Abstract
Beclin-1 and Bcl-2 expression abnormalities have been confirmed in different types of cancer. As important regulators of autophagy and apoptosis, respectively, these molecules serve a complex role in tumorigenesis. However, limited information is currently available regarding the association between Beclin-1 and Bcl-2 in (NSCLC). In the present study, the expression levels of Beclin-1 and Bcl-2 were detected in lung cancer tissues, and their prognostic significance was analyzed for NSCLC. A total of 120 patients with lung cancer who underwent surgical resection were included in the present study. Beclin-1 and Bcl-2 expression was assessed using immunohistochemistry and their associations with the overall survival (OS) in patients with NSCLC was examined. The expression rate of Beclin-1 was significantly lower in NSCLC tissues compared with that in adjacent tissues, whereas the expression rate of Bcl-2 was significantly higher in lung cancer tissues compared with that in adjacent tissues. Additionally, Beclin-1 and Bcl-2 protein expression was strongly associated (P<0.05) in NSCLC. Patients with NSCLC with low Beclin-1 expression were in more advanced stages, with more lymph node metastasis and more poorly differentiated tumors. Similarly, patients with NSCLC with high Bcl-2 expression were also in a more advanced stage and had more lymph node metastasis. Cox regression analysis revealed that the association between Bcl-2 expression and survival was not significant, while a multivariate analysis revealed that Beclin-1 expression was significantly associated with OS. Notably, Beclin-1 expression was significantly associated with OS only in patients with high Bcl-2 expression. In conclusion, the present data indicated that the autophagy activity is decreased in NSCLC. Beclin-1 expression was downregulated, while Bcl-2 expression was upregulated in NSCLC tissues compared with that in adjacent tissues. Additionally, these two proteins were associated with the occurrence and progression of NSCLC. Beclin-1 may be a promising prognostic marker for patients with NSCLC with high Bcl-2 expression. The present findings provided a more accurate prognostic assessment for patients with NSCLC. Furthermore, they may be used to actively follow-up and promptly treat patients with a poor prognosis, which may benefit a greater number of patients with NSCLC.
Keywords: autophagy, Beclin-1, Bcl-2, prognosis, non-small cell lung cancer
Introduction
Lung cancer is the most common malignancy worldwide and has the highest mortality rate (1). Numerous patients present with advanced stages at diagnosis due to concealed symptoms. Additionally, it is the most common type of cancer in China (2). In the United States, there was estimated to be >230,000 new cases of lung cancer in 2018 and lung cancer was suggested to lead to more deaths than breast, prostate and colon cancer combined (3). Non-small cell lung cancer (NSCLC) is the most typical form of lung cancer, accounting for ~85% of all cases (4). The improvement of diagnostic technologies and the emergence of effective new treatment methods, such as targeted therapies and immunotherapy, have improved the therapeutic management of lung cancer; however, the overall 5-year survival rate of this type of cancer remains low at 17.4% (5). Therefore, the early diagnosis and the identification of effective biomarkers are important to improve the prognosis of patients with lung cancer.
Autophagy, also known as type II programmed cell death, is a genetically regulated process that degrades cellular proteins and organelles through lysosomes (6). There is a close and complex association between autophagy and tumors. Furthermore, the role of autophagy in different tumor types depends on the different stages of tumorigenesis. As the first identified mammalian autophagy protein, Beclin-1 has been used to investigate autophagy in cancer (7). However, to the best of our knowledge, there is no research into the association between Beclin-1 and cancer pathogenesis, and the nature of this association and its underlying mechanism remain controversial.
Bcl-2 is a member of the Bcl-2 anti-apoptotic protein family. In addition to being a key regulator of apoptosis, Bcl-2 modulates other important cell functions, such as cell cycle and mitochondrial signaling pathway (8). Additionally, Bcl-2 can bind to Beclin-1 to form the Beclin-1/Bcl-2 complex and then inhibit autophagy. However, the binding of Bcl-2 to Beclin-1 is regulated by a variety of proteins, which enhance or inhibit the Beclin-1/Bcl-2 interaction and further inhibiting or activating autophagy and apoptosis, thus the Beclin-1/Bcl-2 complex act as a crosstalk between autophagy and apoptosis (9). Previous studies have demonstrated that the expression levels of Beclin1 and Bcl-2 in tumor cells depend on the tumor and tissue type (10–12); however, this requires further investigation.
To the best of our knowledge, the association between Beclin-1 and Bcl-2 in lung cancer has not been yet elucidated. Therefore, the present study aimed to evaluate the roles of Beclin-1 and Bcl-2 on the clinicopathological features and survival of patients with NSCLC, and to estimate their value as markers of the development and prognosis of this type of cancer.
Materials and methods
Patients and tissue samples
A total of 120 patients with NSCLC who underwent surgical resection between January 2014 and December 2014 were selected from the archived materials of the Department of Pathology of Ruijin Hospital, Shanghai Jiaotong University School of Medicine (Shanghai, China). All patients were diagnosed by postoperative paraffin pathology, and no neoadjuvant treatment was performed. Patients younger than 18 or older than 75 years old, or patients with distant metastases were excluded from the present study. Of the 120 patients (median age, 61.5 years; age range, 45–75 years), 56 were male and 64 were female. The 2017 Union for International Cancer Control 8th Edition TNM staging (13) as used to classify tumors into stages I, II and III. All patients were followed-up until November 2019 (mean follow-up time, 60.1 months; range, 12–70 months). The present study was approved by the Clinical Ethics Committee of Ruijin Hospital, Shanghai Jiaotong University School of Medicine (approval no. 2017138).
Immunohistochemistry (IHC)
IHC staining was performed on 10% formalin-fixed and paraffin-embedded tumor and adjacent non-tumor tissues (≥3 cm from the margins of tumors). Slides of 4-µm sections were deparaffinized with xylene and antigen retrieval was accomplished by using microwave oven. The sections were then incubated in 3% hydrogen peroxide at room temperature for 15 min to block endogenous peroxidase activity. Slides were then incubated with anti-Beclin-1 (1:200; cat. no. SC-11427; Santa Cruz Biotechnology, Inc.) and anti-Bcl-2 (1:100; cat. no. ab32124; Abcam) primary antibodies at 4°C overnight. The slides were then washed three times in phosphate buffer solution (PBS) for 5 min each and incubated in biotin-labeled secondary antibodies (1:2,000; cat. no. ab205718; Abcam) for 30 min at 37°C. Images were captured with a light microscope. Immunohistochemical readings were performed by two different pathologists. Using a double-blind reading scoring system, five fields (magnification, ×400) were randomly selected and 100 cells were counted in each field. Discordant results were discussed and scored as follows. Score A: 1, ≤10% positive cells; 2, 11–50% positive cells; 3, 51–75% positive cells; and 4, >75% positive cells. The staining intensity was observed under low magnification (×100) and scored according to the staining intensity score B: 0, not stained; 1, light yellow; 2, brownish yellow; and 3, brownish. The final score was calculated as score A × score B. A final score ≤3 was considered low expression, while a score >3 was considered as high expression.
Assessment of clinical outcome
Overall survival (OS) was defined as the survival from the date of surgery to the date of death from any cause.
Statistical analysis
SPSS software (v18.0, SPSS, Inc.) was used to analyze the data. Pearson's χ2 test was used to evaluate the association between Beclin-1 and Bcl-2 expression with several clinicopathological variables. The Kaplan-Meier method was used to determine the probability of survival, and the data were analyzed using the log-rank test. The Cox proportional hazards model was used for univariate and multivariate analyses of prognostic factors. P<0.05 was considered to indicate a statistically significant difference.
Results
Beclin-1 and Bcl-2 expression in NSCLC lesions and adjacent tissues
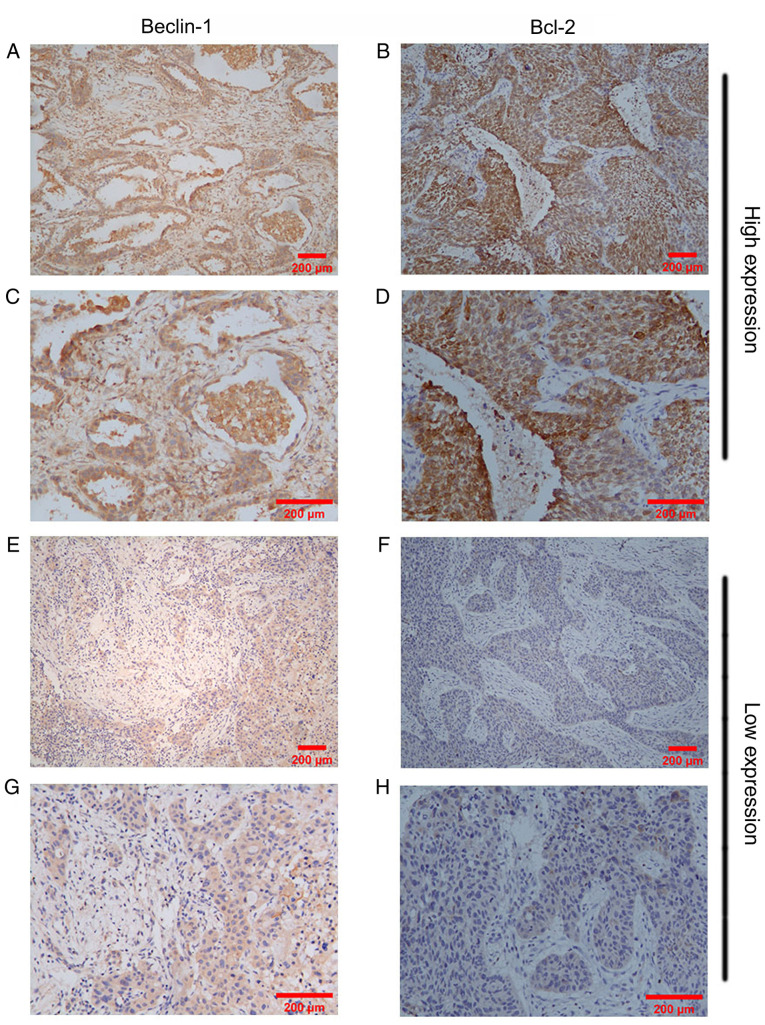
High Beclin-1 expression was observed in 38 (31.67%) NSCLC samples, while 82 (68.33%) NSCLC samples exhibited low Beclin-1 expression. The adjacent tissues presented with significantly higher Beclin-1 expression compared with the NSCLC tissues (P<0.01). By contrast, high Bcl-2 expression was identified in 55 (45.83%) NSCLC samples, while only 26 (21.67%) samples exhibited high Bcl-2 expression in adjacent tissues. NSCLC samples exhibited significantly higher Bcl-2 expression compared with their adjacent tissues (P<0.01; Table I). The expression levels of Beclin-1 and Bcl-2 in NSCLC tissues, as assessed via IHC, are shown in Fig. 1. High or low Beclin-1 and Bcl-2 expression was detected in NSCLC tissues.
Table I.
Beclin-1 and Bcl-2 expression in non-small cell lung cancer tissues and adjacent tissues (n=120).
| Beclin-1 expression | Bcl-2 expression | |||||||
|---|---|---|---|---|---|---|---|---|
| Tissue type | + | − | χ2 | P-value | + | − | χ2 | P-value |
| Lung cancer tissues | 38 | 82 | 7.63 | <0.01a | 55 | 65 | 15.67 | <0.01a |
| Adjacent tissues | 59 | 61 | 26 | 94 | ||||
Statistical significance (P<0.05).
Figure 1.
Immunohistochemical staining of Beclin-1 and Bcl-2 expression in non-small cell lung cancer tissues. (A) ×100 and (B) ×200 magnification for the observation of high Beclin-1 expression. (C) ×100 and (D) ×200 magnification for the observation of high Bcl-2 expression. (E) ×100 and (F) ×200 magnification for the observation of low Beclin-1 expression. (G) ×100 and (H) ×200 magnification for the observation of low Bcl-2 expression Scale bar, 200 µm.
Association between Beclin-1 and Bcl-2 expression
The association between Beclin-1 and Bcl-2 expression in NSCLC tissues was examined using χ2 tests, which revealed a strong association between the two proteins in NSCLC tissues (χ2=4.84; P<0.05; Table II).
Table II.
Association between Beclin-1 and Bcl-2 expression in non-small cell lung cancer tissues (n=120).
| Bcl-2 expression | ||||
|---|---|---|---|---|
| Beclin-1 expression | + | − | χ2 | P-value |
| + | 23 | 15 | 4.84 | 0.05a |
| − | 32 | 50 | ||
Statistical significance (P<0.05).
Association between Beclin-1 and Bcl-2 expression with the clinicopathological characteristics of patients with NSCLC
In the present study, the characteristics of the patients, such as age, sex, smoking history, pathological staging, lymph node metastasis, pathological type, degree of tumor differentiation and preoperative serum carcinoembryonic antigen (CEA) levels, obtained from the patients' medical records, were analyzed. All patients were divided into high and low expression groups regarding Beclin-1 or Bcl-2 expression. As shown in Table III, Beclin-1 expression in NSCLC was not associated with age, sex, smoking history, pathological type and preoperative serum CEA levels (P>0.05). However, Beclin-1 expression was associated with lymph node metastasis, pathological staging and degree of tumor differentiation (P<0.05). Furthermore, Bcl-2 expression in NSCLC was not associated with age, sex, smoking history, pathological staging, pathological type and preoperative serum CEA levels (P>0.05). However, Beclin-1 expression was associated with lymph node metastasis and the degree of tumor differentiation (P<0.05; Table IV).
Table III.
Association between Beclin-1 expression and clinicopathological characteristics in patients with non-small cell lung cancer (n=120).
| Beclin-1 expression | |||||
|---|---|---|---|---|---|
| Pathologic parameter | Cases, n | High | Low | χ2 | P-value |
| Sex | 0.46 | >0.05 | |||
| Male | 56 | 16 | 40 | ||
| Female | 64 | 22 | 42 | ||
| Smoking history | 2.62 | >0.05 | |||
| Yes | 54 | 13 | 41 | ||
| No | 66 | 25 | 41 | ||
| Age, years | 1.56 | >0.05 | |||
| ≤60 | 41 | 16 | 25 | ||
| >60 | 79 | 22 | 57 | ||
| Pathological stage | 6.54 | <0.05a | |||
| I | 59 | 25 | 34 | ||
| II | 42 | 10 | 32 | ||
| III | 19 | 3 | 16 | ||
| Lymph node metastasis | 4.53 | <0.05a | |||
| Positive | 45 | 9 | 36 | ||
| Negative | 75 | 29 | 46 | ||
| Pathological type | 0.89 | >0.05 | |||
| Adenocarcinoma | 83 | 28 | 55 | ||
| Squamous | 31 | 9 | 22 | ||
| Others | 6 | 1 | 5 | ||
| Degree of differentiation | 6.90 | <0.05a | |||
| Well | 26 | 12 | 14 | ||
| Moderate | 61 | 21 | 40 | ||
| Poor | 33 | 5 | 28 | ||
| Carcinoembryonic antigen, ng/ml | 1.99 | >0.05 | |||
| ≤5 | 55 | 21 | 34 | ||
| >5 | 65 | 17 | 48 | ||
Statistical significance (P<0.05).
Table IV.
Association between Bcl-2 expression and clinicopathological characteristics in patients with non-small cell lung cancer (n=120).
| Bcl-2 expression | |||||
|---|---|---|---|---|---|
| Pathologic parameter | Cases, n | High | Low | χ2 | P-value |
| Sex | 0.37 | >0.05 | |||
| Male | 56 | 24 | 32 | ||
| Female | 64 | 31 | 33 | ||
| Smoking history | 0.42 | >0.05 | |||
| Yes | 54 | 23 | 31 | ||
| No | 66 | 32 | 34 | ||
| Age, years | 1.16 | >0.05 | |||
| ≤60 | 41 | 16 | 25 | ||
| >60 | 79 | 39 | 40 | ||
| Pathological stage | 0.02 | >0.05 | |||
| I | 59 | 27 | 32 | ||
| II | 42 | 19 | 23 | ||
| III | 19 | 9 | 10 | ||
| Lymph node metastasis | 4.14 | <0.05a | |||
| Positive | 45 | 26 | 19 | ||
| Negative | 75 | 29 | 46 | ||
| Pathological type | 2.70 | >0.05 | |||
| Adenocarcinoma | 83 | 34 | 49 | ||
| Squamous | 31 | 18 | 13 | ||
| Others | 6 | 3 | 3 | ||
| Degree of differentiation | 9.02 | <0.05a | |||
| Well | 26 | 18 | 8 | ||
| Moderate | 61 | 21 | 40 | ||
| Poor | 33 | 16 | 17 | ||
| Carcinoembryonic antigen, ng/ml | 0.43 | >0.05 | |||
| ≤5 | 55 | 27 | 28 | ||
| >5 | 65 | 28 | 37 | ||
Statistical significance (P<0.05).
Univariate and multivariate analyses of OS in patients with NSCLC
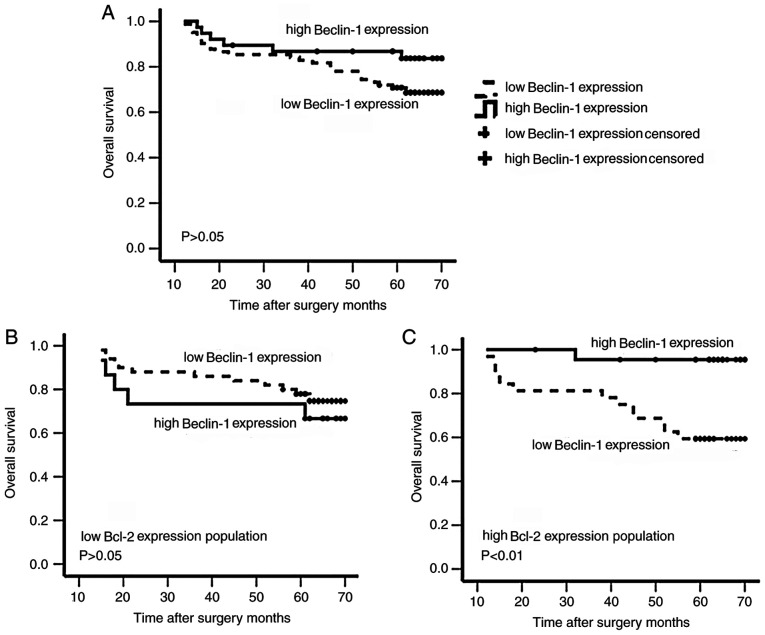
In the present study, the OS time of patients with NSCLC was available for 120 cases; the mean OS time was 60.10 months (range, 12–70 months). Further univariate and multivariate analyses were performed for the main factors associated with OS in patients with NSCLC. The results demonstrated that a lower OS was significantly associated with a more advanced stage (HR, 10.844; 95% CI, 3.885–30.256; P<0.01), poor differentiation (HR, 2.819; 95% CI, 1.425–5.575; P<0.01), high CEA levels (HR, 5.678; 95% CI, 1.035–31.156; P<0.05) and low Beclin-1 expression (HR, 5.319; 95% CI, 1.844–15.348; P<0.01) (Table V). The mean OS time of patients with NSCLC with high Beclin-1 expression was 63.17 months, while it was 58.72 months for patients with low Beclin-1 expression. However, this difference was not significant (63.17±2.75 vs. 58.72±2.15 months; P>0.05; Fig. 2A). Subsequently, the association between the combination of Beclin-1 and Bcl-2 expression status with OS was examined. Patients with NSCLC with high Beclin-1 expression and high Bcl-2 expression had a significantly longer mean OS time than those with high Bcl-2 but low Beclin-1 expression (68.27±1.69 vs. 54.53±3.81 months; P<0.05; Fig. 2C). In patients with low Bcl-2 expression, there was no significant difference in mean OS time according to Beclin-1 expression status (55.40±5.94 vs. 61.42±2.48 months for high and low Beclin-1 expression, respectively; P>0.05; Fig. 2B). However, patients with low Bcl-2 and low Beclin-1 expression tended to have an improved OS compared with patients with low Bcl-2 and high Beclin-1 expression.
Table V.
Univariate and multivariate analyses of prognostic factors in non-small cell lung cancer.
| Variable | Univariate analysis, HR (95% CI) | P-value | Multivariate analysis, HR (95% CI) | P-value |
|---|---|---|---|---|
| Sex | 2.977 (0.612–14.492) | 0.177 | ||
| Male | ||||
| Female | ||||
| Age, years | 1.132 (0.534–3.221) | 0.554 | ||
| ≤60 | ||||
| >60 | ||||
| Smoking history | 0.421 (0.093–1.910) | 0.262 | ||
| Yes | ||||
| No | ||||
| Degree of differentiation | 2.819 (1.425–5.575) | 0.003a | 2.114 (1.185–3.770) | 0.011a |
| Well | ||||
| Moderate and poor | ||||
| Pathological stage | 10.844 (3.885–30.265) | 5.328×10−6a | 13.707 (5.553–33.863) | 1.359×10−8a |
| I | ||||
| II and III | ||||
| Lymph node metastasis | 0.964 (0.295–3.156) | 0.952 | ||
| Positive | ||||
| Negative | ||||
| Pathological type | 1.221 (0.627–2.380) | 0.557 | ||
| Adenocarcinoma | ||||
| Squamous and others | ||||
| Beclin-1 expression | 5.319 (1.844–15.348) | 0.002a | 4.508 (1.753–11.591) | 0.002a |
| High | ||||
| Low | ||||
| Bcl-2 expression | 0.436 (0.166–1.144) | 0.092 | ||
| High | ||||
| Low | ||||
| Carcinoembryonic antigen, ng/ml | 5.678 (1.035–31.156) | 0.046a | 4.373 (0.958–19.954) | 0.057 |
| ≤5 | ||||
| >5 | ||||
Statistical significance (P<0.05). CI, confidence interval; HR, hazard ratio.
Figure 2.
The expression patterns of Beclin-1 and Bcl-2 were used in a survival analysis of patients with non-small cell lung cancer. Kaplan-Meier analysis of overall survival associated with Beclin-1 expression in (A) the whole study population, (B) the population with low Bcl-2 expression and (C) the population with high Bcl-2 expression.
Beclin-1 expression was an independent risk factor for OS in patients with NSCLC [hazard ratio (HR), 4.508; 95% CI, 1.753–11.591; P<0.01], whereas Bcl-2 expression was not an independent biomarker of OS (HR, 0.436; 95% CI, 0.166–1.144; P>0.05; Table V). Furthermore, the present study indicated that earlier pathologic stage (HR, 13.707; 95% CI, 5.553–33.863; P<0.01) and improved differentiation (HR, 2.114; 95% CI, 1.185–3.770; P<0.05) were associated with improved OS in patients with NSCLC (Table V).
Discussion
Autophagy and apoptosis, as type I and II programmed cell death, respectively, are closely associated with tumor progression. Studies of Beclin-1 and Bcl-2, which are key molecules that regulate these two types of programmed cell death, can help shed light on autophagy and apoptosis, as well as the role of cell death in NSCLC (14). The present study focused on the expression levels of Beclin-1 and Bcl-2 in NSCLC, as well as on the analysis of the association between the two proteins, to further explore the roles of autophagy and apoptosis on the biological and clinical behaviors of NSCLC. As a specific marker of autophagy, Beclin-1 has been the focus of previous research. Recent studies have reported that Beclin-1 is downregulated in glioblastoma, liver cancer, bladder cancer and breast cancer (15–18), while it is upregulated in colon cancer (19). The present study revealed that Beclin-1 expression was downregulated in NSCLC tissues, consistent with a study by Zheng et al (20), which demonstrated that the regulation of Beclin-1 may serve a role in the development of this type of cancer.
The association between Beclin-1 expression and the clinicopathological characteristics of patients has different manifestations in different types of tumor. In primary hepatocellular carcinoma, low Beclin-1 protein expression is associated with the degree of tumor cell differentiation and postoperative pathological stage, indicating a poor OS (21). In colon cancer, a meta-analysis of six studies has revealed that high Beclin-1 protein expression is associated with tumor metastasis and predicts a poor OS (22). The present study revealed that Beclin-1 was upregulated in the tumor tissues of 31.7% (38/120) of patients with NSCLC, whereas Beclin-1 was downregulated in 68.3% (82/120) of these patients. Compared with the normal tissues adjacent to the tumors, the positive expression rate of Beclin-1 was significantly lower in NSCLC than in adjacent tissues (χ2=7.63; P<0.01). A subsequent clinicopathological analysis revealed that low Beclin-1 expression was associated with the degree of tumor cell differentiation, postoperative pathological stage and lymphatic metastasis status in patients with NSCLC (P<0.05). Furthermore, a Cox regression analysis demonstrated that low Beclin-1 expression may be used as an independent risk factor for poor prognosis and as an independent predictor of prognosis in patients with NSCLC.
The Bcl-2 protein, an anti-apoptotic protein that helps inhibit apoptosis, has been identified as an oncogenic protein (23); the tumorigenic effect of Bcl-2 has been confirmed in animal model experiments (24). However, in some solid tumors, Bcl-2 appears to have an inhibitory effect, and its expression is associated with good prognostic characteristics, such as in gastric cancer (25) and colorectal cancer (26). A meta-analysis of ~5,892 patients with breast cancer from 17 studies examined the effect of Bcl-2 expression on breast cancer prognosis; its results revealed that Bcl-2 is associated with disease-free survival (DFS) and OS times (27). However, the mechanism via which Bcl-2 exerts its protective effect is unclear. The present study demonstrated that Bcl-2 expression was significantly higher in lung cancer tissues than in adjacent tissues (P<0.01). High Bcl-2 expression was associated with the degree of tumor cell differentiation and lymphatic metastasis in patients with NSCLC (P<0.05). However, a subsequent Cox regression analysis did not reveal its role as an independent risk factor for poor prognosis in patients with NSCLC. Therefore, the present results suggested that Bcl-2 may not be used as an independent predictor of prognosis in patients with NSCLC. However, this observation needs to be confirmed using a larger sample size in future studies.
Beclin-1 and Bcl-2 are the main factors underlying two programmed cell death mechanisms. The association between autophagy and apoptosis is complex and varies according to cell type and stress stage (28). Autophagy may initiate or inhibit apoptosis according to the environment and stimulation of the cell, and inhibition of autophagy may increase the sensitivity of the cell to apoptotic signals (29). Furthermore, the coordination between autophagy and apoptosis may serve an important role in tumorigenesis and tumor development. A previous study has indicated that in breast cancer Beclin-1 may serve a role in the inhibition of the development of breast cancer, which may be due to an interaction with the Bcl-2 protein (30). In pancreatic cancer, a study by Shanshan et al (10) demonstrated that high Bcl-2 and low Beclin-1 expression was associated with an improved DFS and OS. The present study further evaluated the expression levels of Beclin-1 in NSCLC tissue specimens with different expression levels of Bcl-2 via immunohistochemical staining.
It was revealed that in the high Bcl-2 expression group, low Beclin-1 expression in NSCLC tissues indicated a poor prognosis, while high Beclin-1 expression indicated an improved prognosis. In turn, in the low Bcl-2 expression group, Beclin-1 expression was not associated with the prognosis in patients with NSCLC (P>0.05). Therefore, the prognosis of NSCLC was closely associated with Beclin-1 expression only in the presence of high expression levels of Bcl-2. It was hypothesized that, regardless of the functional status of autophagy, tumor cells can be destroyed by apoptosis and that programmed cell death via autophagy may occur when apoptosis is inhibited; by contrast, when apoptosis is activated, autophagy may mainly serve a role in protecting tumor cells from apoptotic death. Nevertheless, the regulatory mechanisms of action behind the tumorigenesis and development of NSCLC are complicated and alternative pathways that are independent of apoptosis or autophagy, or independent of Bcl-2 and Beclin-1, may be involved. Therefore, further studies are required to confirm these observations. It should be noted that there is a limitation in the present study. The results about autophagy activity were only based on Beclin-1 expression, which was not used in conjunction with other autophagy markers, such as LC3-II, to assess autophagy.
In conclusion, the present study revealed that autophagy activity was decreased in NSCLC tumor tissues, and that Beclin-1 was downregulated and Bcl-2 was upregulated in the tumor tissues of these patients. Beclin-1 may be a promising prognostic marker for patients with NSCLC with high Bcl-2 expression. The current findings provide a more accurate prognostic assessment for patients with NSCLC. Additionally, they may be used to actively follow-up and promptly treat patients with a poor prognosis, which may benefit a large number of patients with NSCLC.
Acknowledgements
The authors would like to acknowledge Dr Ruyuan Zhang from Shanghai Ruijin Hospital (Shanghai, China) for his assistance on this manuscript.
Funding
The present study was partly supported by a grant from a project of the Shanghai Jiading District Health Committee (grant no. 2017KY02)
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
QC made contributions towards the conception and design. HD and LC are responsible for the collection of clinical pathological data and patient follow-up. FL carried out the immunohistochemistry experiments. XC and YL performed the statistical analysis. All authors were involved in the writing of the manuscript, and all authors read and approved the final manuscript.
Ethics approval and consent to participate
The present retrospective biomarker study was approved by the Clinical Ethics Committee of Ruijin Hospital, Shanghai Jiaotong University School of Medicine (approval no. 2017138; Shanghai, China). Written informed consent was provided by all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Nasim F, Sabath BF, Eapen GA. Lung cancer. Med Clin North Am. 2019;103:463–473. doi: 10.1016/j.mcna.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Cao M, Chen W. Epidemiology of lung cancer in China. Thorac Cancer. 2019;10:3–7. doi: 10.1111/1759-7714.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoy H, Lynch T, Beck M. Surgical treatment of lung cancer. Crit Care Nurs Clin North Am. 2019;31:303–313. doi: 10.1016/j.cnc.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 5.Mao Y, Yang D, He J, Krasna MJ. Epidemiology of lung cancer. Surg Oncol Clin N Am. 2016;25:439–445. doi: 10.1016/j.soc.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Cotzomi-Ortega I, Aguilar-Alonso P, Reyes-Leyva J, Maycotte P. Autophagy and its role in protein secretion: Implications for cancer therapy. Mediators Inflamm. 2018;2018:4231591. doi: 10.1155/2018/4231591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17:528–542. doi: 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schenk RL, Strasser A, Dewson G. BCL-2: Long and winding path from discovery to therapeutic target. Biochem Biophys Res Commun. 2017;482:459–469. doi: 10.1016/j.bbrc.2016.10.100. [DOI] [PubMed] [Google Scholar]
- 9.Rahman MA, Bishayee K, Habib K, Sadra A, Huh SO. 18α-Glycyrrhetinic acid lethality for neuroblastoma cells via de-regulating the Beclin-1/Bcl-2 complex and inducing apoptosis. Biochem Pharmacol. 2016;117:97–112. doi: 10.1016/j.bcp.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Song S, Wang B, Gu S, Li X, Sun S. Expression of Beclin 1 and Bcl-2 in pancreatic neoplasms and its effect on pancreatic ductal adenocarcinoma prognosis. Oncol Lett. 2017;14:7849–7861. doi: 10.3892/ol.2017.7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang LC, Huang SY, Zhang DS, Zhang SH, Li WG, Zheng PH, Chen ZW. Expression of beclin 1 in primary salivary adenoid cystic carcinoma and its relation to Bcl-2 and p53 and prognosis. Braz J Med Biol Res. 2014;47:252–258. doi: 10.1590/1414-431X20133231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baspinar S, Bircan S, Orhan H, Kapucuoglu N, Bozkurt KK. The relation of Beclin 1 and Bcl-2 expressions in high grade prostatic intraepithelial neoplasia and prostate adenocarcinoma: A tissue microarray study. Pathol Res Pract. 2014;210:412–418. doi: 10.1016/j.prp.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P, Mitchell A, Bolejack V, et al. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Xu HD, Qin ZH. Beclin 1, Bcl-2 and autophagy. Adv Exp Med Biol. 2019;1206:109–126. doi: 10.1007/978-981-15-0602-4_5. [DOI] [PubMed] [Google Scholar]
- 15.Guadagno E, Borrelli G, Pignatiello S, Donato A, Presta I, Arcidiacono B, Malara N, Solari D, Somma T, Cappabianca P, et al. Anti-apoptotic and anti-oxidant proteins in glioblastomas: Immunohistochemical expression of Beclin and DJ-1 and its correlation with prognosis. Int J Mol Sci. 2019;20:4066. doi: 10.3390/ijms20164066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun H, Yu J, Wen Z, Wang M, Chen W. Decreased expression of Beclin-1 in patients with hepatocellular carcinoma. J BUON. 2019;24:634–641. [PubMed] [Google Scholar]
- 17.Chen L, Liu Y, Zhang Q, Zhang M, Han X, Li Q, Xie T, Wu Q, Sui X. p53/PCDH17/Beclin-1 proteins as prognostic predictors for urinary bladder cancer. J Cancer. 2019;10:6207–6216. doi: 10.7150/jca.37335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Wang X, Wang G, Li Z, Wang J, Huang L, Qin Z, Yuan X, Cheng Z, Zhang S, et al. Integrating multiple omics data for the discovery of potential Beclin-1 interactions in breast cancer. Mol Biosyst. 2017;13:991–999. doi: 10.1039/C6MB00653A. [DOI] [PubMed] [Google Scholar]
- 19.Yang Z, Ghoorun RA, Fan X, Wu P, Bai Y, Li J, Chen H, Wang L, Wang J. High expression of Beclin-1 predicts favorable prognosis for patients with colorectal cancer. Clin Res Hepatol Gastroenterol. 2015;39:98–106. doi: 10.1016/j.clinre.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Zheng T, Li D, He Z, Feng S, Zhao S. Prognostic and clinicopathological significance of Beclin-1 in non-small-cell lung cancer: A meta-analysis. Onco Targets Ther. 2018;11:4167–4175. doi: 10.2147/OTT.S164987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu DM, Wang GL, Chen L, Xu YY, He S, Cao XL, Qin J, Zhou JM, Zhang YX, E Q. The expression of Beclin-1, an autophagic gene, in hepatocellular carcinoma associated with clinical pathological and prognostic significance. BMC Cancer. 2014;14:327. doi: 10.1186/1471-2407-14-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han Y, Xue XF, Shen HG, Guo XB, Wang X, Yuan B, Guo XP, Kuang YT, Zhi QM, Zhao H. Prognostic significance of Beclin-1 expression in colorectal cancer: A meta-analysis. Asian Pac J Cancer Prev. 2014;15:4583–4587. doi: 10.7314/APJCP.2014.15.11.4583. [DOI] [PubMed] [Google Scholar]
- 23.Qiu XG, Chen YD, Yuan J, Zhang N, Lei T, Liu J, Yang M. Functional BCL-2 rs2279115 promoter noncoding variant contributes to glioma predisposition, especially in males. DNA Cell Biol. 2019;38:85–90. doi: 10.1089/dna.2018.4318. [DOI] [PubMed] [Google Scholar]
- 24.Chi XX, Zhang T, Chu XL, Zhen JL, Zhang DJ. The regulatory effect of Genistein on granulosa cell in ovary of rat with PCOS through Bcl-2 and Bax signaling pathways. J Vet Med Sci. 2018;80:1348–1355. doi: 10.1292/jvms.17-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Cai H, Huang H, Long Z, Shi Y, Wang Y. The prognostic significance of apoptosis-related biological markers in Chinese gastric cancer patients. PLoS One. 2011;6:e29670. doi: 10.1371/journal.pone.0029670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Q, Li S, Cheng P, Deng M, He X, Wang Z, Yang CH, Zhao XY, Huang J. High expression of anti-apoptotic protein Bcl-2 is a good prognostic factor in colorectal cancer: Result of a meta-analysis. World J Gastroenterol. 2017;23:5018–5033. doi: 10.3748/wjg.v23.i27.5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Callagy GM, Webbe MJ, Pharoah PD, Caldas C. Meta-analysis confirms BCL2 is an independent prognostic marker in breast cancer. BMC Cancer. 2008;8:153. doi: 10.1186/1471-2407-8-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D'Arcy MS. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int. 2019;43:582–592. doi: 10.1002/cbin.11137. [DOI] [PubMed] [Google Scholar]
- 29.Yu Y, Li W, Ren L, Yang C, Li D, Han X, Sun Y, Lv C, Han F. Inhibition of autophagy enhanced cobalt chloride-induced apoptosis in rat alveolar type II epithelial cells. Mol Med Rep. 2018;18:2124–2132. doi: 10.3892/mmr.2018.9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Won KY, Kim GY, Kim YW, Song JY, Lim SJ. Clinicopathologic correlation of Beclin-1 and Bcl-2 expression in human breast cancer. Hum Pathol. 2010;41:107–112. doi: 10.1016/j.humpath.2009.07.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.