Coronary artery obstruction complicates transcatheter aortic valve replacement (TAVR), with an incidence lower than 1% but higher in certain subgroups (1). Patients requiring TAVR for failing transcatheter aortic valves (TAVR-in-TAVR) may be at the highest risk because of the reduced neosinus, tall valve leaflets, and in some cases supra-annular valve design. BASILICA is transcatheter laceration of aortic leaflets to prevent coronary artery obstruction in native and bioprosthetic aortic valves (2,3). Previously we had cautioned against BASILICA for TAVR-in-TAVR (4), predicting that outer TAVR leaflets could get pinned against their frame by the inner TAVR device and thereby fail to splay and allow coronary perfusion. Here we test TAVR-in- TAVR BASILICA in vitro.
Leaflets of 4 common transcatheter heart valves (Evolut R, SAPIEN XT, SAPIEN 3, and Lotus) were split longitudinally on the benchtop to mimic BASILICA laceration. Appropriately sized Evolut R and SAPIEN 3 devices were implanted inside these valves, followed by measurement of splay angle, width, height, and area above stent skirt of the resulting triangular splits. Measurements were taken with the leaflets extended in a worst-case configuration.
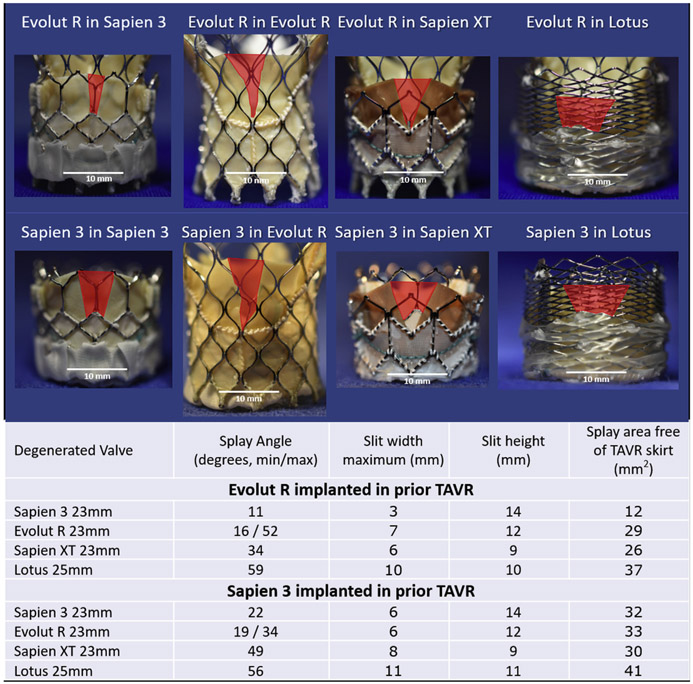
Figure 1 shows that early-generation TAVR devices (SAPIEN XT and Lotus) exhibited wider splay angles (50 ± 11° vs. 17 ± 5°; p < 0.01, Student’s t-test) and slit width (9 ± 2 vs. 5 ± 2; p = 0.05) after BASILICA in vitro than newer generation TAVR devices (SAPIEN 3 and Evolut R). We attribute the broader splay of older TAVR devices to flatter leaflets compared with newer TAVR leaflets. The curved SAPIEN 3 and Evolut R leaflets more completely conform to the outer TAVR frame despite laceration, narrowing the orifice created by BASILICA. Evolut R leaflets exhibit nonlinear rather than triangular splay (Figure 1, leftmost column), further narrowing the orifice.
FIGURE 1. Splay Characteristics After Benchtop BASILICA and TAVR-in-TAVR in 4 Common TAVR Devices.
The orifices created by BASILICA-splayed Leaflets are depicted in red. TAVR = transcatheter aortic valve replacement.
To summarize, effective leaflet splay can be achieved in SAPIEN XT and Lotus valves, but newer generation valves (SAPIEN 3 and Evolut) appear to demonstrate less effective splay, and in the case of Evolut, adequate splay is achieved only high above the annulus.
In addition, as in all cases of BASILICA, the commissure of the new TAVR device might randomly align unfavorably and obstruct the splayed leaflet despite BASILICA laceration. The skirt of the new TAVR device positioned too high might also obstruct the lacerated leaflet. These phenomena are not readily predicted a priori. Superimposed stent frames also reduce effective cell orifice size, exacerbating coronary guiding catheter access challenges. Thus, although it may be feasible to create an adequate leaflet splay with BASILICA in some cases, success or failure will depend on commissural alignment and depth of implantation of the new TAVR device.
For these reasons, we do not advocate BASILICA TAVR-in-TAVR roulette. Although individual attempts may succeed, we believe that BASILICA may not reliably prevent coronary obstruction for TAVR-in-TAVR, especially when the predicted mechanism of obstruction is sinus of Valsalva effacement (4). A better leaflet modification strategy should be developed to prevent coronary obstruction in every at-risk TAVR-in-TAVR case.
Acknowledgments
This work was supported by the Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health (grant Z01-HL006040). Drs. Khan, Rogers, and Lederman are coinventors on patents, assigned to the National Institutes of Health, for catheter devices to lacerate valve leaflets. Dr. Babaliaros is a consultant for Edwards Lifesciences and Abbott Vascular; and his employer has research contracts for clinical investigation of transcatheter aortic and mitral devices from Edwards Lifesciences, Abbott Vascular, Medtronic, St. Jude Medical, and Boston Scientific. Dr. Greenbaum is a proctor for Edwards Lifesciences, Medtronic, and Abbott Vascular; is a consultant for Transmural Systems; and his employer has research contracts for clinical investigation of transcatheter aortic and mitral devices from Edwards Lifesciences, Abbott Vascular, Medtronic, St. Jude Medical, and Boston Scientific. Dr. Robers is a consultant and proctor for Edwards Lifesciences and Medtronic. Dr. Khan is a proctor for Edwards Lifesciences and Medtronic. Dr. Bruce has reported that he has no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Ribeiro HB, Webb JG, Makkar RR, et al. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation: insights from a large multicenter registry. J Am Coll Cardiol 2013;62:1552–62. [DOI] [PubMed] [Google Scholar]
- 2.Khan JM, Dvir D, Greenbaum AB, et al. Transcatheter laceration of aortic leaflets to prevent coronary obstruction during transcatheter aortic valve replacement: concept to first-in-human. J Am Coll Cardiol Intv 2018;11: 677–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan JM, Greenbaum AB, BabaLiaros VC, et al. The BASILICA trial: prospective multicenter investigation of intentional Leaflet Laceration to prevent TAVR coronary obstruction. J Am Coll Cardiol Intv 2019;12:1240–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lederman RJ, BabaLiaros VC, Rogers T, et al. Preventing coronary obstruction during transcatheter aortic valve replacement: from computed tomography to BASILICA. J Am Coll Cardiol Intv 2019;12: 1197–216. [DOI] [PMC free article] [PubMed] [Google Scholar]