23
In the first 2 weeks of treatment, there were documented daily measures of temperature, pulse rate and blood pressure in more than three-quarters of the subsample of patients for whom this information was collected. In the first month of treatment, at least weekly assessment of possible clozapine side effects, such as weight gain and constipation, was documented in almost three-quarters of such cases and for one patient in five a structured side-effect assessment tool was used. The full side-effect burden associated with clozapine treatment may only be ascertained by sensitive questioning and systematic inquiry, which will be facilitated by the use of a structured side-effect assessment tool, allowing side effects to be elicited more systematically and comprehensively.25,26 However, even when side effects had been reviewed in this early treatment period, such tools were used relatively infrequently, limiting the assessment of the side-effect burden as well as the ability to record and communicate this. Further, the limited documentation of the assessment of side effects may represent, in at least a proportion of cases, a missed opportunity for the prescriber to discuss any emergent or worsened side effects with the patient, and to tackle these appropriately. Such discussion, with a patient reporting on their subjective experience of medication in terms of both beneficial and adverse effects, is key to maintaining prescribing as a collaborative process and supporting continued adherence to a medication regimen.27
Monitoring for myocarditis
Clozapine treatment is associated with a range of adverse effects involving the cardiovascular system, including hypotension, tachycardia, cardiac arrhythmias, cardiomyopathy and myocarditis. Clozapine-induced myocarditis is most likely to present in the first 8–12 weeks or so of treatment,12,28 but the reported incidence varies widely, at least partly because the rate of detection seems to be largely dependent on the intensity of cardiac monitoring.29 While some studies conducted in Australia have reported the development of myocarditis early in treatment in up to 3% of patients,30 other studies conducted internationally have found the risk to be considerably lower.31–35
The clinical symptoms and signs of clozapine-induced myocarditis may be rather nonspecific and there are no definitive diagnostic criteria.4,28 Further, treatment guidelines vary regarding the utility of monitoring using markers of inflammation (CRP, erythrocyte sedimentation rate) and cardiac damage (troponin, BNP).15,16,18,36 The current Australian and New Zealand guidelines23 recommend a baseline measure of troponin while the Maudsley prescribing guidelines18 refer to a monitoring algorithm for the detection of treatment-emergent myocarditis15 that includes weekly CRP and troponin measures in the first month of treatment. In those patients in our audit sample who had been receiving clozapine for fewer than 18 weeks, which covers the key risk period for myocarditis, less than half had measures of CRP or CK and just over a quarter had been tested for troponin or BNP levels. A limitation of these audit data is that it is not known whether such tests were conducted as routine monitoring, or prompted by cardiac symptoms or aberrant investigation results, such as raised eosinophils. Further, given the uncertain magnitude of the risk of developing clozapine-induced myocarditis and/or cardiomyopathy and the lack of consensus in clinical guidelines regarding screening and monitoring for these conditions, the practice identified is not open to a judgement on quality and simply reflects current practice in UK mental health services.
Longer-term monitoring
For longer-term monitoring of patients on continuing clozapine treatment, assessments of body weight/BMI, plasma lipids, plasma glucose and ECG are generally recommended annually.15,17,22,23,37 In the subsample of patients prescribed clozapine for at least 1 year, there were documented measures of blood pressure and BMI in the last year for four out of every five patients, while for measures of plasma lipids and plasma glucose this proportion was slightly lower at around three out of every four. For about 1 in 14 of these patients, none of these physical health checks had been documented in the previous year.
There was documented evidence that an ECG had been conducted in the past year for just over half of this subsample of patients receiving maintenance treatment with clozapine. Testing for CRP or CK levels had been conducted for 1 patient in 6 while tests for troponin and BNP levels were conducted in 1 patient in 20. An echocardiogram, a more specific test of cardiac functioning, was documented for 1 patient in 100. While it is likely that some of the less specific tests may have been undertaken as part of routine monitoring, the more specific the test, the higher the probability that it was conducted because the patient had symptoms that were suspected to be cardiac in origin.
The vast majority of patients prescribed clozapine for more than 1 year had a diagnosis of a schizophrenia spectrum disorder, of whom almost a third were coprescribed a second antipsychotic medication. These prescriptions were most commonly for amisulpride or aripiprazole, which could be considered reasonable choices based on their pharmacology and known side-effect profiles: for example, aripiprazole augmentation of clozapine treatment is associated with modest benefits in respect of weight gain and plasma lipids.38 It has become custom and practice to augment clozapine with a second antipsychotic when the response to clozapine monotherapy has proved to be suboptimal. However, the risk–benefit balance for such a strategy for clozapine-refractory schizophrenia is uncertain.5,39,40 The nature of the other psychotropic medications coprescribed with clozapine in these patients suggests that clinicians are using pharmacological strategies to target persistent symptoms and behaviours such as affective disturbance, negative symptoms, aggression and hostility.
At least two-thirds of all patients who had been prescribed clozapine for more than 1 year were prescribed other medications to help manage the side effects of clozapine treatment. Medications that target hypersalivation and constipation were prescribed for more than one patient in four. Treatment for symptomatic oesophageal reflux was prescribed for one patient in five. Weight gain is a very common side effect associated with clozapine treatment, but the data suggest that, aside from the possible use of aripiprazole for this purpose, this was not managed with pharmacological strategies in the majority of cases; such an approach is consistent with the limited effect that anti-obesity medicines have on mitigating clozapine-related weight gain.41
Conclusion
Analysis of the data collected in this national clinical audit suggests that for the majority of patients prescribed clozapine in mental health services, physical health screening and side-effect monitoring are in line with recommended practice, although there was only limited use of structured side-effect assessment tools. Monitoring for clozapine-induced myocarditis during the early risk period, using markers of inflammation such as CRP, and troponin and BNP for cardiac damage, was less consistent, which may be partly a consequence of variations in the guideline recommendations for monitoring for myocarditis and partly because the use of such tests will, in some cases, have been prompted by the emergence of cardiac symptoms.
The relatively common combination of clozapine with another antipsychotic medication and the prescription of additional psychotropic medications for the majority of people on longer-term clozapine treatment may well increase the likelihood of side effects and physical health concerns.42–44 Such polypharmacy may lead to adverse drug–drug interactions and prescribing cascades, where a medication event is misinterpreted as a new medical condition, and a second medication is prescribed.45 Such considerations reinforce the need for continued systematic monitoring of physical health and the side-effect burden for such patients.46
Each participating trust received a customised report of the audit findings, showing its performance against the practice standards, benchmarked against the other participating trusts and also allowing the performance of each service within the trust to be compared.47 Clinicians were invited to reflect on their level of compliance with the practice standards in their clinical service and their performance relative to other services, identify key areas for improvement and consider appropriate QI activity. There are published examples of local service interventions that have been tested to engender more consistent and effective physical health monitoring, usually including the provision of information on recommended clozapine monitoring to clinicians and the introduction of formal systems to support such monitoring.48–51 Whether any improvements seen with such QI interventions are maintained in the long term is unknown. However, the impact of the feedback on the baseline audit data to clinical services in this POMH QI programme will be tested with a re-audit after 2 years.
Acknowledgments
Acknowledgements are due to the clinicians and other staff in participating services who collected and submitted the audit data. Thanks are also due to members of the POMH-UK team aside from the co-authors of this paper: Gavin Herrington, Brittany McConnell and Olivia Rendora.
Footnotes
Conflict of interest statement: TREB has been a member of scientific advisory boards for Lundbeck, Newron and Gedeon Richter/Recordati and received an honorarium for a lecture from Janssen. JMK has received honoraria for lectures and/or consulting from Alkermes, Allergan, Intra-Cellular Therapies, Janssen, LB Pharma, Lundbeck, Merck, Neurocrine Biosciences, Newron, Otsuka, Reviva, Roche, Sunovion, Sumitomo Dainippon and Teva. He is a shareholder in LB Pharma and the Vanguard Research Group.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work of POMH-UK is funded wholly by member subscriptions from mental health services in the UK. JHM has received research funding from Lundbeck and has accepted hospitality from Lundbeck and Saladax Biochemicals.
ORCID iDs: Thomas R. E. Barnes  https://orcid.org/0000-0002-2324-656X
https://orcid.org/0000-0002-2324-656X
Carol Paton  https://orcid.org/0000-0001-7756-1031
https://orcid.org/0000-0001-7756-1031
Contributor Information
Thomas R. E. Barnes, Division of Psychiatry, Imperial College London, 7th Floor Commonwealth Building, Du Cane Road, London W12 0NN, UK.
James H. MacCabe, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, UK, NIHR Biomedical Research Centre for Mental Health, South London, UK, and Maudsley NHS Foundation Trust, London, UK
John M. Kane, Department of Psychiatry, Zucker Hillside Hospital, New York, USA
Oriana Delgado, Prescribing Observatory for Mental Health, Centre for Quality Improvement, Royal College of Psychiatrists, London, UK.
Carol Paton, Division of Psychiatry, Imperial College London, UK, and Prescribing Observatory for Mental Health, Centre for Quality Improvement, Royal College of Psychiatrists, London, UK.
References
- 1. Vermeulen JM, van Rooijen G, van de Kerkhof MPJ, et al. Clozapine and long-term mortality risk in patients with schizophrenia: a systematic review and meta-analysis of studies lasting 1.1-12.5 years. Schizophr Bull 2019; 45: 315–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barnes TR, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol 2020; 34: 3–78. [DOI] [PubMed] [Google Scholar]
- 3. Rubio JM, Kane JM. How and when to use clozapine. Acta Psychiatr Scand 2020; 141: 178–189. [DOI] [PubMed] [Google Scholar]
- 4. Patel RK, Moore AM, Piper S, et al. Clozapine and cardiotoxicity – a guide for psychiatrists written by cardiologists. Psychiatry Res 2019; 282: 112491. [DOI] [PubMed] [Google Scholar]
- 5. Siskind DJ, Lee M, Ravindran A, et al. Augmentation strategies for clozapine refractory schizophrenia: a systematic review and meta-analysis. Aust N Z J Psychiatry 2018; 52: 751–767. [DOI] [PubMed] [Google Scholar]
- 6. de Leon J, Sanz EJ, Norén GN, et al. Pneumonia may be more frequent and have more fatal outcomes with clozapine than with other second-generation antipsychotics. World Psychiatry 2020; 19: 120–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ellis P, Harrison-Woolrych M, McLean R. Clozapine – fatal constipation more common than fatal agranulocytosis. Acta Neuropsychiatr 2006; 18: 285. [DOI] [PubMed] [Google Scholar]
- 8. Every-Palmer S, Newton-Howes G, Clarke MJ. Pharmacological treatment for antipsychotic-related constipation. Cochrane Database Syst Rev 2017; 1: CD011128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ingimarsson O, MacCabe JH, Sigurdsson E. Constipation, ileus and medication use during clozapine treatment in patients with schizophrenia in Iceland. Nord J Psychiatry 2018; 72: 497–500. [DOI] [PubMed] [Google Scholar]
- 10. Lamberti JS, Olson D, Crilly JF, et al. Prevalence of the metabolic syndrome among patients receiving clozapine. Am J Psychiatry 2006; 163: 1273–1276. [DOI] [PubMed] [Google Scholar]
- 11. Ahmed M, Hussain I, O’Brien SM, et al. Prevalence and associations of the metabolic syndrome among patients prescribed clozapine. Ir J Med Sci 2008; 177: 205–210. [DOI] [PubMed] [Google Scholar]
- 12. Citrome L, McEvoy JP, Saklad SR. Guide to the management of clozapine-related tolerability and safety concerns. Clin Schizophr Relat Psychoses 2016; 10: 163–177. [PubMed] [Google Scholar]
- 13. Lappin JM, Wijaya M, Watkins A, et al. Cardio-metabolic risk and its management in a cohort of clozapine-treated outpatients. Schizophr Res 2018; 199; 367–373. [DOI] [PubMed] [Google Scholar]
- 14. Nielsen J, Young C, Ifteni P, et al. Worldwide differences in regulations of clozapine use. CNS Drugs 2016; 30: 149–161. [DOI] [PubMed] [Google Scholar]
- 15. Ronaldson KJ, Fitzgerald PB, Taylor AJ, et al. A new monitoring protocol for clozapine-induced myocarditis based on an analysis of 75 cases and 94 controls. Aust NZ J Psychiatry 2011; 45: 458–465. [DOI] [PubMed] [Google Scholar]
- 16. Knoph KN, Morgan RJ, III, Palmer BA, et al. Clozapine-induced cardiomyopathy and myocarditis monitoring: a systematic review. Schizophr Res 2018; 199: 17–30. [DOI] [PubMed] [Google Scholar]
- 17. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. NICE Clinical Guideline 178. London: National Institute for Health and Care Excellence, 2014. [Google Scholar]
- 18. Taylor DM, Barnes TRE, Young AH. The Maudsley prescribing guidelines in psychiatry. 13th ed. Hoboken, NJ: Wiley Blackwell, 2018. [Google Scholar]
- 19. Formic Software, https://www.formic.com/ (2016, accessed June 2020).
- 20. Health Research Authority 2017. Do I need NHS REC review? Decision tool, http://www.hra-decisiontools.org.uk/research/docs/DefiningResearchTable_Oct2017-1.pdf (2017, accessed June 2020).
- 21. IBM Corp. IBM SPSS Statistics for Windows, version 21.0. Armonk, NY: IBM Corp, 2012. [Google Scholar]
- 22. Scottish Intercollegiate Guidelines Network. SIGN 131: management of schizophrenia: a national clinical guideline. Healthcare Improvement Scotland, www.sign.ac.uk (2013, accessed June 2020).
- 23. Galletly C, Castle D, Dark F, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the management of schizophrenia and related disorders. Aust N Z J Psychiatry 2016; 50: 410–472. [DOI] [PubMed] [Google Scholar]
- 24. Kar N, Barreto S, Chandavarkar R. Clozapine monitoring in clinical practice: beyond the mandatory requirement. Clin Psychopharmacol Neurosci 2016; 14: 323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yusufi BZ, Mukherjee S, Flanagan RJ, et al. Prevalence and nature of side effects during clozapine maintenance treatment and the relationship with clozapine dose and plasma concentration. Int Clin Psychopharmacol 2007; 22: 238–243. [DOI] [PubMed] [Google Scholar]
- 26. Hynes C, Keating D, McWilliams S, et al. Glasgow antipsychotic side-effects scale for clozapine – development and validation of a clozapine-specific side-effects scale. Schizophr Res 2015; 168: 505–513. [DOI] [PubMed] [Google Scholar]
- 27. Barnes TRE, Haddad PM. Working with people with mental health difficulties to improve adherence to medication. In: Hadler A, Sutton S, Osterberg L. (eds) The Wiley handbook of healthcare treatment engagement: theory, research, and clinical practice. Hoboken, NJ: John Wiley & Sons Ltd, 2020. [Google Scholar]
- 28. Bellissima BL, Tingle MD, Cicović A, et al. A systematic review of clozapine-induced myocarditis. Int J Cardiol 2018; 259: 122–129. [DOI] [PubMed] [Google Scholar]
- 29. Anıl Yağcıoğlu AE, Ertuğrul A, Karakaşlı AA, et al. A comparative study of detection of myocarditis induced by clozapine: with and without cardiac monitoring. Psychiatry Res 2019; 279: 90–97. [DOI] [PubMed] [Google Scholar]
- 30. Ronaldson KJ. Cardiovascular disease in clozapine-treated patients: evidence, mechanisms and management. CNS Drugs 2017; 31: 777–795. [DOI] [PubMed] [Google Scholar]
- 31. Hägg S, Spigset O, Bate A, et al. Myocarditis related to clozapine treatment. J Clin Psychopharmacol 2001; 21: 382–388. [DOI] [PubMed] [Google Scholar]
- 32. Haas SJ, Hill R, Krum H, et al. Clozapine-associated myocarditis: a review of 116 cases of suspected myocarditis associated with the use of clozapine in Australia during 1993–2003. Drug Saf 2007; 30: 47–57. [DOI] [PubMed] [Google Scholar]
- 33. Ronaldson KJ, Fitzgerald PB, McNeil JJ. Clozapine-induced myocarditis, a widely overlooked adverse reaction. Acta Psychiatr Scand 2015; 132: 231–240. [DOI] [PubMed] [Google Scholar]
- 34. Curto M, Girardi N, Lionetto L, et al. Systematic review of clozapine cardiotoxicity. Curr Psychiatry Rep 2016; 18: 68. [DOI] [PubMed] [Google Scholar]
- 35. Rohde C, Polcwiartek C, Kragholm K, et al. Adverse cardiac events in out-patients initiating clozapine treatment: a nationwide register-based study. Acta Psychiatr Scand 2018; 137: 47–53. [DOI] [PubMed] [Google Scholar]
- 36. Freudenreich O. Clozapine-induced myocarditis: prescribe safely but do prescribe. Acta Psychiatr Scand 2015; 132: 240–241. [DOI] [PubMed] [Google Scholar]
- 37. Hasan A, Falkai P, Wobrock T, et al. World federation of societies of biological psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry 2012; 13: 318–378. [DOI] [PubMed] [Google Scholar]
- 38. Srisurapanont M, Suttajit S, Maneeton N, et al. Efficacy and safety of aripiprazole augmentation of clozapine in schizophrenia: a systematic review and meta-analysis of randomized-controlled trials. J Psychiatr Res 2015; 62: 38–47. [DOI] [PubMed] [Google Scholar]
- 39. Barnes TRE, Leeson V, Paton C, et al. Amisulpride augmentation of clozapine for treatment-refractory schizophrenia: a double-blind, placebo-controlled trial. Ther Adv Psychopharmacol 2018; 8: 185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wagner E, Löhrs L, Siskind D, et al. Clozapine augmentation strategies – a systematic meta-review of available evidence. Treatment options for clozapine resistance. J Psychopharmacol 2019; 33: 423–435. [DOI] [PubMed] [Google Scholar]
- 41. Cooper SJ, Reynolds GP, Barnes TRE, et al. BAP guidelines on the management of weight gain, metabolic disturbances and cardiovascular risk associated with psychosis and antipsychotic drug treatment. J Psychopharmacol 2016; 30: 717–748. [DOI] [PubMed] [Google Scholar]
- 42. Gallego JA, Nielsen J, De Hert M, et al. Safety and tolerability of antipsychotic polypharmacy. Expert Opin Drug Saf 2012; 11: 527–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Correll CU, Detraux J, De Lepeleire J, et al. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry 2015; 14: 119–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Iversen TSJ, Steen NE, Dieset I, et al. Side effect burden of antipsychotic drugs in real life – impact of gender and polypharmacy. Prog Neuropsychopharmacol Biol Psychiatry 2018; 82: 263–271. [DOI] [PubMed] [Google Scholar]
- 45. Piggott KL, Mehta N, Wong CL, et al. Using a clinical process map to identify prescribing cascades in your patient. BMJ 2020; 368: m261. [DOI] [PubMed] [Google Scholar]
- 46. Baandrup L. Polypharmacy in schizophrenia. Basic Clin Pharmacol Toxicol 2020; 126: 103–111. [DOI] [PubMed] [Google Scholar]
- 47. Barnes TRE, Paton C. The role of the prescribing observatory for mental health. Br J Psychiatry 2012; 201: 428–429. [DOI] [PubMed] [Google Scholar]
- 48. Bolton PJ. Improving physical health monitoring in secondary care for patients on clozapine. The Psychiatrist 2011; 35: 49–55. [Google Scholar]
- 49. Tully J, Sim C, Hemani R, et al. Audit of monitoring of the parameters of metabolic syndrome in patients on clozapine. The Psychiatrist 2012; 36: 466–469. [Google Scholar]
- 50. Ally BAS, Stallman HM. Evaluation of a clozapine decision support tool in a mental health facility. J Pharm Pract Res 2016; 46: 137–138. [Google Scholar]
- 51. Tonkins M, Hardy P, Foster S, et al. Using junior doctors to improve patient care: creating a clinic to monitor the physical health of patients prescribed clozapine. Eur Psychiatry 2017; 41(Suppl.): S383. [Google Scholar]