Abstract
Background:
The prognostic value of the modified Glasgow prognostic score (mGPS) in patients with pancreatic cancer is controversial, based on previous studies. Therefore, this meta-analysis aimed to explore the relationship between mGPS and prognosis in pancreatic cancer.
Methods:
The databases PubMed, Web of Science, Embase, and the Cochrane Library were searched to identify eligible studies. Hazard ratios (HRs) and 95% confidence intervals (CIs) were used to estimate the associations between mGPS score and survival outcomes.
Results:
A total of 26 studies with 5198 patients were included in this meta-analysis. In a pooled analysis, elevated mGPS predicted poorer overall survival (OS; HR = 1.98, 95% CI, 1.65-2.37, P < .001). In addition, elevated mGPS was also significantly associated with worse progression-free survival (PFS), disease-free survival (DFS), and cancer-specific survival (CSS; HR = 1.95, 95% CI, 1.36-2.80, P < .001). Subgroup analyses confirmed a significant association between mGPS and survival outcomes.
Conclusions:
Our meta-analysis demonstrated that high mGPS was correlated to worse OS, PFS, DFS, and CSS in patients with pancreatic cancer. Therefore, mGPS could be employed as an effective prognostic factor for pancreatic cancer in clinical practice.
Keywords: meta-analysis, pancreatic cancer, mGPS, prognosis, survival analysis
Introduction
Pancreatic cancer is a lethal disease and is characterized as one of the most malignant tumors.1 As estimated by the GLOBOCAN 2018 study,2 458 918 cases would be diagnosed with pancreatic cancer, and 432 242 cases will die of the disease worldwide in 2018. The global mortality rate coincides with the incidence rate of pancreatic cancer, which emphasizes poor prognosis.3 Pancreatic cancer is usually diagnosed at an advanced stage and the median overall survival (OS) is <12 months, and the 5-year OS rate is approximately 5%.4 Therapeutic strategies for pancreatic cancer include surgery, chemotherapy, radiotherapy, targeted therapy, and immunotherapy. These treatment methods have improved outcomes during the past years.5 However, patients with pancreatic cancer could also develop chemoresistance because of a lack of efficient predictive and prognostic markers.3,6,7 Therefore, to improve the survival of patients with pancreatic cancer, it is important to identify novel and available prognostic biomarkers.
Recently, growing evidence has shown that tumor-elicited inflammation, immune cells, and inflammatory cytokines play critical roles in pancreatic cancer development, progression, and metastasis.8,9 An increasing number of studies have focused on the prognostic role of inflammatory parameters in various cancers.10 These indicators include the neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, systematic inflammatory index, and the modified Glasgow prognostic score (mGPS). The mGPS is based on serum albumin (Alb) and C-reactive protein (CRP) and is scored as mGPS 0, 1, and 2.11 Previous studies have validated the prognostic value of mGPS in various solid tumors, including small cell lung cancer,12 biliary tract cancer,13 renal cell carcinoma (RCC),11 and soft-tissue sarcoma.14 A variety of studies have also investigated the prognostic significance of mGPS in patients with pancreatic cancer; however, the results were inconsistent.15-40 Therefore, we carried out a meta-analysis to assess the association between mGPS and survival outcomes in patients with pancreatic cancer.
Materials and Methods
Literature Search Strategy
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline.41 A literature search was performed using PubMed, Web of Science, Embase, and the Cochrane Library. The search terms were as follows: “modified Glasgow prognostic score,” “mGPS,” “pancreatic neoplasm,” “pancreatic cancer,” and “pancreatic carcinoma.” Only literature published in English was considered. The search was updated on May 10, 2020. The reference lists of selected studies were checked to identify potential inclusions. All related data were extracted from previously published studies; therefore, no ethical approval or informed consent was necessary for this meta-analysis.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) diagnosis of pancreatic cancer pathologically or histologically confirmed; (2) CRP and Alb measured from peripheral blood samples before treatment; (3) mGPS scored as the following method: patients with Alb ≥ 3.5 g/dL and CRP ≤ 1 mg/dL are defined as mGPS 0, patients with Alb ≥ 3.5 g/dL and CRP > 1 mg/dL or Alb < 3.5 g/dL and CRP ≤ 1 mg/dL are defined as mGPS 1, and patients with Alb < 3.5 g/dL and CRP > 1 mg/dL are defined as mGPS 2; (4) the hazard ratios (HRs) and corresponding 95% confidence intervals (95% CIs) of survival outcomes including OS, progression-free survival (PFS), disease-free survival (DFS), and cancer-specific survival (CSS) supplied in studies or sufficient data were provided; (5) full-text articles published in English. The exclusion criteria were as follows: (1) case reports, reviews, letters, or comments; (2) studies with insufficient information for meta-analysis; (3) overlapping studies; and (4) animal studies.
Data Extraction and Quality Assessment
Two independent investigators (W.F. and R.W.) reviewed eligible studies and extracted data. Any disagreements were resolved by discussion with a third reviewer (T.W.) until consensus was reached. The following information was extracted: name of the first author; year of publication; country; sample size; study period; ethnicity; sex; treatment; follow-up; number of patients with mGPS 0, 1, and 2; and survival outcomes. The HRs and 95%CIs were calculated based on mGPS 1 to 2 versus mGPS 0. For studies that reported HRs for mGPS 1 and 2 separately, we combined these 2 groups into a single group and calculated a combined HR to analyze the prognostic role of the overall elevated GPS as previously reported.42,43 Overall survival was the primary outcome of interest. Progression-free survival, DFS, and CSS were secondary outcomes. The methodological qualities of the included studies were assessed using the Newcastle-Ottawa Quality Assessment Scale (NOS)44 by 2 independent investigators (K.W. and S.Y.). The total NOS scores are in the range of 0 to 9. Studies with NOS scores ≥6 were regarded as of high quality.
Statistical Analysis
The HRs and 95% CIs were used to estimate the associations between mGPS score and survival outcomes. Heterogeneity among studies was evaluated using Cochran Q test and Higgins I 2 statistic. When the Q test (P < .10) or the I 2 test (>50%) indicated significant heterogeneity, a random-effect model would be used to combine the data. Otherwise, a fixed-effect model was applied. Subgroup analysis was performed to detect the source and for further investigation. Sensitivity analysis was carried out to evaluate the robustness of the data by omitting one study at a time. Publication bias was examined using Begg rank correlation test. All analyses were performed using Stata version 12.0 software (STATA Corp). Value of P < .05 was considered statistically significant.
Results
Search Results
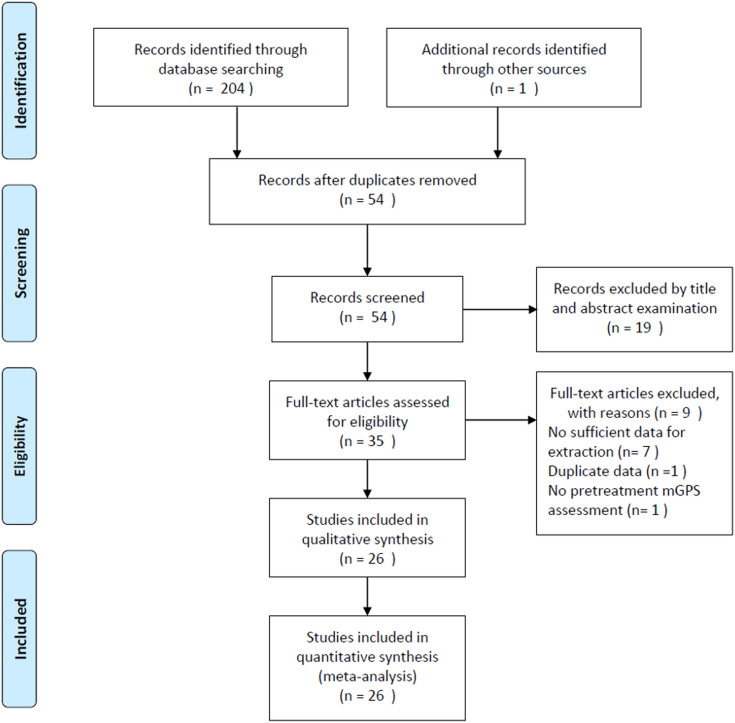
The initial literature search retrieved 205 studies in total (Figure 1). Of these studies, 151 duplicate records were excluded, and 54 studies were screened by titles and abstracts. Then, 19 articles were removed after title and abstract examination, and 35 studies remained for full-text evaluation. After carefully reviewing the full text, 9 articles were excluded for the following reasons: 7 studies without sufficient data for this meta-analysis, 1 study enrolled overlapping patients, and 1 study did not measure pretreatment mGPS. Finally, 26 studies were included in this meta-analysis.15-40 The literature selection process is shown in Figure 1. In the included studies, one study18 recruited 2 independent cohorts of patients receiving chemotherapy or surgical resection, and the HRs and 95% CIs were separately given for these 2 cohorts. These 2 cohorts were independently analyzed and labeled as Stotz(A) and Stotz(B). Therefore, 27 cohorts from 26 studies were included in this meta-analysis.
Figure 1.
Flowchart of the study selection procedure in the meta-analysis.
Characteristics of the Included Studies
The included studies were published from 2012 to 2019, with a total of 5198 cases. The sample sizes ranged from 25 to 807, with a median value of 172. The included studies were conducted in 8 countries, including Japan (n = 16),20-22,24,25,27,29-32,35-40 China (n = 4),17,23,26,28 Austria (n = 2 cohorts),18 Australia (n = 1),19 the United Kingdom (n = 1),15 Greece (n = 1),33 Italy (n = 1),16 and Korea (n = 1).34 A total of 24 studies with 4651 patients15-17,19-34,36-40 provided data on the correlation between mGPS and OS. Seven cohorts presented the data on the prognostic value of mGPS for PFS, DFS, and CSS, including 3 for PFS,29,30,38 2 for DFS,31,35 and 2 for CSS.18 The major characteristics of the enrolled studies are summarized in Table 1. The NOS scores ranged from 6 to 8, and the median value was 7, which suggested that all eligible studies were of high quality.
Table 1.
Characteristics of Studies Included in the Present Meta-Analysis.
| Author | Year | Country | Sample size | Study duration | Ethnicity | Sex (M/F) | TNM stage | Treatment | Follow-up median (months) | mGPS (0/1/2) | Outcomes | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jamieson15 | 2012 | The United Kingdom | 173 | 1997-2009 | Caucasian | 93/80 | I-III | Surgical resection | 27 | 95/37/41 | OS | 8 |
| La Torre16 | 2012 | Italy | 101 | 2003-2009 | Caucasian | 53/48 | I-IV | Surgical resection | 19 | 37/20/25 | OS | 7 |
| Wang17 | 2012 | China | 177 | 2006-2010 | Asian | 120/57 | I-IV | Mixed | 31.33 | 115/46/16 | OS | 7 |
| Stotz(A)18 | 2013 | Austria | 261 | 2004-2010 | Caucasian | 103/158 | III-IV | Chemotherapy | 5 | 117/115/29 | CSS | 7 |
| Stotz(B)18 | 2013 | Austria | 110 | 2004-2010 | Caucasian | 51/59 | I-III | Surgical resection | 18 | 73/21/16 | CSS | 8 |
| Martin19 | 2014 | Australia | 124 | 2008-2012 | Caucasian | 66/58 | III-IV | Chemotherapy | 12 | 46/26/52 | OS | 7 |
| Inoue20 | 2015 | Japan | 440 | 2008-2012 | Asian | 249/191 | I-IV | Mixed | 18.7 | 367/49/24 | OS | 7 |
| Kasuga21 | 2015 | Japan | 61 | 2009-2013 | Asian | 40/21 | IV | Chemotherapy | 6.3 | NA | OS | 6 |
| Imaoka22 | 2016 | Japan | 807 | 2001-2013 | Asian | 473/334 | I-IV | Mixed | 15.8 | 620/153/34 | OS | 7 |
| Wu23 | 2016 | China | 233 | 2011-2014 | Asian | 156/77 | III-IV | Chemotherapy | 4.4 | NA | OS | 6 |
| Yamada24 | 2016 | Japan | 379 | 2002-2014 | Asian | 228/151 | I-IV | Surgical resection | 15.1 | 189/80/36 | OS | 7 |
| Iino25 | 2017 | Japan | 47 | 2010-2015 | Asian | 24/23 | III-IV | Chemotherapy | NA | 35/5/7 | OS | 6 |
| Liu26 | 2017 | China | 386 | 2010-2015 | Asian | 238/148 | I-IV | Mixed | 8.7 | 121/242/13 | OS | 8 |
| Matsumoto27 | 2017 | Japan | 25 | 2007-2013 | Asian | 14/11 | I-IV | Surgical resection | 36.3 | 20/2/3 | OS | 8 |
| Xiao28 | 2017 | China | 66 | 2012-2013 | Asian | 30/36 | III-IV | Chemotherapy | NA | 39/11/16 | OS | 6 |
| Abe29 | 2018 | Japan | 329 | 1996-2014 | Asian | 198/131 | I-III | Surgical resection | NA | 282/27/20 | OS, PFS | 6 |
| Asama30 | 2018 | Japan | 72 | 2006-2016 | Asian | 40/32 | III-IV | Chemotherapy | 8 | 52/11/9 | OS, PFS | 8 |
| Fujiwara31 | 2018 | Japan | 188 | 2000-2015 | Asian | 115/73 | I-IV | Surgical resection | 28.9 | 140/21/27 | OS, DFS | 7 |
| Ikuta32 | 2018 | Japan | 38 | 2011-2018 | Asian | 20/18 | III-IV | Chemotherapy | 39.8 | 30/7/1 | OS | 7 |
| Christos33 | 2019 | Greece | 226 | 2004-2015 | Caucasian | 124/102 | I-IV | Surgical resection | 23.1 | NA | OS | 6 |
| Hwang34 | 2019 | Korea | 203 | Jan-Dec 2016 | Asian | 116/87 | IV | Chemotherapy | 21.5 | 137/19/47 | OS | 8 |
| Ichikawa35 | 2019 | Japan | 176 | 2005-2015 | Asian | 116/60 | III-IV | Chemotherapy | To Dec 2017 | 157/13/6 | DFS | 8 |
| Ikuta36 | 2019 | Japan | 136 | 2005-2017 | Asian | 76/60 | I-IV | Surgical resection | 16.8 | NA | OS | 6 |
| Kubo37 | 2019 | Japan | 119 | 2009-2017 | Asian | 66/53 | I-III | Chemoradiotherapy | NA | NA | OS | 6 |
| Matsumoto38 | 2019 | Japan | 66 | 2013-2014 | Asian | 44/22 | IV | Chemotherapy | 12.7 (1.8-21.1) | NA | OS, PFS | 6 |
| Nakagawa39 | 2019 | Japan | 172 | 2006-2015 | Asian | 102/70 | I-III | Surgical resection | To December 2017 | NA | OS | 7 |
| Shimizu40 | 2019 | Japan | 83 | 2008-2014 | Asian | 57/26 | IV | Chemotherapy | NA | 44/28/11 | OS | 6 |
Abbreviations: CSS, cancer-specific survival; DFS, disease-free survival; F, female; M, male; mGPS, modified Glasgow Prognostic Score; NA, not available; NOS, Newcastle Ottawa Scale; OS, overall survival; PFS, progression-free survival.
Association Between mGPS and OS
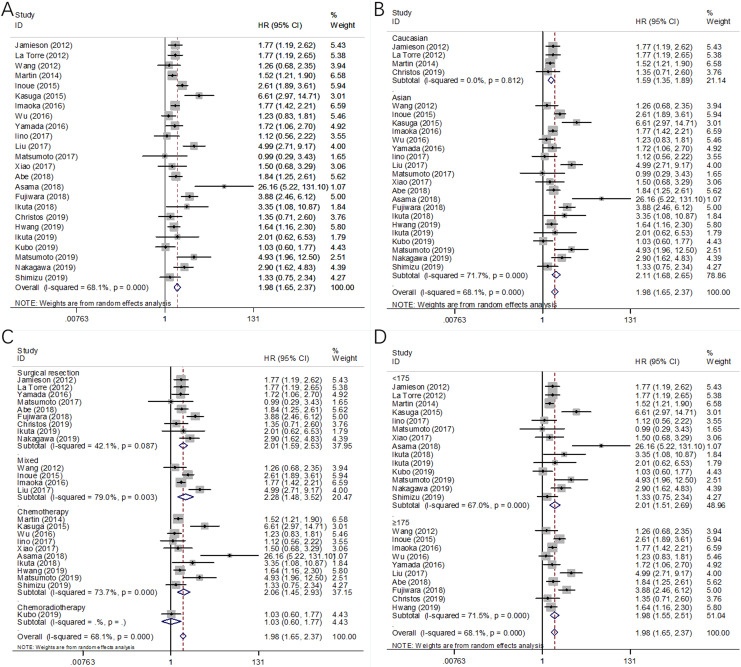
A total of 24 cohort studies consisting of 4651 patients15-17,19-34,36-40 reported the HRs and 95% CIs for the relationship between mGPS and OS in pancreatic cancer. A random-effects model was applied due to significant heterogeneity (I 2 = 68.1%, P < .001, Table 2; Figure 2A). The pooled data demonstrated that pretreatment mGPS was significantly associated with OS, and patients with high mGPS had shorter survival durations (HR = 1.98, 95% CI, 1.65-2.37, P < .001, Table 2; Figure 2A). Subgroup analysis was performed according to ethnicity, treatment, and sample size. The combined data indicated that mGPS remained a significant prognostic factor for OS irrespective of ethnicity (Table 2; Figure 2B) or sample size (Table 2; Figure 2D). In addition, a high mGPS was also associated with poor OS in patients receiving surgical resection, chemotherapy, and mixed treatment (Table 2; Figure 2C).
Table 2.
Subgroup Analysis of the Studies Reporting the Association of mGPS and Prognosis in Pancreatic Cancer.
| Stratified analysis | Number of studies (cohorts) | Number of patients | Effects model | HR (95% CI) | P | Heterogeneity I 2 (%) | P |
|---|---|---|---|---|---|---|---|
| OS | |||||||
| Total | 24 | 4651 | Random | 1.98 (1.65-2.37) | <.001 | 68.1 | <.001 |
| Ethnicity | |||||||
| Asian | 20 | 4027 | Random | 2.11 (1.68-2.65) | <.001 | 71.7 | <.001 |
| Caucasian | 4 | 624 | Fixed | 1.59 (1.35-1.89) | <.001 | 0 | .812 |
| Treatment | |||||||
| Surgical resection | 9 | 1729 | Fixed | 2.01 (1.71-2.38) | <.001 | 42.1 | .087 |
| Chemotherapy | 10 | 993 | Random | 2.06 (1.45-2.93) | <.001 | 73.7 | <.001 |
| Mixed | 4 | 1810 | Random | 2.28 (1.48-3.52) | <.001 | 79.0 | .003 |
| Chemoradiotherapy | 1 | 119 | – | 1.03 (0.60-1.77) | .915 | – | – |
| Sample size | |||||||
| <175 | 14 | 1283 | Random | 2.01 (1.51-2.69) | <.001 | 67.0 | <.001 |
| ≥175 | 10 | 3368 | Random | 1.98 (1.55-2.51) | <.001 | 71.5 | <.001 |
| PFS/DFS/CSS | |||||||
| Total | 7 | 1202 | Random | 1.95 (1.36-2.80) | <.001 | 74.8 | .001 |
| Ethnicity | |||||||
| Asian | 5 | 831 | Fixed | 2.33 (1.84-2.94) | <.001 | 38.1 | .167 |
| Caucasian | 2 | 371 | Fixed | 1.14 (0.90-1.45) | .284 | 0 | .711 |
| Treatment | |||||||
| Surgical resection | 3 | 627 | Random | 1.75 (1.01-3.05) | .048 | 84.4 | .002 |
| Chemotherapy | 4 | 575 | Random | 2.26 (1.24-4.10) | .007 | 72.2 | .013 |
| Sample size | |||||||
| <175 | 3 | 248 | Random | 2.32 (0.90-5.99) | .083 | 82.9 | .003 |
| ≥175 | 4 | 954 | Random | 1.89 (1.27-2.83) | .002 | 71.7 | .014 |
Abbreviations: CI, confidence interval; CSS, cancer-specific survival; DFS, disease-free survival; HR, hazard ratio; mGPS, modified Glasgow prognostic score; OS, overall survival; PFS, progression-free survival; TNM, tumor–node–metastasis.
Figure 2.
Forest plots of the significant correlation of modified Glasgow prognostic score with overall survival in pancreatic cancer: (A) in total patients, (B) subgroup analysis stratified by ethnicity, (C) subgroup analysis stratified by treatment method, and (D) subgroup analysis stratified by sample size.
Correlation Between mGPS and PFS/DFS/CSS
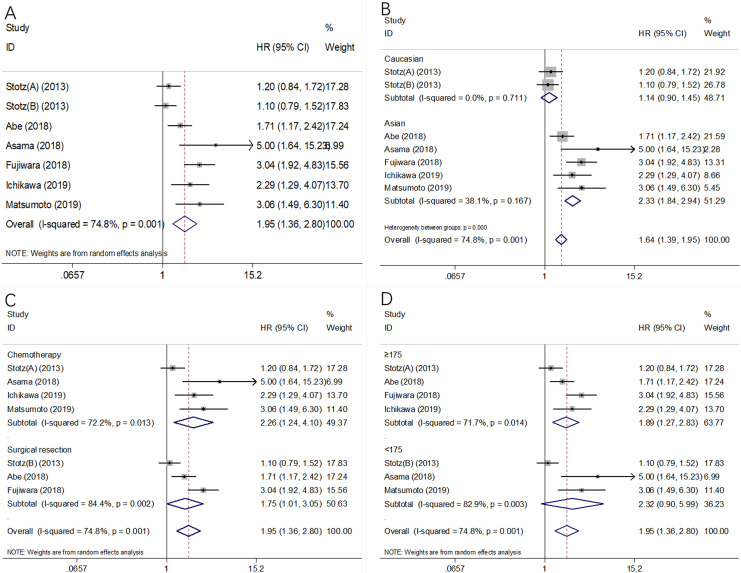
Seven cohorts with 1202 patients18,29-31,35,38 provided data on the prognostic effect of mGPS in PFS/DFS/CSS. There was significant heterogeneity (I 2 = 74.8%, P = .001, Table 2; Figure 3A); therefore, a random-effects model was selected. The pooled HR and 95% CI were HR = 1.95, 95% CI = 1.36-2.80, P < .001 (Table 2; Figure 3A), indicating that a higher mGPS was significantly correlated with poor PFS/DFS/CSS. Subgroup analysis was also carried out, stratified by ethnicity, treatment, and sample size. The results of the subgroup analysis showed that a high mGPS was correlated with inferior PFS/DFS/CSS in Asian patients (HR = 2.33, 95% CI, 1.84-2.94, P < .001, Table 2; Figure 3B), in patients receiving surgical resection (HR = 1.75, 95% CI, 1.01-3.05, P = .048) and receiving chemotherapy (HR = 2.26, 95% CI, 1.24-4.10, P = .007; Table 2; Figure 3C) and in studies with a sample size ≥175 (HR = 1.89, 95% CI, 1.27-2.83, P = .002, Table 2; Figure 3D).
Figure 3.
Forest plots of the significant correlation of mGPS with PFS/DFS/CSS in pancreatic cancer: (A) in total patients, (B) subgroup analysis stratified by ethnicity, (C) subgroup analysis stratified by treatment method, and (D) subgroup analysis stratified by sample size. CSS indicates cancer-specific survival; DFS, disease-free survival; mGPS, modified Glasgow prognostic score; PFS, progression-free survival.
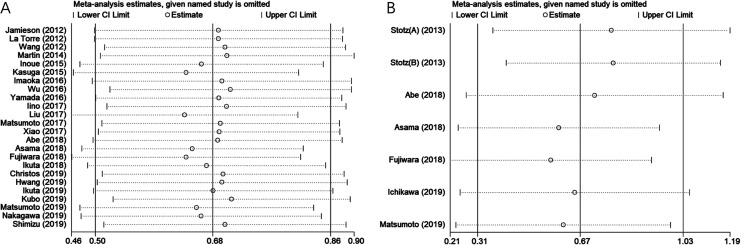
Sensitivity Analysis
To assess the stability of the pooled results, sensitivity analysis was performed by sequentially omitting individual study. The pooled HRs for OS (Figure 4A) and PFS/DFS/CSS (Figure 4B) were not substantially changed, indicating the reliability of our results.
Figure 4.
Sensitivity analysis of included studies for association between mGPS and (A) OS and (B) PFS/DFS/CSS in pancreatic cancer. CSS indicates cancer-specific survival; DFS, disease-free survival; mGPS, modified Glasgow prognostic score; OS, overall survival; PFS, progression-free survival.
Publication Bias
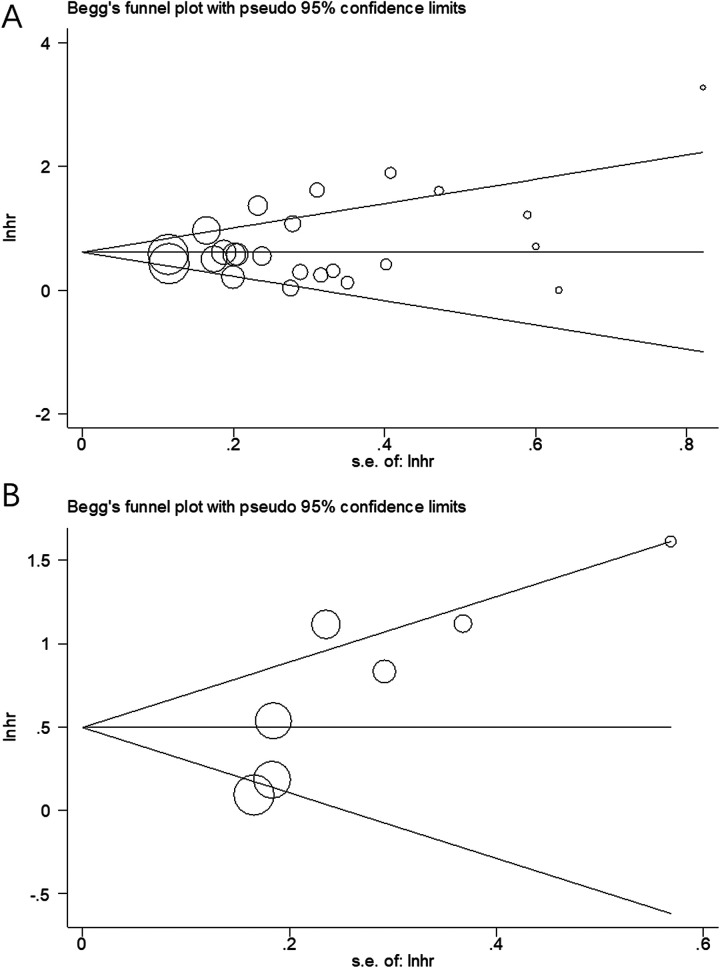
Begg funnel plot was used to evaluate the potential publication bias for OS and PFS/DFS/CSS. As shown in Figure 5, there was no significant publication bias for OS (Begg test, P = .385) or for PFS/DFS/CSS (Begg test, P = .085).
Figure 5.
Publication bias tested by Begg funnel plot. (A) Begg test for OS, P = .385; (B) Begg test for PFS/DFS/CSS, P = .085. CSS indicates cancer-specific survival; DFS, disease-free survival; OS, overall survival; PFS, progression-free survival.
Discussion
The prognostic role of mGPS for patients with pancreatic cancer has been explored in many previous studies,15-40 with the conflicting results presented. Therefore, we aggregated data from 26 eligible studies comprising 5198 patients. Our meta-analysis showed that a high mGPS (score 1-2) was associated with worse OS and PFS/DFS/CSS in pancreatic cancer. To further investigate the association between mGPS and survival in various patient groups, subgroup analysis was conducted, and the data showed that mGPS remained a significant prognostic factor for OS, irrespective of ethnicity or sample size. In addition, a high mGPS was correlated with inferior PFS/DFS/CSS in Asian patients, in patients undergoing surgical resection and receiving chemotherapy. Taken together, our meta-analysis indicated that an elevated mGPS was correlated with poor survival outcomes in patients with pancreatic cancer. Moreover, the reliability of the results was confirmed by quality assessments, sensitivity analysis, and publication bias tests. To the best of our knowledge, the current study was the first meta-analysis to explore the prognostic role of mGPS in pancreatic cancer.
The survival of patients with cancer is associated with nutritional status, and approximately one-third of cancer-related deaths are attributed to malnutrition rather than cancer.45,46 In recent years, much attention has been paid to the prognostic effect of combinational nutritional parameters, such as mGPS, which is derived from Alb and CRP in peripheral blood. Briefly, patients with lower Alb and high CRP levels scored a high mGPS. The mechanisms of association between mGPS and poor survival could be explained by the following aspects. First, Alb is a major protein in the blood. Serum Alb is an objective indicator of nutritional status, and hypoalbuminemia is reflective of malnutrition in patients with cancer. Albumin is also regarded as an acute-phase protein and is downregulated in inflammation.47 Current evidence has shown that serum Alb is associated with anticancer activity, for example, its antioxidant effect.48 When hepatocytes generate Alb normally in patients with cancer, they are more resistant to disease and tumor growth.49 Therefore, reduced serum Alb level is a predictor of poor prognosis in various cancers, including urothelial carcinoma,50 colorectal cancer,51 and non-small cell lung cancer.52 Second, CRP is a common acute-phase serum protein that is synthesized by liver cells.53 Cancer cells secrete inflammatory cytokines, which strongly stimulate CRP production in liver.54 Previous studies indicated that high levels of serum CRP were significantly associated with poor OS in patients with cancer.55-57 The mGPS enables better appreciation of systemic inflammation or malnutrition through changes in Alb and CRP levels. The high mGPS is indicative of low Alb and high CRP, which usually suggests malnutrition and severe systemic inflammation in patients with pancreatic cancer. Thus, mGPS, as a combinational index of Alb and CRP, is efficient in prognostication of survival in patients with cancer. Patients with high mGPS usually have poor survival outcomes.
A variety of meta-analyses have also investigated the prognostic value of mGPS in solid tumors.58-61 A meta-analysis incorporating 12 studies with 2391 patients showed that higher mGPS significantly correlated with worse OS, CSS, recurrence-free survival, and PFS in patients with RCC.60 Subgroup analyses also confirmed the overall results. Moreover, another meta-analysis demonstrated that elevated mGPS predicted poorer OS in patients with lung cancer.58 A recent meta-analysis by Zhang et al62 showed that OS was worse in patients with an mGPS of 1 and 2 compared with those with a score of 0 in patients with gastric cancer. In the current meta-analysis, our findings on pancreatic cancer were in accordance with the results of other types of cancer. In addition, the subgroup analysis also confirmed the consistent prognostic efficiency of mGPS in different subpopulations. In the present meta-analysis, only English studies were included to guarantee the availability of all eligible publications to investigators. English publications are available to most investigators around the world, and the data of included studies can also be extracted and examined. Notably, publications in non-English language were not selected, which may contribute to heterogeneity in this meta-analysis.
This meta-analysis has several limitations. First, all of the included studies were retrospective, which may have introduced selection bias in this meta-analysis. Second, the heterogeneity among the included studies was significant. Although we adopted a random-effects model to calculate the pooled data. The inherent heterogeneity among retrospective studies may still exist. Third, the relationship between mGPS and clinicopathological features in pancreatic cancer could not be analyzed due to insufficient information in recruited studies. Fourth, most eligible studies are from Asian countries, especially Japan, which decreases the study’s significance to the global community. The results may be applicable to Asian patients with pancreatic cancer. Therefore, further large-scale prospective studies are necessary to validate our results.
Conclusions
In summary, our meta-analysis demonstrated that high mGPS correlated with worse OS, PFS, DFS, and CSS in patients with pancreatic cancer. Therefore, mGPS could be employed as an effective prognostic factor for pancreatic cancer in clinical practice.
Footnotes
Authors’ Note: Wen Fu, Kun Wang, and Shan Yan made equal contribution and are co-first authors. T.W. collected and analyzed the data and wrote the article; W.F., K.W., S.Y., X.W., B.T., and J.C. collected and analyzed the data; R.W. and T.W. revised the whole article. All authors reviewed the final article. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (no. 81660407) and the Basic Research on the Application of Yunnan Province (nos. 2017FE468-056 and 2018FE001-234).
ORCID iD: Tao Wu  https://orcid.org/0000-0001-9165-4998
https://orcid.org/0000-0001-9165-4998
References
- 1. Maisonneuve P. Epidemiology and burden of pancreatic cancer. Presse Med. 2019;48(3):E113–E123. [DOI] [PubMed] [Google Scholar]
- 2. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 3. Hasan S, Jacob R, Manne U, Paluri R. Advances in pancreatic cancer biomarkers. Oncol Rev. 2019;13(1):410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Qi ZJ, Yu D, Chen CH, et al. The prognostic value of B7H1 and B7H4 expression in pancreatic cancer: a meta-analysis. Int J Biol Markers. 2019;34(4):373–380. [DOI] [PubMed] [Google Scholar]
- 5. Neoptolemos JP, Kleeff J, Michl P, et al. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. 2018;15(6):332–347. [DOI] [PubMed] [Google Scholar]
- 6. Zeng SY, Pottler M, Lan B, et al. Chemoresistance in pancreatic cancer. Int J Mol Sci. 2019;20(18):4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Amrutkar M, Gladhaug IP. Pancreatic cancer chemoresistance to gemcitabine. Cancers. 2017;9(11):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shi JJ, Xue J. Inflammation and development of pancreatic ductal adenocarcinoma. Chin Clin Oncol. 2019;8(2):19. [DOI] [PubMed] [Google Scholar]
- 9. Padoan A, Plebani M, Basso D. Inflammation and pancreatic cancer: focus on metabolism, cytokines, and immunity. Int J Mol Sci. 2019;20(3):676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. He L, Li H, Cai J, et al. Prognostic value of the Glasgow prognostic score or modified Glasgow prognostic score for patients with colorectal cancer receiving various treatments: a systematic review and meta-analysis. Cell Physiol Biochem. 2018;51(3):1237–1249. [DOI] [PubMed] [Google Scholar]
- 11. Cho DS, Kim SI, Choo SH, et al. Prognostic significance of modified Glasgow Prognostic Score in patients with non-metastatic clear cell renal cell carcinoma. Scand J Urol. 2016;50(3):186–191. [DOI] [PubMed] [Google Scholar]
- 12. Sonehara K, Tateishi K, Komatsu M, et al. Modified Glasgow prognostic score as a prognostic factor in patients with extensive disease-small-cell lung cancer: a retrospective study in a single institute. Chemotherapy. 2019;64(3):129–137. [DOI] [PubMed] [Google Scholar]
- 13. Okano N, Kasuga A, Kawai K, et al. The modified Glasgow prognostic score in patients with gemcitabine-refractory biliary tract cancer. Anticancer Res. 2018;38(3):1755–1761. [DOI] [PubMed] [Google Scholar]
- 14. Morhij R, Mahendra A, Jane M, McMillan DC. The modified Glasgow prognostic score in patients undergoing surgery for bone and soft tissue sarcoma. J Plast Reconstr Aesthet Surg. 2017;70(5):618–624. [DOI] [PubMed] [Google Scholar]
- 15. Jamieson NB, Mohamed M, Oien KA, et al. The Relationship between tumor inflammatory cell infiltrate and outcome in patients with pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2012;19(11):3581–3590. [DOI] [PubMed] [Google Scholar]
- 16. La Torre M, Nigri G, Cavallini M, et al. The Glasgow prognostic score as a predictor of survival in patients with potentially resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2012;19(9):2917–2923. [DOI] [PubMed] [Google Scholar]
- 17. Wang DS, Luo HY, Qiu MZ, et al. Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Med Oncol. 2012;29(5):3092–3100. [DOI] [PubMed] [Google Scholar]
- 18. Stotz M, Gerger A, Eisner F, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer. 2013;109(2):416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martin HL, Ohara K, Kiberu A, et al. Prognostic value of systemic inflammation-based markers in advanced pancreatic cancer. Intern Med J. 2014;44(7):676–682. [DOI] [PubMed] [Google Scholar]
- 20. Inoue D, Ozaka M, Matsuyama M, et al. Prognostic value of neutrophil–lymphocyte ratio and level of C-reactive protein in a large cohort of pancreatic cancer patients: a retrospective study in a single institute in Japan. Jpn J Clin Oncol. 2015;45(1):61–66. [DOI] [PubMed] [Google Scholar]
- 21. Kasuga A, Okano N, Naruge D, et al. Retrospective analysis of fixed dose rate infusion of gemcitabine and S-1 combination therapy (FGS) as salvage chemotherapy in patients with gemcitabine-refractory advanced pancreatic cancer: inflammation-based prognostic score predicts survival. Cancer Chemother Pharmacol. 2015;75(3):457–464. [DOI] [PubMed] [Google Scholar]
- 22. Imaoka H, Mizuno N, Hara K, et al. Evaluation of modified Glasgow prognostic score for pancreatic cancer: a retrospective cohort study. Pancreas. 2016;45(2):211–217. [DOI] [PubMed] [Google Scholar]
- 23. Wu M, Guo J, Guo L, Zuo Q. The C-reactive protein/albumin ratio predicts overall survival of patients with advanced pancreatic cancer. Tumour Biol. 2016;37(9):12525–12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yamada S, Fujii T, Yabusaki N, et al. Clinical implication of inflammation-based prognostic score in pancreatic cancer Glasgow prognostic score is the most reliable parameter. Medicine. 2016;95(18):e3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iino C, Shimoyama T, Igarashi T, et al. Biliary drainage improves the predictive value of modified Glasgow Prognostic Scores in inoperable pancreatic cancer. PLoS One. 2017;12(6):e0178777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu ZQ, Jin KZ, Guo M, et al. Prognostic value of the CRP/Alb ratio, a novel inflammation-based score in pancreatic cancer. Ann Surg Oncol. 2017;24(2):561–568. [DOI] [PubMed] [Google Scholar]
- 27. Matsumoto M, Nakabayashi Y, Fujiwara Y, et al. Duration of preoperative biliary drainage as a prognostic factor after pancreaticoduodenectomy for pancreatic head cancer. Anticancer Res. 2017;37(6):3215–3219. [DOI] [PubMed] [Google Scholar]
- 28. Xiao YY, Xie ZH, Shao ZY, et al. Prognostic value of postdiagnostic inflammation-based scores in short-term overall survival of advanced pancreatic ductal adenocarcinoma patients. Medicine. 2017;96(50):e9247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abe T, Nakata K, Kibe S, et al. Prognostic value of preoperative nutritional and immunological factors in patients with pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2018;25(13):3996–4003. [DOI] [PubMed] [Google Scholar]
- 30. Asama H, Suzuki R, Takagi T, et al. Evaluation of inflammation-based markers for predicting the prognosis of unresectable pancreatic ductal adenocarcinoma treated with chemotherapy. Mol Clin Oncol. 2018;9(4):408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fujiwara Y, Haruki K, Shiba H, et al. C-Reactive protein-based prognostic measures are superior at predicting survival compared with peripheral blood cell count-based ones in patients after curative resection for pancreatic cancer. Anticancer Res. 2018;38(11):6491–6499. [DOI] [PubMed] [Google Scholar]
- 32. Ikuta S, Sonoda T, Aihara T, Nakajima T, Yamanaka N. The preoperative modified Glasgow prognostic score for the prediction of survival after pancreatic cancer resection following non-surgical treatment of an initially unresectable disease. Contemp Oncol (Pozn). 2018;22(4):229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Christos S, Georgios T, Chatzis I, Michael A, Euthymmios C. Prognostic factors in patients with pancreatic adenocarcinoma and the impact of pancreatic fistula on oncologic outcomes. J Pancreas. 2019;20(5):142–150. [Google Scholar]
- 34. Hwang I, Kang J, Ip HNN, et al. Prognostic factors in patients with metastatic or recurrent pancreatic cancer treated with first-line nab-paclitaxel plus gemcitabine: implication of inflammation-based scores. Invest New Drugs. 2019;37(3):584–590. [DOI] [PubMed] [Google Scholar]
- 35. Ichikawa K, Mizuno S, Hayasaki A, et al. Prognostic nutritional index after chemoradiotherapy was the strongest prognostic predictor among biological and conditional factors in localized pancreatic ductal adenocarcinoma patients. Cancers. 2019;11(4):514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ikuta S, Aihara T, Yamanaka N. Preoperative C-reactive protein to albumin ratio is a predictor of survival after pancreatic resection for pancreatic ductal adenocarcinoma. Asia Pac J Clin Oncol. 2019;15(5):E109–E114. [DOI] [PubMed] [Google Scholar]
- 37. Kubo H, Murakami T, Matsuyama R, et al. Prognostic impact of the neutrophil-to-lymphocyte ratio in borderline resectable pancreatic ductal adenocarcinoma treated with neoadjuvant chemoradiotherapy followed by surgical resection. World J Surg. 2019;43(12):3153–3160. [DOI] [PubMed] [Google Scholar]
- 38. Matsumoto I, Kamei K, Omae K, et al. FOLFIRINOX for locally advanced pancreatic cancer: results and prognostic factors of subset analysis from a nation-wide multicenter observational study in Japan. Pancreatology. 2019;19(2):296–301. [DOI] [PubMed] [Google Scholar]
- 39. Nakagawa K, Sho M, Akahori T, et al. Significance of the inflammation-based prognostic score in recurrent pancreatic cancer. Pancreatology. 2019;19(5):722–728. [DOI] [PubMed] [Google Scholar]
- 40. Shimizu T, Taniguchi K, Asakuma M, et al. Lymphocyte-to-monocyte ratio and prognostic nutritional index predict poor prognosis in patients on chemotherapy for unresectable pancreatic cancer. Anticancer Res. 2019;39(4):2169–2176. [DOI] [PubMed] [Google Scholar]
- 41. Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Int Med. 2009;151(4):264–W264. [DOI] [PubMed] [Google Scholar]
- 42. Fang E, Wang X, Feng J, Zhao X. The Prognostic role of Glasgow prognostic score and C-reactive protein to albumin ratio for sarcoma: a system review and meta-analysis. Dis Markers. 2020;2020:8736509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dong JY, Zhang YH, Qin LQ. Erectile dysfunction and risk of cardiovascular disease: meta-analysis of prospective cohort studies. J Am Coll Cardiol. 2011;58(13):1378–1385. [DOI] [PubMed] [Google Scholar]
- 44. Stang A. Critical evaluation of the Newcastle-Ottawa Scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. [DOI] [PubMed] [Google Scholar]
- 45. Garcia-Luna PP, Campos JP, Cunill JLP. Causes and impact of hyponutrition and cachexia in the oncologic patient. Nutr Hosp. 2006;21(suppl 3):10–16. [PubMed] [Google Scholar]
- 46. Osorio Y, Vielma N, Mora CJ, et al. Assessment of nutritional status in patients hospitalized with cancer. MedULA. 2016;25(2):83–88. [Google Scholar]
- 47. McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12(3):223–226. [DOI] [PubMed] [Google Scholar]
- 48. Namiesnik J, Vearasilp K, Nemirovski A, et al. In vitro studies on the relationship between the antioxidant activities of some berry extracts and their binding properties to serum albumin. Appl Biochem Biotechnol. 2014;172(6):2849–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li X, Qin SD, Sun X, et al. Prognostic significance of albumin-globulin score in patients with operable non-small-cell lung cancer. Ann Surg Oncol. 2018;25(12):3647–3659. [DOI] [PubMed] [Google Scholar]
- 50. Liu J, Wang F, Li SH, et al. The prognostic significance of preoperative serum albumin in urothelial carcinoma: a systematic review and meta-analysis. Biosci Rep. 2018;38(4):BSR20180214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chiang JM, Chang CJ, Jiang SF, et al. Pre-operative serum albumin level substantially predicts post-operative morbidity and mortality among patients with colorectal cancer who undergo elective colectomy. Eur J Cancer Care (Engl). 2017;26(2). [DOI] [PubMed] [Google Scholar]
- 52. Tanriverdi O, Avci N, Oktay E, et al. Pretreatment serum albumin level is an independent prognostic factor in patients with stage IIIB non-small cell lung cancer: a study of the Turkish Descriptive Oncological Researches Group. Asian Pac J Cancer Prev. 2015;16(14):5971–5976. [DOI] [PubMed] [Google Scholar]
- 53. Morris-Stiff G, Gomez D, Prasad KR. C-reactive protein in liver cancer surgery. Eur J Surg Oncol. 2008;34(7):727–729. [DOI] [PubMed] [Google Scholar]
- 54. Kinoshita T, Ito H, Miki C. Serum interleukin-6 level reflects the tumor proliferative activity in patients with colorectal carcinoma. Cancer. 1999;85(12):2526–2531. [DOI] [PubMed] [Google Scholar]
- 55. Huang Y, Feng J-F, Liu J-S, Chen Q-X. Prognostic role of serum C-reactive protein in esophageal cancer: a systematic review and meta-analysis. Ther Clin Risk Manag. 2015;11:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhou L, Cai X, Liu Q, et al. Prognostic role of C-reactive protein in urological cancers: a meta-analysis. Sci Rep. 2015;5:12733–12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fang Y, Xu C, Wu P, et al. Prognostic role of C-reactive protein in patients with nasopharyngeal carcinoma: a meta-analysis and literature review. Medicine. 2017;96(45):e8463–e8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jin J, Hu K, Zhou Y, Li W. Clinical utility of the modified Glasgow prognostic score in lung cancer: a meta-analysis. PLoS One. 2017;12(9):e0184412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhou GT, Chen JJ, Hong Z, Tong HF, Wang JS. Prognostic role of modified Glasgow prognostic score in patients with cholangiocarcinoma: a meta-analysis and literature review. Int J Clin Exp Med. 2017;10(6):8767–8772. [Google Scholar]
- 60. Hu X, Wang Y, Yang WX, et al. Modified Glasgow prognostic score as a prognostic factor for renal cell carcinomas: a systematic review and meta-analysis. Cancer Manag Res. 2019;11:6163–6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kimura S, D’Andrea D, Soria F, et al. Prognostic value of modified Glasgow Prognostic Score in non-muscle-invasive bladder cancer. Urol Oncol. 2019;37(3):179.e19-179.e28. [DOI] [PubMed] [Google Scholar]
- 62. Zhang X, Chen X, Wu T, et al. Modified Glasgow prognostic score as a prognostic factor in gastric cancer patients: a systematic review and meta-analysis. Int J Clin Exp Med. 2015;8(9):15222–15229. [PMC free article] [PubMed] [Google Scholar]