Abstract
Various species of Trypanosoma parasites are known to infect several wild and domestic animals worldwide. A 7-year-old Holstein cow from Baneh, Kurdistan province, was examined by a private veterinarian due to anorexia and depression. Physical examination revealed fever, enlarged subscapular lymph node, and pale mucosa. Blood samples were taken for hemato-logical, parasitological, and PCR examination. The large Trypanosoma spp. was microscopically observed in a stained blood smear. Decreased red blood cells (RBCs) count, packed cell volume and hemoglobin concentration were observed through complete blood cell count. Nucleated RBCs were also found in this case. Species-specific PCR assay confirmed T. theileri infection. Treatment was performed subcutaneously with diminazene aceturate. The clinical signs were improved after two days. Two-month follow-up showed no recurrence. In conclusion, T. theileri is characterized by anemia and pyrexia in a cow. To our knowledge, the present case report describes the first molecular evidence of T. theileri in Kurdistan, West of Iran.
Key Words: Anemia, Cow, Iran, Trypanosoma theileri
Introduction
Trypanosoma theileri is assigned to the subgenus Megatrypanum by a classification originally based on its morphology. This species is one of the largest mammalian blood trypanosomes. The large trypomastigote form T. theileri can be revealed in peripheral blood having a long pointed posterior end with kinetoplast typically situated near the nucleus.1,2 The main form of transmission is probably through blood-sucking arthropods such as Tabanus.3 Although T. theileri is mostly associated with mild or no pathogenicity, evidence of regenerative anemia, fever, and progressive weight loss has been revealed in sporadic clinical cases in domestic animals of several countries such as Spain, Ireland, Germany, and Italy.4-10 The present case report describes a cow infected with T. theileri in Kurdistan, West of Iran.
Case Description
A 7-year-old Holstein cow from Baneh county (35° 59′ 51″ N 45° 53′ 07″ E), located at about 249 km north of Sanandaj (Kurdistan province) in the West of Iran, was examined by a private veterinarian due to fever, anorexia, and depression. Enlarged subscapular lymph nodes, strong heart sounds, and pale mucosa were also recorded. The blood samples from the jugular vein were collected in EDTA-containing blood collection tubes (Kendall Co., Mansfield, USA) for hematological and molecular examinations. First, Giemsa-stained blood smears were prepared for precise detection of the parasite under a light microscope (Olympus SZ61; Olympus Corporation, Tokyo, Japan) using morphological features and taxonomical key of Kaufmann.11 Then, a part of the blood sample was assigned to routine hematological parameters including red blood cell (RBC) count, packed cell volume (PCV) value, and hemoglobin concentration by automated hematology analyzer (Exigo, Stockholm, Sweden).
The DNA from the blood sample was extracted using a DNA extraction kit (MBST, Tehran, Iran). A pair of primers, forward 5′-CGTCTCTGGCTCCGGTCAAAC-3' and reverse 5’-TTAAAGCTTCCACGAGTTCTTGATGATCCAGTA-3’ was used to amplify a 289 bp fragment of cathepsin L-like protein (CATL) gene from T. theileri according to the method described by Yokoyama et al.12 Polymerase chain reaction (PCR) was carried out in 50.00 µL total reaction volume containing 5.00 µL of 10x PCR buffer, 2.00 mM MgCl2 (Cinnagen, Tehran, Iran), 250 µM of each of the four deoxynucleotide triphosphates, 1.25 U Taq DNA poly-merase (Fermentas Life Science, St. Leon-Rot, Germany), 50.00 pmol of each primer and 50.00 ng of extracted DNA. Amplification of parasite DNA was done in a thermocycler (CP2-003; Corbett Research, Sydney, Australia). Cycling condition for T. theileri was 95.00 ˚C for 5 min, followed by 45 cycles at 95.00 ˚C for 30 sec, 55.00 ˚C for 60 sec and 72.00 ˚C for 1 min with a final extension step of 72.00 ˚C for 10 min. The PCR products extracted from the agarose gel were sent for sequencing (SinaClon, Tehran, Iran). The T. theileri positive control was confirmed by GenBank under the accession number of MK393794. The negative control contained all essential components of the amplification reaction except the template. The study was approved by the Ethics Committee of Faculty of Veterinary Medicine, Urmia University, Urmia, Iran (AECVU-190-2019).
Results
Light microscopic examination of Giemsa-stained peripheral blood films revealed the presence of Trypanosoma spp. trypomastigotes (85.00 ± 5.00 μm in length) freely scattered between erythrocytes having kinetoplast typically situated near the nucleus (Fig. 1). The PCR analysis showed an expected 289 bp fragment of the CATL gene of T. theileri amplified from a blood sample (Fig. 2). Negative control reactions of each PCR run did not yield amplification products. The PCR product was purified, sequenced, and registered under the accession number of MK393794 in GenBank. The sequences of the CATL gene of T. theileri obtained in the present study were compared with other CATL sequences retrieved from GenBank. Sequence analysis demonstrated the highest homology of 98.00-100% between obtained sequences and registered sequence of T. theileri in GenBank.
Fig. 1.
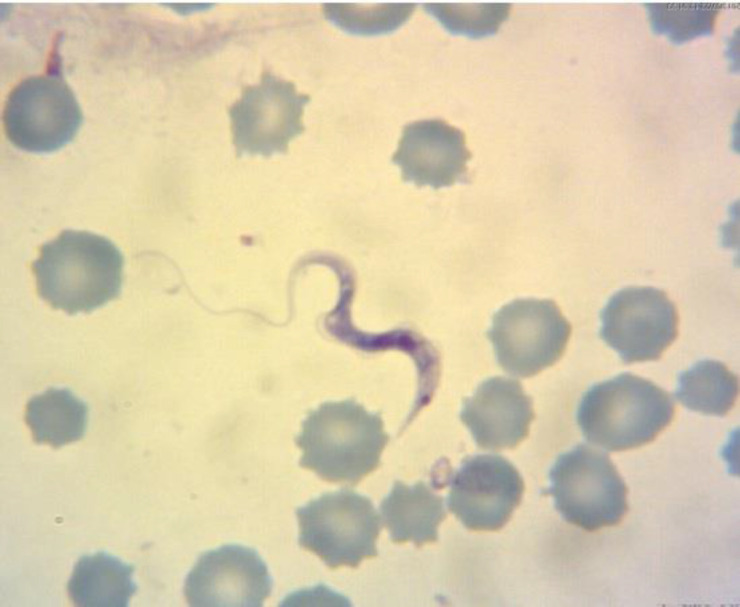
Giemsa-stained blood smear showing large trypomastigote of Trypanosoma spp (100x)
Fig. 2.
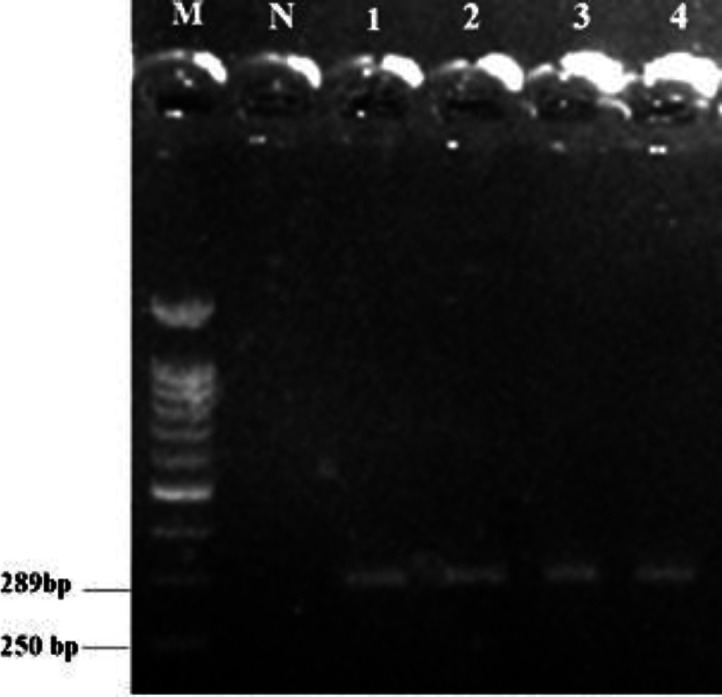
Agarose gel electrophoresis of T. theileri cathepsin L-like protein gene products by PCR. Lane M: 100 bp DNA molecular marker; Lane N: Negative control; Lanes 2 to 4: PCR product of T. Theileri samples
Hematological examinations indicated low PCV (18.00%; reference interval: 24.00 - 46.00%), RBC count (3.55 × 106 μL-1; reference interval: 5.00-10.00 × 106 μL-1) and hemoglobin (6.10 g dL-1; reference interval: 8.00 - 15.00 g dL-1).13 In addition, nucleated RBCs (four per 100 white blood cells) were found in this case.
Discussion
Microscopic examinations such as Giemsa-stained blood smears and cultivation of blood samples are mostly used as a confirmatory diagnosis of vertebrate host suffering from Trypanosoma infections. Based on micro-scopic examinations, there are two reports of T. theileri infection in cattle of Iran.14,15 However, this study was focused on molecular confirmation of T. theileri, which is the lesser-studied species. The PCR has been proven to be more sensitive than a blood examination regarding trypanosome genomic DNA detection in the animal or vector host. Also, this method is sensitive enough to detect even one trypanosome genomic DNA per mL of blood. Also, DNA-based diagnostic methods have generally facilitated epidemiological studies of trypanosomes.12
Prevalence of T. theileri varies considerably in different countries ranging from 10.00 to 90.00%.7 In the previous study in Iran, a culture of blood was used and the prevalence of T. theileri was determined as 26.47%.14 Infection of a cow by T. theileri has been reported previously in cattle of Urmia, Iran using the cytologic examination.15 However, to the best of the authors' knowledge, the present report is the first molecular-confirmed case of T. theileri infection in cattle of Iran.
Trypanosoma theileri is commonly associated with mild or no clinical signs and it is thought to have very low pathogenicity.8 In this case report, pyrexia, enlarged lymph nodes, and decreased RBCs, PCV, and hemoglobin concentration are in agreement with clinical and hematological findings reported in other studies.5,9,15 Enlargement of the lymph node in infected cow could be due to extravascular localization of trypomastigotes in the lymph node.16
In summation, the findings recorded here demonstrated that T. theileri should be considered in the differential diagnosis of diseases characterized by anemia and pyrexia. The PCR evaluation for T. theileri DNA should be included in the investigation of such cases to enable the rapid definitive detection of this infection, which may be more common than the previous estimation. Besides, diminazene aceturate might be effective in the treatment of T. theileri in cattle, however; conducting further case studies are necessary to recommend successful treatment. Our findings also indicate that T. theileri should be considered as an occasional pathogen of cattle in Iran.
Acknowledgments
The authors would like to thank the Research Dean of Urmia University, Urmia, Iran for the financial support of this study.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Votýpka J, d'Avila-Levy CM, Grellier P, et al. New approaches to systematics of Trypanosomatidae: Criteria for taxonomic (re) description. Trends Parasitol. 2015;31(10):460–469. doi: 10.1016/j.pt.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 2.Reichenow E. East African observations on trypanosomids. Arch Protistenk. 1940;94:267–287. [Google Scholar]
- 3.Böse R, Heister NC. Development of Trypanosoma (M) theileri in tabanids. J Eukaryot Microbiol. 1993;40(6):788–792. doi: 10.1111/j.1550-7408.1993.tb04475.x. [DOI] [PubMed] [Google Scholar]
- 4.Sood NK, Singla LD, Singh RS, et al. Association of Trypanosoma theileri with peritonitis in a pregnant cross-bred cow: A case report. Vet Med. 2011;56(2):82–84. [Google Scholar]
- 5.Villa A, Gutierrez C, Gracia E, et al. Presence of Trypanosoma theileri in Spanish cattle. Ann N Y Acad Sci. 2008;1149:352–354. doi: 10.1196/annals.1428.016. [DOI] [PubMed] [Google Scholar]
- 6.Fölsch DW. Simplified, contamination-free, cultural demonstration of Trypanosoma theileri (Laveran, 1902) in cattle blood in North Germany. Isolation and culture. Acta Trop. 1971;28(2):170–174. [PubMed] [Google Scholar]
- 7.Polidori GA, Grelloni V, Moretti A, et al. Trypanosoma theileri infection in cattle in central Italy. Atti Soc Ital Sci Vet. 1982;36:650–652. [Google Scholar]
- 8.Greco A, Loria GR, Dara S, et al. First isolation of Trypanosoma theileri in Sicilian cattle. Vet Res Commun. 2000;24(7):471–475. doi: 10.1023/a:1006403706224. [DOI] [PubMed] [Google Scholar]
- 9.Doherty ML, Windle H, Voorheis HP, et al. Clinical disease associated with Trypanosoma theileri infection in a calf in Ireland. Vet Rec. 1993;132(26):653–656. doi: 10.1136/vr.132.26.653. [DOI] [PubMed] [Google Scholar]
- 10.Desquesnes M, McLaughlin G, Zoungrana A, et al. Detection and identification of Trypanosoma of African livestock through a single PCR based on internal transcribed spacer 1 of rDNA. Int J Parasitol. 2001;31(5-6):610–614. doi: 10.1016/s0020-7519(01)00161-8. [DOI] [PubMed] [Google Scholar]
- 11.Kaufmann J. Parasitic infections of domestic animals: A diagnostic manual. 1st ed. Berlin, Germany: Birkhäuser ; 1996. pp. 61–62. [Google Scholar]
- 12.Yokoyama N, Sivakumar T, Fukushi S, et al. Genetic diversity in Trypanosoma theileri from Sri Lankan cattle and water buffaloes. Vet Parasitol. 2015;207(3-4):335–341. doi: 10.1016/j.vetpar.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Blood DC. Pocket companion to veterinary medicine. 8th ed. London, UK: WB Saunders; pp. 1997–484. [Google Scholar]
- 14.Niak A. The incidence of Trypanosoma theileri among cattle in Iran. Trop Anim Health Prod. 1978;10(1):26–27. doi: 10.1007/BF02235297. [DOI] [PubMed] [Google Scholar]
- 15.Seifi HA. Clinical trypanosomosis due to Trypanosoma theileri in a cow in Iran. Trop Anim Health Prod. 1995;27(2):93–94. doi: 10.1007/BF02236319. [DOI] [PubMed] [Google Scholar]
- 16.Luckins AG, Gray AR. Trypanosomes in the lymph nodes of cattle and sheep infected with Trypanosoma congolaise. Res Vet Sci. 1979;27(1):129–131. [PubMed] [Google Scholar]


