Abstract
Newcastle disease (ND) causes severe economic losses in poultry production. Despite the intensive vaccination regimes of NDV in Egypt, many outbreaks are being reported. The present study focused on the preparation and evaluation of inactivated velogenic Newcastle disease virus vaccine (genotype VII) isolated from Egyptian broiler chicken during 2015-2016. Fifty-five tissue samples including trachea, lung, liver, proventriculus, intestine, and kidney collected from commercial broiler chickens were used for virus isolation in specific pathogen-free embryonated chicken eggs (ECE) and identified using RT-PCR and sequencing. The isolates were classified by sequencing as velogenic NDV genotype VIId containing F0 protein cleavage site motifs (112RRQKRF117). A selected isolate was served as a master seed for the preparation of inactivated NDV vaccine with or without Montanide ISA70 adjuvant and evaluated in SPF chicks. Nine NDV isolates were isolated on ECE and the highest infectivity titer of the virus was 7.50 log10 EID50 mL-1 by the 5th passage. Vaccinated chicks with NDV-Montanide ISA70 adjuvanted vaccine exhibited antibody titer of 5.20 log2 at the 3rd-week-post-vaccination (WPV) with the highest titer (8.90 log2 mL-1) at the 6th-WPV. Protective antibodies values were persisted to 12th WPV followed by a gradual decrease to the end of the experiment (16th weeks). Vaccination of chicks with inactivated NDV isolate without adjuvant failed to induce protective HI antibodies all over the experiment. Chickens vaccinated with the ISA70 adjuvant vaccine were passed homologous challenge tests with 100% protective efficiency, while the unadjuvanted vaccine could not provide any protective efficiency. In conclusion, the preparation of inactivated oil adjuvant vaccine from NDV field circulating strains was efficient in controlling the disease in Egypt.
Key Words: Egypt, Genotypes VIId, Inactivated vaccine, Newcastle disease virus
Introduction
Newcastle disease (ND) is considered one of the most important poultry viral diseases in terms of worldwide distribution and economic impacts. The NDV is classified into lentogenic, mesogenic, and velogenic strains according to the pathogenicity in chickens.1 The NDV is classified as a member of the orthoavulavirus genus of subfamily Avulavirinae in the Paramyxoviridae family. Its genome is single-stranded negative-sense RNA of approximately 15.20 kb in length that encodes 6 genes, namely, hemagglutinin-neuraminidase (HN), matrix (M), fusions (F) proteins that associated with virus, envelope polymerase protein (L), nucleoprotein (NP), and phosphoprotein (P) that are associated with the RNA genome.2 The virulence of NDV is influenced by the amino acids sequence motifs at the cleavage site of F-glycoprotein.3
The highly pathogenic form of ND may act as enzootic disease or make regular, frequent epizootics throughout Asia, Africa, Central America, and parts of South America.4 In Egypt, NDV is endemic and causes severe economic losses since the first outbreak in 1948.5 Based on the F gene sequence, NDV strains have two classes (I and II). Avirulent strains with a single genotype present in Class I while virulent (fifteen genotypes I-XV) and a virulent vaccine strain (LaSota, and Hitchner B1) present in class II.6-8 Genotypes V, VI, and VII of class II are the current worldwide circulating strains.9,10 Genotype VII was divided into VIIa and VIIb sub-genotypes,9 later divided into VIIc, d, and e for isolates from Kazakhstan, China, and South Africa,11 and VIIf, g, and h of African isolates.12 The genotype VIId is prevalent in Egypt causing several outbreaks in poultry farms.13
Despite Intensive vaccination regimes carried out in Egypt, outbreaks of NDV is being reported so frequently with high losses in infected flocks. Many outbreaks of NDV may be due to the random use of intensive vaccines, frequent mutations, and emerging of new pathotypes of NDV.14 Furthermore, some commercially used live vaccines of NDV were a source of infections in Egypt.15 However, the inactivated vaccine is characterized by safety, efficacy, and economic control of the disease prevalence. The serologic response and vaccine efficacy of inactivated vaccines are affected by antigen content.16
Several ND outbreaks were reported in vaccinated flocks due to genetic and phylogenetic divergence between current NDV vaccine strains and circulating strains. The formulation of ND vaccines from the recent circulating isolates followed by evaluation of vaccine efficacy may be very important to protect against the disease morbidity, mortality and virus transmission. Depending on this strategy, the present study was aimed to assess the causative agent of NDV outbreaks even in vaccinated chicken flocks, prepare, and evaluate the inactivated oil-adjuvanted vaccine from the obtained isolates.
Materials and Methods
Ethical approval. The use of animal and protocol was approved by the animal care and use committee of Veterinary Serum and Vaccine Research Institute (VSVRI), Abbasia, Cairo, Egypt according to the recommendations and guidelines of the European Communities Council Directive 1986 (86/609/EEC).
Samples. Fifty-five tissue samples collected aseptically from different organs including trachea, lung, proventriculus, intestine, liver, and kidney from broiler chickens flocks showed NDV suspected signs. These flocks represented five Egyptian governorates (Ismailia, El_Sharkia, El_Gharbia, Kafr El_Shaikh, and El_Giza). These flocks were of various brands like Sasso, Cobb, Ross, Avian 48, and were vaccinated with Hitchner vaccine and/or LaSota vaccines.
Specific pathogen-free embryonated chicken eggs (SPF ECE). The SPF ECEs used for virus propagation, virus titration, and assurance of complete inactivation was obtained from Nile SPF Farm, Kom Oshiem, Fayoum, Egypt.
Vaccine preparation (Newcastle disease master seed virus propagation and titration). Isolation of virus from tissue homogenate of each bird) in the allantoic cavity of 9-11 days old SPF ECE was carried out according to OIE.17 Five blind passages were done to all samples, hemagglutination (HA) and hemagglutination inhibition (HI) test were carried out on allantoic fluid.17 The collected harvest of each virus was titrated in SPF ECEs and calculated according to Reed and Muench.18 The titrated virus was used as a master virus seed for the preparation of inactivated vaccines.
RT-PCR and sequencing. RT-PCR followed by sequencing was conducted to molecular characterization to confirm the identity of the isolated strains. The purity of the master seed used in this study was conducted in a molecular laboratory of Animal Health Research Institute, El-Dokki, Giza, Egypt using PCR against extraneous agents. The RNA extraction was done using a QIAamp Viral RNA Mini Kit (Qiagen, Redwood City, USA) according to the manufacturer’s instructions. The primers used and the RT-PCR was performed to amplify 766 bp of NDV according to Mase et al.19 The PCR product was separated by gel electrophoresis using 1.50% agarose gel stained with ethidium bromide (Merck, Darmstadt, Germany) and visualized under ultraviolet light using a gel documentation system. The purified PCR product was sequenced in forward and reverse directions.
Virus inactivation. The inactivation process was carried out according to Razmaraii et al. 20 using binary ethyl-enimine (BEI; Merck) at a final concentration of 3.00% (v/v). Sodium thiosulfate solution (20.00%; Merck) at a concentration of 10 times of the BEI final concentration was used to stop the reactions. Samples from the inactivated virus before the addition of adjuvant were tested by at least two successive blind-passages in 9-11-days-old SPF ECE (0.10 mL per egg) via allantoic cavity route. All embryos that died or remained alive after 24 hr and up to six days post-inoculation were examined for the presence of the virus by rapid HA on the allantoic fluid and confirmed by HI test using specific reference serum from Newcastle department, Veterinary Serum and Vaccine Research Institute, Abbasia, Cairo, Egypt. The complete inactivation of the virus was considered if no mortality or HA activities were observed.
Inactivated NDV vaccine emulsion. Inactivated NDV vaccine was prepared as water in oil emulsion (W/O) using Montanide ISA 70 at a ratio of 3/7 (v/v) aqueous /oil ratio. The manufacturing process was carried out according to the standard protocol of SEPPIC, France.
Quality control of the prepared vaccines (Sterility and safety test). The prepared vaccines were tested for its freedom from any fungal or bacterial contaminants by culturing on specific media according to OIE.21 Two groups of 3-week-old chickens (10 chickens/group) were inoculated subcutaneously with two field doses (1.00 mL) of each prepared vaccine. These chicks were observed for two weeks for any clinical signs or appearance of local reaction. After 5 days post-inoculation, some birds were subjected to post-mortem examinations to detect pathological lesions.21
Experimental design for evaluating the potency of prepared vaccines. Five groups (25 each) of one-day-old SPF chicks were used in this study: G1: Inactivated NDV vaccine-challenged group, G2: Inactivated NDV-ISA 70 vaccine-challenged group, G3: Served as non-vaccinated-challenged control, G4: Non-vaccinated-non-challenged control, and G5: Served as the vaccinated-non-challenged control group, vaccinated with the prepared inactivated NDV vaccine). Serum samples were collected from all chicks weekly to 8th-weeks post-vaccination then every 4 weeks to 16th weeks. The sera were inactivated for 30 min at 56.00 ˚C, then stored at – 20.00 ˚C until used in the hemagglutination inhibition (HI) test.
Evaluation of humoral immune response in vaccinated chicks. It was carried out by HI test using 4 HA units of homologous antigen to estimate antibody titers in sera of vaccinated and non-vaccinated chickens according to OIE.17
Challenge test. After three weeks of NDV vaccine single immunization, chickens were intramuscularly challenged with 1.00 mL of NDV virus isolated strain (105.50 EID50 mL-1). Daily observation of clinical symptoms and mortality was carried out for 14-days post-challenge. The average clinical scores for each group were calculated and reported daily according to OIE manual and previous reports as follows: Normal chickens (score 0), mild depression (score 1), neurological signs, and/or severe depression (score 2), and death (score 3).21,22,23
Results
Clinical signs and gross findings. Newcastle disease was reported in different governorates of Egypt between 2015 and 2016 despite the regular application of the vaccination program. The signs include depression, ruffled feather, respiratory distress, greenish diarrhea, facial swelling, and high mortalities, especially in young chicks. Postmortem examination of dead birds revealed facial hemorrhage, edema in the neck, hemorrhage in trachea and lungs, hemorrhagic spots in proventriculus and gizzard, and severe enlargement of kidneys.
NDV virus isolation and titration in SPF ECE. Among the examined five flocks, nine field samples from two flocks resulted in the death of an embryo within 72 hr post-inoculation in ECE with hemorrhage, congestion, and edema of the embryo that were characteristics of NDV. Allantoic fluids collected from these embryos were found positive for NDV by the direct HA test with 4.00 and 5.00 log2 HA U mL-1 in Kafr-El Shaikh and El_Giza governorate, respectively. After five blind passages in the SPF embryonated eggs, the NDV isolate was titrated. It was noticed that the highest infectivity titer of the virus was 7.50 log10 EID50 mL-1.
RT-PCR. The F gene fragment of NDV isolates was successfully amplified and gave band with an expected size of 724 bp in length on agarose gel electrophoresis.
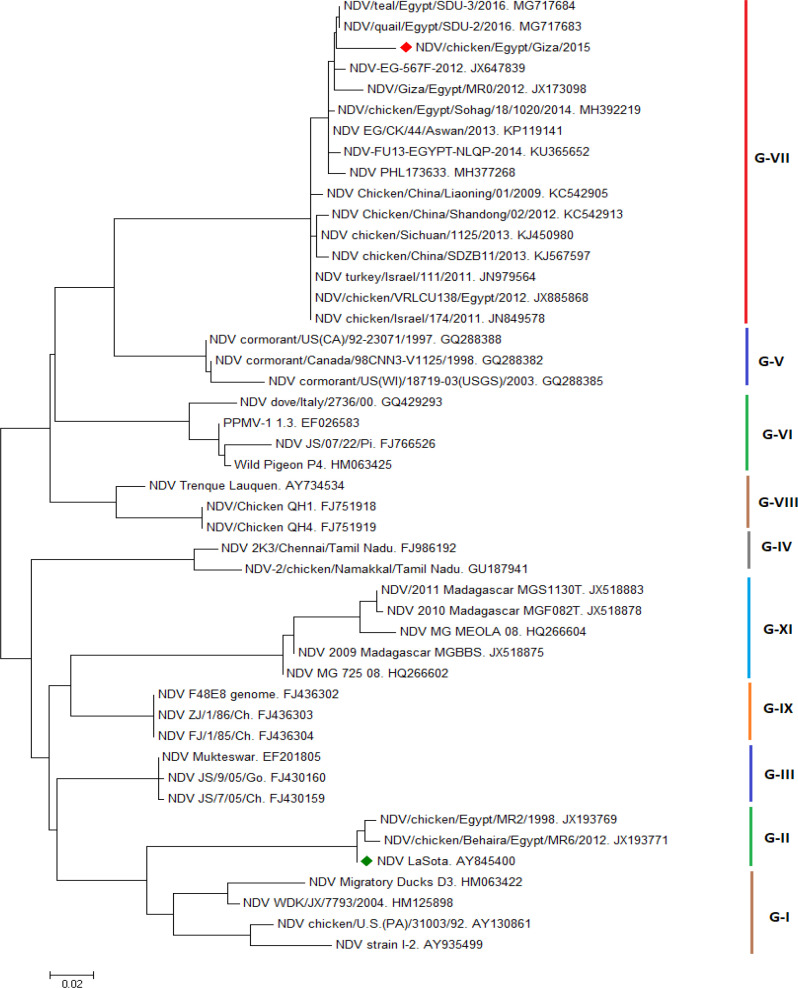
Nucleotide and amino acid sequence analysis of the F gene of NDV. The phylogenetic analysis of partial F gene of isolated strain revealed that the isolate NDV/chicken/Egypt/Giza/2015 was velogenic type as it carried the motif 112RRQKRF117 at the amino acid sequence surrounding the fusion glycoprotein cleavage site. The isolate also was belonging to the genotype VIId strains (possesses characteristic 101K and 121V), (Fig. 1).
Fig. 1.
Phylogenetic analysis of F gene sequence of isolated NDV/Chicken/Egypt/Giza/2015 (red circle) in comparison with LaSota vaccinal strain (green circle) and representative strains of NDV different genotypes from Genbank. The phylogenetic tree was constructed by MEGA version 7 using the neighbor-joining method of tree construction
Inactivation of the propagated virus suspension with binary ethylenimine (BEI) and infectivity assay. The prepared inactivated NDV sample was checked for their pathogenicity by inoculation into BHK cell culture and ECE. Complete inactivation of the ND virus suspension of BEI was found at 30.00 ˚C in 18 hr post-treatments.
Sterility and safety test for the prepared vaccine. It was found that the vaccines were sterile and safe as they were free from any bacterial and fungal contaminants. No local or systemic reactions were observed in both prepared vaccines. No clinical signs or mortality were recorded in inoculated chicks and no pathological lesions were observed by postmortem examination.
Evaluation of the humoral immune response. The humoral immune response was assessed for chicks vaccinated with Inactivated NDV isolate alone and with Montanide ISA 70 oil as an adjuvant by HI test (Fig. 2). The vaccinated chicks with the adjuvanted vaccine produced a titer of (5.20) log2 at three weeks post-vaccination. The highest titer of 8.90 log2 was recorded at the 6th weeks’ post-vaccination, and 3rd weeks post-challenge test. However, the titer showed higher value till 12th weeks post-NDV-Montanide ISA 70 vaccine followed by a gradual decrease in HI titers till the end of the experiment (16th weeks). On the other hand, vaccination of chicks with inactivated NDV isolate without adjuvant failed to induce protective HI antibody titers all over the experiment. Non-vaccinated-challenged control chickens had no antibody against NDV until three weeks-old, all chicks died within 4-5-days post-challenge. Also, the non-vaccinated-non-challenged control group did not have an antibody against NDV during the experiment. However, vaccinated-non-challenged control exhibited antibody titers of 5.50 log2 for seven weeks post-vaccination, followed by a gradual decrease of the titers to be 3 log2 16 weeks post-vaccination.
Fig. 2.
Humoral immune response of chicks vaccinated with inactivated NDV infected fluid and inactivated NDV with Montanide ISA 70 oil measured by hemagglutination inhibition test
Study of challenge and protection. After challenge with NDV virulent virus isolate, chickens vaccinated with ISA 70 adjuvant vaccine group did not show any signs of disease (score 0) and no mortalities, providing 100% protective efficiency to SPF chickens. However, the group vaccinated with antigen alone showed severe clinical signs (score 3) and a 100% mortality rate within 4-5 days after the challenge. Also, non-vaccinated-challenged control group chickens died within 3-4 days post-challenge with clinical signs of score 3.
Virus shedding. The virus shedding data on oropharyngeal (tracheal) swaps were evaluated based on a number of shedders and amount of shedding (quantitative RT-PCR converted to EID50) and the chicken monitored at 3, 5, 7, and 9days post-challenge. The RT-PCR results of oro-pharyngeal (tracheal) viral shedding in the newly developed vaccine showed a significant reduction in a number of shedders and the amount of virus shedding in comparison with vaccine without Montanide ISA adjuvant or non-vaccinated challenged group (p ≤ 0.05), (Table 1).
Table 1.
RT-PCR results of oropharyngeal (tracheal) viral shedding
| Days Post-infection |
Group-1
(Montanide vaccine) |
|
Group-2
(Antigen vaccine) |
|
Group-3
(Non-vaccinated) |
p - value | |||
|---|---|---|---|---|---|---|---|---|---|
| Positive tracheal swabs | Shedding as EID 50 | Positive tracheal swabs | Shedding as EID 50 | Positive tracheal swabs | Shedding as EID 50 | ||||
| 3 | 10/25(40%)b | 2.20 ± 0.40c | 25/25(100%)a | 4.10 ± 0.60 b | 25/25(100%)a | 4.80 ± 0.70a | 0.00* | ||
| 5 | 3/25 (12%)c | 1.80 ± 0.60b | 16/16(100%)a | 4.00 ± 0.80 a | 13/13(100%)b | 4.30 ± 0.60a | 0.00* | ||
| 7 | 0/25 (0%) | NA | 0/25 (0%) | NA | 0/25 (0%) | NA | - | ||
| 9 | 0/25 (0%) | NA | 0/25 (0%) | NA | 0/25 (0%) | NA | - | ||
NA: not applicable, EID50: Egg infective dose fifty. Some birds died so no swabs could be collected to measure shedding rate.
abc Different letters indicate significant differences between the groups day (p ≤ 0.05), and * denotes significance between groups on the same day (p ≤ 0.05).
Discussion
In Egypt, several outbreaks of Newcastle disease are still frequently occurring despite intensive vaccination programs.24 The circulating Newcastle disease virus in Egypt belongs to Genotype-VII subtyped and the available commercial vaccines in Egypt did not provide suitable protection.25,26
It is widely accepted for a long time that the commercial conventional NDV vaccines are effective in controlling ND, however, they could not prevent completely infection and virus shedding due to difference in genotypes between used vaccines and the circulating ND virus genotypes.27 Investigation of the current situation of NDV in Egypt that represent the Middle East, and Africa by molecular characterization and phylogenetic analysis are important to develop effective control measures.28
In the present study, several chicken flocks of different brands and ages in five governorates were subjected to virus isolation on SPF ECE identification using hem-agglutination tests followed by molecular characterization by RT-PCR. The isolates from two infected flocks were positive to both slide and micro-plate hemagglutination (HA) test post propagation in ECE. Grimes suggested that two flocks were infected with NDV.29 In the present study, analysis of the F gene nucleotide sequence of the NDV/ chicken/Egypt/Giza/2015 suggested that the isolates belonged to velogenic NDV Genotype VIId, this data matched to the previous studies since 2011,30-33 reported circulating NDV G-VIId in the Egyptian poultry flocks.
In the present study, NDV was completely inactivated by BEI at a final concentration of 3% when the infected harvest was incubated at 30.00 ˚C for 18 hr. Multiple studies showed that the BEI is a good inactivating agent even in the lower concentrations.34-36 Razmaraii et al. found that incubation of aziridines at 30.00 ˚C was more effective than the reaction at lower temperatures as high temperature allowed faster insertion of the chemical agents into viral particles.20 Mudasser et al. observed that infectious bursal disease virus vaccine inactivated with BEI was highly immunogenic and stable than other inactivants.37Another study conducted that NDV and AVI vaccines inactivated by formalin and BPL showed lower HI titers than BEI.38
Assessment of Quality control measures for the prepared vaccines indicated that they were completely sterile with no bacterial or fungal contaminants. Also, no local and systemic reactions or mortalities were recorded in inoculated chicks, and these guaranteed the safety of the prepared antigen. These were in agreement with the recommendation of OIE.21
Montanide adjuvants are patented for SEPPIC company (Paris, France) containing its surfactant which enables easy manufacturing of vaccines by mixing the aqueous medium into the montanide oil at room temperature manually, however, vigorous stirring and the use of a high shear mixer is necessary for mass production. Many commercial vaccine producers use montanide adjuvants for poultry vaccine preparations. Mohammadi et al. developed and manufactured inactivated oil emulsion against ND or other pathogens.39 Montanide vaccine adjuvants bear mineral oil that modulates the cell mediates immune response in chickens.
In the present study, the humoral immune response was assessed by HI test for chicks vaccinated with Inactivated NDV isolate alone and with Montanide ISA 70 oil as an adjuvant. The results revealed that the higher titer of (8.90 log2) was recorded 6 weeks post-vaccination in NDV-Montanide ISA 70-challenged group. However, high titers ≥ 4.00 log2 were seen to 12 weeks post-vaccination followed by a gradual decrease to the end of the experiment (16th weeks). On the other hand, vaccination of chicks with inactivated NDV isolate without adjuvant failed to induce protective HI antibodies all over the experiment. These results were in agreement with Liu et al. who concluded that a potent vaccine needed not only good antigens,40 but selected adjuvant to stimulate cellular and humoral immune responses of the antigen.
In the present study, the inactivated vaccine adjuvanted with ISA 70 showed the ability to protect all the chickens from the challenge virus morbidity or mortality. However, the group vaccinated with an inactivated NDV vaccine without adjuvant could not provide any protective efficiency. These results were agreement with previous reports that suggested an ND inactivated vaccine must be prepared from current local circulating NDV strains.41 Our results were similar to Miller et al. who observed 100% mortality for non-vaccinated chicks and 100% survival for four weeks-old SPF chicks vaccinated subcutaneously with a single dose of inactivated NDV vaccine after three weeks post-challenge with NDV.42 The NDV vaccines formulated with the homologous genotype of the challenge virus, for both genotype II and genotype V, not only decreased the number of birds shedding the virus, but the number of viruses shed from individual birds.43 The newly developed vaccine not only protected mortalities, but it reduced the number of shedders birds and the quantity of the virus in comparison with vaccine without Montanide ISA adjuvant or non-vaccinated challenge group (Table 1).
The main drawbacks of virulent NDV vaccine are risk of dissemination and low virus titer that make it commercially not profitable, hence, the companies are directed to produce reassortant Lassota strain vaccine carrying hemagglutinin (HN) and fusion (F) gene of NDV genotype VII for better protection.16,44 Continuous genetic and phylogenetic characterization of circulating NDV isolates causing worldwide outbreaks are important to understand the NDV epidemiology, evolution and to develop rapid diagnostic,45 and control strategies.46 In conclusion, the results of the present study confirmed that the production of an inactivated oil-adjuvanted vaccine from the local circulating velogenic NDV was efficient to protect the vaccinated birds from morbidity and mortality against the challenge test.
Acknowledgments
The authors want to thank staff members of the Department of Newcastle Disease, Veterinary Serum and Vaccine Research Institute, Abbassia, Cairo, Egypt for continuous support during the practical work of this study.
Conflict of interest
The authors have any conflict of interest to declare.
References
- 1.Nordin M, Lorenz T, Campello M. Biomechanics of tendons and ligaments. In: Nordin M, editor. Frankel VH . Basic biomechanics of the musculoskeletal system London, UK: Lippincott Williams & Wilkins; 2001. pp. 102–125. [Google Scholar]
- 2.Lamb RA, Collins PL, Kolakofsky D, et al. Family Paramyxoviridae. In: In: Fauquet CM, Mayo J, Maniloff J, et al., editors. Virus taxonomy. London, UK: Elsevier/ Academic Press ; 2005. pp. 655–668. [Google Scholar]
- 3.Römer-Oberdörfer A, Werner O, Veits J, et al. Contribution of the length of the HN protein and the sequence of the F protein cleavage site to Newcastle disease virus pathogenicity. J Gen Virol. 2003;84:3121–3129. doi: 10.1099/vir.0.19416-0. [DOI] [PubMed] [Google Scholar]
- 4.Alders RG, Spradbrow PB. Controlling Newcastle Disease in Village Chickens: a field manual. Canberra, Australian Centre for International Agricultural Research. 2001. Monograph 82.112. [Google Scholar]
- 5.Doubney R, Mansy W. The occurrence of Newcastle disease in Egypt. J Comp Pathol. 1948;58(1):189–200. doi: 10.1016/s0368-1742(48)80019-6. [DOI] [PubMed] [Google Scholar]
- 6.Diel DG, da Silva LH, Liu H, et al. Genetic diversity of avian paramyxovirus type 1: proposal for a unified nomenclature and classification system of Newcastle disease virus genotypes. Infect Genet Evol. 2012;12(8):1770–1779. doi: 10.1016/j.meegid.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Huang Y, Wan HQ, Liu HQ, et al. Genomic sequence of an isolate of Newcastle disease virus isolated from an outbreak in geese: a novel six nucleotide insertion in the non-coding region of the nucleoprotein gene. Brief report. Arch Virol. 2004;149(7):1445–1457. doi: 10.1007/s00705-004-0297-8. [DOI] [PubMed] [Google Scholar]
- 8.Tsai HJ, Chang KH, Tseng CH, et al. Antigenic and genotypical characterization of Newcastle disease viruses isolated in Taiwan between 1969 and 1996. Vet Microbiol. 2004;104(1-2):19–30. doi: 10.1016/j.vetmic.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Aldous EW, Mynn JK, Banks J, et al. A molecular epidemiological study of avian paramyxovirus type 1 (Newcastle disease virus) isolates by phylogenetic analysis of a partial nucleotide sequence of the fusion protein gene. Avian Pathol. 2003;32(3):239–256. doi: 10.1080/030794503100009783. [DOI] [PubMed] [Google Scholar]
- 10.Lin MY, Liu HJ, Ke GM. Genetic and antigenic analysis of Newcastle disease viruses from recent outbreaks in Taiwan. Avian Pathol. 2003;32(4):345–350. doi: 10.1080/0307945031000121086. [DOI] [PubMed] [Google Scholar]
- 11.Bogoyavlenskiy A, Berezin V, Prilipov A, et al. Newcastle disease outbreaks in Kazakhstan and Kyrgyzstan during 1998, 2000, 2001, 2003, 2004, and 2005 were caused by viruses of the genotypes VIIb and VIId. Virus Genes. 2009;39(1):94–101. doi: 10.1007/s11262-009-0370-1. [DOI] [PubMed] [Google Scholar]
- 12.Snoeck CJ, Ducatez MF, Owoade AA, et al. Newcastle disease virus in West Africa: new virulent strains identified in non-commercial farms. Arch Virol. 2009;154(1):47–54. doi: 10.1007/s00705-008-0269-5. [DOI] [PubMed] [Google Scholar]
- 13.Radwan MM, Darwish SF, El-Sabagh IM, et al. Isolation and molecular characterization of Newcastle disease virus genotype ІІ and VΠd in Egypt between 2011 and 2012. Virus Genes. 2013;47(2):311–316. doi: 10.1007/s11262-013-0950-y. [DOI] [PubMed] [Google Scholar]
- 14.Pchelkina IP, Manin TB, Kolosov SN, et al. Characteristics of pigeon paramyxovirus serotype-1 isolates (PPMV-1) from the Russian Federation from 2001 to 2009. Avian Dis. 2013;57(1):2–7. doi: 10.1637/10246-051112-reg.1. [DOI] [PubMed] [Google Scholar]
- 15.Aly SE. phylogenetic analysis of lentogenic strains of Newcastle disease virus. Master thesis Cairo University. 2012:83–84. [Google Scholar]
- 16.Hu Z, Hu S, Meng C, et al. Generation of a genotype VII Newcastle disease virus vaccine candidate with high yield in embryonated chicken eggs. Avian Dis. 2011;55(3):391–397. doi: 10.1637/9633-122410-Reg.1. [DOI] [PubMed] [Google Scholar]
- 17.OIE Manual of diagnostic tests and vaccines for terrestrial animals. Newcastle Disease. 2012 Chapter 2. [Google Scholar]
- 18.Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. The Am J Hyg. 1938;27(3):493–497. [Google Scholar]
- 19.Mase M, Imai K, Sanada Y, et al. Phylogenetic analysis of Newcastle disease virus genotypes isolated in Japan. J Clin Microbiol. 2002;40(10):3826–3830. doi: 10.1128/JCM.40.10.3826-3830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Razmaraii N, Toroghi R, Babaei H, et al. Immuno-genicity of commercial, formaldehyde and binary ethyl-enimine inactivated Newcastle disease virus vaccines in specific pathogen free chickens. Arch Razi Inst. 2012;67(1):21–25. [Google Scholar]
- 21.OIE Manual of diagnostic tests and vaccines for terrestrial animals. Newcastle Disease. 2014 Chapter 2. [Google Scholar]
- 22.Oyebanji VO, Emikpe OB, Oladele OA, et al. Clinico-pathological evaluation of Newcastle disease virus vaccination using gums from Cedrela odorata and Khaya senegalensis as delivery agents in challenged chickens. Int J Vet Sci Med. 2017;5(2):135–142. doi: 10.1016/j.ijvsm.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarcheshmei M, Dadras H, Mosleh N, et al. Comparative evaluation of protective efficacy of different vaccination programs against a virulent field strain of the Newcastle disease virus in broilers. Brazilian Journal of Poultry Science. 2016;18(3):363–370. [Google Scholar]
- 24.Nabila O, Sultan S, Ahmed AI, et al. Isolation and patho-typing of Newcastle disease viruses from field outbreaks among chickens in the southern part of Egypt 2011-2012. Global Veterinaria. 2014;12(2):237–243. [Google Scholar]
- 25.Orabi A, Hussein A, Saleh AA, et al. Evolutionary insights into the fusion protein of Newcastle disease virus isolated from vaccinated chickens in 2016 in Egypt. Arch Virol. 2017;162(10):3069–3079. doi: 10.1007/s00705-017-3483-1. [DOI] [PubMed] [Google Scholar]
- 26.Sedeik ME, Elbestawy AR, El-Shall NA, et al. Comparative efficacy of commercial inactivated Newcastle disease virus vaccines against Newcastle disease virus genotype VII in broiler chickens. Poult Sci. 2019;98(5):2000–2007. doi: 10.3382/ps/pey559. [DOI] [PubMed] [Google Scholar]
- 27.Shahar E, Haddas R, Goldenberg D, et al. Newcastle disease virus: Is an updated attenuated vaccine needed? Avian Pathol. 2018;47(5):467–478. doi: 10.1080/03079457.2018.1488240. [DOI] [PubMed] [Google Scholar]
- 28.Fringe R, Bosman AM, Ebersohn K, et al. Molecular characterisation of Newcastle disease virus isolates from different geographical regions in Mozambique in 2005. Onderstepoort J Vet Res. 2012;79(1):E1–7. doi: 10.4102/ojvr.v79i1.409. [DOI] [PubMed] [Google Scholar]
- 29.Grimes SE. A basic laboratory manual for the small-scale production and testing of I-2 Newcastle disease vaccine. In FAO Regional Office for Asia and the Pacific publication. Bangkok, Thailand; 2002. p. 139. [Google Scholar]
- 30.El-Bagoury GF, El-Nahas EM, Abd-El-Monem MM, et al. Detection and pathotyping of a recent Newcastledisease virus outbreak in Egypt. Benha Vet Med J. 2015;29(2):297–302. [Google Scholar]
- 31.Mohamed MHA, Kumar S, Paldurai A, et al. Sequence analysis of fusion protein gene of Newcastle disease virus isolated from outbreaks in Egypt during 2006. Virol J. 2011;8 doi: 10.1186/1743-422X-8-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hassan KE, Shany SA, Ali A, et al. Prevalence of avian respiratory viruses in broiler flocks in Egypt. Poult Sci. 2016;95(6):1271–1280. doi: 10.3382/ps/pew068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abd El Aziz M, Abd El-Hamid H, Ellkany H, et al. Biological and molecular characterization of Newcastle disease virus circulating in chicken flocks, Egypt, during 2014-2015. Zag Vet J. 2016;44(1):9–20. [Google Scholar]
- 34.Mondal SK, Neelima M, Seetha S, et al. Validation of the inactivant binary ethylenimine for inactivating rabies virus for veterinary rabies vaccine production. Biologicals. 2005;33(3):185–189. doi: 10.1016/j.biologicals.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Kamaraj G, Lakshmi Narasu M, Srinivasan VA. Validation of betapropiolactone (BPL) as an inactivant for infectious bovine rhinotracheitis (IBR) virus. Res Vet Sci. 2008;85(3):589–594. doi: 10.1016/j.rvsc.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 36.Burrage T, Kramer E, Brown F. Structural differences between foot-and-mouth disease and papillomyelitis viruses influence their inactivation by aziridines. Vaccine. 2000;18(22):2454–2461. doi: 10.1016/s0264-410x(99)00542-3. [DOI] [PubMed] [Google Scholar]
- 37.Mudasser H, Iftikhar H, Hamid I, et al. Immunogenicity of formaldehyde and binary ethylenimine inactivated infectious bursal disease virus in broiler chicks. J Zhejiang Univ Sci B. 2006;7(8):660–664. doi: 10.1631/jzus.2006.B0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.King DJ. Evaluation of different methods of inactivation of Newcastle disease virus and avian influenza virus in egg fluids and serum. Avian Dis. 1991;35(3):505–514. [PubMed] [Google Scholar]
- 39.Mohammadi AA, Hooshamand-Rad P, Ahourai P, et al. Development and manufacture of an inactive oilemulsion Newcastle disease vaccine in Iran. Arch Inst Razi. 1996;1(46/47):91–99. [Google Scholar]
- 40.Liu CG, Liu M, Liu F, et al. Evaluation of several adjuvants in avian influenza vaccine to chickens and ducks. Virol J. 2011;8:321. doi: 10.1186/1743-422X-8-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang JY, Liu WH, Ren JJ et al. Characterization of emerging Newcastle disease virus isolates in China. Virol J. 2015;12:119. doi: 10.1186/s12985-015-0351-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller PJ, Afonso CL, El Attrache J, et al. Effects of Newcastle disease virus vaccine antibodies on the shedding and transmission of challenge viruses. Dev Comp Immunol. 2013;41(4):505–513. doi: 10.1016/j.dci.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 43.Miller PJ, King DJ, Afonso CL, et al. Antigenic differences among Newcastle disease virus strains of different genotypes used in vaccine formulation affect viral shedding after a virulent challenge. Vaccine. 2007;25(41):7238–7246. doi: 10.1016/j.vaccine.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 44.Xiao S, Nayak B, Samuel A, et al. Generation by reverse genetics of an effective, stable, live-attenuated Newcastle disease virus vaccine based on a currently circulating highly virulent Indonesian strain. PLoS One. 2012;7(12):e52751. doi: 10.1371/journal.pone.0052751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mandour MF, Abd-Eldaim MM, Abdelwahab SAM, et al. Rapid detection of foot and mouth disease virus from tongue epithelium of cattle and buffaloes in Suez Canal area, Egypt from 2009 to 2011. Int J Virol. 2014;10(1):55–62. [Google Scholar]
- 46.Diel DG, Susta L, Cardenas Garcia S, et al. Complete genome and clinicopathological characterization of a virulent Newcastle disease virus isolate from South America. J Clin Microbiol. 2012;50(2):378–387. doi: 10.1128/JCM.06018-11. [DOI] [PMC free article] [PubMed] [Google Scholar]