Abstract
Capparis spinosa L. has many biological effects such as antioxidant properties. In the present study, we compared the effects of the hydro-alcoholic extract of Capparis spinosa fruit, quercetin (Q), and vitamin E (Vit E) on monosodium glutamate (MSG)-induced toxicity. The following groups were designed: Control groups (normal saline and/or corn oil); MSG group (4.00 g kg-1 MSG); MSG + low dose extract group (4.00 g kg-1 MSG with 100.00 mg kg-1 extract); MSG + high dose extract (HDE) group (4.00 g kg-1 MSG with 300.00 mg kg-1 extract); MSG + Q group (4.00 g kg-1 MSG with 10.00 mg kg-1 Q); MSG + Vit E group (4.00 g kg-1 MSG with 200.00 mg kg-1 Vit E). All chemicals were orally administered for 14 consecutive days. Tissue specimens from the heart, kidney, and liver tissues and blood samples were collected for histopathological and biochemical evaluations. The results showed that the MSG-induced tissue edema, congestion, and inflammatory cell infiltration were resolved by HDE, Q, and Vit E treatments. These chemicals also restored tissue malondialdehyde level and superoxide dismutase activity. Besides, alterations induced by MSG in serum levels of aspartate transaminase, alanine aminotransferase, urea, lactate dehydrogenase, and creatine kinase-MB were also resolved. It is concluded that Capparis spinosa fruit extract, Q and Vit E can produce approximately similar protective effects on tissue function through oxidative stress alleviation and antioxidant mechanisms restoration.
Key Words: Capparis spinosa, Monosodium glutamate, Quercetin, Toxicity, Vitamin E
Introduction
Monosodium glutamate (MSG), a glutamic acid salt consists of 78.00% glutamic acid and 22.00% sodium salt and water, is a common food additive.1,2 It is frequently used by various food industries and Asian cuisine to increase palatability.3 In animals, chronic higher doses of MSG were found to be neurotoxic as it destructs neurons in the cerebellum.4 Moreover, other animal studies have also shown toxic effects of MSG in various organs such as liver and kidney.5 Chronic administration of MSG (4.00 g kg-1 body weight) produced histopathological and biochemical changes in the heart tissue.6 Also, the toxic effects of MSG on thyroid tissue function and histology have been reported previously.7 In this context, MSG, through elevation of reactive oxygen species (ROS) production, is capable to induce genotoxicity in a rat bone marrow micronuclei model.8
Caper (Capparis spinosa L.), a member of the genus Capparis (Capparidaceae family), is widely distributed in different parts of the world.9 In traditional medicine, different parts of C. spinosa have been frequently used for treatment of various human diseases like diabetes, asthma, convulsion, and rheumatism.10-12 It contains glucosinolates, coumarins, saponins, tannins, and flavonoids such as rutin, kaempferol, quercetin and quercetin derivatives.13 Caper flavonoids are known to have anti-allergic, anti-diabetic, anti-inflammatory, antibacterial, and anti-hepatotoxic properties.11 Reportedly, caper extract exhibits a notable activity in protection against oxidative stress and interruption of the ROS-ERK1/2-Ha-Ras signal loop in systemic sclerosis.14 Panico et al. have reported the chondro-protective effect of caper extract in inflammatory joint diseases.15 Also, caper extract exhibited a potential protective effect against learning and memory damages induced by chronic administration of lipopolysaccharide.16
Quercetin (3, 3', 4', 5, 7-pentahydroxyflavone), a well-known antioxidant agent, is one of the most important bioflavonoids being present in more than 20 plants material such as Morus alba, Moringa oleifera, Hypericum perforatum, Lactuca sativa, and C. spinosa.17 The C. spinosa contains more quercetin/weight than any other plant.13 The E-vitamins are lipid-soluble antioxidants found in all cellular membranes. In addition to their anti-oxidative properties, molecules of the vitamin E family exert neuroprotective, anti-inflammatory, and hypo-cholesterolemic activities.18
Although no reports are showing the effects of caper on MSG-induced toxicity, potential antioxidant effects of vitamin C, vitamin E, and quercetin against MSG-induced genotoxicity have been reported in rats.8
The present study was aimed to investigate the effect of caper on MSG-induced toxicity using tissue histopathology and tissue and serum biochemical evaluations. Also, we compared the effects of caper with a well-known antioxidant agent (vitamin E) and also with quercetin (one of the biologically active constituents of caper).8,11,17
Materials and Methods
Plant preparation and extraction. The fruits of caper (C. spinosa) were collected from the mountains around Khoy city, West Azerbaijan province, Iran in July 2016. To prepare extract, 300 g of caper fruit powder was mixed with water/ethanol (30/70) and the mixture was placed on the shaker for 24 hr at room temperature. Then, the supernatant was filtered and the remnant was placed in suxuseleh (50.00 ˚C) to concentrate the extraction. After that, the extracts were collected and placed within the rotator (50.00 ˚C) to volatile the water and alcohol from extracts. To dry fully, finally concentrated extracts were incubated for 48 hr inside the incubator (40.00 ˚C). The resulting powder before doing experiments was placed in 20.00 ˚C conditions.19
Chemicals. Chemicals used in the present study included MSG, quercetin, and vitamin E. The chemicals were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). All the analytical chemicals were purchased from Merck Chemical Co. (Darmstadt, Germany). Also, the hydroalcoholic extract of C. spinosa fruit was prepared and used in this study.
Animals. Male Wistar rats weighing 180-200 g were obtained from the Central Animal House of Urmia University, Urmia, Iran. They were housed in a standard animal facility under controlled temperature (22.00 ± 0.50 ˚C) and 12/12 hr light/dark cycle with food and water provided ad libitum. Veterinary Ethics Committee of the Faculty of Veterinary Medicine of Urmia University (AECVU-159-2018) approved all research and animal care procedures used in the present study.
Treatment groups. Forty rats were used in the present study. To explore the effect of corn oil as a vehicle for quercetin and vitamin E 10 rats were divided into two subgroups, one of which received normal saline (n = 5) and the other one treated with corn oil (n = 5). The other 30 rats were divided into five groups with six rats in each as follows: MSG group received 4.00 g kg-1 body weight MSG, MSG + LDE group received MSG with a low dose extract (100 mg kg-1), MSG + HDE group received MSG with a high dose extract (300 mg kg-1), MSG + Q group received MSG and 10.00 mg kg-1 quercetin and MSG + vitamin E group received MSG plus 200 mg kg-1 vitamin E. The MSG and plant extract were dissolved in normal saline and quercetin and vitamin E were suspended in corn oil. All chemicals were administered by gavages daily for 14 consecutive days. The chemical doses and the duration (14 days) of treatments used here were per previous studies.6,8,20,21
Tissue sampling. On day15, the animals were anesthetized using ketamine (100 mg kg-1; Alfasan, Woerden, Netherlands) and xylazine (10.00 mg kg-1; Alfasan), and blood samples were taken directly from the heart and then the rats were euthanized by intracardiac injection of 0.50 mL xylazine. Sera were obtained by blood centrifuging and stored at – 80.00 ˚C for further analyses. Heart, liver, and kidney tissues were dissected out and half of them were fixed in 10.00% formalin for 72 hr for histopathological evaluation, and the other half was weighed, rinsed in ice-cold saline solution and stored for following biochemical analyses.
Histopathological evaluation. The formalin-fixed samples were processed through paraffin embedding, cut by rotary microtome, and stained with Hematoxylin and Eosin. The evaluation of the tissue sections was based on the severity of the pathological changes including congestion, edema, and fiber discontinuity in the heart sections; congestion, microhemorrhage and fatty degeneration in the liver sections and congestion, cloudy swelling and glomerular shrinkage in the kidney sections. The following scores were given to the severity of histopathological lesions: 0: None, 1: Mild, 2: Moderate, and 3: Severe.22
Biochemical assay. For the preparation of heart, liver, and kidney tissue homogenates, their segments were cut into small pieces and homogenized at 4.00 ˚C in 2.00 mL of ice-cold phosphate-buffered saline with a glass homogenizer. The resulting homogenate was passed through a cellulose filter to remove impurities and divided into aliquots for biochemical analysis. Lipid peroxidation was estimated by measuring malondialdehyde (MDA) levels spectrophotometrically (Novaspec II; Pharmacia LKB, Buckinghamshire, UK) as described by Ohkawa et al.23 For measuring tissue MDA level, 0.30 mL of 10.00% trichloroacetic acid (TCA) and 0.30 mL of 0.67% of an aqueous solution of thiobarbituric acid (TBA) were added to 0.15 mL of supernatant, then mixed and incubated at 100 ˚C for 60 min. After cooling on ice, samples were centrifuged at 4,000 rpm for 10 min and then the absorbance of the organic layer was measured at 535 nm. The MDA in the supernatant can react with freshly prepared TBA to form a colored complex, which has the maximum absorbance at 535 nm. The MDA level (nmol mg-1 protein) of the tissue was calculated from the plotted standard curve prepared from 1,1,3,3-Tetraethoxypropane. Superoxide dismutase (SOD) activity of the heart, liver, and kidney tissues was determined according to the method described previously.24 The principle of the method is based on the inhibition of nitroblue tetrazolium (NBT) reduction by the xanthine oxidase system as a superoxide generator. The activity was assessed in the ethanol phase of the supernatant after one mL ethanol/chloroform mixture (5/3, v/v) was added to the same volume of sample and centrifuged. One unit of SOD was defined as the enzyme amount causing 50.00% inhibition in the NBT reduction rate. The SOD activity was expressed as U mg-1 protein. The protein concentration of the heart, liver, and kidney tissue homogenates was measured by the method of Lowry et al.25 To analyze aspartate transaminase (AST), alanine aminotransferase (ALT), urea, creatinine, lactate dehydrogenase (LDH) and creatine kinase-MB (CK-MB) of serum, blood samples collected from the heart into non-heparin containing tubes, were centrifuged at 3,500 rpm for 10 min. After separation of serum, the samples were transferred to Eppendorf tube. Serum levels of AST, ALT, urea, LDH, and CK-MB were measured spectrophotometrically (LKB Ultrasepec, Vienna, Austria) using their test kits obtained from Man Co., Tehran, Iran.
Statistical analysis. Statistical comparisons were performed using the GraphPad Prism software (version 5.0; GraphPad Software Inc., San Diego, USA). Because of the semi-quantitative nature of data obtained from histopathological changes, Kruskal-Wallis and post hoc Mann-Whitney tests were performed. Values of tissue MDA level, SOD activity, and serum biochemical markers were analyzed using one-way ANOVA followed by Tukeyʼs test. Values of histopathological changes were presented as quartiles minimum value, first quartile, median, third quartiles, and maximum value, and the other data were expressed as the mean ± SEM. Significance at p < 0.05 has been given receptive in all tests.
Results
In the present study, oral administration of corn oil for 14 consecutive days did not produce any significant effects on histology of the heart, liver, and kidney in intact animals compared to the normal saline-treated group. It also did not change the tissue MDA level and SOD activity as well as serum biochemical markers (data are not shown). Thus, we considered both of them as a single control group.
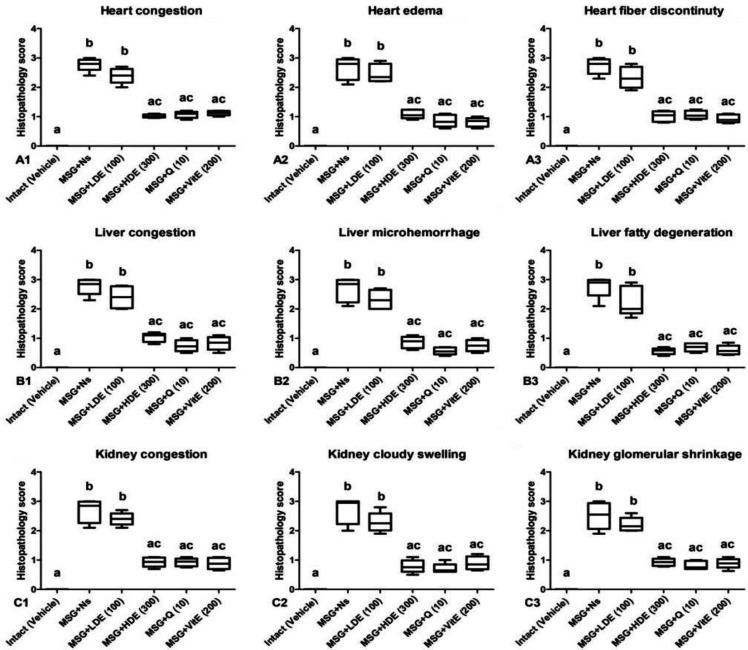
Figures 1 and 2 show the normal histoarchitecture (Figs. 1A1, 1B1, and 1C1) and histological scores (Figs. 2A1, 2B1, and 2C1) of the heart, liver and kidney tissues, respectively. Chronic oral administration of MSG produced congestion, edema and fiber discontinuity in the heart tissue (Figs. 1A2, 2A1, 2A2 and 2A3), congestion, microhemorrhage and fatty degeneration in the liver tissue (Figs. 1B2, 2B1, 2B2 and 2B3) and congestion, cloudy swelling and glomerular shrinkage in the kidney tissue (Figs. 1C2, 2C1, 2C2 and 2C3). Fruit extract of C. spinosa at a dose of 100 mg kg-1 produced no significant effects on the heart (Figs. 1A3 and 2A), liver (Figs. 1B3 and 2B) and kidney (Figs. 1C3 and 2C), whereas at a dose of 300 mg kg-1, it significantly (p < 0.05) reduced histological changes and scores induced by MSG in the heart (Figs. 1A4 and 2A), liver (Figs. 1B4 and 2B) and kidney (Figs. 1C4 and 2C) tissues. Quercetin (10.00 mg kg-1) significantly (p < 0.05) attenuated MSG-induced histopathological changes and scores in the heart (Figs. 1A5 and 2A), liver (Figs. 1B5 and 2B) and kidney (Figs. 1C5 and 2C) tissues. Vitamin E (200 mg kg-1) also produced significant (p < 0.05) improving effects on MSG-induced histological changes and scores in the heart (Figs. 1A6 and 2A), liver (Figs. 1B6 and 2B) and kidney (Figs. 1C6 and 2C) tissues. No significant (p > 0.05) differences were observed for the improving effects of the high dose of the extract, quercetin, and vitamin E on histological changes induced by MSG.
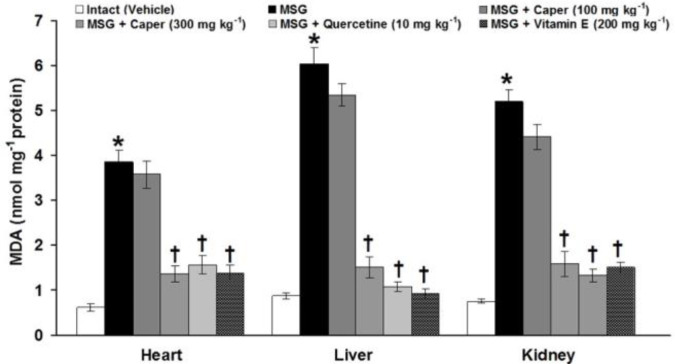
In control groups, the heart, liver, and kidney tissue MDA levels were 0.61 ± 0.07, 0.87 ± 0.07, and 0.74 ± 0.04 nmol mg-1 protein, respectively. In the MSG-treated group, this parameter was significantly (p < 0.05) elevated to 3.86 ± 0.23, 6.03 ± 0.36, and 5.21 ± 0.26 in the heart, liver, and kidney tissues, respectively. The C. spinosa fruit extract at the low dose (100 mg kg-1) had no effects, whereas, at the high dose (300 mg kg-1), it significantly (p < 0.05) recovered tissue level of MDA. After chronic treatment with caper (300 mg kg-1), quercetin (10.00 mg kg-1) and vitamin E (200 mg kg-1), no significant (p > 0.05) differences were observed in the tissue MDA level (Fig. 3).
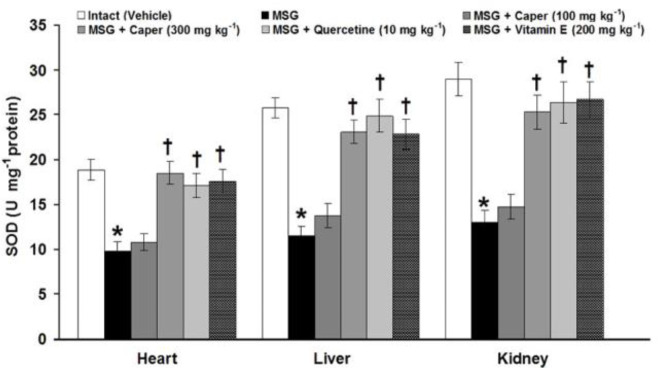
The SOD activities in the heart, liver, and kidney tissues in control groups were 18.85 ± 1.19, 25.80 ± 1.16, and 28.95 ± 1.89 U mg-1 protein, respectively. The SOD activity of the heart, liver, and kidney tissues was significantly (p < 0.05) decreased to 9.80 ± 1.02, 11.53 ± 1.02, and 12.98 ± 1.35 U mg-1 protein, respectively, after chronic administration of MSG. The C. spinosa fruit extract at the low dose (100 mg kg-1) had no effects, whereas, at the high dose (300 mg kg-1), it significantly (p < 0.05) normalized tissue activity of SOD. Quercetin (10.00 mg kg-1) and vitamin E (200 mg kg-1), produced better-improving effects on the tissue SOD activity. No significant (p > 0.05) differences were observed among caper (300 mg kg-1), quercetin (10.00 mg kg-1) and vitamin E (200 mg kg-1) treatments in tissue activity of SOD (Fig. 4).
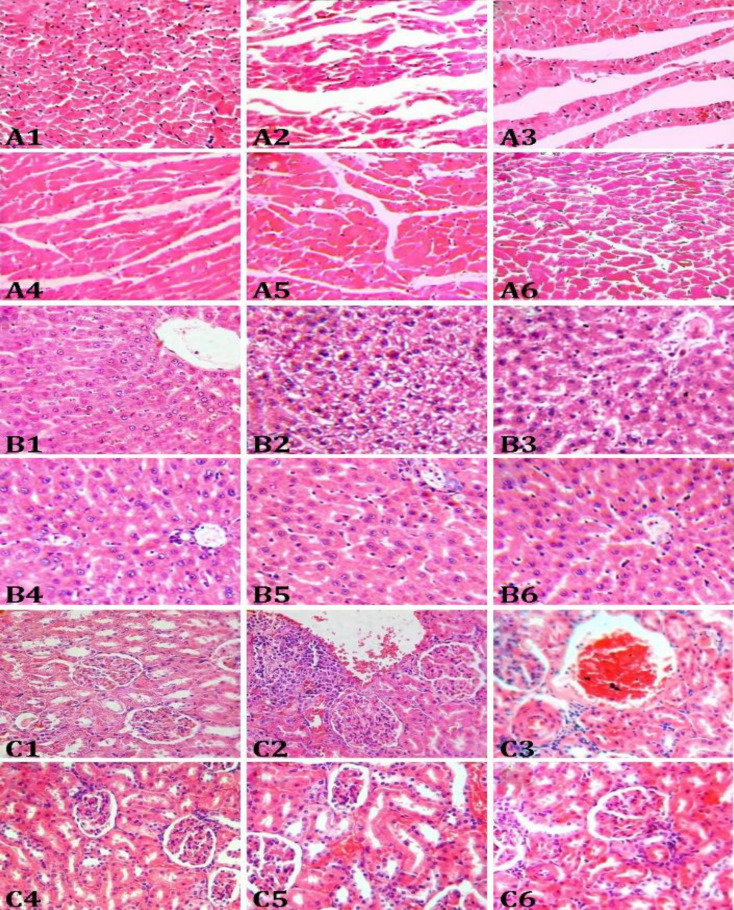
Fig. 1.
Micrographs of the heart (A), liver (B), and kidney (C) tissues in rats. Normal histoarchitecture of A1) heart, B1) liver, and C1) kidney tissues are seen. Monosodium glutamate (MSG)-treated rats show congestion, edema, and fiber discontinuity in A2) heart, congestion, microhemorrhage, and fatty degeneration in B2) liver and congestion, cloudy swelling and glomerular shrinkage in C2) kidney tissues. Oral administration of a low dose (100 mg kg-1) caper fruit extract had no effects on A3) heart, B3) liver, and C3) kidney histopathological changes induced by MSG. Oral administration of a high dose (300 mg kg-1) caper fruit extract reduced MSG-induced histopathological changes in A4) heart, B4) liver, and C4) kidney tissues. The MSG-induced histopathological changes in A5) heart, B5) live, and C5) kidney were attenuated by oral administration of quercetin (10.00 mg kg-1). Oral administration of vitamin E (200 mg kg-1) reduced MSG-induced histopathological changes in A6) heart, B6) liver, and C6) kidney tissues, (H & E, 400×)
Fig.2.
Effects of Caper extract, quercetin, and vitamin E on the heart (A), liver (B) and kidney (C) tissue histological scores induced by monosodium glutamate (MSG) in rats. MSG+Ns: monosodium glutamate + normal saline; MSG+LDE: monosodium glutamate + low dose extract; MSG+HDE: monosodium glutamate + high dose extract; MSG+ Q: monosodium glutamate + quercetin; MSG + Vit E: monosodium glutamate + vitamin E. Different letters indicate significant differences among groups at p < 0.05
Fig. 3.
Effects of Caper extract, quercetin, and vitamin E on the heart, liver, and kidney tissue-level changes of malondialdehyde (MDA) induced by monosodium glutamate (MSG) in rats
* indicates significant differences compared to the control group at p < 0.05; and † indicates significant differences compared to the MSG-treated group at p < 0.05.
Fig. 4.
Effects of Caper extract, quercetin, and vitamin E on the heart, liver, and kidney tissue-level changes of superoxide dis-mutase (SOD) induced by monosodium glutamate (MSG) in rats. * indicates significant differences compared to the control group at p < 0.05; and † indicates significant differences compared to the MSG-treated group at p < 0.05
Table 1.
Effects of Caper extract, quercetin, and vitamin E on serum level changes of ALT, AST, urea, creatinine, LDH, and CK-MB induced by MSG in rats
| Groups | ALT (U L -1 ) | AST (U L -1 ) | Urea (U L -1 ) | Creatinine (U L -1 ) | LDH (U L -1 ) | CK-MB (U L -1 ) |
|---|---|---|---|---|---|---|
| Intact (Vehicle) | 23.15 ± 1.51a | 44.31 ± 3.62a | 36.63 ± 1.72a | 0.28 ± 0.02a | 24.98 ± 2.62a | 86.00 ± 9.94a |
| MSG (4.00 mg g -1 ) | 48.66 ± 2.87b | 78.36 ± 2.56b | 51.48 ± 2.34b | 0.52 ± 0.04b | 50.68 ± 3.14b | 183.00 ± 13.66b |
| MSG + Caper (100 mg kg -1 ) | 44.63 ± 2.81b | 70.80 ± 3.86b | 47.23 ± 1.74b | 0.46 ± 0.03b | 48.05 ± 3.06b | 160.83 ± 11.02b |
| MSG + Caper (300 mg kg -1 ) | 25.86 ± 1.92ac | 49.21 ± 2.79ac | 33.75 ± 2.58ac | 0.30 ± 0.02ac | 27.25 ± 2.08ac | 104.66 ± 9.09ac |
| MSG + Quercetin (10.00 mg kg -1 ) | 21.28 ± 1.92ac | 44.95 ± 2.42ac | 35.35 ± 1.86ac | 0.29 ± 0.02ac | 25.96 ± 1.70ac | 110.00 ± 7.80ac |
| Vitamin E (200 mg kg -1 ) | 25.01 ± 2.07ac | 43.28 ± 2.44ac | 34.30 ± 2.54ac | 0.30 ± 0.02ac | 26.13 ± 1.46ac | 108.50 ± 8.92ac |
ALT: Alanine aminotransferase; AST: Aspartate transaminase; LDH: Lactate dehydrogenase; CK-MB: Creatine kinase-MB; MSG: Monosodium glutamate. Different letters indicate significant differences among groups (p < 0.05).
Discussion
The present study results showed that oral administration of MSG (4.00 g kg-1) produced histological changes including congestion, edema and fiber discontinuity in the heart tissue, congestion, micro-hemorrhage, and fatty degeneration in liver tissue and congestion, cloudy swelling and glomerular shrinkage in kidney tissue. Also, the heart, liver, and kidney tissue biochemical changes including elevation of MDA level and reduction of SOD activity after oral administration of MSG were also observed in the present study. Moreover, our results revealed that the levels of serum biomarkers of tissue function including ALT, AST, urea, creatinine, LDH, CK-MB were also increased in MSG-treated animals. Histological alterations such as fiber separation and vascular congestion in the heart tissue, tubular swelling and capillary congestion in the kidney tissue and central veins dilation and degenerated vacuolated cytoplasm in the liver tissue have been reported in previous studies.6,26,27 The heart, liver, and kidney tissues elevation of MDA and reduction of SOD have been also reported after oral administration of MSG in rats.6,26,28 The effects of MSG on serum markers of organ function are in accordance with the other research findings5,6,26 indicating that oxidative stress is involved in MSG-induced organ toxicity and abnormal function. Although the underlying mechanisms of MSG-induced organ toxicity are still unclear, the involvement of α-ketoglutarate dehydrogenase, glutamate receptors, and cysteine-glutamate antiport in up-regulation of oxidative stress in renal toxicity has been reported formerly.3 The circulating MSG is dissociated in sodium ion and L-glutamate.2 Beside to central nervous system, glutamate receptors are widely distributed in peripheral organs such as gut, lungs, spleen, ovaries, liver, kidneys and heart.29 Overstimulation of these receptors by glutamate or glutamate analogs can lead to ROS production, DNA damage, lipid peroxidation and caspase activation through triggering Ca2+ influx and subsequent activation of many intracellular enzymes such as protein kinase C.29 Consequently, these changes can lead to functional impairments of the organs and tissue enzyme disturbances.5,6,26
The results of the present study showed that oral administration of hydroalcoholic extract of C. Spinosa fruit attenuates MSG-induced histological alterations in heart, liver and kidney tissues. This treatment also recovered the increased MDA level and the decreased SOD activity in the heart, liver, and kidney tissues. Besides, our findings also revealed improving the effects of caper extract on serum biomarkers. These indicate that caper fruits can improve histological and functional changes by reducing oxidative stress in MSG-treated animals. Different parts of caper including flowers, seeds, buds, and fruits have been found to possess potent antioxidant activities and the major contributors to the antioxidant activity of caper fruits are believed to be water-soluble compounds such as phenolic acids and flavonoids.11 Mousavi et al., have reported a cardioprotective effect of C. spinosa against doxorubicin-induced toxicity in cardiomyoblast cells by increasing cell viability and decreasing cell apoptosis.30 Also, Yu et al., have reported that C. spinosa produces improving effects on doxorubicin-induced cardiac toxicity by normalizing serum LDH and CK levels and activation of antioxidant and anti-apoptotic mechanisms in mice.31 Administration of C. spinosa fruit extract decreased liver triglyceride and cholesterol as well as mRNA expression and enzyme activity of glucose-6-phosphate in streptozotocin-treated diabetic rats.32 Besides, its leaves methanolic extract treatment reduced the elevated MDA level and restored the kidney damage provoked by cisplatin. It also decreased the amount of hepatic MDA formation, elevated the activity of SOD and recovered liver injury in acute liver damage induced by CCl4.33 Moreover, C. spinosa fruit extract inhibited the secretion of interleukins (ILs) such as IL-12p40, IL-6 and IL-1β as well as tumor necrosis factor alpha-induced by lipopolysaccharide.34
In the present study, oral administration of quercetin improved histological and biochemical alterations induced by MSG in the heart, liver, and kidney tissues. Quercetin is a versatile antioxidant known to possess protective abilities against tissue injury induced by various drug toxicities.17,34-36 The results of our study are following other findings in which the antioxidant and tissue-protective effects of quercetin have been reported in the liver, kidney, and brain of MSG-treated rats.8 It has been reported that quercetin exerts protective effects on various cells including myocytes and testicular, renal and liver cells in ischemia and reperfusion injury.37 Quercetin also found to scavenge ROS and inhibit the activation of extracellular signal-regulated and mitogen-activated protein kinases in ROS-induced cardiomyopathy.38 It has been reported that quercetin recovers the increased levels of ALT, AST, ALP, bilirubin and leads to increase in the concentration of total protein and albumin in cadmium-induced toxicity in rats.39 Our present results showed that the histological, biochemical and functional changes induced by MSG in the heart, liver and kidney tissues were recovered by oral administration of vitamin E. Vitamins E is comprised of eight fat-soluble compounds including α-, β-, γ- and δ-tocopherol and α-, β-, γ- and δ-tocotrienol synthesized in plant organisms and represented to different extents in fat-rich food such as edible oils and seeds.18 Co-administration of vitamin E and MSG reduced lipid peroxidation level in liver, kidneys, and brain of rats, probably by scavenging ROS in the membrane.8,40 It also recovered serum concentration of ALT and AST, urea and creatinine in MSG-treated rats.21,41
In conclusion, the results of the present study showed that MSG produced detrimental effects on the heart, liver, and kidney through oxidative stress activation. Also, caper fruit extract, quercetin, and vitamin E improved the histological, biochemical, and functional alterations induced by MSG in these organs. Caper fruit extract, quercetin, and vitamin E produced approximately equal tissue-protective and antioxidant effects. The exact mechanisms of the antitoxic effects of other parts of C. spinosa such as leaves, roots, and seeds beside MSG-induced toxicity need to be evaluated in future studies.
Acknowledgments
This work was financially supported by the Faculty of Veterinary Medicine, Urmia University, Urmia, Iran.
Conflict of interest
The authors declared that there are no conflicts of interest.
References
- 1.Samuels A. The toxicity/safety of free glutamic acid (MSG): a study in suppression of information. Account Res. 1999;6(4):259–310. doi: 10.1080/08989629908573933. [DOI] [PubMed] [Google Scholar]
- 2.Walker R, Lupien JR. The safety evaluation of monosodium glutamate. J Nutr. 2000;130(4S suppl):1049S–1052S. doi: 10.1093/jn/130.4.1049S. [DOI] [PubMed] [Google Scholar]
- 3.Sharma A. Monosodium glutamate-induced oxidative kidney damage and possible mechanisms: a mini-review. J Biomed Sci. 2015;22:93. doi: 10.1186/s12929-015-0192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashem HE, El-Din Safwat MD, Algaidi S. The effect of monosodium glutamate on the cerebellar cortex of male albino rats and the protective effects of vitamin C (histopathological and immunohistochemical study) J Mol Histol. 2012;43(2):179–186. doi: 10.1007/s10735-011-9380-0. [DOI] [PubMed] [Google Scholar]
- 5.Oritz GG, Bitzer-Quintero OK, Zárate CB, et al. Monosodium glutamate-induced damage in liver and kidney: a morphological and biochemical approach. Biomed Pharmacother. 2006;60(2):86–91. doi: 10.1016/j.biopha.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Paul S, Mohanan A, Varghese MV, et al. Ameliorative effect of α-tocopherol on monosodium glutamate-induced cardiac histological alterations and oxidative stress. J Sci Food Agric. 2012;92(15):3002–3006. doi: 10.1002/jsfa.5714. [DOI] [PubMed] [Google Scholar]
- 7.Khalaf HA, Arafat EA. Effect of different doses of monosodium glutamate on the thyroid follicular cells of adult male albino rats: a histological study. Int J Clin Exp Pathol. 2015;8(12):15498–15510. [PMC free article] [PubMed] [Google Scholar]
- 8.Farombi EO, Onyema OO. Monosodium glutamate-induced oxidative damage and genotoxicity in the rat: Modulatory role of vitamin C, vitamin E and quercetin. Hum Exp Toxicol. 2006;25(5):251–259. doi: 10.1191/0960327106ht621oa. [DOI] [PubMed] [Google Scholar]
- 9.Tlili N, Elfalleh W, Saadaoui E, et al. The caper (Capparis L): ethnopharmacology, phytochemical and pharmacological properties. Fitoterapia. 2011;82(2):93–101. doi: 10.1016/j.fitote.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Kazemian M, Abad M, Haeri MR, et al. Anti-diabetic effects of Capparis spinosa L root extract in diabetic rats. Avicenna J Phytomed. 2015;5(4):325–332. [PMC free article] [PubMed] [Google Scholar]
- 11.Nabavi SF, Maggi F, Daglia M, et al. Pharmacological effects of Capparis spinosa L. Phytother Res. 2016;30(11):1733–1744. doi: 10.1002/ptr.5684. [DOI] [PubMed] [Google Scholar]
- 12.Eddouks M, Lemhadri A, Hebi M, et al. Capparis spinosaL aqueous extract evokes antidiabetic effect in streptozotocin-induced diabetic mice. Avicenna J Phytomed. 2017;7(2):191–198. [PMC free article] [PubMed] [Google Scholar]
- 13.Tlili N, Khaldi A, Triki S, et al. Phenolic compounds and vitamin antioxidants of caper (Capparis spinosa) Plant Foods Hum Nutr. 2010;65(3):260–265. doi: 10.1007/s11130-010-0180-6. [DOI] [PubMed] [Google Scholar]
- 14.Cao YL, Li X, Zheng M. Capparis spinosa protects against oxidative stress in systemic sclerosis dermal fibroblasts. Arch Dermatol Res. 2010;302(5):349–355. doi: 10.1007/s00403-009-0998-7. [DOI] [PubMed] [Google Scholar]
- 15.Panico AM, Cardile V, Garufi F, et al. Protective effect of Capparis pinosa on chondrocytes. Life Sci. 2005;77(20):2479–2488. doi: 10.1016/j.lfs.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 16.Goel A, Digvijaya , Garg A, et al. Effect of Capparis spinosa Linn extract on lipopolysaccharide-induced cognitive impairment in rats. Indian J Exp Biol. 2016;54(2):126–132. [PubMed] [Google Scholar]
- 17.Anand David AV, Arulmoli R, Parasuraman S. Overview of biological importance of quercetin: a bioactive flavonoid. Pharmacogn Rev. 2016;10(20):84–89. doi: 10.4103/0973-7847.194044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galli F, Azzi A, Birringer M, et al. Vitamin E: Emerging aspects and new directions. Free Radic Biol Med. 2017;102:16–36. doi: 10.1016/j.freeradbiomed.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 19.Rahmani R, Mahmoodi M, Karimi M, et al. Effect of hydroalcoholic extract of Capparis spinosa fruit on blood sugar and lipid profile of diabetic and normal rats. Zahedan J Res Med Sci. 2013;15(11):34–38. [Google Scholar]
- 20.Hashemnia M, Oryan A, Hamidi AR, et al. Blood glucose levels and pathology of organs in alloxan-induced diabetic rats treated with hydro-ethanol extracts of Allium sativum and Capparis spinosa. Afr J Pharm Pharmacol. 2012;6(21):1559–1564. [Google Scholar]
- 21.Tawfik MS, Al-Badr N. Adverse effects of monosodium glutamate on liver and kidney functions in adult rats and potential protective effect of vitamins C and E. Food Nutr Sci. 2012;3:651–659. [Google Scholar]
- 22.Farshid AA, Tamaddonfard E, Moradi-Arzeloo M, et al. The effects of crocin, insulin and their co-administration on the heart function and pathology in streptozotocin-induced diabetic rats. Avicenna J Phytomed. 2016;6(6):658–670. [PMC free article] [PubMed] [Google Scholar]
- 23.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxidase in normal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 24.Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34(3):497–500. [PubMed] [Google Scholar]
- 25.Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 26.Paul MV, Abhilash M, Varghese MV, et al. Protective effects of α-tocopherol against oxidative stress related to nephrotoxicity by monosodium glutamate in rats. Toxicol Mech Methods. 2012;22(8):625–630. doi: 10.3109/15376516.2012.714008. [DOI] [PubMed] [Google Scholar]
- 27.El-Meghawry El-Kenawy A, Osman HE, Daghestani MH. The effect of vitamin C administration on monosodium glutamate-induced liver injury: An experimental study. Exp Toxicol Pathol. 2013;65(5):513–521. doi: 10.1016/j.etp.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Diniz YS, Fernandes AA, Campos KE, et al. Toxicity of hypercaloric diet and monosodium glutamate: oxidative stress and metabolic shifting in hepatic tissue. Food Chem Toxicol. 2004;42(2):313–319. doi: 10.1016/j.fct.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Gill SS, Pulido OM. Glutamate receptors in peripheral tissues: current knowledge, future research and implications for toxicology. Toxicol Pathol. 2001;29(2):208–223. doi: 10.1080/019262301317052486. [DOI] [PubMed] [Google Scholar]
- 30.Mousavi SH, Hosseini A, Bakhtiari E, et al. Capparis spinosa reduces doxorubicin-induced cardio-toxicity in cardiomyoblast cells. Avicenna J Phytomed. 2016;6(5):488–494. [PMC free article] [PubMed] [Google Scholar]
- 31.Yu L, Yang J, Wang X, et al. Antioxidant and antitumor activities of Capparis spinosa L and the related mechanisms. Oncol Rep. 2017;37(1):357–367. doi: 10.3892/or.2016.5249. [DOI] [PubMed] [Google Scholar]
- 32.Jalali MT, Mohammadtaghvaei N, Larky DA. Investigating the effects of Capparis spinosa on hepatic gluconeogenesis and lipid content in streptozotocin-induced diabetic rats. Biomed Pharmacother. 2016;84:1243–1248. doi: 10.1016/j.biopha.2016.10.061. [DOI] [PubMed] [Google Scholar]
- 33.Tlili N, Feriani A, Saadoui E, et al. Capparis spinosa leaves extract: source of bioantioxidants with nephroprotective and hepatoprotective effects. Biomed Pharmacother. 2017;87:171–179. doi: 10.1016/j.biopha.2016.12.052. [DOI] [PubMed] [Google Scholar]
- 34.Hamuti A, Li J, Zhou F, et al. Capparis spinose fruit ethanol extracts exert different effects on the maturation of dendritic cells. Molecules. 2017;22(1):E97. doi: 10.3390/molecules22010097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Şengül E, Gelen V, Gedikli S, et al. The protective effect of quercetin on cyclophosphamide-induced lung toxicity in rats. Biomed Pharmacother. 2017;92:303–307. doi: 10.1016/j.biopha.2017.05.047. [DOI] [PubMed] [Google Scholar]
- 36.Baltaci BB, Uygur R, Caglar V, et al. Protective effects of quercetin against arsenic-induced testicular damage in rats. Andrologia. 2016;48(10):1202–1213. doi: 10.1111/and.12561. [DOI] [PubMed] [Google Scholar]
- 37.Abarikwu SO. Protective effect of quercetin on atrazine-induced oxidative stress in the liver, kidney, brain, and heart of adult wistar rats. Toxicol Int. 2014;21(2):148–155. doi: 10.4103/0971-6580.139794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boots AW, Haenen GR, Bast A. Health effects of quercetin: From antioxidant to nutraceutical. Eur J Pharmacol. 2008;583(2-3):325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 39.Chen JY, Hu RY, Chou HC. Quercetin-induced cardio-protection against doxorubicin cytotoxicity. J Biomed Sci. 2013;20:95. doi: 10.1186/1423-0127-20-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sedky A, Mahboub F, Elsawy H, et al. Protective potential of quercetin on Cd-induced hepatorenal damage. Pol J Environ Stud. 2017;26(5):2197–2205. [Google Scholar]
- 41.Onyema OO, Farombi EO, Emerole GO, et al. Effect of vitamin E on monosodium glutamate induced hepatotoxicity and oxidative stress in rats. Indian J Biochem Biophys. 2006;43(1):20–24. [PubMed] [Google Scholar]