Abstract
There are worldwide urgency, efforts, and uncertainties for the discovery of a vaccine against SARS CoV2. If successful, it will take its own time till useful for the humans. Till the specific vaccine is available, there are evidences for repurposing existing other vaccines. It is observed that countries having a routine BCG vaccination programme, have shown to have lower incidence of COVID-19, suggesting some protective mechanisms of BCG against COVID-19 in such countries. In countries like India despite vast population density and other adversities, and growing numbers of COVID19 infections, the mortality rate and severity of COVID has been low in comparison to some TB non-endemic countries (like Europe and USA). In addition, there are evidences that BCG vaccination offers partial protection and survival in low-income countries where tuberculosis is prevalent. The nonspecific effects (NSEs) of immune responses induced by BCG vaccination protect against other infections seem to be due to its immunological memory eliciting lymphocytes response and trained immunity. The protective effect on other viral infection in humans are believed to be mediated by heterologous lymphocyte activation and the initiation of innate immune memory may be applicable to SARS CoV2. The BCG vaccination at birth does not have a protective effect beyond childhood against COVID-19. In adults, there might be other factors dampening the virulence and pathogenicity of COVID-19. In the TB endemic countries like India, with high population density, similar to BCG vaccination, the environmental Mycobacteria might be imparting some immune-protection from severity and deaths of COVID-19.
Keywords: BCG vaccination, Protection, COVID-19, Corona virus, Non-tubercular mycobacteria
1. Introduction
The COVID-19 pandemic continues to ravage India and the world with its high transmissibility and varying degree of virulence. As the causative agent SARS CoV2 is a novel coronavirus, prior infection with other endemic coronaviruses does not confer any protection. Due to the immune naivety of the population and ease of international travel, the world faces the greatest ever global pandemic of the century. While uncertainty of an effective vaccine against SARS CoV2 persists, protective attributes of century old Bacillus Calmette-Guérin (BCG) vaccine is currently the hot topic of attention.
At the beginning of the pandemic, it was predicted that the developing countries will face the maximum case fatalities because of poor health infrastructure, lack of preparedness and poor health regulations. However, till now, the number of deaths have been higher in developed nations like USA and Europe. Surprisingly less number of cases have been reported from Africa, the majority of cases recovered with mild diseases. Even if the number is increasing in India the severity and mortality is less compared to some countries. In India, over 80% of the patients have mild symptoms are asymptomatic. Hence, the inter-regional variation in the clinical severity and mortality of COVID-19 is speculated to be through immune response impact. It is also claimed that BCG vaccination offers partial protection and survival in low-income countries where tuberculosis is prevalent.1
There is much variation of severity of inflammatory process of COVID-19 across geographical locations in world. The possible reasons could be individual's age and viral factors modified by population density, environment, ambient air temperature and humidity. High temperature and higher relative humidity have been demonstrated to have a negative effect on the spread of the virus.2 , 3 The increase of temperature from spring to summer could facilitate the containment and the cold season may witness an upsurge in infections during 2020–2021 winter season.3 In addition to geographic variation influencing COVID 19 spread and severity, there are other established factors like advanced age, inflammatory comorbidities, and immune compromised conditions for severe illness.4
Two basic steps by which the virus overcomes host immune response are recognition and evasion of SARS-CoV-2 to circumvent the cytosolic pathogen recognition receptors (PRR) and subsequent invasion of the host.5 SARS-CoV-2 causes severe damage rapidly by excessive cytokine production (storm) or slowly through innate immune resistance manifesting as fever in order to provoke a delayed over inflammation.6 More severe disease have inflammation-based sequelae due to uncontrolled systemic inflammatory response resulting from the release of large amounts proinflammatory cytokines that further affects the immune system, which contributes to severity of the disease. The excessive production and secretion of cytokines like tumour necrosis factor (TNF), IL-6, and IL-1β results in a critical state which is described as a cytokine storm. The cytokine storm leads to an increased vascular (hyper)permeability, multi-organ failure, and eventually death when the cytokine concentrations are unrestricted and high over time.7 Therefore, controlling the immune evasion of SARS-CoV-2 is an important step in management.
2. Evidences of protection due to BCG vaccination against viral infections
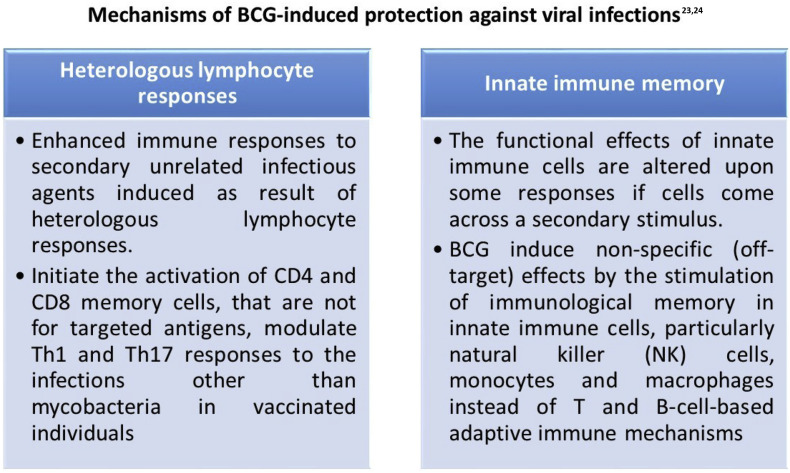
BCG vaccine, a live attenuated strain derived from Mycobacterium bovis, has the ability to induce potent nonspecific immunity also known as so-called ‘off-target’ protection against bacterial and viral pathogens. BCG has shown to diminish the susceptibility to various respiratory tract infections. Such protection is mediated by the non-specific boosting of innate immunity. However the mechanisms of the beneficial effects of the BCG vaccine are now better understood. Protective effects of BCG vaccine against COVID 19 disease severity is partially explained by the different national policies respect to BCG vaccination.
The cellular and molecular mechanisms of the non-specific protective effects of BCG vaccination against various DNA and RNA viruses, including herpes and influenza viruses have been studied in mice recently.8 BCG vaccination is also shown to protect from herpes simplex virus type 2 (HSV2) infection in new-born mice.9 Subcutaneous injection of component of the mycobacterial cell wall (muramyl dipeptide) protects against vaccinia virus and HSV2 infections in mice.10 Such protection is facilitated by peritoneal macrophages.10 The above effect suggests that there is substantial effects of BCG on the innate immune system. BCG administration acts through macrophages and have reduced viral titres of influenza A virus in the injected mice.11
Several studies underscored reduction in respiratory tract infections and risk of pneumonia upon BCG vaccination of elderly people.12 , 13 However, limited period time of the acquired protection remains a caveat for trained immunity boosters.
A randomized study showed that BCG vaccination prior to influenza vaccination in healthy individuals resulted in a significantly higher antibody response against influenza A (H1N1) compared to placebo.14
The effectiveness of BCG in the promotion of long-lasting T cell immunity to human respiratory syncytial virus (hRSV) antigens was experimentally demonstrated without any observable adverse effects.15
In severe combined immunodeficiency (SCID) mice, with functionally depleted T- and B-cells, BCG vaccination had protected against a secondary non-Mycobacterial challenge, which highlights the importance of innate immune cells in protecting from diseases.16
Several studies have shown protective action of BCG against unrelated respiratory infections both in children and adults. A comparable protection effect of BCG on respiratory infections was shown among elderly population in Indonesia.12 Prospective clinical trial performed in Japan has shown BCG vaccine to protect from pneumonia in tuberculin negative elderly populations.13 Randomised controlled trials have demonstrated that the BCG vaccine have immunomodulatory effects to protect partially against respiratory infections. In South Africa, BCG-Danish reduced respiratory tract infections by 73% (95% CI 39–88) in adolescents.17 In a randomized placebo controlled trial, BCG vaccination showed to induce significant reduction in viremia in an experimental infection with live attenuated yellow fever virus vaccine strain. The level of lowering of viremia was correlated with heterologous IL1-β production which was attributed for protection induced by BCG.18
3. Evidences of protection due to BCG vaccination against SARS Cov-2 infections
Miller et al19 (Correlation between universal BCG vaccination policy) compared number of countries with BCG vaccination policies with the morbidity and mortality for COVID-19. They found that countries without universal policies of BCG vaccination (like Italy, USA) have been more severely affected compared to countries with universal BCG policies. They proposed that BCG vaccination attributed for reduced morbidity and mortality in countries with universal BCG policies.19 , 20 Countries with high BCG vaccination coverage have shown lower incidence of COVID-19, suggesting some protective mechanisms in TB-endemic areas.21 Ozdemir et al have shown proportionately less cases, milder illness and a lower death rate in BCG vaccinated population as compared to BCG non-vaccinated across countries and hemispheres.22 BCG vaccination might alter a secondary innate immune response upon viral infection over month apart resulting in improved antiviral responses and lowering viremia.18 This is so far proven that the countries more prone to be severely affected SARS-CoV-2 didn't adopt universal policies of BCG vaccination like Italy and Spain. The BCG vaccine likely reduces cytokine storm after SARS-COV-2 exposure, resulting mild COVID-19 and early recovery.
4. Mechanisms of BCG-induced protection against viral infections23,24
The protective effect on viral infection in humans are believed to be mediated by heterologous lymphocyte activation and the initiation of innate immune memory.
5. COVID-19: children are protected?
The children might have some immune characteristic to incite the triggering active innate immune response to stop cytokine storm and progression to pneumonia/illness.30 The immune dysfunction and extent of cytokine overproduction are minimal in children compared to adults. Lymphopenia in the majority of paediatric COVID-19 patients is uncommon in contrast to adults.31 It is possible that the innate immunity plays a protective role in the pathogenesis of COVID-19 in the paediatric age group as the adaptive immunity is not well-developed in early ages. There could be a cross-protective immunity developed in response to regionally prevalent viral infections and repeated mild upper respiratory tract infections. Some protection due to an immune system activation as a result of frequent viral infections decreases the susceptibility to severe illness.
A reasonable explanation is that children of the country where routine childhood BCG vaccination is a policy and BCG vaccinated children have some degree of protection from infection with SARS- CoV-2, and less severe diseases among those who infected. Ultimately there is less transmissibility of the virus. The children also seem to be better protected from COVID-19 than adults.
In late adulthood, there is an involution of the thymus, and the decrease in regulatory T cells can be a contributory mechanism underlying severe COVID-19 illness. In older ages, generalized inability to fine-control inflammation favours propensity toward sustaining the cytokine release syndrome (CRS).
6. Should BCG vaccination be given to all?
Considering the above evidences of BCG regarding its non-specific beneficial effect on non-tubercular infections including respiratory and other viral infections, reduction in mortality due to pneumonia and sepsis, a thought is worth given for indiscriminate BCG vaccination (in non BCG vaccinated countries) to reduce severity of Covid-19. Two separate multi-centre placebo-controlled parallel group randomized trials are currently underway in the Netherlands and Australia to assess whether BCG-Danish reduces health care workers absenteeism and to reduce hospital admission among the elderly during the COVID-19 pandemic through BCG vaccination.32 These trials are ‘BCG Vaccination to Protect Healthcare Workers against COVID-19 (BRACE) and, reducing health care workers absenteeism in COVID-19 pandemic through BCG vaccine (BCG-CORONA).32
In the present scenario WHO does not recommend BCG vaccination for the prevention of COVID-19. Curtis et al have explained the reasons why it is important to comply with the WHO's recommendation regarding the use of BCG vaccination only for COVID-19 trials till the results are complete,1 i) there is no robust evidence regarding effectiveness of BCG against COVID19. According to WHO, the studies showing correlation between BCG vaccination and COVID-19 protection are grossly confounded by national demographics data, testing rate, disease burden and stage of pandemic. ii) The BCG vaccine is of limited supply, and unselective use could affect the supply of routine vaccination for children in high risk countries. Iii) It is unlikely that a BCG vaccine given during childhood will be effective to protect COVID-19 in adult age. Iv) If BCG vaccination is done for COVID-19, it could create a false sense of immunity, and v) The possibility of up-regulation of immunity to exacerbate the severity of COVID-19 infection by BCG, though remote cannot be ruled out.1
7. Possible role of Environmental Mycobacteria
On the flipside of the flimsy evidence, the BCG vaccination in childhood may not have protective effect against COVID-19 in adulthood as the effect of BCG vaccination is moderate and lasts for nearly 20 years.33
So there might be some other factors modifying the virulence and pathogenicity of COVID-19. Is it the endemic infections like dengue, chikungunya, malaria and other tropical infections, have an inverse correlation with severity of COVID-19? It is to hypothesize that endemic infections in the community may protect through various interferons which retard subsequent disease progression through viral interference.34 , 35
We hypothesise on the possible mechanism behind the exact “environment-antiviral immunity” interplay on the pathogenesis of COVID-19.36 In the TB endemic countries like India, with high population density, the environmental Mycobacteria play great role as over half of the population are positive for tuberculin skin test (TST). A study from southern India has shown that immune responses of non-vaccinated tuberculin reactors to have significantly higher than the vaccinated tuberculin non-reactors,36 In addition there was no significant difference in the responses among the BCG-vaccinated tuberculin reactors when compared with the non-vaccinated tuberculin reactors.36 , 37 Trials have described the protective effects of nontuberculous Mycobacterium species (like, Mycobacterium vaccae) in up-regulation of IFN-gamma secretion.38 Again BCG vaccination used give a moderate protection against tuberculosis and only for up to 20 years, not lifelong. So the protection of BCG against COVID-19 if any looks to be applicable only to certain portion of population. On the other hand, the environmental mycobacteria are ubiquitous and are sustained in environment since long. The development of nonspecific partial immunity is likely from environmental mycobacteria as people from TB endemic countries like India get infected from time to time. For the same reason most of the people are TST positive.36 Factually these conditions impact some degree of general immunity for new infections. TB endemicity or environmental mycobacteria seems to be correlated with reduced disease burden and severity of COVID-19.39
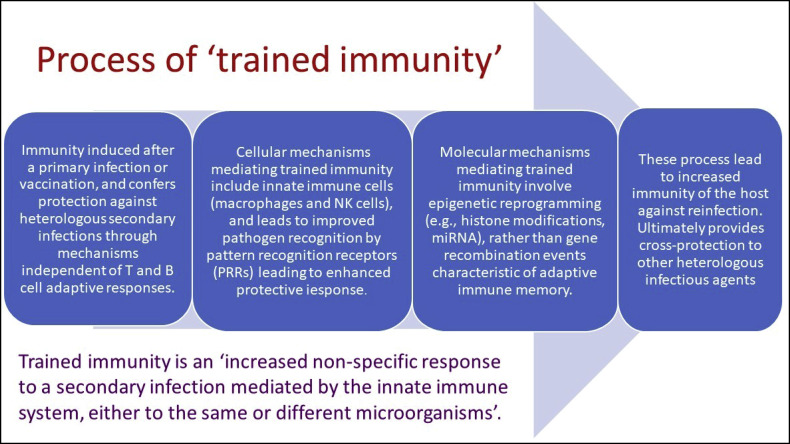
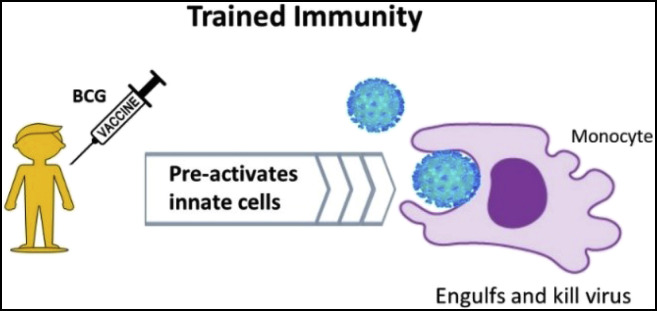
Like BCG, it is hypothesized that environmental Mycobacteria induce prolonged alteration in the immune system that results in increased level of innate and adaptive immunity.39 The environmental Mycobacteria might have induced similar immunological memory eliciting lymphocytes response (Fig. 1 ) and trained immunity (Fig. 2 ) making epigenetic alterations in the similar mechanism to BCG at the promotor sites of various genes encoding inflammatory cytokines such as interleukin (IL)-1, IL-6, and tumour necrosis factor (TNF), resulting less cytokine storms compared to naïve population.40
Fig. 3.
Severity of the COVID-19 depends on the level of cytokine storm and T cell lymphopenia and both are associated with pulmonary damage, respiratory distress and higher mortality. BCG induces epigenetic and functional reprogramming in human monocytes and increases immunity for un-related viral infections and Interleukin (IL)-1β plays as mediator of trained immunity responses.8 Hence, durable epigenetic alterations to increase antiviral function of innate immune cells is observed after live vaccines, facilitating a faster and better responsiveness if exposed to re-infections.16,29
Fig. 1.
Vaccines excite the initiation of the adaptive immune response and the development of immunological memory.25,26 The immunological memory consists of the developments of antigen-specific T and B cells which protects against subsequent insult by the pathogen. This is thought to be due to secondary innate immune response induced by BCG vaccination, coined as nonspecific effects (NSEs). The efficacy to protect against other infections can be linked to its immunological memory eliciting lymphocytes response (Fig. 1) and trained immunity (Fig. 2, Fig. 3).
Fig. 2.
The NSE is a consequence of the type of non-specific immune memory induced after vaccination as part of protective “trained immunity”.27 This type of immunological memory (of past insults) is developed by innate immune cells, like monocytes, macrophages, and natural killer cells, and can be efficiently induced by BCG.16,28
It is possible that people from TB endemic countries like India despite vast population and growing numbers of COVID19 infection, have acquired some protections from severity and deaths from COVID-19 in comparison to TB non-endemic countries (like Europe and USA). Although it appears the immunity may not able to stop COVID 19 infections, but is likely to diminish its impact on severity and mortality.
If the BCG vaccine as an inducer of trained immunity induces non-specific protection to bridge the gap before a real specific vaccine is developed, this would be an important tool in the response to COVID-19 and future pandemics. Better understandings of the molecular mechanisms are still evolving. By identifying the factors that impact the non-specific effects of BCG, can be an important step towards novel therapeutic options and vaccination strategies, which might lead to a reduction in severe morbidity and mortality associated with viral infections.
Conflicts of interest
The authors have none to declare.
References
- 1.Curtis N., Sparrow A., Ghebreyesus T.A., Netea M.G. Considering BCG vaccination to reduce the impact of COVID-19. Lancet (London, England) 2020;395(10236):1545–1546. doi: 10.1016/S0140-6736(20)31025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y.C., Bai W.Z., Hashikawa T. Response to Commentary on "The neuroinvasive potential of SARS-CoV-2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92(7):707–709. doi: 10.1002/jmv.25824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neher R.A., Dyrdak R., Druelle V., Hodcroft E.B., Albert J. Potential impact of seasonal forcing on a SARS-CoV-2 pandemic. Swiss Med Wkly. 2020;150:w20224. doi: 10.4414/smw.2020.20224. [DOI] [PubMed] [Google Scholar]
- 4.Kang S.J., Jung S.I. Age related morbidity and mortality among patients with COVID-19. Infect Chemother. 2020;52(2):154–164. doi: 10.3947/ic.2020.52.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peeples L. News Feature: avoiding pitfalls in the pursuit of a COVID-19 vaccine. Proc Natl Acad Sci USA. 2020;117(15):8218–8221. doi: 10.1073/pnas.2005456117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jose R.J., Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8(6):e46–e47. doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moorlag S., Arts R.J.W., van Crevel R., Netea M.G. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect. 2019;25(12):1473–1478. doi: 10.1016/j.cmi.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Starr S.E., Visintine A.M., Tomeh M.O., Nahmias A.J. Effects of immunostimulants on resistance of newborn mice to herpes simplex type 2 infection. Proc Soc Exp Biol Med (New York, NY) 1976;152(1):57–60. doi: 10.3181/00379727-152-39327. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda S., Negishi T., Nishimura C. Enhancement of non-specific resistance to viral infection by muramyldipeptide and its analogs. Antivir Res. 1985;5(4):207–215. doi: 10.1016/0166-3542(85)90025-7. [DOI] [PubMed] [Google Scholar]
- 11.Spencer J.C., Ganguly R., Waldman R.H. Nonspecific protection of mice against influenza virus infection by local or systemic immunization with Bacille Calmette-Guérin. J Infect Dis. 1977;136(2):171–175. doi: 10.1093/infdis/136.2.171. [DOI] [PubMed] [Google Scholar]
- 12.Wardhana, Datau E.A., Sultana A., Mandang V.V., Jim E. The efficacy of Bacillus Calmette-Guerin vaccinations for the prevention of acute upper respiratory tract infection in the elderly. Acta Med Indones. 2011;43(3):185–190. [PubMed] [Google Scholar]
- 13.Ohrui T., Nakayama K., Fukushima T., Chiba H., Sasaki H. Prevention of elderly pneumonia by pneumococcal, influenza and BCG vaccinations. Nihon Ronen Igakkai zasshi Jpn J Geriatr. 2005;42(1):34–36. doi: 10.3143/geriatrics.42.34. [DOI] [PubMed] [Google Scholar]
- 14.Leentjens J., Kox M., Stokman R., et al. BCG vaccination enhances the immunogenicity of subsequent influenza vaccination in healthy volunteers: a randomized, placebo-controlled pilot study. J Infect Dis. 2015;212(12):1930–1938. doi: 10.1093/infdis/jiv332. [DOI] [PubMed] [Google Scholar]
- 15.Céspedes P.F., Rey-Jurado E., Espinoza J.A., et al. A single, low dose of a cGMP recombinant BCG vaccine elicits protective T cell immunity against the human respiratory syncytial virus infection and prevents lung pathology in mice. Vaccine. 2017;35(5):757–766. doi: 10.1016/j.vaccine.2016.12.048. [DOI] [PubMed] [Google Scholar]
- 16.Kleinnijenhuis J., Quintin J., Preijers F., et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci USA. 2012;109(43):17537–17542. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nemes E., Geldenhuys H., Rozot V., et al. Prevention of M. tuberculosis infection with H4:IC31 vaccine or BCG revaccination. N Engl J Med. 2018;379(2):138–149. doi: 10.1056/NEJMoa1714021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arts R.J.W., Moorlag S., Novakovic B., et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23(1):89–100. doi: 10.1016/j.chom.2017.12.010. e105. [DOI] [PubMed] [Google Scholar]
- 19.Miller C.L., Morris J., Pollock T.M. PHLS inquiry into current BCG vaccination policy. Br Med J (Clin Res Ed) 1984;288(6416):564. doi: 10.1136/bmj.288.6416.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otu A., Ebenso B., Labonte R., Yaya S. Tackling COVID-19: can the African continent play the long game? J Glob Health. 2020;10(1) doi: 10.7189/jogh.10.010339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madan M., Pahuja S., Mohan A., et al. TB infection and BCG vaccination: are we protected from COVID-19? Publ Health. 2020;185:91–92. doi: 10.1016/j.puhe.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozdemir C., Kucuksezer U.C., Tamay Z.U. Is BCG vaccination affecting the spread and severity of COVID-19? Allergy. 2020;75(7):1824–1827. doi: 10.1111/all.14344. [DOI] [PubMed] [Google Scholar]
- 23.Goodridge H.S., Ahmed S.S., Curtis N., et al. Harnessing the beneficial heterologous effects of vaccination. Nat Rev Immunol. 2016;16(6):392–400. doi: 10.1038/nri.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Netea M.G., Joosten L.A., Latz E., et al. Trained immunity: a program of innate immune memory in health and disease. Science (New York, NY) 2016;352(6284):aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rey-Jurado E., Soto J., Gálvez N., Kalergis A.M. A safe and efficient BCG vectored vaccine to prevent the disease caused by the human Respiratory Syncytial Virus. Hum Vaccines Immunother. 2017;13(9):2092–2097. doi: 10.1080/21645515.2017.1334026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Netea M.G., Quintin J., van der Meer J.W. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9(5):355–361. doi: 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Arts R.J., Blok B.A., Aaby P., et al. Long-term in vitro and in vivo effects of γ-irradiated BCG on innate and adaptive immunity. J Leukoc Biol. 2015;98(6):995–1001. doi: 10.1189/jlb.4MA0215-059R. [DOI] [PubMed] [Google Scholar]
- 29.Benn C.S., Netea M.G., Selin L.K., Aaby P. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends Immunol. 2013;34(9):431–439. doi: 10.1016/j.it.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Qiu H., Wu J., Hong L., Luo Y., Song Q., Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20(6):689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao Q., Chen Y.C., Chen C.L., Chiu C.H. SARS-CoV-2 infection in children: transmission dynamics and clinical characteristics. J Formos Med Assoc. 2020;119(3):670–673. doi: 10.1016/j.jfma.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ten Doesschate T., Moorlag S., van der Vaart T.W., et al. Two Randomized Controlled Trials of Bacillus Calmette-Guérin Vaccination to reduce absenteeism among health care workers and hospital admission by elderly persons during the COVID-19 pandemic: a structured summary of the study protocols for two randomised controlled trials. Trials. 2020;21(1):481. doi: 10.1186/s13063-020-04389-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mangtani P., Nguipdop-Djomo P., Keogh R.H., et al. The duration of protection of school-aged BCG vaccination in England: a population-based case-control study. Int J Epidemiol. 2018;47(1):193–201. doi: 10.1093/ije/dyx141. [DOI] [PubMed] [Google Scholar]
- 34.Chan K.F., Carolan L.A., Korenkov D., et al. Investigating viral interference between influenza A virus and human respiratory syncytial virus in a ferret model of infection. J Infect Dis. 2018;218(3):406–417. doi: 10.1093/infdis/jiy184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laurie K.L., Horman W., Carolan L.A., et al. Evidence for viral interference and cross-reactive protective immunity between influenza B virus lineages. J Infect Dis. 2018;217(4):548–559. doi: 10.1093/infdis/jix509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Periasamy M., Datta M., Kannapiran M., Ramanathan V.D., Venkatesan P. Neonatal bacillus Calmette-Guerin vaccination and environmental mycobacteria in sensitizing antimycobacterial activity of macrophages. Am J Med Sci. 2014;348(1):57–64. doi: 10.1097/MAJ.0000000000000206. [DOI] [PubMed] [Google Scholar]
- 37.Chandrasekaran P., Mave V., Thiruvengadam K., et al. Tuberculin skin test and QuantiFERON-Gold in Tube assay for diagnosis of latent TB infection among household contacts of pulmonary TB patients in high TB burden setting. PloS One. 2018;13(8) doi: 10.1371/journal.pone.0199360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akkoc T., Eifan A.O., Ozdemir C., et al. Mycobacterium vaccae immunization to OVA sensitized pregnant BALB/c mice suppressed placental and postnatal IL-5 and inducing IFN-gamma secretion. Immunopharmacol Immunotoxicol. 2008;30(1):1–11. doi: 10.1080/08923970701812159. [DOI] [PubMed] [Google Scholar]
- 39.Mohapatra P.R., Mishra B., Behera B. Immunity and protection from COVID-19 - environmental mycobacteria play a role. J Med Virol. Jun 24 2020 doi: 10.1002/jmv.26214. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Netea M.G., Domínguez-Andrés J., Barreiro L.B., et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20(6):375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]