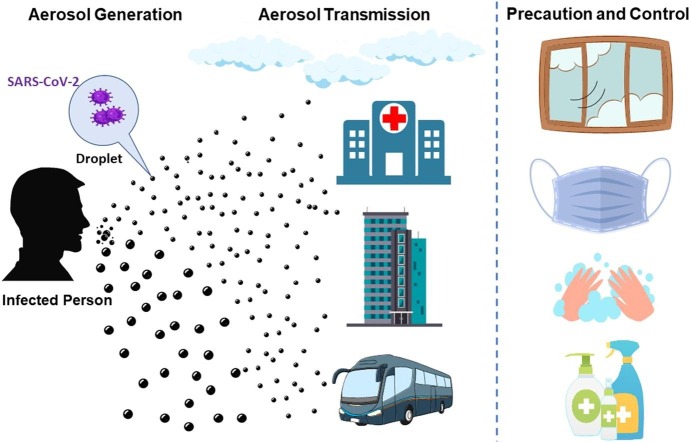
Graphical abstract
Keywords: COVID-19, Airborne transmission, Hospital, Precaution, Mask, Respiratory protection
Abstract
As public health teams respond to the pandemic of coronavirus disease 2019 (COVID-19), containment and understanding of the modes of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission is of utmost importance for policy making. During this time, governmental agencies have been instructing the community on handwashing and physical distancing measures. However, there is no agreement on the role of aerosol transmission for SARS-CoV-2. To this end, we aimed to review the evidence of aerosol transmission of SARS-CoV-2. Several studies support that aerosol transmission of SARS-CoV-2 is plausible, and the plausibility score (weight of combined evidence) is 8 out of 9. Precautionary control strategies should consider aerosol transmission for effective mitigation of SARS-CoV-2.
1. Introduction
An unprecedented pandemic of coronavirus disease 2019 (COVID-19) has created a global public health threat. As of August 2020, the cumulative number of confirmed cases of COVID-19 has exceeded 20 million, with over 740,000 deaths worldwide (WHO, 2020). SARS-CoV-2, which causes COVID-19, is the seventh coronavirus documented to infect humans. The guidance of different countries and organizations about modes of transmission of SARS-CoV-2 mostly stipulate the droplet, contact or fomite routes (MOH, 2020, CDC, 2020, MHLW, 2020, ECDC, 2020, WHO, 2020), except for China, which also stipulates the airborne route (NHC, 2020). There is growing evidence that in addition to contact and drople spread, the transmission of SARS-CoV-2 via aerosols is plausible under favorable conditions, particularly in relatively confined settings with poor ventilation and long duration exposure to high concentrations of aerosols, causing the World Health Organization (WHO) and other agencies to review their guidance. Recently WHO acknowledged aerosol transmission of SARS-CoV-2, especially in closed indoor settings, and that aerosol transmission could not be ruled out from some reported outbreaks (WHO, 2020). The aim of this review was to synthesize the evidence for aerosol transmission of COVID-19 and highlight the localities and vulnerable populations where SARS-CoV-2 aerosols may be particularly pertinent to COVID-19 transmission. Based on the synthesis of evidence, we summarized precautions and infection control strategies to mitigate the possible aerosol transmission of SARS-CoV-2, so as to inform scientific countermeasures for combatting COVID-19 globally.
1.1. Characteristics of viral aerosol transmission
Virus-containing body secretions and excreta can be aerosolized into infectious virus-containing droplets or particles through a variety of ways. Respiratory secretions are known to be aerosolized through daily activities (e.g. exhaling, talking, coughing, and sneezing) and medical procedures (e.g. tracheal intubation, non-invasive ventilation, bronchoscopy, and tracheotomy) (Zietsman et al., 2019). Excreta can also be aerosolized through toilet flushing (Johnson et al., 2013). Material that has deposited onto surfaces can be re-aerosolized by human activities (e.g. walking, cleaning a room, and door opening) (Khare and Marr, 2015). Biological specimens can be aerosolized through improper laboratory procedures (Mustaffa-Babjee et al., 1976). In all of these contexts, infectious aerosols can pose infection risks to people, influenced by complex environmental factors which affect the survival, transport and fate of aerosolized virus.
Aerosols are generally poly-dispersed droplets and particles which have many different sizes. Classical airborne aerosol hygiene research described droplets of respiratory secretions evaporating to become “droplet nuclei”, which remain suspend in air currents or turbulence and may drift away considerable distances (>1 m) (Keene, 1955). Modern researchers generally use the phrase “droplet nuclei” to refer to respiratory aerosol droplets with aerodynamic diameter <5 μm, and some disease transmission research now refers to respiratory droplets in this size range as “aerosols”. Particles and droplets with aerodynamic diameter <5 μm have the ability to readily penetrate deep into the alveolar region of the lungs of a bystander (Buonanno et al., 2020). In contrast, relatively large droplets are thought to arise from the upper respiratory tract and settle quickly and relatively close to their source. For example, 100 μm droplets take about 10 s, whereas 10 μm droplets take 17 min to fall to the floor (Knight, 1980), and 5 μm droplets originating from an average height (160 cm) of speaking or coughing take 9 min to reach the ground (Somsen et al., 2020). Droplets that settle more slowly have increased opportunity to travel in the air from the source. The 1 m limit of safe spatial separation is based on limited and dated epidemiologic and simulation studies of some selected infections (Feigin et al., 1982; Siegel et al., 2007), but more recent studies suggest droplets can travel much further than 2 m (Wei and Li, 2015, Parienta et al., 2011, Liu et al., 2017). For a person near the source, large droplets may project onto the facial mucous membranes or be inspired into the upper airways. Modern technology confirms that aerosolized respiratory secretions vary widely in size. The size and concentration of influenza virus aerosol droplets and particles to which a susceptible person may be exposed is mainly under 2.5 μm and an average person can generate over 500 particles per liter of air (Fabian et al., 2008, Yang et al., 2011, Milton et al., 2013).
Existing epidemiological and experimental research demonstrates a wide variety of respiratory viruses, including Severe Acute Respiratory Syndrome coronavirus (SARS-CoV), Middle East Respiratory Syndrome coronavirus (MERS-CoV), influenza virus, and norovirus, could be transmitted by aerosols under many conditions (de Wit et al., 2016, Xiao et al., 2018, Brankston et al., 2007, Lopman et al., 2012). A striking example of long range aerosol transmission inside a building and to adjacent buildings were the clusters of SARS cases at Amoy Gardens and Prince of Wales Hospital in Hong Kong in 2003 (Li et al., 2005, Chu et al., 2005, Yu et al., 2004, Lee et al., 2003). Influenza virus remains infectious in aerosols across a broad range of relative humidity (Coleman and Sigler, 2020), and this route has been used to explain transmission in hospitals and aircrafts (Moser et al., 1979; Blachere et al., 2009), which has been confirmed by epidemiological investigation, fluid dynamic models and animal models (Coleman and Sigler, 2020; Wong et al., 2010, Mubareka et al., 2009). Influenza virus was also identified in the air of an emergency department 3 h after an infectious patient has left the area (Blachere et al., 2009). In norovirus outbreaks in kindergartens and schools in China and the United Kingdom (Marks et al., 2003; Wu et al., 2012), the timing and spatial patterns of the cases were consistent with aerosol diffusion processes. Norovirus can also form aerosols during the floor cleaning and can be detected in air (Bonifait et al., 2015). For many pathogens, transmission is multi-modal, and the contribution of aerosol route may rely on the environmental conditions, proximity of susceptible people, human behavior, and other factors. A recent study detected exhaled aerosols which indicated the possibility of aerosol transmission through tidal breathing, in the absence of coughing, of seasonal human coronaviruses OC43, HKU1 and NL63 (Leung et al., 2020).
1.2. Evidence of SARS-CoV-2 aerosol transmission
To evaluate evidence of aerosol transmission of SARS-CoV-2, we apply the criteria of Jones and Brosseau (Jones and Brosseau, 2015), which are that aerosol transmission is plausible when (1) virus-containing aerosols are generated by or from an infectious person, (2) virus remains viable and infective in the aerosols for some period of time, and (3) the target tissues where virus initiates infection are accessible to the aerosol with enough load.
For the first criterion, it is established that infectious SARS-CoV-2 may be discharged into the surrounding environment through respiratory emissions, body fluids or excreta. SARS-CoV-2 genetic material and/or viable viruses have been frequently detected in throat swabs, anal swabs, conjunctival swabs, blood, sputum, feces, and urine of infected cases (Holshue et al., 2020, Guan et al., 2020, Xie et al., 2020, Wölfel et al., 2020, Jeong et al., 2020). Several studies show that the viral load of SARS-CoV-2 is higher in the lungs compared to the upper respiratory tract (Zou et al., 2020). This is consistent with smaller aerosolized particles being emitted from the lungs. Infections with a higher viral load in the upper respiratory tract may be more likely to be droplet spread. A cough can produce approximately 3000 droplets while a sneeze releases about 40,000 (Dhand and Li, 2020), most of which were small droplets (1–10 μm). During normal breathing and talking, 80–90% droplet sizes are <1 μm that are subject to aerosol transport (Morawska et al., 2009). Since breathing and speaking occur more frequently than coughs and sneezes, they have a critical role in viral transmission, particularly from asymptomatic cases. For COVID-19, the average virus RNA load in oral fluid was 7 × 106 copies/mL (Wölfel et al., 2020), but some patients may exceed that by more than two orders of magnitude. There is a 37% probability that a 50 μm droplet prior to dehydration contains at least one virus, and this probability is reduced to 0.37% for 10 μm droplets (Wölfel et al., 2020). Although very few particles actually carry viruses, the number of small particles far exceeds the number of larger sized droplets (Wölfel et al., 2020; Rothe et al., 2020). By using a laser light scattering observation, at an average viral load of 7 × 106 per mL (Wölfel et al., 2020), 1 min of loud speaking could produce thousands of oral droplets per second, of these at least 1000 virus-containing droplet nuclei that could remain airborne for more than 8 min (Stadnytskyi et al., 2020). Thus, these are likely to be inhaled by others and hence trigger new infections.
During COVID-19 pandemic, toilets are a daily necessity but may promote fecal-derived aerosol transmission if used improperly, particularly in hospitals (Ding et al., 2020). A fluid dynamics simulation suggests that during toilet flushing, massive upward transport of virus aerosol particles was observed, with 40–60% of particles rising above the toilet seat, leading to large-scale virus spread indoors (Li et al., 2020). Past tests confirmed that SARS-CoV-2 genetic material was found on toilets used by COVID-19 patients, in the air in hospital nurses’ stations, in air handling grate, on surfaces, on multiple air outlet vents, and in the air in patient rooms as well as airborne infection isolation rooms (AIIRs) in general wards (GW) (Chia et al., 2020, Santarpia et al., 2020, Ding et al., 2020, Jiang et al., 2020; Ong et al., 2020). A Singapore study revealed SARS-CoV-2 particles with sizes >4 μm and 1–4 μm containing a 1.8–3.4 viral RNA copies/m3 were found in two AIIRs rooms, despite these rooms having 12 air changes per hour (Chia et al., 2020). Swabs taken from air exhaust outlets in a Singapore hospital room of a symptomatic patient was positive, indicating small virus-laden aerosols have been displaced by airflows and deposited on vents (Ong et al., 2020). Moreover, study in Renmin Hospital, Fangcang Shelter Hospital, and surrounding public areas in Wuhan, China found traces of SARS-CoV-2 RNA in the air inside the patient mobile toilet room (19 copies/m3) and in medical staff areas (18–42 copies/m3 in protective apparel removal rooms) (Liu et al., 2020). The peak concentrations of SARS-CoV-2 RNA in air appear in two distinct size ranges of 0.25–1.0 μm and >2.5 μm aerodynamic diameter, indicating the virus-containing aerosols are small enough to remain suspended in air for a long period of time, and be inhaled (Liu et al., 2020). This study also documented SARS-CoV-2 virus on protective apparel or floor surface, which was found to be resuspended as a source of aerosols by the movements of medical staff. Another study in Huoshenshan Hospital in Wuhan has a similarly finding showing that contamination of SARS-CoV-2 was greater in intensive care units (ICU) than GW with a widely distribution on surfaces of floors, computer mice, trash cans, and sickbed handrails as well as in air about 4 m (13 feet) from patients (Guo et al., 2020). Floor swab samples of ICU showed relatively high positive rates of 70%, indicating virus droplets from the aerosol falling due to gravity and air flow to the floor (Guo et al., 2020). In addition, SARS-CoV-2 RNA was found on airborne particulate matter (PM) obtained over a 3-week period from an industrial site of Bergamo Province, Italy (Setti et al., 2020). A limitation of studies measuring SARS-CoV-2 virus in the environment to date is the reliance on polymerase chain reaction (PCR) to identify and quantify the virus, in part owing to ease of PCR analyses relative to culture for SARS-CoV-2. However, a very recent study firstly pointed out that viable SARS-CoV-2 has been detected in the air in hospital wards with COVID-19 patients by using cell culture method (Santarpia et al., 2020).
For the second criterion, the viability of SARS-CoV-2 has been demonstrated experimentally in air and on surfaces. As a hypothetical example, after 7 days, SARS-CoV-2 could still be found viable on the outer layer of a surgical mask (22 ℃; relative humidity 65%) (Chin et al., 2020). SARS-CoV-2 can survive for more than 3 h in the air, with a half-life of 1.1 h in aerosols (21–23 °C; relative humidity 65%) (van Doremalen et al., 2020). A more recent study found a UK variant of SARS-CoV-2 could remain viable in aerosols for at least 90 min under experimental conditions (artificial saliva and tissue culture media) (Smither et al., 2020). Another study suggests SARS-CoV-2 in respirable-sized aerosols could persist and maintain infectivity for up to 16 h (Fears et al., 2020). Santarpia et al. have reported measuring viable SARS-CoV-2 in air collected in hospital wards with COVID-19 patients(Santarpia et al., 2020), which consistent with detection of airborne SARS-CoV-2 RNA in patient areas. Altogether, these results indicate that SARS-CoV-2 could survive in aerosols for a relative long time under favorable conditions and potentially spread through aerosols.
For the third criterion, epidemiological studies are difficult to interpret with respect to role of transmission unless other routes can be ruled out. In particular, when people are close together, they can be simultaneously exposed to an infectious disease through multiple routes. However, by analyzing the trend and mitigation measures in Wuhan of China, Italy, and New York City of USA, a recent study indicated airborne transmission contributed to the spread of COVID-19 (Zhang et al., 2020). Some outbreaks of COVID-19 in which aerosol transmission may have a role are summarized in Table 1 . For example, on Feb 3, 2020, in Inner Mongolia of China, a case of COVID-19 was reported in a person who passed the door of a symptomatic patient several times but did not have direct contact, suggesting airborne transmission (Wang and Du, 2020). Another study compared risks of COVID-19 outbreak among 126 passengers taking two buses (59 from Bus #1 and 67 from #2) on a 100-minute round trip in Ningbo, Zhejiang Province (Shen et al., 2020). Compared to individuals in the non-exposed bus (Bus #1), those in the exposed bus (Bus #2) were 41.5 times more likely to be infected. Evidence from this outbreak suggesting airborne transmission of SARS-CoV-2, particularly in this closed environment with air re-circulation and no contact between passengers. Air-conditioning ventilation also explained the aerosol transmission of a outbreak among diners at adjacent tables following the direction of airflow in a restaurant at Guangzhou, China. The distances between patient zero and patients at other tables in this outbreak were all >1 m, and in the review of video records from the restaurant, no evidence of direct or indirect contact were found between the three parties (Lu et al., 2020). An experimental study also showed that high quanta emission rates can be reached by an asymptomatic infectious SARS-CoV-2 subject performing vocalization during light activities, which highlight the key role played by airflow in indoor environments (Buonanno et al., 2020). In February 2020 in Guangzhou, Guangdong Province, China, SARS-CoV-2 RNA was found on surface samples (e.g. sink, faucet, and shower handle) collected from a bathroom in a long-vacant 16th floor apartment, which was right above the bathroom of the apartment of five persons with COVID-19 (confirmed between Jan 26 and 30; Source: unpublished data from China CDC). The possibility of aerosol transport through sewage pipe after flushing the toilet at the 15th floor restroom was confirmed by an onsite tracer simulation experiment showing that aerosols were found in the restroom of apartments on the 25th floor (two cases confirmed on Feb 1) and 27th floor (two cases were confirmed on Feb 6 and 13, respectively) of the building (Source: unpublished data from China CDC). Although transmission via the shared elevator cannot be excluded, this event is consistent with the findings of the Amoy Gardens SARS outbreak in Hong Kong in 2003 (Chu et al., 2005, Lee et al., 2003, Li et al., 2005, Yu et al., 2004).
Table 1.
Empirical and laboratory studies indicating the possible aerosol transmission of SARS-CoV-2 to as July 30th, 2020.
| Type | Place | Date | Main Finding(s) | Country | Reference(s) |
|---|---|---|---|---|---|
| Environmental samples | Apartment | 2020/Feb/14 | SARS-CoV-2 was detected in surface samples (e.g. sink, faucet, and shower handle) of the restroom in a long-term vacant apartment at 16-floor, which is right above the restroom of an apartment with five persons with COVID-19 (confirmed between Jan 26 and 30) at 15-floor in Guangzhou. The possibility of aerosol diffusion through sewage pipe after flushing the toilet at the 15-floor restroom was further confirmed by an onsite tracer simulation experiment showing aerosols were found in the restroom of apartments at 25-floor (two cases confirmed on Feb 1) and 27-floor (two cases confirmed on Feb 6 and 13) | China | Unpublished data of China CDC |
| Lab | 2020/Mar/17 | SARS-CoV-2 remained viable in aerosols throughout the duration of experiment (3 h) | USA | NEJM (van Doremalen et al., 2020) | |
| Lab | 2020/Apr/13 | SARS-CoV-2 maintained infectivity in aerosols for up to 16 h | USA | Emerg Infect Dis (Fears et al., 2020) | |
| Hospital | 2020/Feb/25 | SARS-CoV-2 was detected on the surfaces of the nurse station in the isolation area with suspected patients and in the air of the isolation ward with an intensive care patient at the First Hospital of Jilin University | China | MedRxiv (Jiang et al., 2020) | |
| Hospital | 2020/Mar/4 | Samples with 13 (87%) of 15 room sites (including air outlet fans) and 3 (60%) of 5 toilet sites (toilet bowl, sink, and door handle) were positive by using RT-PCR at the dedicated SARS-CoV-2 outbreak center in Singapore | Singapore | JAMA (Ong et al., 2020) | |
| Hospital | 2020/Mar/8 | Deposition samples inside ICU and air sample in Makeshift Hospital patient toilet were positive for SARS-CoV-2. SARS-CoV-2 was also found in the air in outdoor areas at the hospital entrance and in front of a department store | China | Nature (Liu et al., 2020) | |
| Hospital | 2020/Mar/8 | Of the 163 surface and aerosol samples collected in the University of Nebraska Medical Center (UNMC), 126 (77.3%) had a positive result for SARS-CoV-2 with the highest concentration from an air handling grate. In room air samples were 63.2% positive with mean concentration 2.86 copies/L of air. Viable SARS-CoV-2 has been detected in the air in hospital wards using cell culture method | USA | Sci Rep (Santarpia et al., 2020) | |
| Hospital | 2020/Apr/3 | Of 107 surface samples, 4 samples were positive (2 ward door door-handles, 1 bathroom toilet toilet-seat cover and 1 bathroom door door-handle). Three were weakly positive from a bathroom toilet seat, 1 bathroom washbasin tap lever and 1 bathroom ceiling exhaust louvre. One of the 46 corridor air samples was weakly positive, and virus was also found on the surface of the exhaust grilles in the bathroom | China | medRxiv (Ding et al., 2020) | |
| Hospital | 2020/Apr/7 | Air sampling is performed in three of the 27 AIIRs in the general ward, and detects SARS-CoV-2 positive particles of sizes > 4 μm and 1–4 μm in two rooms, despite these rooms having 12 air changes per hour | Singapore | Nat Commun (Chia et al., 2020) | |
| Hospital | 2020/Apr/10 | A study in Huoshenshan Hospital in Wuhan found contamination was greater in ICU than general wards. Virus was widely distributed on surface samples of floors, computer mice, trash cans, and sickbed handrails and was detected in air samples ≈4 m from patients | China | Emerg Infect Dis (Guo et al., 2020) | |
| Outdoor air | 2020/Apr/15 | SARS-CoV-2 RNA was detected on outdoor particulate matter (PM) for the first time, suggesting that, in conditions of atmospheric stability and high concentrations of PM, SARS-CoV-2 could create clusters with outdoor PM and, by reducing their diffusion coefficient, enhance the persistence of the virus in the atmosphere | Italy | Environ Res (Setti et al., 2020) | |
| Cases | Public transportation | 2020/Mar/4 | 24 of 68 people were infected at a bus in Ningbo City, Zhejiang Province. Compared to individuals in the non-exposed bus (Bus #1), those in the exposed bus (Bus #2) were 41.5 (95% CI, 2.6–669.5) times more likely to be infected with COVID-19 | China | ResearchGate (Shen et al., 2020) |
| Public transportation | 2020/Feb/11 | Two died and at least 103 people were infected among 1,111 crew and 2,460 passengers in Grand Princess cruise ship | USA | US CDC (Moriarty et al., 2020) | |
| Apartment | 2020/Feb/3 | In Inner Mongolia, a case of COVID-19 reported positive when a person has passed the door of a symptomatic patient several times | China | Ir J Med Sci (Wang and Du, 2020) | |
| Shopping Mall | 2020/Jan/21 | 40 people were infected at a shopping mall in Tianjin | China | Chinese Journal of Epidemiology (Wu et al., 2020) | |
| Shopping Mall | 2020/Jan/21 | Indirect Virus Transmission (e.g. virus aerosolization in a confined public space such as restrooms or elevators) in Cluster of COVID-19 Cases in Wenzhou | China | Emerg Infect Dis (Cai et al., 2020) | |
| Restaurant | 2020/Jan/26 | Air-conditioned ventilation explained the aerosol transmission of an outbreak in a restaurant in Guangzhou, China. The distances between patient zero and others in this outbreak were all >1 m | China | Emerg Infect Dis (Lu et al., 2020) | |
| Choir | 2020/Mar/10 | A superspreading events occurred in a 2.5 h choir rehearsal at Skagit Valley Chorale (SVC) of Mount Vernon, WA of USA where 53 out of 61 attendees were infected and two were dead, even though adequate caution measures for fomite and droplet transmission being taken and none presented symptoms. | USA | MedRxiv (Read, 2020;Miller et al., 2020) |
Airborne route appeared to be a major contributor for superspreading events in a 2.5 h choir rehearsal on March 10, at Skagit Valley Chorale (SVC) of Mount Vernon, WA, USA where 53 out of 61 attendees were infected and two died, even though adequate precautions for fomite and droplet transmission were taken and no-one had symptoms (Read, 2020; Miller et al., 2020). It is suspected that during singing, the forceful exhalation and inhalation may have aerosolized SARS-CoV-2, leading to high levels of disease transmission. This indoor transmission risk may have been increased because of high occupancy, long duration, loud vocalization, and poor ventilation (Miller et al., 2020). A recent study addressed the potential long distances covered by SARS-CoV-2 through cough and sneeze and revealed that small droplets, emitted during a sneeze, could reach distances of 7–8 m (Bourouiba, 2020). Similarly, Paules et al. recently demonstrated that the airborne transmission of SARS-CoV-2 may occur in addition to close contact transmission (Paules et al., 2020). On April 1, 2020, National Academy of Sciences (NAS) committee on emerging infectious diseases and 21st century health threats letter has pointed out that “While the current SARS-CoV-2 specific research is limited, the results of available studies are consistent with aerosolization of virus from normal breathing” (Medicine, 2020). In addition, there have been some other outbreaks, mostly involving confirmed cases in relatively confined or crowded environments (e.g. hospitals, shopping malls, public transportation vehicles, offices, and prisons) (Table 1). For example, a case study of South China Seafood Market (Zhang et al., 2020) showed that the median risk of a customer acquiring SARS-CoV-2 infection via the aerosol route after 1 hr exposure in the market with one infected shopkeeper was about 2.23 × 10−5. With the assumption of one infected shopkeeper in the market, the 97.5% percentile infection risk by aerosol transmission was about 2.34 × 10−4 and could be reduced to about 10−4 with a ventilation rate of 1 ACH, for customers with 1 h exposure in poorly ventilated markets. The risk was about 5–10 times lower than the manageable risk (1.17 × 10−3), but it could be increased by several times if multiple infected shopkeepers were simultaneously in the market, becoming close to the manageable risk. Poor ventilation for a relatively long time, and lack of mask use may have increased the risk of aerosolized infection. Taken together, this suggests the possibility of aerosol transmission, especially in confined settings after exposure to high concentrations of viral aerosols for a long time.
A biological argument can also be made for COVID-19 transmission through aerosols. Like SARS-CoV, SARS-CoV-2 binds the ACE-2 receptor to gain entry into human cells (Chowdhury and Maranas, 2020, Letko et al., 2020). SARS-CoV-2 has a higher affinity for this receptor than SARS-CoV (Wrapp et al., 2020). The ACE-2 receptor is widely expressed in some types of human epithelial cells in the respiratory tract, including alveolar type 2 cells (Smith and Sheltzer, 2020) and transient secretory cells in the subsegmental bronchial branches (Lukassen et al., 2020), which are in the lower respiratory tract. Thus, it is biologically plausible that inhaled SARS-CoV-2 aerosol can gain direct access to alveolar surface ACE-2 receptors and initiate infection in the lung under suitable biological, physical and environmental conditions (Xu et al., 2020). Further, a Rhesus macaque exposed to SARS-CoV-2 via intratracheal instillation had greater viral replication in the lungs than animals exposed to the virus via ocular conjunctiva and intragastric routes (Deng et al., 2020).
Recent studies in animal models have demonstrated SARS-CoV-2 transmission in the absence of contact between animals, including among naive ferrets (Kim et al., 2020), golden Syrian hamsters(Chan et al., 2019, Sia et al., 2020), and mice (Bao et al., 2020). In addition, placing surgical mask material between animal cages significantly reduced the transmission of SARS-CoV-2 from experimentally-infected hamsters to the naive hamsters (Chan et al., 2019). These studies in animal models affirm that SARS-CoV-2 infection can be transmitted through the air, in the absence of contact.
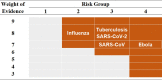
We ranked the plausibility (weight of evidence) of aerosol transmission of SARS-CoV-2 as 8 out of 9 (Table 2 ), according to the criteria assigned by Jones and Brosseau (Jones and Brosseau, 2015). This is based on the following three conditions. First, aerosol generation was ranked level 3 (out of 3) because viable SARS-CoV-2 has been founded in the air around COVID-19 patients; extensive studies have found SARS-CoV-2 genetic material in the air around patients and after toilet flushing. Second, viability in the environment was ranked level 2 (out of 3) because viable SARS-CoV-2 has been found to survive in aerosols for 16 h and there is epidemiologic evidence of aerosol transmission in a variety of settings such as between apartments, within a restaurant, a choir, and a bus (Table 1). Third, access to target tissue was ranked level 3 (out of 3) because SARS-CoV-2 can reach receptors in the respiratory tract, where the ACE-2 receptor is located, through inhalation and animal models have demonstrated SARS-CoV-2 transmission in the absence of contact, as well as replication in the respiratory tract.
Table 2.
The plausibility of aerosol transmission (Weight of Evidence) of SARS-CoV-2 with consequences of infection (Risk Group) according to the criteria of Jones and Brosseau (Jones and Brosseau, 2015). High concern indicated by dark orange and low concern by light gray.
 |
Current evidence on SARS-CoV-2 has limitations, but is strongly indicative of aerosols as one of several routes of COVID-19 transmission. It should be noted that the equivalent evidence for contact and large droplet transmission is not available, but has been an unproven assumption from the outset. Epidemiologic and experimental data continues to be obtained at a rapid pace, and the role of aerosols in COVID-19 transmission should be revisited in light of the emerging evidence. Considering the high percentage of asymptomatic and pre-symptomatic individuals among COVID-19 patients (Oran and Topol, 2020), well people may contact with aerosols produced by infected people even though they do not cough or sneeze to any appreciable extent. This leaves direct or indirect contact modes and aerosol transmission as the main possible modes of transmission of SARS-CoV-2 (Asadi et al., 2020). Future work should consider more wide-spread use of culture-based detection methods to detect SARS-CoV-2 in the environment, transmission dynamics of SARS-CoV-2 aerosols in the environment, SARS-CoV-2 emission from infected persons, and exposure modeling and microbial risk assessment to characterize the relative importance of different transmission routes and impact of environmental conditions and of different groups (e.g. age, gender, etc.) in transmission (Cowling and Leung, 2020). We believe that the evidence to date, however, is ample to acknowledge and address the aerosol transmission of COVID-19 in healthcare settings, other workplaces, and in the community.
2. Precautionary and control strategies
Precautionary control strategies that are important for public health protection are needed to avoid aerosol transmission of SARS-CoV-2. As long duration of viral shedding was reported in asymptomatic cases (Zhou et al., 2020; Tan et al., 2020), with high infectivity relative to symptomatic cases (Chen et al., 2020), the virus could spread via aerosols during breathing and talking before awareness is triggered by symptoms. This poses risks, particularly in confined and poor ventilated environments with prolonged person to person contact. Settings with a large proportion of infected people or contaminated samples, such as hospitals, healthcare institutions and laboratories are the highest risk, especially to HCWs, who should be provided airborne precautions. The general public and vulnerable populations should be made aware that confined, crowded and poor ventilation environments may pose a medium risk when an infected person is present. Prevention and control countermeasures are proposed to reduce the potential aerosol transmission under different occasions (Table 3 ).
-
(1)
Control and elimination of aerosol transmissions
Table 3.
Precautionary and control strategies to mitigate the possible aerosol transmission of SARS-CoV-2 under different occasions based on risks. The classification is mainly based on the population density, environmental hygiene quality, occupational characteristics, and accessibility of PPE and hand hygiene products.
| Places | Scenarios | Key Populations | Risk | Suggestions |
|---|---|---|---|---|
| Hospitals and healthcare settings | Closed environment with frequent AGPs (e.g. ICU, ward, emergency department, operating room) | Healthcare workers (e.g. doctors and nurses), patients and staff | High | Proper PPE (e.g. gloves, eye protection, N95 respirator, and fluid repellent long sleeved gown) |
| Scrupulous hand-washing and personal hygiene | ||||
| AGPs performed by qualified personnel | ||||
| Isolating suspected patients in single rooms with negative pressure | ||||
| Ultraviolet systems, ionization units or air filtration devices (HEPA) for air cleaning and disinfection Increasing outdoor air ventilation rates Managing air flow direction and speed Avoiding air recirculation | ||||
| Thorough environmental cleaning and disinfection process | ||||
| Avoidance of patients/visitors/staff who have or have been exposed to COVID-19 entering hospitals without a reason | ||||
| Minimizing unnecessary patient contact during patient care | ||||
| Appropriate waste management | ||||
| Education, training and seminars | ||||
| Laboratory | Viral detection and research | Phlebotomists collecting samples and laboratory professionals | High | Proper PPE (e.g. gloves, eye protection, N95 respirator, and fluid repellent long sleeved gown) |
| Operate in proper bio-safety level of laboratories (PC3, BSL3 and higher) | ||||
| Operations follow SOPs | ||||
| Ultraviolet systems, ionization units or air filtration devices (HEPA) can be used Managing air flow direction and speed Avoiding air recirculation | ||||
| Appropriate waste management | ||||
| Practice of PPE wearing | ||||
| Education, training and seminars | ||||
| Public transportation Vehicles or naval vessels | Closed and crowded settings (e.g. subway, airplane, cruise ship, bus, train, aircraft carrier) or naval vessels | Public, crew/drivers/pilot, passengers or soldiers | Medium | Avoiding over-crowding Wearing facial masks |
| Adequate natural or mechanical ventilation | ||||
| Increase the frequency of disinfection of public utilities, floors and surfaces | ||||
| Reduce use of central AC, increase fresh air volume when use, use filters with antimicrobial function or HEPA in ventilation systems | ||||
| Cover the lid before flushing the toilet | ||||
| Timely collection and clean of garbage and disinfection of garbage container | ||||
| Inform relevant knowledge through media or display screens and billboards (e.g. etiquette of cough and mask wearing) | ||||
| Public places | Closed and crowded with mobile population (e.g. shopping mall, bar, restaurant, club, hotel, bank, conference room, and cinema) | Staff, customer or public | Low-Medium | Wearing facial masks |
| Reduce social activities, avoid crowded and poorly ventilated spaces | ||||
| Adequate natural or mechanical ventilation | ||||
| Reduce the use of central AC, increase fresh air volume when use | ||||
| Cover the lid before flushing the toilet | ||||
| Flow control | ||||
| Timely disinfection (e.g. surfaces, floors, and elevator buttons) | ||||
| Timely collection and clean of garbage and disinfection of garbage container | ||||
| Maintain social distance | ||||
| Hand hygiene | ||||
| Closed and crowded with fixed population (e.g. office,) | Staff | Low-Medium | Wearing facial masks | |
| Adequate natural or mechanical ventilation | ||||
| Reduce the use of central AC, increase fresh air volume when use | ||||
| Maintain social distance | ||||
| Reduce face-to-face meetings | ||||
| Hand hygiene | ||||
| Restrooms | Public restroom (e.g. in hotel, shopping mall, markets, hospital) | Passenger or customer | Medium | Wearing facial masks, Close the lid before flushing |
| Adequate natural or mechanical ventilation | ||||
| Increase the frequency of disinfection of public utilities, floors and surfaces | ||||
| Cleaning and disinfection processes | ||||
| Residential restroom | Household members | Low-Medium | Increase the frequency of disinfection | |
| Adequate natural or mechanical ventilation, fresh air system is recommended | ||||
| Drains in floors and other sanitary devices should have adding water to ensure water frequently to ensure seals work all time | ||||
| Specific places | Fixed population (e.g. prison, church, military barracks) | Prisoner, staff or believers | Low-Medium | Adequate natural or mechanical ventilation, fresh air system is recommended |
| Reduce use of central AC | ||||
| Education | ||||
| Reduce gathering activities, reduce face-to-face meetings | ||||
| Wearing mask (apply to staff or visitors) | ||||
| Timely disinfection (e.g. surfaces, floors, and elevator buttons) | ||||
| Personal hygiene | ||||
| Fixed but vulnerable population (e.g. orphanage, school, nursing home, kindergarten) | Children or older people | Low-Medium | Adequate natural or mechanical ventilation, fresh air system is recommended | |
| Reduce use of central AC | ||||
| Education and seminars | ||||
| Personal hygiene | ||||
| Timely disinfection (e.g. surfaces and floors) | ||||
| Wearing mask (apply to staff) | ||||
| Reduce gathering activities in closed environment | ||||
| Mobile population (e.g. slums) | Poor or unhealthy people | Low-Medium | Adequate natural or mechanical ventilation, fresh air system | |
| Personal hygiene | ||||
| Timely disinfection (e.g. surfaces and floors) | ||||
| Reduce gathering activities in closed environment |
In the hospital and healthcare settings, ventilation is a primary control strategy for infectious diseases, which promotes the air dilution around a source and the removal of respiratory viruses (Francisco et al., 2014). In an optimally ventilated room, the number of droplets could halved after 30 s, whereas with poorly ventilated and no ventilation rooms this could take 1–4 min and 5 min, respectively (Somsen et al., 2020). Diagnosis and subsequent isolation measures should be arranged rapidly using single rooms with negative pressure and ventilation capacity (e.g. AIIR). Infected patients should ideally be placed in single rooms, but it is acceptable to co-locate with infected patients. Unless necessary, patients should be restricted to their room and keep windows and doors closed. If not in AIIR (e.g. transport), infected or suspected patients should wear facial masks to as a physical barrier to droplets or aerosols (Bourouiba, 2020). Education of patients is necessary to encourage adherence to guidelines. HCWs should be provided respirators and airborne precautions, and the precautionary principle should be followed to protect their occupational health and safety (Campell, 2006). This means proper protection for HCWs should not await scientific certainty, a major lesson from the SARS commission which investigated the nosocomial outbreak of SARS in Toronto in 2003, where over 300 HCWs denied a respirator were infected, and three died.
Safety-compliant operation is necessary in PC3 or BSL3 and higher virus laboratories, and should be considered for COVID-19 wards. Ultraviolet systems, ionization units or air filtration devices (HEPA) can be used for air cleaning to effectively reduce the hazardous viral aerosol concentrations in high-risk areas (REHVA, 2020). This is evident from a recent study demonstrating that UVB levels representative of natural sunlight rapidly inactivate SARS-CoV-2 on surfaces (Ratnesar-Shumate et al., 2020). Surface sanitization of apparel before removal may help reduce the infection risk for medical staff, and alcohol or chlorine-containing disinfectants could be used to keep floors or surfaces clean that may also help reduce secondary aerosol transmission. Design of joint anterooms as an additional buffer between common areas and protected spaces can be considered in future (Dietz et al., 2019).
In public settings, there is wide variety in the design and use of ventilation systems, with many settings focusing on comfort, not the control of airborne contaminants. In general, ventilation will clear the viral aerosols fairly quickly (Cook, 2020). Therefore, adequate natural ventilation, reduced use of central air conditioning, increasing air exchange rates, and use of common or antimicrobial filters in ventilation systems are recommended. The frequency of disinfection of public transportation vehicles should be increased, as virus was found 17 days later on surfaces in the Diamond Princess cruise ship. Restrooms, owing to the shedding of SARS-CoV-2 in fecal material and aerosolization during toilet flushing, should be thoroughly cleaned regularly (e.g. ventilation and sterilization). If the toilet seat is equipped with a lid, it is recommended to close the lid before flushing the toilet, especially in hospitals. Floor drains and other outlets of sewer should have adding water frequently to ensure seals work at all time. The role of sewer pipes in aerosol transmission should also be taken into account in future architectural design. In slums, inadequate sewage and drainage systems may increase the risk of formation of aerosol and spread. Hence, disinfection processes should be conducted frequently.
-
(2)
Protection of HCWs and laboratory workers
Frontline HCWs who come in direct contact with potentially infected patients, such as doctors, nurses, allied health workers, phlebotomists collecting medical laboratory specimens, food service staff, cleaners and laboratory professionals in open-space laboratories should wear proper personal protective equipment (PPE) (Tellier et al., 2019), specifically waterproof gowns, N95/KN95 (and above) particle protective respirator or powered air purifying respirators, face shields or goggles, and gloves, in addition to the usual contact-transmission prevention precautions (e.g. handwashing and respiratory hygiene) to avoid potential infection. Recommendations that restrict airborne precautions only to Aerosol Generating Procedures (AGPs, e.g., intubation, bronchoscopy, physiotherapy, and suctioning) and stipulate surgical masks for general care of COVID-19 patients will likely place HCWs at occupational risk of infection in COVID-19 wards. In other areas of hospitals, universal face mask use can reduce the risk of transmission. N95 masks could block nearly all outward emissions of pseudo SARS-CoV-2 (avian influenza virus) in aerosol, while standard medical masks blocked about 97% of the virus (Ma et al., 2020). Earlier implementation of prevention can drastically lower the risk to other staff and patients. AGPs should be performed by the most qualified personnel in the room with a minimal number of personnel present. The use and exposure to any respiratory assistance devices (high-flow oxygen masks, nebulizers) should be managed by only allowing their use in designated, containment areas by staff using airborne precautions. Virus positivity could be higher when PPE was worn for longer duration or after HCWs cared for many patients. As such, special protocols and precautions are necessary when removing PPE. Education of guidelines and barrier precautions is necessary for the protections of HCWs.
-
(3)
Protection of general public and vulnerable populations
The Federation of European Heating, Ventilation and Air-Conditioning associations (REHVA) has updated the guidance on how to operate and use building services to prevent the spread of SARS-CoV-2 in workplaces, and the recommendations are mostly to stop air recirculation and to increase the inflow of outdoor air (Kurnitski et al., 2020). To reduce aerosol transmission of SARS-CoV-2 in the general public in relatively confined and poor ventilation settings such as public transportation vehicles (e.g. bus, cruise, train, subway, and plane) and some public places (e.g. shopping malls, bars, restaurants, clubs, hotels, banks, offices, libraries, conference rooms and cinemas), and vulnerable populations in high density living areas or institutions (e.g. nursing home, orphanage, welfare, kindergarten, school, and urban slums), individuals should reduce social activities and avoid crowded and poorly ventilated spaces. Since there is no single measure that provides complete protection, facial mask should be used in conjunction with hand hygiene (e.g. hand washing, using alcohol hand rub or hand sanitizer) when going to public settings or taking public transportations, coupled with social and physical distancing. Additionally, the government should guide society to broadcast the relevant prevention and control knowledges via media tools (e.g. TV, broadcasts, newspapers, messages, social media such as Twitter, Facebook, Instagram, display screens, billboards, and brochures). The recognition of aerosol spread and asymptomatic transmission may have influenced the decision in the United States to recommend universal face mask use for the general public (Bai et al., 2020).
3. Contributions
ST and XS had the idea for and designed the study. ST, YM and RJ contributed equally to this work. ST, YM, RJ, QT, NL, JJ, CRM and XS drafted the paper, and all authors critically revised the manuscript and gave final approval for the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to any part of the work are appropriately investigated and resolved.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors acknowledge all healthcare workers involved in the diagnosis and treatment of patients during the pandemic of COVID-19 globally. We thank Xia Li, Cheng Ding, Yueyun Luo, Mingyuan Zhang and Bing Wu from NIEH, China CDC as well as Daitao Zhang and Yi Zhang from Beijing CDC for their tremendous help and insightful suggestions for this study. The present perspective has not been subjected to the peer and policy review from China CDC, and therefore does not necessarily reflect the views of the China CDC and no official endorsement should be inferred.
We acknowledge financial support from the Young Scholar Scientific Research Foundation of National Institute of Environmental Health, China CDC (2020YSRF_03), and the National Key Research and Development Program of China (No. 2017YFC0702800).
Handling Editor: Xavier Querol
References
- Asadi S., Bouvier N., Wexler A.S., Ristenpart W.D. The coronavirus pandemic and aerosols: does COVID-19 transmit via expiratory particles? Aerosol Sci. Technol. 2020;54:635–638. doi: 10.1080/02786826.2020.1749229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Yao L., Wei T., et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L., Gao H., Deng W., et al. Transmission of severe acute respiratory syndrome coronavirus 2 via close contact and respiratory droplets among human angiotensin-converting enzyme 2 mice. J. Infect. Dis. 2020;222:551–555. doi: 10.1093/infdis/jiaa281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachere F.M., Lindsley W.G., Pearce T.A., et al. Measurement of airborne influenza virus in a hospital emergency department. Clin. Infect. Dis.: Off. Publ. Infect. Dis. Soc. Am. 2009;48:438–440. doi: 10.1086/596478. [DOI] [PubMed] [Google Scholar]
- Bonifait L., Charlebois R., Vimont A., et al. Detection and quantification of airborne norovirus during outbreaks in healthcare facilities. Clin. Infect. Dis.: Off. Publ. Infect. Dis. Soc. Am. 2015;61:299–304. doi: 10.1093/cid/civ321. [DOI] [PubMed] [Google Scholar]
- Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.4756. [DOI] [PubMed] [Google Scholar]
- Brankston G., Gitterman L., Hirji Z., Lemieux C., Gardam M. Transmission of influenza A in human beings. Lancet. Infect. Dis. 2007;7:257–265. doi: 10.1016/S1473-3099(07)70029-4. [DOI] [PubMed] [Google Scholar]
- Buonanno G., Stabile L., Morawska L. Estimation of airborne viral emission: quanta emission rate of SARS-CoV-2 for infection risk assessment. Environ. Int. 2020;141 doi: 10.1016/j.envint.2020.105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J., Sun W., Huang J., Gamber M., Wu J., He G. Indirect virus transmission in cluster of COVID-19 cases, Wenzhou, China, 2020. Emerg. Infect. Dis. 2020;26:1343–1345. doi: 10.3201/eid2606.200412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campell A. Government of Ontario; Canada: 2006. SARS Commission Final Report: Spring of Fear. [Google Scholar]
- CDC, 2020. Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings. (Accessed April 1, 2020, at https://www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html).
- Chan J.F., Yuan S., Zhang A.J., et al. Surgical mask partition reduces the risk of non-contact transmission in a golden Syrian hamster model for Coronavirus Disease 2019 (COVID-19) Clin. Infect. Dis.: Off. Publ. Infect. Dis. Soc. Am. 2020 doi: 10.1093/cid/ciaa644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Wang A., Yi B., et al. The epidemiological characteristics of infection in close contacts of COVID-19 in Ningbo city. Chinese J. Epidemiol. 2020;41 doi: 10.3760/cma.j.cn112338-20200304-00251. [DOI] [PubMed] [Google Scholar]
- Chia P.Y., Coleman K.K., Tan Y.K., et al. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat. Commun. 2020;11:2800. doi: 10.1038/s41467-020-16670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin A., Chu J., Perera M., et al. Stability of SARS-CoV-2 in different environmental conditions. The Lancet Microbe. 2020;1(1) doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury R., Maranas C.D. Biophysical characterization of the SARS-CoV2 spike protein binding with the ACE2 receptor explains increased COVID-19 pathogenesis. bioRxiv. 2020;2020(03):30.015891. doi: 10.1016/j.csbj.2020.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C.M., Cheng V.C., Hung I.F., et al. Viral load distribution in SARS outbreak. Emerg. Infect. Dis. 2005;11:1882–1886. doi: 10.3201/eid1112.040949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman K.K., Sigler W.V. Airborne influenza A virus exposure in an elementary school. Sci. Rep. 2020;10:1859. doi: 10.1038/s41598-020-58588-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook T.M. Personal protective equipment during the COVID-19 pandemic – a narrative review. Anaesthesia. 2020 doi: 10.1111/anae.15158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowling B.J., Leung G.M. Epidemiological research priorities for public health control of the ongoing global novel coronavirus (2019-nCoV) outbreak. Euro Surveill.: Bull. Europeen sur les maladies transmissibles = European Commun. Dis. Bull. 2020;2 doi: 10.2807/1560-7917.ES.2020.25.6.2000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W., Bao L., Gao H., et al. Ocular conjunctival inoculation of SARS-CoV-2 can cause mild COVID-19 in Rhesus macaques. bioRxiv. 2020;2020(03):13.990036. doi: 10.1038/s41467-020-18149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhand R., Li J. Coughs and sneezes: their role in transmission of respiratory viral infections, including SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020 doi: 10.1164/rccm.202004-1263PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz Leslie, Horve Patrick F., Coil David A., Fretz Mark, Eisen Jonathan A., Van Den Wymelenberg Kevin, Gilbert Jack A. 2019 Novel coronavirus (COVID-19) pandemic: built environment considerations to reduce transmission. mSystems. 2019;5(2) doi: 10.1128/mSystems.00245-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, Z., Qian, H., Xu, B., et al. 2020. Toilets dominate environmental detection of SARS-CoV-2 virus in a hospital. medRxiv 2020:2020.04.03.20052175.
- ECDC, 2020. Q & A on COVID-19: 4. What is the mode of transmission? How (easily) does it spread? (Accessed March 31, 2020, at https://www.ecdc.europa.eu/en/covid-19/questions-answers).
- Fabian P., McDevitt J.J., DeHaan W.H., et al. Influenza virus in human exhaled breath: an observational study. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fears Alyssa C., Klimstra William B., Duprex Paul, Hartman Amy, Weaver Scott C., Plante Kenneth S., Mirchandani Divya, Plante Jessica Ann, Aguilar Patricia V., Fernández Diana, Nalca Aysegul, Totura Aysegul, Dyer David, Kearney Brian, Lackemeyer Matthew, Bohannon J. Kyle, Johnson Reed, Garry Robert F., Reed Doug S., Roy Chad J. Persistence of severe acute respiratory syndrome coronavirus 2 in aerosol suspensions. Emerg. Infect. Dis. 2020;26(9) doi: 10.3201/eid2609.201806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigin R.D., Baker C.J., Herwaldt L.A., Lampe R.M., Mason E.O., Whitney S.E. Epidemic meningococcal disease in an elementary-school classroom. N. Engl. J. Med. 1982;307:1255–1257. doi: 10.1056/NEJM198211113072007. [DOI] [PubMed] [Google Scholar]
- Francisco, P.W., Emmerich, S.J., 2014. ASHRAE Position Document on Airborne Infectious Diseases.
- Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z.-D., Wang Z.-Y., Zhang S.-F., et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg. Infect. Dis. J. 2020;26:1583. doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., et al. First Case of 2019 Novel Coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong Hye Won, Kim Se-Mi, Kim Hee-Sung, Kim Young-Il, Kim Jun Hyoung, Cho Jun Yeon, Kim Sun-hyung, Kang Hyeran, Kim Seong-Gyu, Park Su-Jin, Kim Eun-Ha, Choi Young Ki. Viable SARS-CoV-2 in various specimens from COVID-19 patients. Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y., Wang, H., Chen, Y., et al., 2020. Clinical Data on Hospital Environmental Hygiene Monitoring and Medical Staffs Protection during the Coronavirus Disease 2019 Outbreak. medRxiv 2020.02.25.20028043; doi:https://doi.org/10.1101/2020.02.25.20028043.
- Johnson D., Lynch R., Marshall C., Mead K., Hirst D. Aerosol generation by modern flush toilets. Aerosol Sci. Technol.: J. Am. Assoc. Aerosol Res. 2013;47:1047–1057. doi: 10.1080/02786826.2013.814911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R.M., Brosseau L.M. Aerosol transmission of infectious disease. J. Occup. Environ. Med. 2015;57:501–508. doi: 10.1097/JOM.0000000000000448. [DOI] [PubMed] [Google Scholar]
- Keene C.H. Airborne contagion and air hygiene. William firth wells. J. Sch. Health. 1955;25:249. [Google Scholar]
- Khare P., Marr L.C. Simulation of vertical concentration gradient of influenza viruses in dust resuspended by walking. Indoor Air. 2015;25:428–440. doi: 10.1111/ina.12156. [DOI] [PubMed] [Google Scholar]
- Kim Y.I., Kim S.G., Kim S.M., et al. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe. 2020;27(704–9) doi: 10.1016/j.chom.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight V. Viruses as agents of airborne contagion. Ann. N. Y. Acad. Sci. 1980;353:147–156. doi: 10.1111/j.1749-6632.1980.tb18917.x. [DOI] [PubMed] [Google Scholar]
- Kurnitski, J., Boerstra, A., Franchimon, F., Mazzarella, L., Hogeling, J., Hovorka, F., 2020. REHVA COVID-19 guidance document , March 17, 2020 (updates will follow as necessary) How to operate and use building services in order to prevent the spread of the coronavirus disease (COVID-19) virus (SARS-CoV-2) in workplaces. 2020, 1–6.
- Lee N., Hui D., Wu A., et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung N.H.L., Chu D.K.W., Shiu E.Y.C., et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 2020 doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Duan S., Yu I.T., Wong T.W. Multi-zone modeling of probable SARS virus transmission by airflow between flats in Block E, Amoy Gardens. Indoor Air. 2005;15:96–111. doi: 10.1111/j.1600-0668.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- Li Y.Y., Wang J.X., Chen X. Can a toilet promote virus transmission? From a fluid dynamics perspective. Phys. fluids. 2020;32 doi: 10.1063/5.0013318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Ning Z., Chen Y., et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- Liu L., Wei J., Li Y., Ooi A. Evaporation and dispersion of respiratory droplets from coughing. Indoor Air. 2017;27:179–190. doi: 10.1111/ina.12297. [DOI] [PubMed] [Google Scholar]
- Lopman B., Gastanaduy P., Park G.W., Hall A.J., Parashar U.D., Vinje J. Environmental transmission of norovirus gastroenteritis. Curr. Opin. Virol. 2012;2:96–102. doi: 10.1016/j.coviro.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Lu J., Gu J., Li K., et al. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg. Infect. Dis. 2020;26:1628–1631. doi: 10.3201/eid2607.200764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukassen S., Chua R.L., Trefzer T., et al. SARS-CoV-2 receptor ACE2 and TMPRSS2 are predominantly expressed in a transient secretory cell type in subsegmental bronchial branches. bioRxiv. 2020;2020(03):13.991455. [Google Scholar]
- Ma Q.X., Shan H., Zhang H.L., Li G.M., Yang R.M., Chen J.M. Potential utilities of mask-wearing and instant hand hygiene for fighting SARS-CoV-2. J. Med. Virol. 2020 doi: 10.1002/jmv.25805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks P.J., Vipond I.B., Regan F.M., Wedgwood K., Fey R.E., Caul E.O. A school outbreak of Norwalk-like virus: evidence for airborne transmission. Epidemiol. Infect. 2003;131:727–736. doi: 10.1017/s0950268803008689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, S.L., Nazaroff, W.W., Jimenez, J.L., et al.,2020. Transmission of SARS-CoV-2 by inhalation of respiratory aerosol in the Skagit Valley Chorale superspreading event. 2020:2020.06.15.20132027. [DOI] [PMC free article] [PubMed]
- Milton D.K., Fabian M.P., Cowling B.J., Grantham M.L., McDevitt J.J. Influenza virus aerosols in human exhaled breath: particle size, culturability, and effect of surgical masks. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MHLW, 2020. Q & A on coronavirus 2019 (COVID-19): How does human to human transmission of the novel coronavirus (2019-nCOV) happen? (Accessed March 14, 2020, at https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/dengue_fever_qa_00014.html#Q2).
- MOH, 2020. Clarifications on Misinformation regarding COVID-19. 2020. (Accessed April 1, 2020, at https://www.moh.gov.sg/covid-19/clarifications).
- Morawska L., Johnson G., Ristovski Z., Hargreaves M., Mengersen K., Corbett S. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J. Aerosol Sci. 2009;40:256–269. doi: 10.1016/j.jaerosci.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty L.F., Plucinski M.M., Marston B.J., et al. Public health responses to COVID-19 outbreaks on cruise ships - worldwide, february-march 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69:347–352. doi: 10.15585/mmwr.mm6912e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M.R., Bender T.R., Margolis H.S., Noble G.R., Kendal A.P., Ritter D.G. An outbreak of influenza aboard a commercial airliner. Am. J. Epidemiol. 1979;110:1–6. doi: 10.1093/oxfordjournals.aje.a112781. [DOI] [PubMed] [Google Scholar]
- Mubareka S., Lowen A.C., Steel J., Coates A.L., Garcia-Sastre A., Palese P. Transmission of influenza virus via aerosols and fomites in the guinea pig model. J. Infect. Dis. 2009;199:858–865. doi: 10.1086/597073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustaffa-Babjee A., Ibrahim A.L., Khim T.S. A case of human infection with Newcastle disease virus. Southeast Asian J. Trop. Med. Public Health. 1976;7:622–624. [PubMed] [Google Scholar]
- National Academies of Sciences E, Medicine, 2020. Rapid Expert Consultation on the Possibility of Bioaerosol Spread of SARS-CoV-2 for the COVID-19 Pandemic (April 1, 2020). The National Academies Press, Washington, DC.
- NHC, 2020. Diagnosis and treatment plan of COVID-19 (trial version 7). (in Chinese) (Accessed March 3, 2020, at http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf).
- Ong S.W.X., Tan Y.K., Chia P.Y., et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020 doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oran D.P., Topol E.J. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann. Intern. Med. 2020 doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parienta D., Morawska L., Johnson G.R., et al. Theoretical analysis of the motion and evaporation of exhaled respiratory droplets of mixed composition. J. Aerosol Sci. 2011;42:1–10. [Google Scholar]
- Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections—more than just the common cold. JAMA. 2020;323:707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- Ratnesar-Shumate S., Williams G., Green B., et al. Simulated sunlight rapidly inactivates SARS-CoV-2 on surfaces. J. Infect. Dis. 2020;222:214–222. doi: 10.1093/infdis/jiaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read, R., 2020. A choir decided to go ahead with rehearsal. Now dozens of members have COVID-19 and two are dead. 2020. (Accessed April 13, 2020, at www.latimes.com/world-nation/story/2020-03-29/coronavirus-choir-outbreak).
- Rothe C., Schunk M., Sothmann P., et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REHVA, 2020. REHVA COVID-19 guidance document, April 3, 2020. (Accessed March 17, 2020, at https://www.rehva.eu/fileadmin/user_upload/REHVA_covid_guidance_document_2020-03-17.pdf).
- Santarpia J.L., Rivera D.N., Herrera V.L., et al. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Sci. Rep. 2020;10:12732. doi: 10.1038/s41598-020-69286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setti L., Passarini F., De Gennaro G., et al. SARS-Cov-2RNA found on particulate matter of Bergamo in Northern Italy: First evidence. Environ. Res. 2020;188 doi: 10.1016/j.envres.2020.109754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Li C., Dong H., et al. Airborne transmission of COVID-19: epidemiologic evidence from two outbreak investigations. ResearchGate. 20202020 https://www.researchgate.net/publication/340418430 [Google Scholar]
- Sia S.F., Yan L.M., Chin A.W.H., et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020 doi: 10.1038/s41586-020-2342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J.D., Rhinehart E., Jackson M., Chiarello L. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am. J. Infect. Control. 2007;35:S65–S164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, J.C., Sheltzer, J.M., 2020. Cigarette smoke triggers the expansion of a subpopulation of respiratory epithelial cells that express the SARS-CoV-2 receptor ACE2. bioRxiv 2020:2020.03.28.013672.
- Smither S.J., Eastaugh L.S., Findlay J.S., Lever M.S. Experimental aerosol survival of SARS-CoV-2 in artificial saliva and tissue culture media at medium and high humidity. Emerging Microbes Infect. 2020;9:1415–1417. doi: 10.1080/22221751.2020.1777906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somsen G.A., van Rijn C., Kooij S., Bem R.A., Bonn D. Small droplet aerosols in poorly ventilated spaces and SARS-CoV-2 transmission. Lancet Respir. Med. 2020;8:658–659. doi: 10.1016/S2213-2600(20)30245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadnytskyi V., Bax C.E., Bax A., Anfinrud P. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. PNAS. 2020;117:11875–11877. doi: 10.1073/pnas.2006874117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L., Kang X., Zhang B., et al. A special case of COVID-19 with long duration of viral shedding for 49 days. medRxiv. 2020;2020(03) doi: 10.1002/mco2.2. 22.20040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellier R., Li Y., Cowling B.J., Tang J.W. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect. Dis. 2019;19:101. doi: 10.1186/s12879-019-3707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen, N., Bushmaker, T., Morris, D., et al., 2020. Aerosol and surface stability of HCoV-19 (SARS-CoV-2) compared to SARS-CoV-1. medRxiv 2020:2020.03.09.20033217.
- Wang J., Du G. COVID-19 may transmit through aerosol. Ir. J. Med. Sci. 2020:1–2. doi: 10.1007/s11845-020-02218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J., Li Y. Enhanced spread of expiratory droplets by turbulence in a cough jet. Build. Environ. 2015;93:86–96. [Google Scholar]
- WHO, 2020. WHO Coronavirus Disease (COVID-19) Dashboard. 2020. (Accessed July 30, 2020, at https://covid19.who.int/).
- WHO, 2020. Q&A on coronaviruses (COVID-19) : Is COVID-19 airborne? (Accessed March 9, 2020, at https://www.who.int/news-room/q-a-detail/q-a-coronaviruses).
- WHO, 2020. Q&A: How is COVID-19 transmitted? What do we know about aerosol transmission? (Accessed July 9, 2020, at https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/q-a-how-is-covid-19-transmitted).
- Wölfel R., Corman V.M., Guggemos W., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Wong B.C., Lee N., Li Y., et al. Possible role of aerosol transmission in a hospital outbreak of influenza. Clin. Infect. Dis.: Off. Publ. Infect. Dis. Soc. Am. 2010;51:1176–1183. doi: 10.1086/656743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D., Wang N., Corbett K.S., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Z., Zhang, D., Lei, Y., 2012. An outbreak of norovirus in school transmitted by aerosol. Chinese Journal of School Health 33, 244–5. (in Chinese).
- Wu, W., Li, Y., Wei, Z., et al., 2020. Investigation and analysis on characteristics of a cluster of COVID-19 associated with exposure in a department store in Tianjin. Chinese Journal of Epidemiology 41, 489–93. (in Chinese). [DOI] [PubMed]
- Xiao S., Li Y., Sung M., Wei J., Yang Z. A study of the probable transmission routes of MERS-CoV during the first hospital outbreak in the Republic of Korea. Indoor Air. 2018;28:51–63. doi: 10.1111/ina.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C., Jiang L., Huang G., et al. Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests. Int. J. Infect. Dis. 2020;93:264–267. doi: 10.1016/j.ijid.2020.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Zhong L., Deng J., et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Elankumaran S., Marr L.C. Concentrations and size distributions of airborne influenza A viruses measured indoors at a health centre, a day-care centre and on aeroplanes. J. R. Soc. Interface. 2011;8:1176–1184. doi: 10.1098/rsif.2010.0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I.T., Li Y., Wong T.W., et al. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N. Engl. J. Med. 2004;350:1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- Zhang X., Ji Z., Yue Y., Liu H., Wang J. Infection risk assessment of COVID-19 through aerosol transmission: a case study of South China seafood market. Environ. Sci. Technol. 2020 doi: 10.1021/acs.est.0c02895. [DOI] [PubMed] [Google Scholar]
- Zhang R., Li Y., Zhang A.L., Wang Y., Molina M.J. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc. Natl. Acad. Sci. USA. 2020;117:14857–14863. doi: 10.1073/pnas.2009637117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zietsman M., Phan L.T., Jones R.M. Potential for occupational exposures to pathogens during bronchoscopy procedures. J. Occup. Environ. Hygiene. 2019;16:707–716. doi: 10.1080/15459624.2019.1649414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Ruan F., Huang M., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]