Abstract
Background and objectives.
Donated blood is not currently screened for human T-cell lymphotropic virus (HTLV) in South Africa. Several small studies have detected HTLV-1 in South Africa, but prevalence by geographic region or population group are unavailable.
Materials and Methods.
We performed a large seroprevalence study of South African blood donors during three months in 2013. All geographic regions except the Western Cape were included and Black and Coloured (local term for mixed-race) donors were oversampled. Identity-unlinked plasma samples were screened with the Abbott Prism HTLV-1/2 assay and repeatedly reactive samples were tested by the Inno-LIA HTLV-1/2 Score confirmatory assay. Odds ratios were calculated with multivariable logistic regression.
Results.
Of 46,716 donors tested, 133 (0.28%) were initially reactive, 111 (0.24%) repeatedly reactive and 57 (0.12%) confirmed positive for HTLV-1; none were HTLV-2 positive. Prevalence was 0.062% weighted to annual blood donations but highly concentrated in the Black population group (OR=20.24 CI 2.77-147.88); higher in females than males (OR=1.81 CI 1.06-3.08); and in donors aged >50 years compared to ages 16-19 (OR=6.4 CI 2.95-13.86). After controlling for age, sex and population group there was no difference in prevalence between new and repeat blood donors or among geographic regions within South Africa.
Conclusions.
We conclude that HTLV-1 infection is widespread among the Black population of South Africa and its epidemiology is similar to other endemic areas. Because South Africa is increasing its recruitment of Black blood donors the implications for blood screening require further consideration.
Introduction
Human T-cell Lymphotropic Virus types 1 and 2 (HTLV-1/2) are closely related retroviruses first reported in the early 1980’s[1, 2]. HTLV-1 is the causative agent of Adult T-Cell Leukaemia (ATL)[3] and has been associated with Tropical Spastic Paraparesis (TSP) also called HTLV-1 Associated Myelopathy (HAM)[4]. It is endemic in Southern Japan, the Caribbean Islands and parts of central Africa.[5] Transmission is by sexual contact[6], intravenous drug abuse, from an infected mother to her child, mainly via breast milk[7] and by non-leukoreduced blood transfusion[8–10]. HTLV-1 causes ATL in 2-4% of infected individuals and typically after long latency periods[11, 12]. Once diagnosed with ATL life expectancy is typically less than a year. HAM/TSP occurs in approximately 0.25-4% of HTLV-1 infected individuals usually after a latency period of up to 20 years, although HAM/TSP may occur after a few months when HTLV-1 infection is acquired through a blood transfusion[13–16]. Patients with HAM/TSP may live with significant disability for 20-30 years post HAM/TSP diagnosis.[2] HTLV-2 infrequently causes HAM/TSP, increased incidence of pneumonia and bronchitis and perhaps higher all-cause and cancer mortality[17].
In 1993 Bhigjee et al. reported a seroprevalence of HTLV-1 in the predominantly Black Ngwelezana area of KwaZulu-Natal of 2.6% (95% confidence interval (CI) 1.62–3.58)[18]. An age-related rise in HTLV-1 seropositivity from 1.3% in the 15–24 year age group to 6.1% in the over 55-year-old group was also noted. In a study performed by van der Ryst et al. in 1996 in the Free State region of South Africa[19] it was reported that 2% (95% CI: 0.5 to 5 %) of asymptomatic urban Blacks and 1.1% (95% CI: 0.14 to 4%) of asymptomatic rural Blacks had HTLV-1 antibodies.An HTLV-1/2 seroprevalence study was conducted in 1996 among KwaZulu-Natal blood donors by the Natal Blood Transfusion Service (Sykes, W Personal communication). Donations were tested with an HTLV-1/2 enzyme immunoassay over a 3-month period from March to June 1996. Of 37,422 donations tested (22000 were from white donors) 3 were confirmed positive, for an overall prevalence of 0.008%. Of the three positives detected in this study 2 (0.016%) were female and 1 (0.004%) was male.
With increased donor recruitment in the Black community, current HTLV-1 prevalence data are needed for decision making about blood screening within the South Africa National Blood Service (SANBS). In this study we aim to determine the prevalence of HTLV-1 and -2 in the South African (SA) donor population and ascertain associations with demographic characteristics and geography
Methods
Case Report.
In October 2013, a potential HTLV transmission was reported to SANBS[20]. A 65 year old Indian-descent male had undergone surgery for carcinoma of the bladder in 2011 and had required 6 units of blood. In October 2013 the patient presented with a three month history of progressive lower limb weakness. Examination revealed a spastic paraparesis in the lower limbs. Upper limbs were normal, with normal sensation and intact bowel function. Western blot testing in the blood and polymerase chain reaction assay in the CSF was positive for HTLV-1. Upon trace back SANBS was able to identify one of the six blood donors as being HTLV-1 positive.
Phylogenetic analysis
DNA sequencing of HTLV-1 provirus and phylogenetic analysis was performed on the donor and transfusion recipient samples. High-molecular weight DNA was extracted from peripheral blood mononuclear cells (PBMC) using the QIAamp DNA minikit (Qiagen, Hilden, Germany). The two PBMC samples were first subjected to polymerase chain reaction (PCR) using human beta-globin specific primers to ensure that DNA was amplifiable. Both samples were then amplified by PCR using “env” primers, which were designed to amplify a 885-bp long fragment of the envelop gene: Env11: 5’-TGGCACGTCCTRTACTCTCCCAAC-3’ and Env22: 5’-GGCGAGGTGGAGTCCTTGGAGGC-3’ corresponding to nucleotides 5,911 to 5,934 and 6,774 to 6,796 respectively of the prototype ATK-1 sequence (Genbank: J02029). From each sample, 250 ng of DNA was amplified under the following conditions: 98°C, 1mn; 40 X (98°C, 5 s; 72°C, 20 s); 72°C, 1 mn. Reactions tubes were prepared in a dedicated room outside the laboratory with a final volume of 50 μl (DNA matrix, 250 ng; dNTP mix (Roche, Basel, Switzerland), 40 mM; 5X Phire II reaction buffer which contains 1.5 mM MgCl2 at final reaction concentration (Ozyme, Saint Quentin-en-Yvelines, France), 10 μl; Phire II hot start DNA polymerase (Ozyme, Saint Quentin-en-Yvelines, France), 2 U and 0.5 mM of each oligonucleotide primer (Eurofins MWG, Ebersberg, Germany). Ten microliters of amplified DNA was size fractionated by 1.5% agarose gel electrophoresis. The PCR products (40 μl) were sent for purification and sequencing reactions to the MWG Platform at Cochin Hospital, Paris, France. Each PCR product was sequenced using the Env11/Env22 pair of primers plus an additional inner pair of primers. A comparison of each generated segment by an alignment of the forward and reverse sequences using the ClustalW algorithm (Mac Vector 14.0.6 software, Oxford Molecular) was implemented to derive a consensus sequence. Then, phylogenetic trees were generated, using both neighbour- joining and maximum likelihood methods, from multiple alignments using the CLC Main Workbench 7.6.4 (Qiagen) software.
Sampling and Testing.
An identity-unlinked cross sectional study to determine the prevalence of HTLV-1/2 in SA blood donors was performed between August 2013 and November 2013. A sample size of 50,000 donations was planned; Black and Coloured (local term for mixed-race) donors were oversampled in a ratio of 4:1 as compared to White/Asian donors to increase statistical power in expected high prevalence population groups (however the over-sampling of Black and Coloured donors was not correctly implemented in the Eastern Cape). Donor samples were collected from all areas of South Africa, excluding the Western Cape Province where another blood service collects and tests donated blood. The donor record was tagged when donations were selected to ensure that if the donor presented to donate again during the study period they were excluded. Donor demographic information (race, gender, age, region and whether the donor was a first time, repeat or lapsed donor) and virology test results were uploaded into the study dataset and linked to a de novo study ID. The donation identifier number was then removed from the specimen prior to testing. The protocol was approved by the SANBS Human Research Ethics Committee (Clearance certificate number 12/01).
All samples were tested for HTLV-1/ 2 using the Abbott PRISM HTLV 1/2 chemiluminescent assay (ChLIA) (Abbott Diagnostics, Delkenheim, Germany). Initially reactive samples were repeated in duplicate on the same testing platform and repeatedly reactive samples were tested by a confirmatory assay using the Inno-LIA HTLV-1/2 Score Line ImmunoAssay (Fujirebio, Ghent, Belgium) method. Six confirmed positive samples were sent to the National Health Laboratory Service Clinical pathology department at Groote Schuur Hospital in Cape Town for Proviral DNA using a hemi-nested in house PCR targeting a region of the pol gene to validate the Inno-LIA results.
Statistical analysis.
All demographic, donation and laboratory data were captured electronically. HTLV prevalence was calculated overall and by subgroup, and 95% confidence intervals were calculated. Differences in prevalence between groups were assessed with chi-square tests. Multiple logistic regression was performed to determine factors independently associated with HTLV. A p-value of <0.05 was considered significant. Finally, prevalence was extrapolated to annual blood donations at SANBS by weighting according to the original oversampling of Black and Coloured donors. Here we multiplied the HTLV prevalence of each race group in the study by the number of blood donations by that race group annually to determine the number of HTLV positive donations that would be detected annually per race group. These were then added up and the overall prevalence was determined as the total number of HTLV positive donations predicted annually divided by the number of donations collected annually.
Results
A total of 46,752 blood donors (Black 73%, Coloured 13%, White 12% and Asian 2%) were tested for HTLV-1/2 antibodies (Table 1). Of 133 (0.28% of total) initial reactive samples, 111 (0.24%) tested repeat reactive and 57 (0.12%) were confirmed positive. There were 5 samples that could not be repeated by the Inno-LIA assay due to insufficient volume; when adjusted for these we estimate that a total of 60 (0.128%) would have confirmed positive. All positives were HTLV-1 according to Inno-LIA and all of the 6 Inno-LIA positive samples tested by PCR were found to contain HTLV-1 provirus. There was one co-infection with HIV and no co-infections with either HBV or HCV.
Table 1:
HTLV-1 prevalence, by demographic and geographic characteristics as well as donor status. Zones indicate SANBS blood collection regions that correspond roughly to South African Provinces.
| N Tested | HTLV-1 Positive, n (%) | P-VALUE(Adjusted) | ||
|---|---|---|---|---|
| All donors | 46 752 | 57 (0.12%) | ||
| Age | 16-19 | 9 521 | 10 (0.11%) | <.0001 |
| 20-29 | 14 254 | 12 (0.08%) | ||
| 30-39 | 9 904 | 4 (0.04%) | ||
| 40-49 | 7 426 | 9 (0.12%) | ||
| 50+ | 5 647 | 22 (0.39%) | ||
| Gender | Male | 26 701 | 25 (0.09%) | 0.03 |
| Female | 20 051 | 32 (0.16%) | ||
| Race | White | 5 643 | 1 (0.02%) | 0.0023 |
| Asian | 909 | 0 (0.00%) | ||
| Coloured | 6 033 | 1 (0.02%) | ||
| Black | 34 166 | 55 (0.16%) | ||
| Other | 1 | 0 (0.00%) | ||
| Zone | Egoli | 11 318 | 15 (0.13%) | 0.0568 |
| Eastern Cape | 4 604 | 0 (0.00%) | ||
| Free State | 2 287 | 3 (0.13%) | ||
| Kwa-Zulu-Natal | 7 826 | 10 (0.13%) | ||
| Mpumalanga | 4 750 | 12 (0.25%) | ||
| Northern | 10 109 | 12 (0.12%) | ||
| Vaal | 5 858 | 5 (0.09%) | ||
| Donor status | New | 7 214 | 8 (0.11%) | 0.6885 |
| Re-Join | 5 548 | 8 (0.14%) | ||
| Repeat | 33 966 | 41 (0.12%) |
In South Africa, Coloured ethnicity is a multiracial group made up of five source populations namely: African San, African non-San, European, South Asian, and East Asian[44]
Lapsed donors have made at least one donation but none within the past year.
HTLV-1 prevalence was 0.16% (95%CI 0.14%-0.23%) in Black donors, 0.02% (95%CI 0%-0.06%) in Coloured donors, 0.02% (95%CI 0%-0.05%) in White donors and 0% (95%CI 0%-0.6%) in Asian donors (Table 1). Female donors showed a significantly higher prevalence than did males (0.16% vs. 0.09% (p=0.03)). There was no difference in prevalence between first time (0.11%) and repeat (0.12%) donors. Focusing on the Black population group, HTLV-1 prevalence increased with age and especially ages over 50 years, and females were more likely to be positive than males at all ages (Figure 1). Geographically, there was no significant difference in prevalence among the operational zones of SANBS, which generally correspond to the provinces of South Africa (Figure 2) except that no HTLV-1 positives were found in the Eastern Cape where oversampling of Black and Coloured was not properly implemented.
Figure 1. HTLV-1 prevalence by age and sex, South African blood donors from the Black population group only.
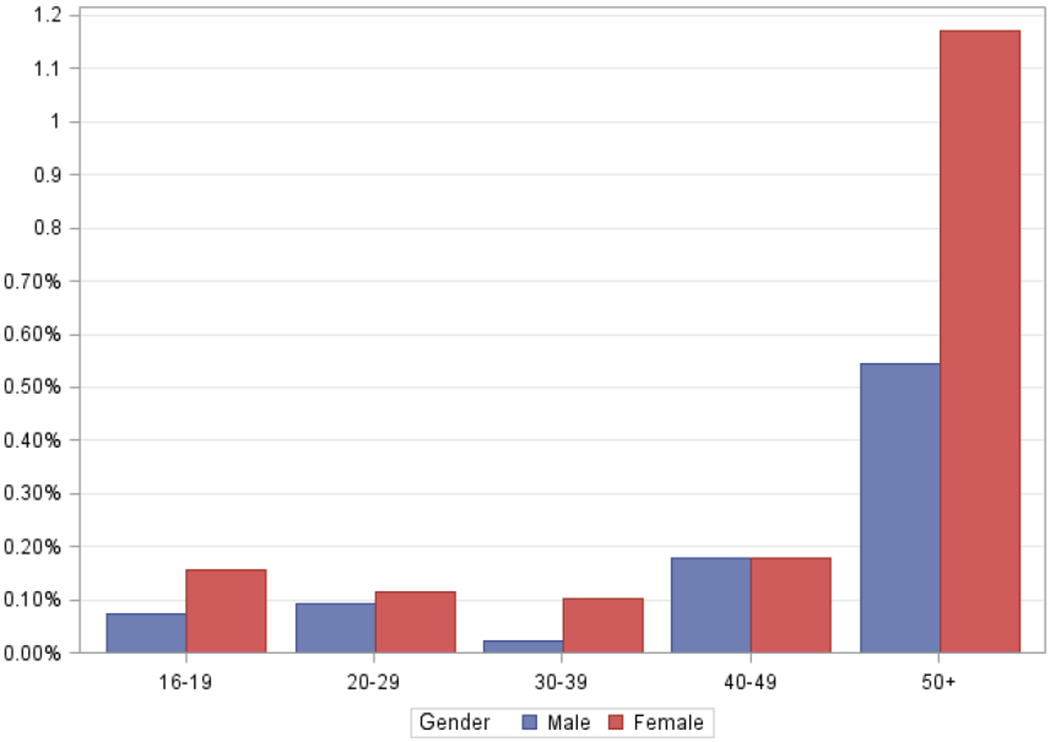
HTLV-1 seroprevalence by age and sex, South African blood donors, 2013.
Figure 2. HTLV-1 prevalence by SANBS collection zones, which correspond generally to South African Provinces.
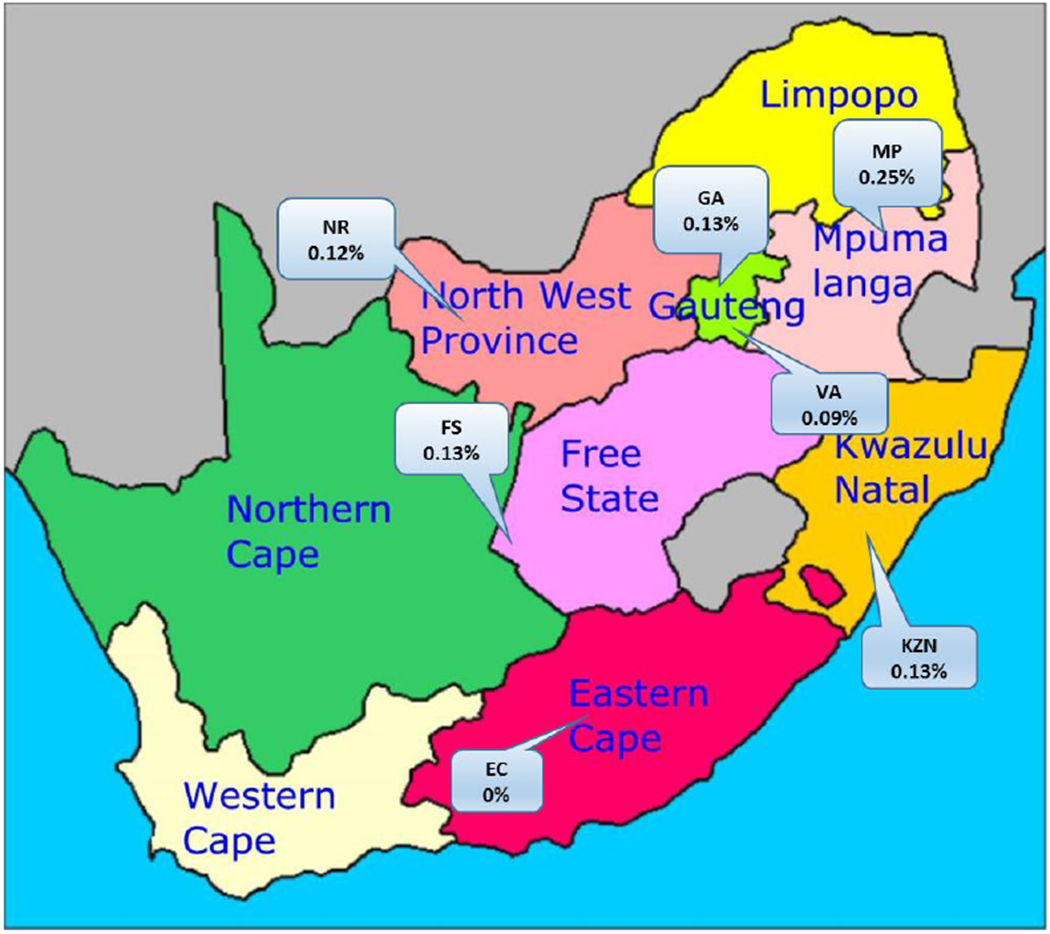
HTLV-1 seroprevalence according to province of blood collection, South African blood donors, 2013.
After extrapolating the study sample back into the population group distribution of current SANBS donations in 2015, the overall number of confirmed infections and estimated prevalence in SANBS donations would be 509 or 0.062% (95%CI 0.0568%−0.068%). The Initial reactive specificity of the antibody screening assay compared to the Inno-LIA (assuming all screen negatives were true negatives) was 99.84% (95%CI 99.75%-99.93%) which would result in 1277 false positives per annum if all 818,000 donations were tested.
Logistic regression analysis was used to adjust for confounding between variables (Table 2). The odds of infection rose substantially with age (odds ratio = 6.40 for those aged over 50 compared to those aged under 20). Females had nearly twice the odds of HTLV-1 infection compared to males. Black donors had 20 times the odds of HTLV-1 infection compared to White donors, but there was no difference by new versus repeat donor status. Due to the relatively small numbers of positive subjects in any one zone or province, there were no significant differences in the prevalence by geography. Mpumalanga had the highest odds of infection with KwaZulu-Natal having odds similar to Egoli (the Johannesburg/Pretoria region), and lower odds observed in Eastern Cape and Vaal.
Table 2:
Logistic regression model of factors associated with HTLV-1 infection. Adjusted odds ratios and 95% confidence intervals are shown. Zones indicate SANBS blood collection regions that correspond roughly to South African Provinces.
| Variable | Groups | Odds Ratio | 95%CI | |
|---|---|---|---|---|
| Age | 16-19 | 1.00 | --- | |
| 20-29 | 0.80 | 0.34 | 1.88 | |
| 30-39 | 0.44 | 0.14 | 1.41 | |
| 40-49 | 1.68 | 0.65 | 4.13 | |
| 50+ | 6.40 | 2.95 | 13.86 | |
| Gender | Male | 1.00 | --- | |
| Female | 1.81 | 1.06 | 3.08 | |
| Race | White and Asian | 1.00 | --- | |
| Black | 20.24 | 2.77 | 147.88 | |
| Coloured | 1.65 | 0.10 | 26.49 | |
| Zone / Province | Egoli | 1.00 | --- | |
| Eastern Cape/Vaal | 0.36 | 0.13 | 1.00 | |
| Free State | 1.33 | 0.38 | 4.61 | |
| Kwazulu-Natal | 1.04 | 0.46 | 2.33 | |
| Mpumalanga | 1.84 | 0.85 | 3.97 | |
| Northern | 0.75 | 0.35 | 1.60 | |
| Donor type | First time | 1.00 | --- | |
| Repeat | 1.25 | 0.57 | 2.75 | |
| Lapsed | 1.56 | 0.57 | 4.24 | |
HTLV transmission case
The blood donor and recipient HTLV-1 strains were found identical on a 772-bp long env fragment, which comprises the 522 bp fragment used for env phylogenetic analyses. These two new sequences (SANBS480 and SANBS900 accession numbers MK496634 and MK496635 respectively) are closely related, but different (1 to 4 bp difference/522bp), from those previously characterized from South Africa and available in GenBank (afs1, 2, 3 and afs 911). Furthermore, several non-South African sequences (i.e. PH757, PH1494 and Ar55 from the West-Indies and Argentina respectively) were identical to the two novel sequences generated in this study. The phylogenetic analysis performed on a 522-bp-long env region with 1,000 bootstrap replicates showed that both tree topologies were comparable for the neighbour-joining and maximum likelihood methods (data not shown). The main HTLV-1 subtypes (a-d) were identifiable and the two new viral strains (SANBS480 and SANBS900) belong to the HTLV-1 Cosmopolitan a-subtype and the transcontinental clade (Figure 3). Furthermore, we also amplified the complete LTR fragment (757 bp) of the donor and the recipient HTLV-1 strains and these were found to be identical. This sequence is slightly different (4 nucleotides difference) from the only other LTR sequence available from an HTLV-1 strain from South Africa (afs911) (see supplemental data).
Figure 3. Phylogenetic analysis.
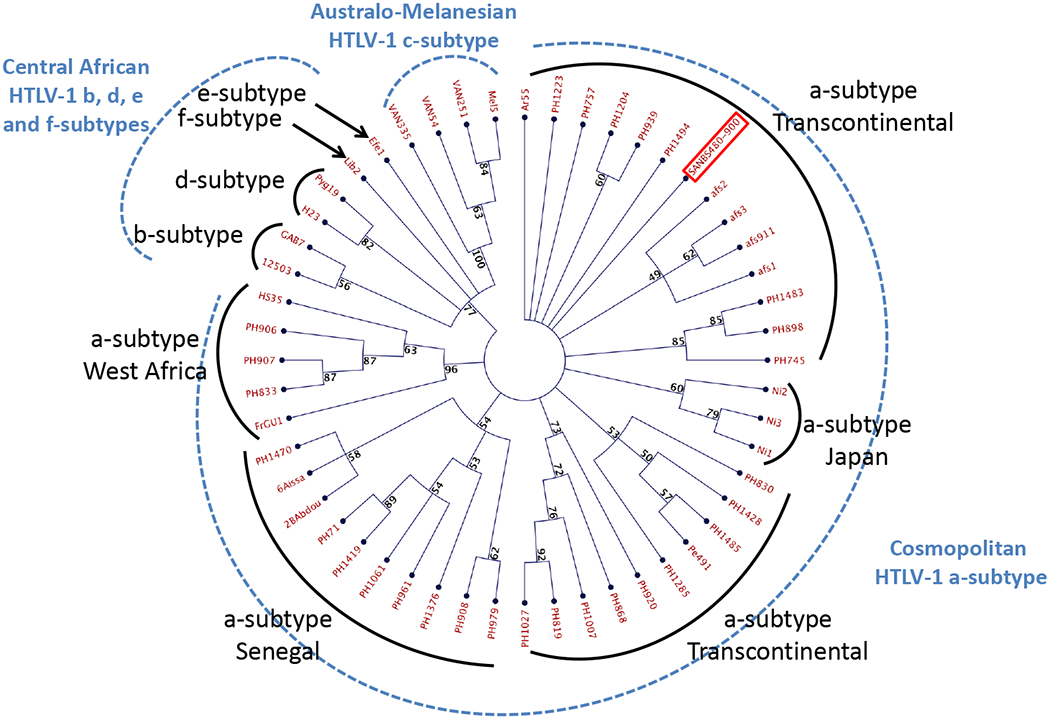
Phylogenetic analysis of HTLV-1 env sequences. Phylogenetic comparison was performed on 522-nucleotide-long env gene fragment of 53 HTLV-1 isolates, including the two sequences generated in this study (SANBS 480 and SANBS 900; in red frame); other South African isolates afs1, afs2, afs3, afs911 and 47 previously published sequences. The phylogeny was derived by the neighbour-joining method using the GTR model. Numbers on each node indicate the percentage of bootstrap samples (of 1,000) in which the cluster is supported (threshold value ≥ 50%).
Thus, genetic comparison and phylogenetic analyses, performed on both a fragment of the env gene and the complete LTR sequence, are compatible with HTLV-1 transmission from the donor to the recipient, but evidence falls short of proof as the two identified HTLV-1 strains belong to the frequent and widespread a-TC genotype, which exhibits a very low genetic variability.
Discussion
This study found measurable levels of HTLV-1 infection among South African blood donors, with an adjusted prevalence of 0.062% among blood donations. HTLV-1 infection was localized almost entirely to the Black population group with a prevalence of 0.16%. Associations with female gender (OR = 1.82 versus males) and older age (OR = 6.40 in those over 50 years versus those under 20 years) were similar to reports in other populations [21–24].
The finding of endemic HTLV-1 among Black South Africans is consistent with data from other countries in Africa. HTLV-1 prevalence in small studies of non-blood donors ranged from 1-2% in Ghana[21], 2–3% in Mozambique, Uganda and Egypt[18, 25–27] and as high as 5% and 9.1% in Guinea and Gabon respectively[23, 28]. Caution must be used in comparing these prevalence rates because different population groups were studied and the uncertain use of confirmatory testing.
Among blood donors, Senegal reported a HTLV-1/2 prevalence of 0.16%[29] of which 88% were HTLV-1, Guinea reported 1.2% [30] and Mozambique reported 0.89% of which all were HTLV-1[31]. In smaller studies with questionable confirmatory methods, Zimbabwe reported a prevalence of 0.1% [32], Ethiopia reported HTLV-1 and HTLV-2 prevalences of 0.19% and 0.25%, respectively[27], Mali had an unconfirmed blood donor prevalence of 1.4%[33] and Tunisia found no HTLV positives in 500 blood donors[27]. In Mali, Diarra et al. showed the prevalence of HTLV in multi-transfused patients to be 2 and 5.3 fold higher (2.8% and 7.5%) in patients that received 2 and 3 blood transfusions respectively than in blood donors from the same region (1.4%)[33].
Others have suggested that there is about a 6-fold reduction in HTLV-1 prevalence in blood donors versus the general population due to their younger age[34], selective recruitment and pre-donation risk questioning[35]. HTLV prevalence in the general population is mainly in the elderly whereas at SANBS 80% of the donations are made by donors under the age of 50. In the early 1990s, Bhigjee et al. demonstrated a prevalence increasing from 1.3% in 15 to 24 years olds to 6.1% in those aged over 55 years in a mostly black community-based sample in the KwaZulu-Natal province of South Africa, a province that has the highest prevalence of HIV[18]. Overall, this is about 10-fold higher than the age-specific prevalence we found in Black blood donors, demonstrating selection for safer donors as noted above. If we were to apply a similar factor of 6-10 to extrapolate the current data, it would suggest that the prevalence of HTLV-1 in the general Black adult population of South Africa is at least 1%, suggesting endemic infection but at perhaps a lower prevalence than in certain countries in sub-Saharan Africa.
SANBS implemented a strategic objective in 2005 to increase blood donations from the majority Black population to correct historical racial imbalances and improve sustainability. At the time of this study in 2013, the proportion of Black donors was 39% compared to 6% in 2005[36]. Because our study found HTLV-1 infection to be evident in the Black population, efforts to increase donations from Black donors may be expected to increase the overall prevalence in blood donations above 0.062% and this will need to be monitored prospectively.
During this study SANBS was informed of a potential HTLV-1 transmission. Findings based on sequence comparison and phylogenetic analyses on both a fragment of the env gene and the complete LTR, are compatible with transmission from the donor to the recipient. There is one other previously reported potential blood-borne HTLV-1 transmission in the late 80’s from South Africa however this case was not studied molecularly.[37]. Possible reasons for so few reported transmission events are many: 1) lack of HTLV awareness among health care providers and a poor haemovigilance system with under reporting; 2) an assumed 50% mortality after transfusion[38], asymptomatic infection in most patients[2], and a prolonged asymptomatic phase before rare disease outcomes[34]; and 3) aspects of blood processing may reduce transmission including storage time prior to transfusion but SANBS transfuses 80% of its red cell products in less than 11 days following collection[34] and white cell reduction by buffy coat removal (84%) or filter leukoreduction (16%)[39].
HTLV-1 antibody testing of all blood donations has been implemented in a number of high income countries however some have questioned the cost effectiveness of these strategies considering that money spent to prevent rare HTLV-1 infections is diverted from other health priorities[40–43].
Strengths of the study include its large sample size, oversampling of the endemic Black and Coloured populations, broad geographic scope, and use of state-of-the-art assays for HTLV-1 antibody screening and confirmation. Weaknesses include relatively low power for subgroup analyses due to the limited number of positives and the lack of more detailed risk factor information due to its unlinked design. In addition, the over-sampling of Black and Coloured donors was not correctly implemented in Eastern Cape, which likely explains the observed zero prevalence in this Province. Cellular blood samples were not stored from positives and so molecular epidemiologic studies to compare HTLV-1 subtypes are not possible. Finally, as mentioned above, the data need to be extrapolated to the general population with caution because blood donors are selected to be low risk and healthy.
In conclusion, this large study has allowed the measurement of contemporary HTLV-1 prevalence in South African blood donors and provides strong evidence that the virus is endemic in the South African Black population and is not limited to KwaZulu-Natal province. It raises the question as to whether HTLV-1 antibody screening or other measures should be implemented to prevent transfusion transmitted infections in the country. The findings from this study along with a budget impact tool were used to assess implications of different blood screening options for HTLV in South Africa using the Alliance of Blood Operators Risk Based Decision Making framework (see Vermeulen et al in this issue). At this time SANBS has decided not to implement screening for HTLV due to financial constraints in the South African health sector.
Supplementary Material
Acknowledgements
We would like to thank Vitalant Research Institute for partial funding and Abbott Diagnostics for providing a portion of the HTLV test kits. A special thanks to all the Donation Testing staff at SANBS for performing the testing in addition to their normal work. We would also like to thank Dr. Diana Hardie of the South African National Health Laboratory Service for performing the HTLV proviral DNA testing for confirmation of the Inno-LIA results.
Research support: Vitalant Research Institute and Abbott Diagnostics for the HTLV reagents
REFERENCES
- 1.Poiesz BJ, Ruscetti FW, Reitz MS, et al. : Isolation of a new type C retrovirus (HTLV) in primary uncultured cells of a patient with Sezary T-cell leukaemia. Nature 1981; 294: 268–71. [DOI] [PubMed] [Google Scholar]
- 2.Proietti FA, Carneiro-Proietti AB, Catalan-Soares BC, et al. : Global epidemiology of HTLV-I infection and associated diseases. Oncogene 2005; 24: 6058–68. [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi K, Seiki M, Yoshida M, et al. : The detection of human T cell leukemia virus proviral DNA and its application for classification and diagnosis of T cell malignancy. Blood 1984; 63: 1235–40. [PubMed] [Google Scholar]
- 4.Gessain A, Barin F, Vernant JC, et al. : Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet 1985; 2: 407–10. [DOI] [PubMed] [Google Scholar]
- 5.Gessain A, Cassar O: Epidemiological Aspects and World Distribution of HTLV-1 Infection. Front Microbiol 2012; 3: 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy EL, Figueroa JP, Gibbs WN, et al. : Sexual transmission of human T-lymphotropic virus type I (HTLV-I). Annals of internal medicine 1989; 111: 555–60. [DOI] [PubMed] [Google Scholar]
- 7.Percher F, Jeannin P, Martin-Latil S, et al. : Mother-to-Child Transmission of HTLV-1 Epidemiological Aspects, Mechanisms and Determinants of Mother-to-Child Transmission. Viruses 2016; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inaba S: [HTLV-I transmission by blood transfusion]. Rinsho byori The Japanese journal of clinical pathology 1991; Suppl 88: 171–5. [PubMed] [Google Scholar]
- 9.Hjelle B, Mills R, Mertz G, et al. : Transmission of HTLV-II via blood transfusion. Vox sanguinis 1990; 59: 119–22. [DOI] [PubMed] [Google Scholar]
- 10.Okochi K, Sato H: Transmission of ATLV (HTLV-I) through blood transfusion. Princess Takamatsu symposia 1984; 15: 129–35. [PubMed] [Google Scholar]
- 11.Wyld PJ, Tosswill JH, Mortimer PP, et al. : Sporadic HTLV-I associated adult T-cell leukaemia (ATL) in the U.K. British journal of haematology 1990; 76: 149–50. [DOI] [PubMed] [Google Scholar]
- 12.Hinuma Y: Preleukemia and typical adult T-cell leukemia (ATL) etiologically associated with a retrovirus (HTLV/ATLV). Haematologica 1987; 72: 72–4. [PubMed] [Google Scholar]
- 13.Inaba S, Okochi K, Sato H, et al. : Efficacy of donor screening for HTLV-I and the natural history of transfusion-transmitted infection. Transfusion 1999; 39: 1104–10. [DOI] [PubMed] [Google Scholar]
- 14.Gout O, Baulac M, Gessain A, et al. : Rapid development of myelopathy after HTLV-I infection acquired by transfusion during cardiac transplantation. The New England journal of medicine 1990; 322: 383–8. [DOI] [PubMed] [Google Scholar]
- 15.Manns A, Wilks RJ, Murphy EL, et al. : A prospective study of transmission by transfusion of HTLV-I and risk factors associated with seroconversion. Int J Cancer 1992; 51: 886–91. [DOI] [PubMed] [Google Scholar]
- 16.Toro C, Rodes B, Poveda E, et al. : Rapid development of subacute myelopathy in three organ transplant recipients after transmission of human T-cell lymphotropic virus type I from a single donor. Transplantation 2003; 75: 102–4. [DOI] [PubMed] [Google Scholar]
- 17.Murphy ELB R: Principles and Practice of Infectious Diseases. Elsevier 2014; Chapter 170. [Google Scholar]
- 18.Bhigjee AI, Vinsen C, Windsor IM, et al. : Prevalence and transmission of HTLV-I infection in Natal/KwaZulu. S Afr Med J 1993; 83: 665–7. [PubMed] [Google Scholar]
- 19.van der Ryst E, Joubert G, Smith MS, et al. : HTLV-I infection in the Free State region of South Africa: a sero-epidemiologic study. Cent Afr J Med 1996; 42: 65–8. [PubMed] [Google Scholar]
- 20.Hoosain P, Bhigjee AI: Health policy implications of blood transfusion-related human T-cell lymphotropic virus type 1 infection and disease. South African Journal of Infectious Diseases 2015; 30: 145–6. [Google Scholar]
- 21.Biggar RJ, Neequaye JE, Neequaye AR, et al. : The prevalence of antibodies to the human T lymphotropic virus (HTLV) in Ghana, West Africa. AIDS research and human retroviruses 1993; 9: 505–11. [DOI] [PubMed] [Google Scholar]
- 22.Eshima N, Iwata O, Iwata S, et al. : Age and gender specific prevalence of HTLV-1. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology 2009; 45: 135–8. [DOI] [PubMed] [Google Scholar]
- 23.van Tienen C, van der Loeff MF, Peterson I, et al. : HTLV-1 in rural Guinea-Bissau: prevalence, incidence and a continued association with HIV between 1990 and 2007. Retrovirology 2010; 7: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang YB, Kaidarova Z, Hindes D, et al. : Seroprevalence and demographic determinants of human T-lymphotropic virus type 1 and 2 infections among first-time blood donors--United States, 2000-2009. J Infect Dis 2014; 209: 523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caterino-de-Araujo A, Magri MC, Costa EA, et al. : Prevalence of human T-cell lymphotropic virus (HTLV-1/2) in individuals from public health centers in Mozambique. AIDS research and human retroviruses 2010; 26: 559–61. [DOI] [PubMed] [Google Scholar]
- 26.El-ghazzawi E, Hunsmann G, Schneider J: Low prevalence of antibodies to HIV-1 and HTLV-I in Alexandria, Egypt. AIDS-Forschung : AIFO = Acquired immune deficiency syndrome research 1987; 2: 639. [PubMed] [Google Scholar]
- 27.Vrielink H, Reesink HW: HTLV-I/II prevalence in different geographic locations. Transfus Med Rev 2004; 18: 46–57. [DOI] [PubMed] [Google Scholar]
- 28.Delaporte E, Dupont A, Peeters M, et al. : Epidemiology of HTLV-I in Gabon (Western Equatorial Africa). International journal of cancer Journal international du cancer 1988; 42: 687–9. [DOI] [PubMed] [Google Scholar]
- 29.Diop S, Calattini S, Abah-Dakou J, et al. : Seroprevalence and molecular epidemiology of human T-Cell leukemia virus type 1 (HTLV-1) and HTLV-2 in blood donors from Dakar, Senegal. J Clin Microbiol 2006; 44: 1550–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gessain A, Fretz C, Koulibaly M, et al. : Evidence of HTLV-II infection in Guinea, West Africa. J Acquir Immune Defic Syndr 1993; 6: 324–5. [PubMed] [Google Scholar]
- 31.Gudo ES, Abreu CM, Mussa T, et al. : Serologic and molecular typing of human T-lymphotropic virus among blood donors in Maputo City, Mozambique. Transfusion 2009; 49: 1146–50. [DOI] [PubMed] [Google Scholar]
- 32.Houston S, Thornton C, Emmanuel J, et al. : Human T cell lymphotropic virus type 1 in Zimbabwe. Trans R Soc Trop Med Hyg 1994; 88: 170–2. [DOI] [PubMed] [Google Scholar]
- 33.Diarra AB, Kouriba B, Guindo A, et al. : Prevalence of HTLV-I virus in blood donors and transfusion in Mali: Implications for blood safety. Transfusion clinique et biologique : journal de la Societe francaise de transfusion sanguine 2014; 21: 139–42. [DOI] [PubMed] [Google Scholar]
- 34.Murphy EL: Infection with human T-lymphotropic virus types-1 and -2 (HTLV-1 and -2): Implications for blood transfusion safety. Transfus Clin Biol 2016; 23: 13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor GP, Bodeus M, Courtois F, et al. : The seroepidemiology of human T-lymphotropic viruses: types I and II in Europe: a prospective study of pregnant women. Journal of acquired immune deficiency syndromes 2005; 38: 104–9. [DOI] [PubMed] [Google Scholar]
- 36.Vermeulen M, Lelie N, Coleman C, et al. : Assessment of HIV transfusion transmission risk in South Africa: a 10-year analysis following implementation of individual donation nucleic acid amplification technology testing and donor demographics eligibility changes. Transfusion 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhigjee AI, Harvey MM, Windsor I, et al. : Blood transfusion and HTLV-I-associated myelopathy. S Afr Med J 1989; 76: 700. [PubMed] [Google Scholar]
- 38.Borkent-Raven BA, Janssen MP, van der Poel CL, et al. : The PROTON study: profiles of blood product transfusion recipients in the Netherlands. Vox sanguinis 2010; 99: 54–64. [DOI] [PubMed] [Google Scholar]
- 39.Hewitt PE, Davison K, Howell DR, et al. : Human T-lymphotropic virus lookback in NHS Blood and Transplant (England) reveals the efficacy of leukoreduction. Transfusion 2013; 53: 2168–75. [DOI] [PubMed] [Google Scholar]
- 40.O’Brien SF, Yi QL, Goldman M, et al. : Human T-cell lymphotropic virus: A simulation model to estimate residual risk with universal leucoreduction and testing strategies in Canada. Vox sanguinis 2018; 113: 750–9. [DOI] [PubMed] [Google Scholar]
- 41.Stigum H, Magnus P, Samdal HH, et al. : Human T-cell lymphotropic virus testing of blood donors in Norway: a cost-effect model. International journal of epidemiology 2000; 29: 1076–84. [DOI] [PubMed] [Google Scholar]
- 42.Styles CE, Hoad VC, Seed CR: Estimation of human T-lymphotropic virus incidence in blood donors from observed prevalence. Vox sanguinis 2018. [DOI] [PubMed] [Google Scholar]
- 43.Styles CE, Seed CR, Hoad VC, et al. : Reconsideration of blood donation testing strategy for human T-cell lymphotropic virus in Australia. Vox sanguinis 2017; 112: 723–32. [DOI] [PubMed] [Google Scholar]
- 44.Daya M, Van der Merwe L, Galal U, et al. : A panel of ancestry informative markers for the complex five-way admixed South African coloured population. PLoS One 2013; 8: e82224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


