Abstract
BACKGROUND:
Fine needle aspiration (FNA) is commonly used for the preoperative evaluation of salivary gland tumors. Tumor grade is a key factor influencing clinical management of salivary gland carcinomas (SGCs). To assess the ability to grade nonbasaloid SGCs in FNA specimens, an international panel of cytopathologists convened to review and score SGC cases.
METHODS:
The study cohort included 61 cases of primary SGC from the pathology archives of 3 tertiary medical centers. Cases from 2005 to 2016 were selected, scanned, and digitized. Nineteen cytopathologists blinded to the histologic diagnosis reviewed the digitized cytology slides and graded them as low, high, or indeterminate. The panelists’ results were then compared to the tumor grades based on histopathologic examination of the corresponding resection specimens.
RESULTS:
All but 2 of the 19 (89.5%) expert panelists review more than 20 salivary gland FNAs per year; 16 (84.2%) of the panelists work at academic medical centers, and 13 (68.4%) have more than 10 years’ experience. Participants had an overall accuracy of 89.4% in the grading of SGC cases, with 90.2% and 88.3% for low- and high-grade SGC, respectively. Acinic cell carcinoma and mucoepidermoid carcinoma had the highest degree of accuracy, while epithelial-myoepithelial carcinoma and salivary duct carcinoma had the lowest degree of accuracy. As expected, the intermediate-grade SGC cases showed the greatest variability (high-grade, 42.1%; low-grade, 37.5%, indeterminate, 20.4%).
CONCLUSION:
This study confirms the high accuracy of cytomorphologic grading of primary SGC by FNA as low- or high-grade. However, caution should be exercised when a grade cannot be confidently assigned.
Keywords: cancer, cytology, FNA, grading, Milan system, salivary gland
INTRODUCTION
Salivary gland carcinomas (SGCs) are uncommon and represent an unusually heterogeneous group of tumors.1–5 The 2017 World Health Organization (WHO) classification recognizes over 30 different epithelial salivary gland neoplasms.6 A majority of salivary gland tumors occurs in the parotid or submandibular glands facilitating pretreatment cytologic evaluation by palpationor ultrasound-guided fine needle aspiration (FNA).2–15 The results of FNA interpretation can have significant implications for the clinical and surgical management of salivary gland lesions.6,16–25 Reactive and inflammatory conditions (eg, chronic sialadenitis, lymphoepithelial sialadenitis) are typically managed by clinical observation or treated medically. Benign neoplasms such as Warthin tumor and pleomorphic adenoma are either excised by a conservative surgical approach or, in some cases, patients may opt for close clinical monitoring that may include occasional sampling by FNA. Low-grade SGCs are usually treated with conservative resection with negative margins, while high-grade SGCs are often managed by a more radical resection16,18,22–25 that may include neck dissection16,19,23–28 and facial nerve sacrifice pre- or intraoperatively.29
The Milan System for Reporting Salivary Gland Cytopathology (MSRSGC) is designed to facilitate standardized reporting of salivary gland FNA across institutions.30 In addition, the MSRSGC also provides a risk of malignancy for each diagnostic category and a pathway to clinical management.28–31 Initial studies have shown the MSRSGC to be effective.31–37 The MSRSGC includes the diagnostic category “malignant” for those cases wherein cytomorphologic features combined with results of any ancillary studies are clearly diagnostic of cancer. Given the potentially significant impact on clinical management, the MSRSGC recommends that whenever possible, the cytopathologist assigns the SGC a grade of high or low, as well as a specific classification if feasible.30
Although it is generally accepted that salivary gland FNA can effectively differentiate between lowand high-grade carcinomas, the evidence in the literature is limited.17,38–41 This is most likely due to the low incidence of primary salivary gland malignancies restricting the institutional ability to acquire a significant case load. Additionally, most studies on salivary gland FNA are retrospective and do not address the ability to grade primary carcinomas.42–45 In order to assess the effectiveness of grading SGCs by FNA, we organized an international panel of cytopathologists blinded to the final histologic diagnosis to grade a large FNA cohort of primary SGCs.
MATERIALS AND METHODS
The study was approved by the IRB committee of the Partners Healthcare System. Three pathologists (J.F.K., E.D.R., W.C.F.) provided FNA cases of primary SGC with corresponding surgical follow-up from the archives (2005 to 2016) of their institutions. The cytopathology slides from 66 SGCs were initially screened for this study, from which 61 cases were selected to be included in the final cohort. One representative cellular slide from each FNA case was selected, scanned, and digitized using the Aperio CS digital pathology scanner system at a magnification of ×400 (CS, Leica Biosystems, Buffalo Grove, Illinois). The scanned images retained no patient identifiers. Selected slides included a mix of Diff-Quik (n = 12) and Papanicolaou stains (n = 54) comprising both smears (n = 61) and monolayer preparations (n = 5). Three cases were excluded from the study cohort due to technical difficulties in digitization of the cytopathology slides (obscuring blood and large dark 3-dimensional clusters difficult to resolve on 2 FNA smear slides and scant cellularity and poor focus on single cells and clusters on 1 monolayer preparation). In addition, 2 cases were excluded from the cohort because the corresponding histologic classification was of an indeterminate tumor type. FNA cases of adenoid cystic carcinoma and other basaloid matrix-producing neoplasms were not included in the cohort, since grading of these carcinomas is controversial and clinical management is based less on the tumor grade than on the specific entity. In addition, adenoid cystic carcinoma and its differential diagnostic cohort present a separate set of diagnostic issues under the general category of “basaloid matrix-producing neoplasm” that distinguishes them from most other primary SGCs.46–48
The gold standard for tumor grading of cases in our cohort was the corresponding histopathologic examination and final diagnosis of the subsequent resection specimens as determined by 3 pathologists (J.F.K., E.D.R., W.C.F.). While several classical salivary gland carcinomas are associated with an inherent grade (eg, salivary duct carcinoma and acinic cell carcinoma), the criteria for the histologic grading of others such as mucoepidermoid carcinoma and adenocarcinoma not otherwise specified (NOS) can present challenges, especially for intermediate-grade classifications. For this reason, only classical histological examples of low- and high-grade mucoepidermoid carcinomas (using the Brandwein and Armed Forces Institute of Pathology [AFIP] grading schemes) and other SGCs were selected for this study. A subset of our cohort cases designated as intermediate-grade are defined by their final histologic diagnosis; the intermediate-grade subset of cases did not fall clearly into a low- or high-grade histologic category. The histologic diagnosis and grading of cases in our cohort followed the WHO Classification of Head and Neck Tumours, 4th edition.6
Our final study cohort of primary SGCs consisted of 22 high-grade, 31 low-grade, and 8 intermediate-grade FNA cases. The corresponding histologic diagnoses were: acinic cell carcinoma (n = 14 [all low-grade without high-grade transformation]), mucoepidermoid carcinoma (n = 12 [6 low-grade, 4 intermediate-grade, 2 high-grade]), salivary duct carcinoma (n = 13 [all high-grade]), epithelial-myoepithelial carcinoma (n = 5 [all low-grade]), polymorphous adenocarcinoma (n = 2 [both low-grade]), oncocytic carcinoma (n = 1 [low-grade]), myoepithelial carcinoma (n = 2 [both high-grade]), primary salivary gland squamous cell carcinoma (n = 1 [high-grade]), secretory carcinoma (n = 1 [low-grade]), neuroendocrine carcinoma (n = 1 [low-grade]), and adenocarcinoma, not otherwise specified (n = 9 [1 low-grade, 4 intermediate-grade, 4 high-grade]).
The 19 panelists were asked to anonymously provide data in response to 3 online survey questions about professional work characteristics: cytopathology experience (number of years in practice [<5, 5–10, >10]), number of salivary gland FNAs diagnosed per year ([<10, 10–20, >20]), and practice setting (academic or other). For each case, panelists were asked to classify the lesion as high-grade, low-grade, or indeterminate. Grading criteria were based on panelists’ training, experience, and according to features described for the “malignant” category in MSRSGC. Finally, panelists were optionally encouraged to provide their specific classification of each cohort case in a separate field.
Statistical analysis was performed using the MedCalc software package (https://www.medcalc.org) and VassarStats (http://VassarStats.net/kappa.html). Values of kappa can range between −1.0 and 1.0, with −1.0 indicating perfect disagreement below chance, 0.0 indicating agreement equal to chance, and 1.0 indicating perfect agreement above chance. Values less than .40 are classified as poor, values from .40 to .75 are classified as intermediate to good, and values above .75 are classified as excellent.49–54
RESULTS
The participating panel of 19 cytopathologists achieved an overall accuracy of 89.4% (n = 781/874) on grading the 53 low- and high-grade SGC cases. For low-grade SGC cases, participants had an accuracy of 90.2% (n = 450/499), with 90 indeterminate responses, and for high-grade SGC cases, the accuracy was 88.3% (n = 331/375), with 43 indeterminate responses (Table 1). The panelists assigned an indeterminate grade at an overall rate of 14.2% (164 indeterminate grading responses out of 1159): 14.8% (90/589) for low-grade SGCs, 10.2% (43/418) for high-grade SGCs, and 20.4% (31/152) for intermediate-grade SGCs.
TABLE 1.
Overall Accuracy by Final Grade on Histology
| Participant Grading | |||||
|---|---|---|---|---|---|
| Final Grade on Histology | Low | Indeterminate | High | Total | Accuracya |
| Low | 450 (76.4) | 90(15.3) | 49 (8.3) | 589(100) | 90.2% |
| Intermediate | 57 (37.5) | 31 (20.4) | 64(42.1) | 152(100) | - |
| High | 44 (10.5) | 43(10.3) | 331 (79.2) | 418(100) | 88.3% |
| Total | 551 | 164 | 444 | 1159 | 89.4% |
Data are presented as n (%).
Correct grading responses over incorrect grading responses (excluding indeterminate responses); otherwise, the percentages given are the proportion of responses given for each grade of carcinoma based on final histology.
Low-Grade SGCs
Among the low-grade SGCs, acinic cell carcinomas and low-grade mucoepidermoid carcinomas were the most accurately graded (Figs. 1–3). The accuracy for grading acinic cell carcinoma was 93.4% (n = 227/243), with 23 indeterminate responses, while the accuracy for grading low-grade mucoepidermoid carcinoma was 92.2% (n = 95/103), with 11 indeterminate responses. In contrast, epithelial-myoepithelial carcinoma had the least accurate grading among low-grade SGCs, with an accuracy of 76.8% (n = 53/69) and 26 indeterminate responses. One secretory carcinoma, 1 low-grade neuroendocrine carcinoma, and 2 polymorphous adenocarcinomas had the highest rate of indeterminate responses of 42.1%, 31.6%, and 31.6%, respectively.
FIGURE 1.
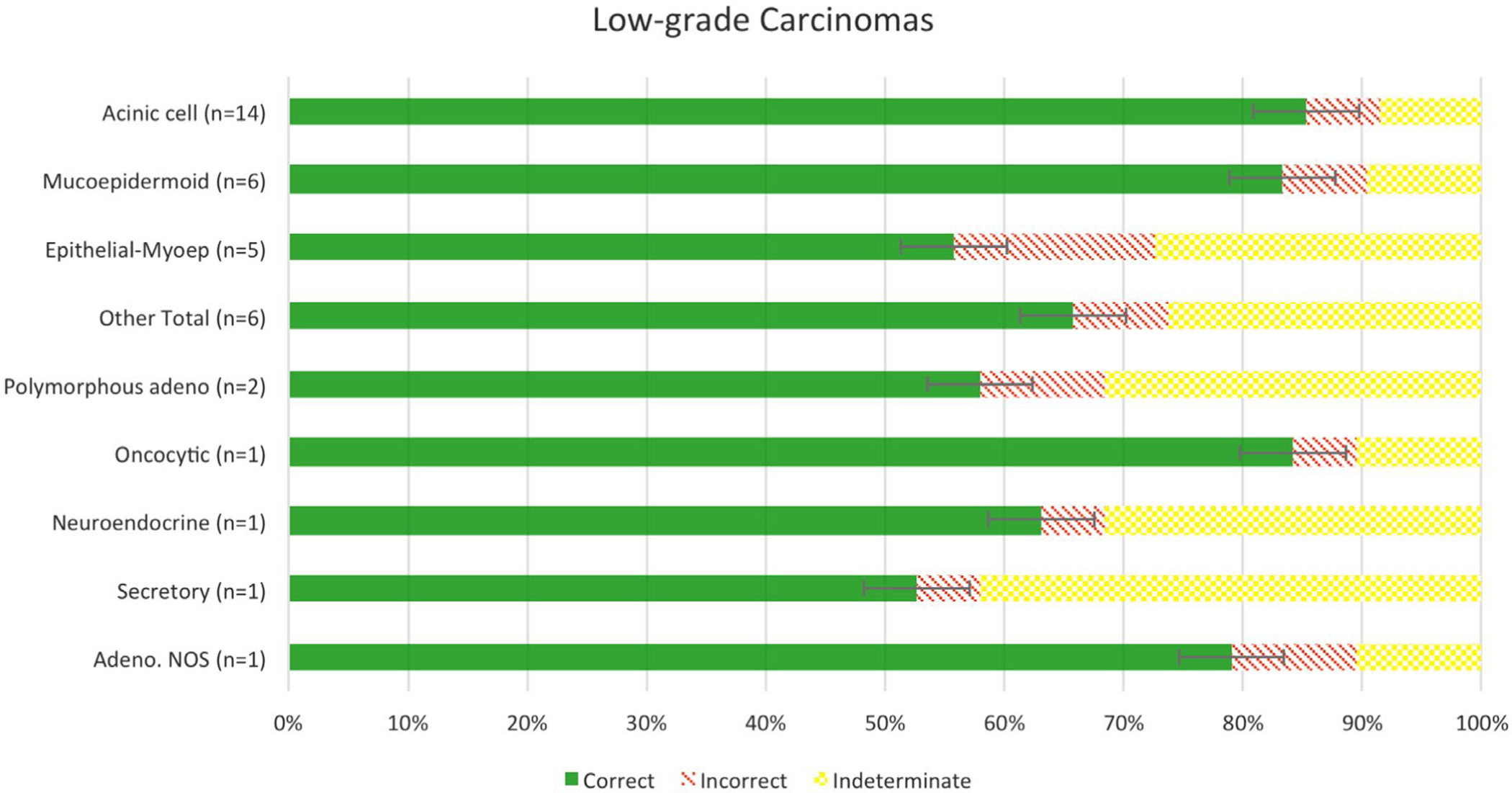
Accuracy of grading of low-grade salivary gland carcinomas.
FIGURE 3.
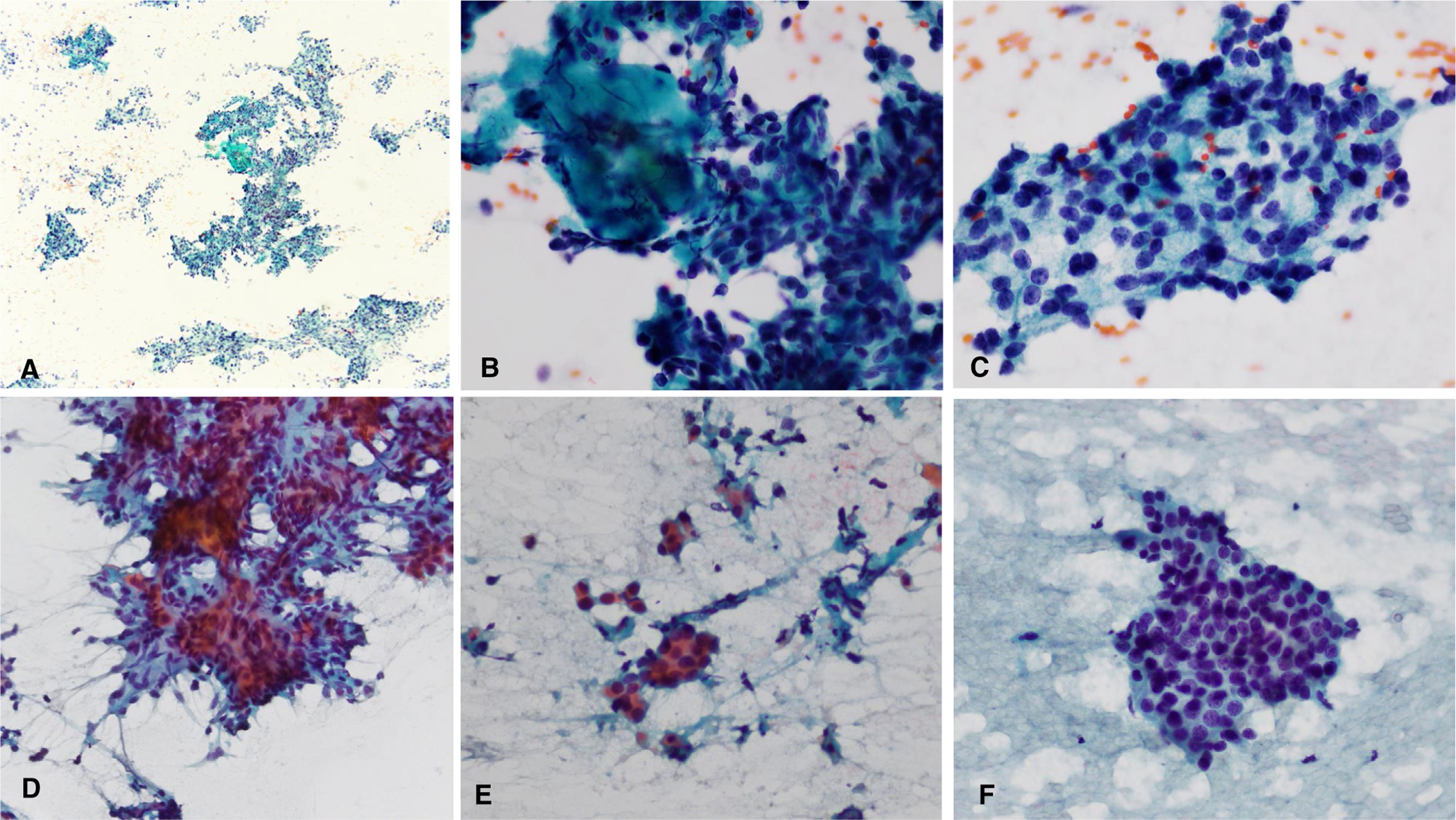
Two difficult low-grade salivary gland carcinoma cases. (A-C) Acinic cell carcinoma with hypercellularity with crowded groups, focal fibrous tissue, and variable hyperchromasia that likely led to overgrading. (D-F) Epithelial-myoepithelial carcinoma with hypercellularity, small crowded groups, background debris, and mild hyperchromasia that likely were problematic for accurate grading. (Papanicolaou stain, A, original magnification ×40, B-F, original magnification ×400.)
High-Grade SGCs
Among specific high-grade SGCs, high-grade mucoepidermoid carcinoma, like its low-grade counterpart, was the most accurately graded at 97.1% (n = 33/34), with 4 indeterminate responses. The only case of primary high-grade squamous cell carcinoma was accurately graded by 18 of the 19 participants (94.7%). For conventional salivary duct carcinoma, which by definition is high-grade, the participants accurately graded it at a rate of 86.3% (n = 189/219) with 28 indeterminate responses (Figs. 4–6).
FIGURE 4.
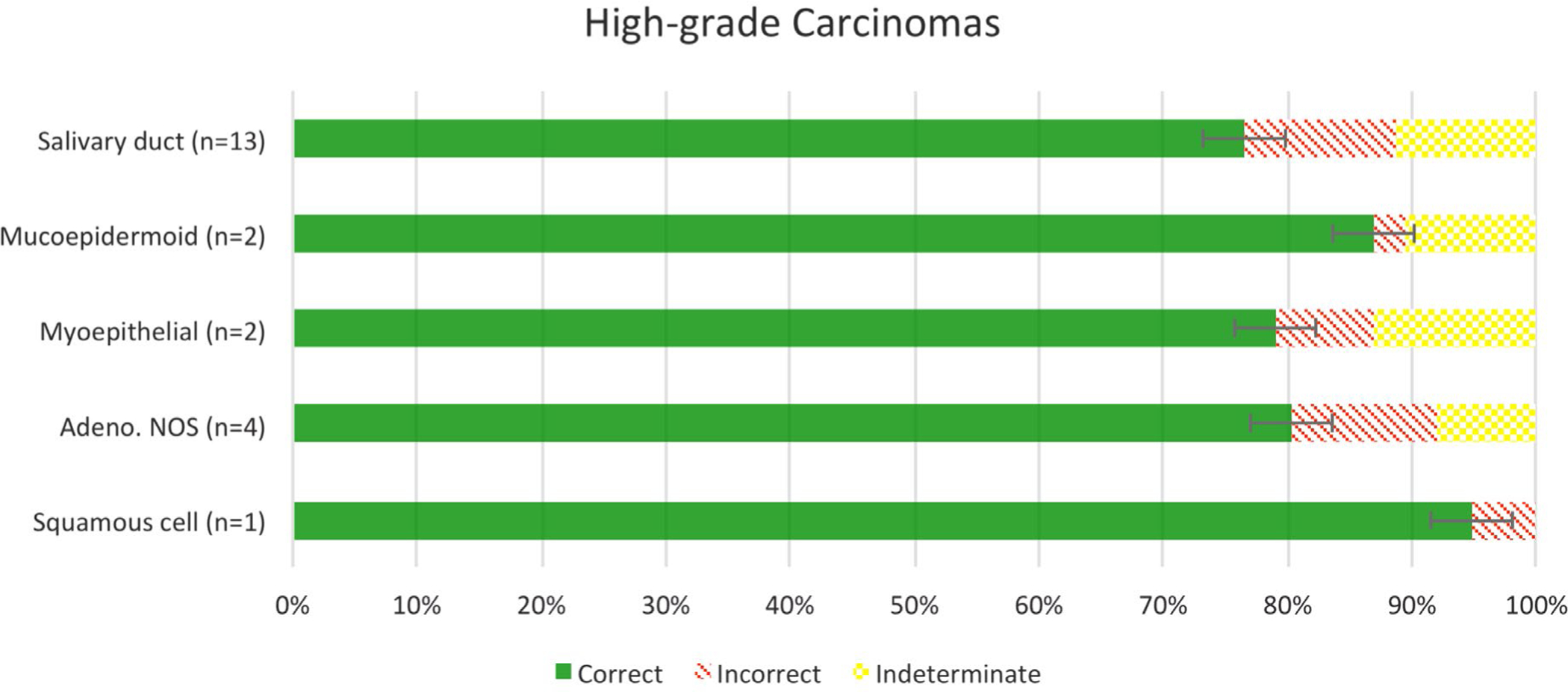
Accuracy of grading of high-grade salivary gland carcinomas.
FIGURE 6.
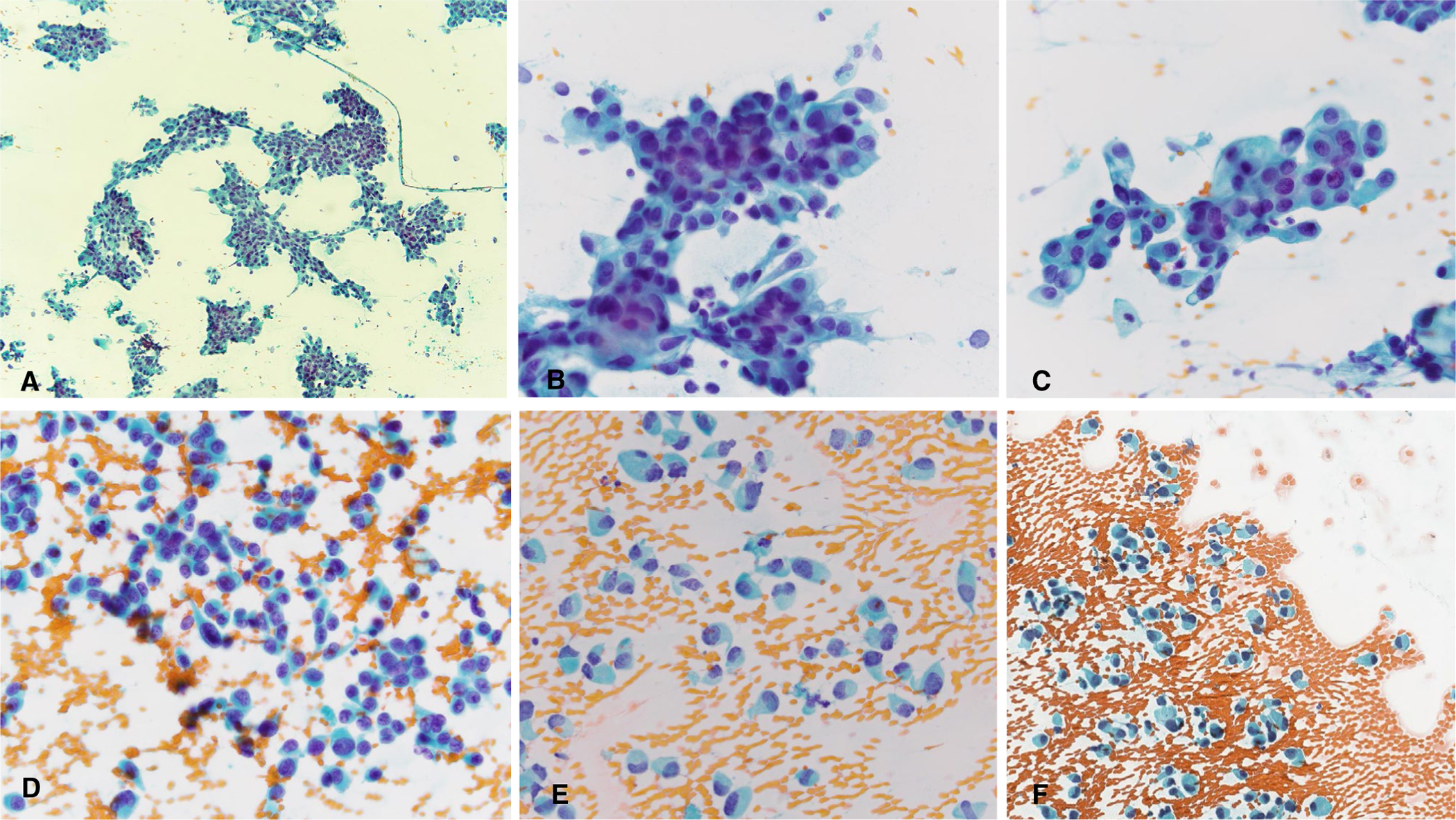
Two difficult high-grade salivary gland carcinomas. (A-C) Salivary duct carcinoma with low nuclear-cytoplasmic ratios and uniform atypia. (D-F) Salivary duct carcinoma with dispersed cell population, plasmacytoid appearance, low nuclear-cytoplasmic ratios, and uniform atypia. (Papanicolau stain, A, original magnification ×40; B-F, Papanicolaou stain, original magnification ×400.)
Intermediate-Grade SGCs
Intermediate-grade cases of SGC (Fig. 7) comprised 13.1% (n = 8/61) of the SGC cohort. The intermediate-grade SGC cases received a range of diagnoses, including 42.1% (n = 64/152) as high-grade, 37.5% (n = 57/152) as low-grade, and 20.4% (n = 31/152) as indeterminate.
FIGURE 7.
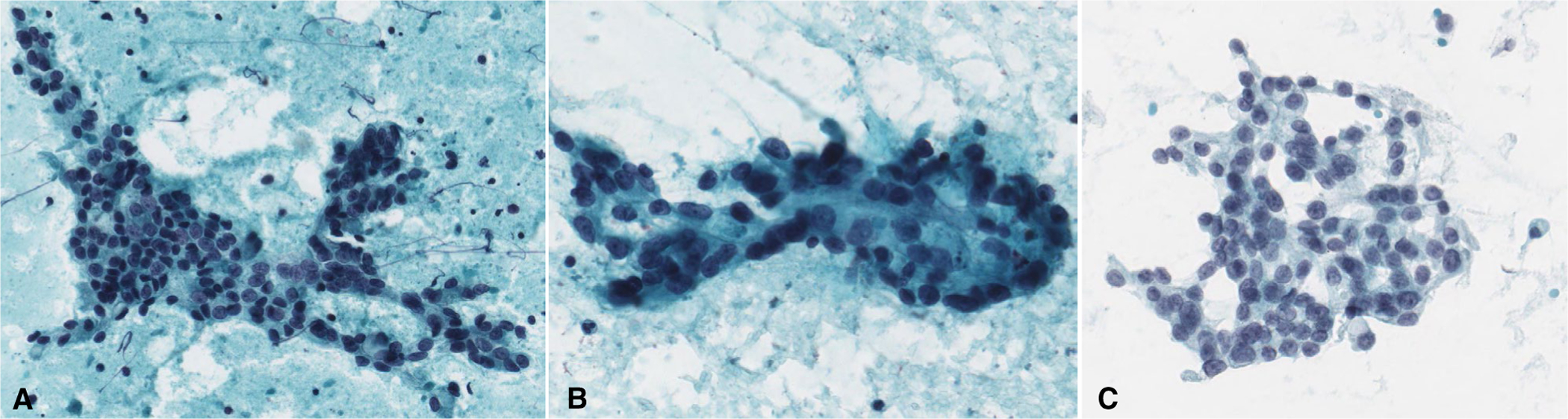
Intermediate-grade salivary gland carcinomas. (A) Mucoepidermoid carcinoma. (B, C) Adenocarcinoma not otherwise specified. (Papanicolaou stain, original magnification ×400.)
Individual Cases of SGC
Among individual cases of SGC, the low-grade carcinomas which were graded with 100% accuracy by the 19 panelists were 7 acinic cell carcinomas and 2 mucoepidermoid carcinomas (Figs. 1 and 2; Table 2). High-grade SGCs graded with 100% accuracy included 5 salivary duct carcinomas, 1 high-grade adenocarcinoma NOS, 1 high-grade mucoepidermoid carcinoma, and 1 poorly differentiated myoepithelial carcinoma (Figs. 4 and 5; Table 2). In total, 32% (n = 17/53) of the low- and high-grade SGC cases yielded 100% accurate grading, and 5 SGC cases (3 salivary duct carcinomas, 1 high-grade adenocarcinoma NOS, and 1 acinic cell carcinoma) from this group received no indeterminate responses from any of the 19 panelists.
FIGURE 2.
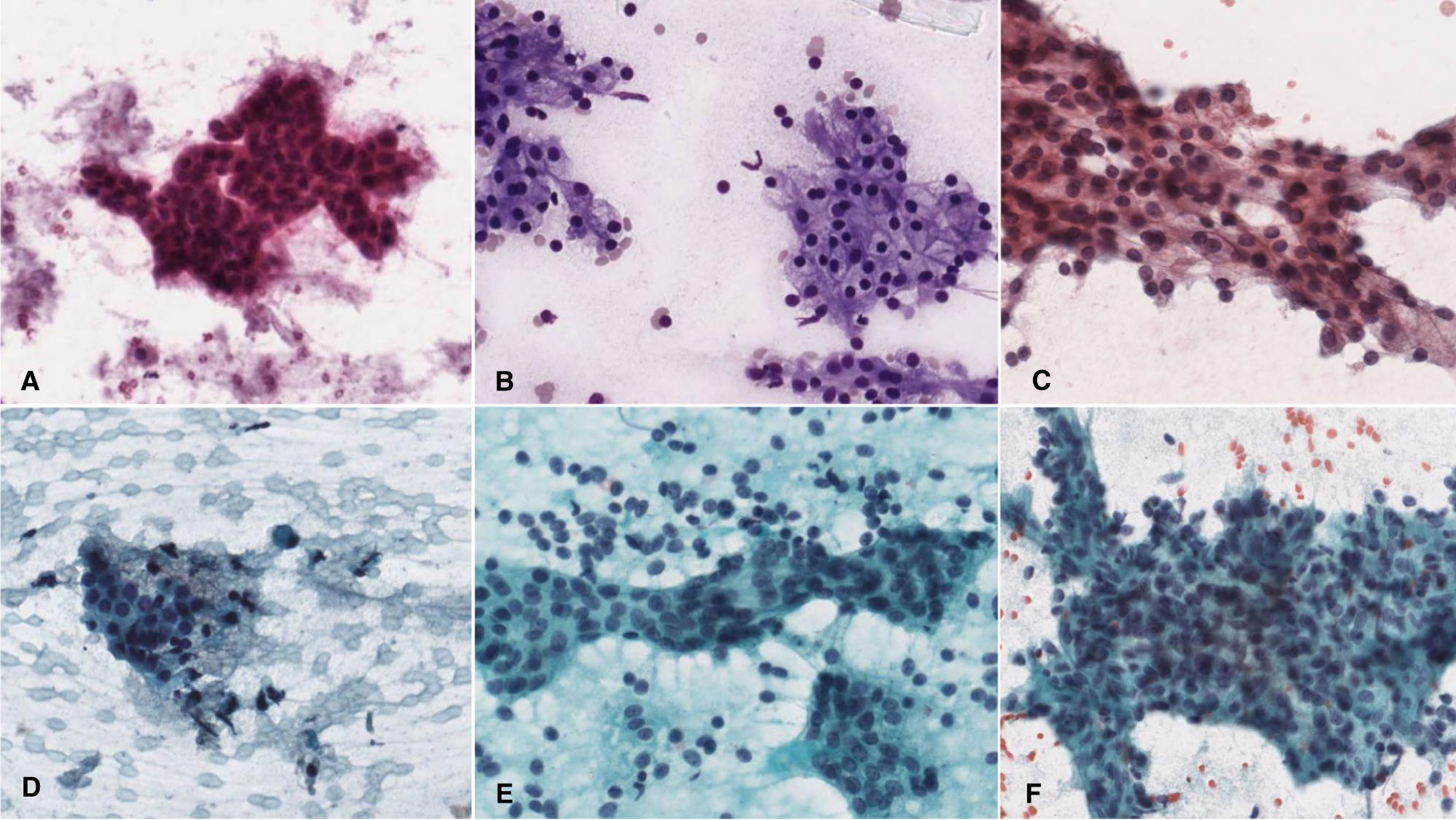
Low-grade salivary gland carcinomas with high (A-C) and low accuracy (D-F). (A) Low-grade mucoepidermoid carcinoma. (B, C) Acinic cell carcinoma. (D) Epithelial-myoepithelial carcinoma with somewhat irregular organization within a cluster of cells. (E) Epithelial-myoepithelial carcinoma with single cells, some with nuclear hyperchromasia. (F) Acinic cell carcinoma exhibiting a large, irregularly shaped cluster. (A, C-F, Papanicolaou stain, original magnification ×400; B, Diff Quik, original magnification ×400.)
TABLE 2.
Accuracy of Cytopathologic Grading by Tumor Type
| Carcinoma Type | Correct | Incorrect | Indeterminate | Accuracy (%) | Number 100% accurate |
|---|---|---|---|---|---|
| Low-Grade | |||||
| Adenocarcinoma NOS (n = 1) | 15 | 2 | 2 | 88.2 | 0 |
| Secretory (n = 1) | 10 | 1 | 8 | 90.9 | 0 |
| Neuroendocrine (n = 1) | 12 | 1 | 6 | 92.3 | 0 |
| Oncocytic (n = 1) | 16 | 1 | 2 | 94.1 | 0 |
| Polymorphous adenocarcinoma (n = 2) | 22 | 4 | 12 | 84.6 | 0 |
| Other total (n = 6) | 75 | 9 | 30 | 89.3 | 0 |
| Epithelial-Myoepithelial (n = 5) | 53 | 16 | 26 | 76.8 | 0 |
| Mucoepidermoid (n = 6) | 95 | 8 | 11 | 92.2 | 2 |
| Aciniccell(n = 14) | 227 | 16 | 23 | 93.4 | 7 |
| Total | 450 | 49 | 90 | 90.2 | 9 |
| High-Grade | |||||
| Squamous cell (n = 1) | 18 | 1 | 0 | 94.7 | 0 |
| Adenocarcinoma NOS (n = 4) | 61 | 9 | 6 | 87.1 | 1 |
| Myoepithelial (n = 2) | 30 | 3 | 5 | 90.9 | 1 |
| Mucoepidermoid (n = 2) | 33 | 1 | 4 | 97.1 | 1 |
| Salivary duct (n = 13) | 189 | 30 | 28 | 86.3 | 5 |
| Total | 331 | 44 | 43 | 88.3 | 8 |
Abbreviation: NOS, not otherwise specified.
FIGURE 5.
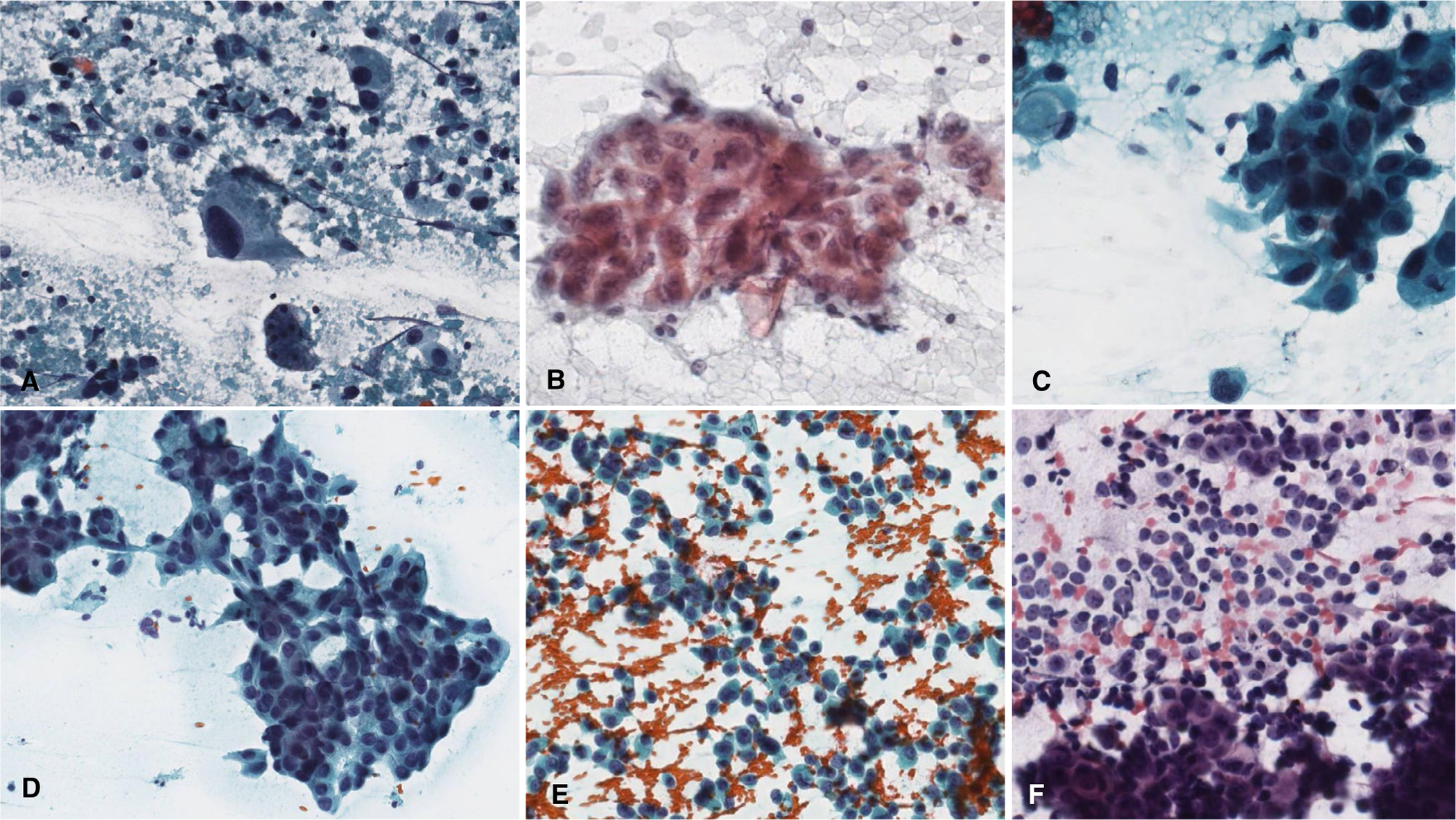
High-grade salivary gland carcinomas with high (A-C) and low (D-F) accuracy. (A) Salivary duct carcinoma with overt pleomorphism, single atypical cells, and background necrosis. (B) High-grade mucoepidermoid carcinoma. (C) Salivary duct carcinoma. (D) Salivary duct carcinoma showing somewhat subtle disorganized clusters known as “drunken honey-combing.” (E) Salivary duct carcinoma with abundant single cells and somewhat uniform-appearing nuclear atypia. (F) Adenocarcinoma, not otherwise specified, high-grade with plasmacytoid single cells potentially mistaken for myoepithelial cells. (Papanicolaou stain, original magnification ×400.)
Based on stain type (Papanicolaou vs Diff-Quik), slight differences in grading accuracy were observed, but the differences did not meet statistical significance. Cases with representative slides stained with Diff-Quik had an accuracy of 91.7% (n = 188/205), with 23 indeterminate responses. Those cases with conventional Papanicolaou-stained smears had an accuracy of 88.6% (n = 593/669), with 106 indeterminate responses. Liquid-based preparations were used for 1 acinic cell carcinoma and 1 secretory carcinoma and had an accuracy of 93.3%. The other 3 liquid-based cases were of intermediate grade.
Panelist Agreement for Grading of SGCs
In addition to accuracy, the panelists had an intermediate to good agreement grading the SGC cohort, with an overall agreement of 67.4% (CI, 64.0%–70.7%). When only the low- and high-grade SGC cases are considered (n = 53), the overall agreement was 71.67% (CI, 68.3%–75.0%) and improved to 77.08% (CI, 73.6%–80.6%) if indeterminate responses were excluded. Low-grade SGCs (n = 31) yielded an overall agreement of 76.4% (CI, 0.7272–0.7973), whereas there was better agreement for high-grade SGCs (n = 22; 79.19% [CI, 0.7491–0.8291]).
SGC Classification
Only a subset of the panelists provided a classification for the cohort SGCs. The low-grade carcinomas were more accurately classified as well as graded. The 4 cases that had the best classification accuracy (73%–100%) by the panelists were each acinic cell carcinoma. Of the top 9 cases (64%–100%), 7 were acinic cell carcinoma and 2 were mucoepidermoid carcinomas (1 low-grade, 1 intermediate-grade).
Participant Characteristics
A majority of the 19 panelists had significant experience interpreting salivary gland FNAs, and 84.2% (n = 16) were affiliated with academic medical centers. Twelve participants had >20 years of experience, 5 had between 10 and 20 years of experience, and 2 participants had <10 years of experience. All but 2 of the 19 (89.5%) participants reported reviewing more than 20 salivary gland FNA cases per year. The 2 panelists with the greatest accuracy grading SGCs practiced at tertiary academic medical centers. The panelists with the most experience (>10 years, n = 12) had an accuracy of 91.5% (484/531; 105 indeterminate), while the panelists with 5 to 10 years of experience (n = 5) had an accuracy of 84.6% (208/246; 19 indeterminate), and the panelists with the least experience (<5 years, n = 2) achieved an accuracy of 91.8% (89/97; 9 indeterminate). When compared with each other, panelists with >10 years of experience had significantly higher accuracy than those with 5 to 10 years of experience (P = .0019) and for all panelists with <10 years of experience (P = .0119). However, there were no statistically significant differences between the panelists with <5 years of experience compared with those with >10 years of experience (P = .9686).
DISCUSSION
This study contributes to the limited but growing literature on salivary gland FNA by demonstrating its utility for accurately grading nonbasaloid, non–matrix-producing SGCs.7–14,17,20,22,29–48 This work specifically examines the subject of grading SGCs using a broad range of types of SGC as well as employing a diverse panel of cytopathologists. Our data demonstrate a high accuracy for grading primary SGCs by FNA while also revealing important limitations.
Overall, intermediate to good rates of interobserver agreement (kappa = 0.45–0.53) were observed in our study, suggesting that most of the inaccuracies seen are due to inherent limitations of salivary gland cytopathology. Interestingly, greater accuracy (86.9%–90.2%) was observed when cytopathologists were allowed to abstain from grading SGC cases when they were uncertain of the grade by classifying the cohort case as indeterminate (ie, cannot grade). This finding has important implications for salivary gland FNA practice; it suggests that a greater degree of accuracy would be obtained when cytologists are grading SGC cases on a 2-tiered scheme of either low or high grade. In addition, the current study indicates that grading of SGC FNA cases may suffer from inaccuracies when a specific cytologic diagnosis cannot be rendered due to limited cellularity, focal nuclear pleomorphism, lack of high-grade features (eg, necrosis), and certain tumor types that can be difficult to grade, such as epithelial-myoepithelial carcinoma and secretory carcinoma. For those salivary gland FNA cases where the cytopathologist is not confident of the tumor grade, it would be better to avoid grading the SGC and defer specific grading to intraoperative frozen section or the final surgical pathology.
Considering the inherent limitations of this type of blinded review study, we would speculate that the results of our cohort of SGC FNA cases most likely represents an underestimate of the accuracy for grading.55 The limitations of our study design include providing only 1 representative digitized cytology slide, and stain (Papanicolaou versus Diff-Quik), or preparation type (conventional smear versus liquid-based) per digitized case. While the digitized slide selected for blinded review was representative of the salient cytomorphologic features for that particular lesion, it is possible that this study would have had greater accuracy if the cytopathologist panel had been given access to all available slides for each case. An important limitation of our study is the use of digitized SGC FNA cases lacking multiple focal planes and limited ability to compensate for nuclear hyperchromasia or dark staining. These pose a greater challenge for cytologic specimens where 3-dimensional aspects are difficult to interpret.
This study did not include basaloid matrix-producing salivary gland neoplasms. It is well-known that there is a morphologic overlap between both benign and malignant basaloid neoplasms such as basal cell adenoma, basal cell adenocarcinoma, cellular pleomorphic adenoma, and adenoid cystic carcinoma. Recently, Gargano and colleagues47 studied the group of basaloid matrix-producing salivary gland neoplasms and reported that this group of tumors is diagnostically challenging and not amenable to grading.48 In fact, grading of adenoid cystic carcinoma is not typically practiced and does not have the same significance for clinical management as does grading of cases such as mucoepidermoid carcinoma and other nonbasaloid, nonmatrix producing cancers.
An important aspect of salivary gland FNA that was not incorporated into our study is the use of ancillary testing for diagnostic purposes.56–59 We did not include cell blocks or ancillary studies given the complexity that this would add to this type of digitized online slide review. In addition, many of the SGCs, including our study cohort, did not have cell blocks or ancillary studies available, thus their inclusion would lack uniformity. The use of ancillary testing might improve the ability to cytologically grade SGCs, since a majority of salivary gland tumors have known molecular and/or immunocytochemical signatures. In clinical practice, ancillary studies can be used to specifically classify a salivary gland neoplasm.5,56–59 Often, the precise subclassification of an SGC, such as acinic cell carcinoma, secretory carcinoma, or salivary duct carcinoma will have inherent implications for tumor grade and clinical management.
Apart from cytologic grading, even the histologic grading of SGCs, especially intermediate-grade cases, is known to be challenging and in some cases controversial. Some SGCs, such as salivary duct carcinoma, are an inherently specific grade based on the specific tumor classification. The WHO Classification of Head and Neck Tumours, 4th edition, includes general information pertaining to the grading of certain SGCs, although a defined set of grading criteria for each type of carcinoma is not provided.6 In particular, 4 schemes for grading mucoepidermoid carcinoma are available: the AFIP grading scheme tends to downgrade cases, while the Brandwein method tends to upgrade these carcinomas, and the Healey and Katabi methods are variable.3,60,61 In practice, a majority of mucoepidermoid carcinomas, including cases in our study cohort, can be easily classified histologically as low- or high-grade regardless of the grading method used. The intermediate-grade SGCs would be the most challenging and least reproducible both histologically and cytologically.
With the publication in 2018 of the MSRSGC, it was recommended that salivary gland FNA cases that are classified as malignant be designated further, when feasible, as a specific tumor type and grade.30 This study shows that based upon cytomorphology alone, without the benefit of ancillary studies, and despite recognized limitations of this type of study, cytopathologists are still able to achieve a relatively high degree of accuracy grading FNA specimens of nonbasaloid, non–matrix-producing SGCs as low or high. In addition, when a tumor grade cannot be confidently assigned, it is important to designate the cancer grade as “not gradable” or “indeterminate.”
FUNDING SUPPORT
No specific funding was disclosed.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.Tyagi R, Dey P. Diagnostic problems of salivary gland tumors. Diagn Cytopathol. 2015;43:495–509. [DOI] [PubMed] [Google Scholar]
- 2.Rooper LM. Challenges in minor salivary gland biopsies: a practical approach to problematic histologic patterns. Head and Neck Pathol. 2019;13:476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seethala RR. An update on grading of salivary gland carcinomas. Head and Neck Pathol. 2009;3:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seethala RR. Basaloid/blue salivary gland tumors. Mod Pathol. 2017;30(suppl 1):S84–S95. [DOI] [PubMed] [Google Scholar]
- 5.Skálová A, Gnepp DR, Lewis JS, et al. Newly described entities in salivary gland pathology. Am J Surg Pathol. 2017;41:e33–e47. [DOI] [PubMed] [Google Scholar]
- 6.El-Naggar AK, Chan JK, Grandis JR, Takata T, Slootweg PJ, eds. WHO Classification of Head and Neck Tumours. 4th ed. International Agency for Research on Cancer, World Health Organization; 2017. [Google Scholar]
- 7.Ahn S, Kim Y, Oh YL. Fine needle aspiration cytology of benign salivary gland tumors with myoepithelial cell participation: an institutional experience of 575 cases. Acta Cytol. 2013;57:567–574. [DOI] [PubMed] [Google Scholar]
- 8.Kechagias N, Ntomouchtsis A, Valeri R, et al. Fine-needle aspiration cytology of salivary gland tumours: a 10-year retrospective analysis. Oral Maxillofac Surg. 2011;16:35–40. [DOI] [PubMed] [Google Scholar]
- 9.Riley N, Allison R, Stevenson S. Fine-needle aspiration cytology in parotid masses: our experience in Canterbury, New Zealand. ANZ J Surg. 2005;75:144–146. [DOI] [PubMed] [Google Scholar]
- 10.Stewart CJR, MacKenzie K, McGarry GW, Mowat A. Fine-needle aspiration cytology of salivary gland: a review of 341 cases. Diagn Cytopathol. 2000;22:139–146. [DOI] [PubMed] [Google Scholar]
- 11.Layfield LJ, Glasgow BJ. Diagnosis of salivary gland tumors by fine-needle aspiration cytology: a review of clinical utility and pitfalls. Diagn Cytopathol. 1991;7:267–272. [DOI] [PubMed] [Google Scholar]
- 12.Salehi S, Maleki Z. Diagnostic challenges and problem cases in salivary gland cytology: A 20-year experience. Cancer Cytopathol. 2017;126:101–111. [DOI] [PubMed] [Google Scholar]
- 13.Maleki Z, Miller JA, Arab SE, et al. “Suspicious” salivary gland FNA: risk of malignancy and interinstitutional variability. Cancer Cytopathol. 2017;126:94–100. [DOI] [PubMed] [Google Scholar]
- 14.Brennan PA, Davies B, Poller D, et al. Fine needle aspiration cytology (FNAC) of salivary gland tumours: repeat aspiration provides further information in cases with an unclear initial cytological diagnosis. Br J Oral Maxillofac Surg. 2010;48:26–29. [DOI] [PubMed] [Google Scholar]
- 15.Omhare A, Singh SK, Nigam JS, Sharma A. Cytohistopathological study of salivary gland lesions in Bundelkhand region, Uttar Pradesh, India. Patholog Res Int. 2014:804265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelley DJ, Spiro RH. Management of the neck in parotid carcinoma. Am J Surg. 1996;172:695–697. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt RL, Hall BJ, Wilson AR, Layfield LJ. A systematic review and meta-analysis of the diagnostic accuracy of fine-needle aspiration cytology for parotid gland lesions. Am J Clin Pathol. 2011;136:45–59. [DOI] [PubMed] [Google Scholar]
- 18.Lim YC, Lee SY, Kim K, et al. Conservative parotidectomy for the treatment of parotid cancers. Oral Oncol. 2005;41:1021–1027. [DOI] [PubMed] [Google Scholar]
- 19.Stennert E, Kisner D, Jungehuelsing M, et al. High incidence of lymph node metastasis in major salivary gland cancer. Arch Otolaryngol Head Neck Surg. 2003;129:720–723. [DOI] [PubMed] [Google Scholar]
- 20.Mueller JS, Schultenover S, Simpson J, Ely K, Netterville J. Value of rapid assessment cytology in the surgical management of head and neck tumors in a Nigerian mission hospital. Head Neck. 2008;30:1083–1085. [DOI] [PubMed] [Google Scholar]
- 21.Croonenborghs TM, Van Hevele J, Scheerlinck J, Nout E, Schoenaers J, Politis C. A multicentre retrospective clinico-histopathological review of 250 patients after parotidectomy. Int J Oral Maxillofac Surg. 2020;49:149–156. [DOI] [PubMed] [Google Scholar]
- 22.Lewis AG, Tong T, Maghami E. Diagnosis and management of malignant salivary gland tumors of the parotid gland. Otolaryngol Clin North Am. 2016;49:343–380. [DOI] [PubMed] [Google Scholar]
- 23.Ettl T, Gosau M, Brockhoff G, et al. Predictors of cervical lymph node metastasis in salivary gland cancer. Head Neck. 2013;36:517–523. [DOI] [PubMed] [Google Scholar]
- 24.Green B, Rahimi S, Brennan PA. Current management of the neck in salivary gland carcinomas. J Oral Pathol Med. 2016;46:161–166. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Luo Y, Li M, Yan H, Sun M, Fan T. Management of salivary gland carcinomas—a review. Oncotarget. 2017;8:3946–3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armstrong JG, Harrison LB, Thaler HT, et al. The indications for elective treatment of the neck in cancer of the major salivary glands. Cancer. 1992;69:615–619. [DOI] [PubMed] [Google Scholar]
- 27.Frankenthaler RA, Byers RM, Luna MA, Callender DL, Wolf P, Goepfert H. Predicting occult lymph node metastasis in parotid cancer. Arch Otolaryngol Head Neck Surg. 1993;119:517–520. [DOI] [PubMed] [Google Scholar]
- 28.Thiryayi SA, Low YX, Shelton D, Narine N, Slater D, Rana DN. A retrospective 3-year study of salivary gland FNAC with categorisation using the Milan reporting system. Cytopathology. 2018;29:343–348. [DOI] [PubMed] [Google Scholar]
- 29.McGurk M, Thomas BL, Renehan AG. Extracapsular dissection for clinically benign parotid lumps: reduced morbidity without oncological compromise. Br J Cancer. 2003;89:1610–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faquin WC, Rossi ED, Baloch Z. The Milan System for Reporting Salivary Gland Cytopathology. Springer International Publishing; 2018. [Google Scholar]
- 31.Farahani SJ, Baloch Z. Retrospective assessment of the effectiveness of the Milan system for reporting salivary gland cytology: a systematic review and meta-analysis of published literature. Diagn Cytopathol. 2018;47:67–87. [DOI] [PubMed] [Google Scholar]
- 32.Pusztaszeri M, Baloch Z, Vielh P, Faquin WC. Application of the Milan system for reporting risk stratification in salivary gland cytopathology. Cancer Cytopathol. 2018;126:69–70. [DOI] [PubMed] [Google Scholar]
- 33.Liu H, Ljungren C, Lin F, Zarka MA, Chen L. Analysis of histologic follow-up and risk of malignancy for salivary gland neoplasm of uncertain malignant potential proposed by the Milan System for Reporting Salivary Gland Cytopathology. Cancer Cytopathol. 2018;126:490–497. [DOI] [PubMed] [Google Scholar]
- 34.Kaushik R, Bhatia K, Sarin H, Gautam D, Sarin D. Incorporation of the Milan system in reporting salivary gland fine needle aspiration cytology—an insight into its value addition to the conventional system. Diagn Cytopathol. 2020;48:17–29. [DOI] [PubMed] [Google Scholar]
- 35.Rossi ED, Wong LQ, Bizzarro T, et al. The impact of FNAC in the management of salivary gland lesions: institutional experiences leading to a risk-based classification scheme. Cancer Cytopathol. 2016;124:388–396. [DOI] [PubMed] [Google Scholar]
- 36.Rossi ED, Faquin WC, Baloch Z, et al. The Milan System for Reporting Salivary Gland Cytopathology: analysis and suggestions of initial survey. Cancer Cytopathol. 2017;125:757–766. [DOI] [PubMed] [Google Scholar]
- 37.Pusztaszeri M, Rossi ED, Baloch ZW, Faquin WC. Salivary gland fine needle aspiration and introduction of the Milan Reporting System. Adv Anat Pathol. 2019;26:84–92. [DOI] [PubMed] [Google Scholar]
- 38.Wei S, Layfield LJ, LiVolsi VA, Montone KT, Baloch ZW. Reporting of fine needle aspiration (FNA) specimens of salivary gland lesions: a comprehensive review. Diagn Cytopathol. 2017;45:820–827. [DOI] [PubMed] [Google Scholar]
- 39.Wang H, Malik A, Maleki Z, et al. “Atypical” salivary gland fine needle aspiration: Risk of malignancy and interinstitutional variability. Diagn Cytopathol. 2017;45:1088–1094. [DOI] [PubMed] [Google Scholar]
- 40.Pantanowitz L, Thompson LDR, Ross ED. Diagnostic approach to fine needle aspirations of cystic lesions of the salivary gland. Head Neck Pathol. 2018;12:548–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim BY, Hyeon J, Ryu G, et al. Diagnostic accuracy of fine needle aspiration cytology for high-grade salivary gland tumors. Ann Surg Oncol. 2013;20:2380–2387. [DOI] [PubMed] [Google Scholar]
- 42.Zbären P, Nuyens M, Loosli H, Stauffer E. Diagnostic accuracy of fine-needle aspiration cytology and frozen section in primary parotid carcinoma. Cancer. 2004;100:1876–1883. [DOI] [PubMed] [Google Scholar]
- 43.Colella G, Cannavale R, Flamminio F, Foschini MP. Fine-needle aspiration cytology of salivary gland lesions: a systematic review. J Oral Maxillofac Surg. 2010;68:2146–2153. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt RL, Hall BJ, Layfield LJ. A systematic review and meta-analysis of the diagnostic accuracy of ultrasound-guided core needle biopsy for salivary gland lesions. Am J Clin Pathol. 2015;136:516–526. [DOI] [PubMed] [Google Scholar]
- 45.Seethala RR, LiVolsi VA, Baloch ZW. Relative accuracy of fine-needle aspiration and frozen section in the diagnosis of lesions of the parotid gland. Head Neck. 2005;27:217–223. [DOI] [PubMed] [Google Scholar]
- 46.Tabatabai ZL, Auger M, Kurtycz DFI, et al. Performance characteristics of adenoid cystic carcinoma of the salivary glands in fine-needle aspirates: results from the College of American Pathologists Nongynecologic Cytology Program. Arch Pathol Lab Med. 2015;139: 1525–1530. [DOI] [PubMed] [Google Scholar]
- 47.Gargano SM, Sebastiano C, Solomides CC, Griffith CC, HooKim K. Cytohistologic correlation of basaloid salivary gland neoplasms: can cytomorphologic classification be used to diagnose and grade these tumors? Cancer Cytopathol. 2019;2:166–168. [DOI] [PubMed] [Google Scholar]
- 48.Faquin WC. Diagnosis and grading of basaloid salivary gland tumors using the Milan System for Reporting Salivary Gland Cytopathology. Cancer Cytopathol. 2020;128:87–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brennan RL, Prediger DJ. Coefficient kappa: some uses, misuses, and alternatives. Educ Psychol Meas. 1981;41:687–699. [Google Scholar]
- 50.Fleiss JL. Measuring nominal scale agreement among many raters. Psychol Bull. 1971;16:378–382. [Google Scholar]
- 51.Fleiss JL. Statistical Methods for Rates and Proportions. Wiley; 1981. [Google Scholar]
- 52.Gwet KL. Handbook of Interrater Reliability. 2nd ed. Advanced Analytics; 2010. [Google Scholar]
- 53.Warrens MJ. Inequalities between multi-rater kappas. Adv Data Anal Classif. 2010;4:271–286. [Google Scholar]
- 54.Randolph JJ. Online kappa calculator. Published 2008. Accessed March 10, 2019 http://justus.randolph.name/kappa
- 55.Whiting PF, Rutjes AWS, Westwood ME, Mallett S, QUADAS-2 Steering Group. A systematic review classifies sources of bias and variation in diagnostic test accuracy studies. J Clin Epidemiol. 2013;66:1093–1104. [DOI] [PubMed] [Google Scholar]
- 56.Griffith CC, Siddiqui MT, Schmitt AC. Ancillary testing strategies in salivary gland aspiration cytology: a practical pattern-based approach. Diagn Cytopathol. 2017;45:808–819. [DOI] [PubMed] [Google Scholar]
- 57.Skálová A, Stenman G, Simpson RHW, et al. The role of molecular testing in the differential diagnosis of salivary gland carcinomas. Am J Surg Pathol. 2018;42:e11–e27. [DOI] [PubMed] [Google Scholar]
- 58.Darras N, Mooney KL, Long SR. Diagnostic utility of fluorescence in situ hybridization testing on cytology cell blocks for the definitive classification of salivary gland neoplasms. J Am Soc Cytopathol. 2019;8:157–164. [DOI] [PubMed] [Google Scholar]
- 59.Dubucs C, Basset C, D’Aure D, Courtade-Saïdi M, Evrard SM. A 4-year retrospective analysis of salivary gland cytopathology using the Milan System for Reporting Salivary Gland Cytology and Ancillary Studies. Cancers (Basel). 2019;11:1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nance MA, Seethala RR, Wang Y, et al. Treatment and survival outcomes based on histologic grading in patients with head and neck mucoepidermoid carcinoma. Cancer. 2008;113:2082–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cipriani NA, Lusardi KK, McElherne J, et al. Mucoepidermoid carcinoma: a comparison of histologic grading systems and relationship to MAML2 rearrangement and prognosis. Am J Surg Pathol. 2019;43:885–897. [DOI] [PMC free article] [PubMed] [Google Scholar]


