Abstract
Myoglobin, besides its role in oxygen turnover, has gained recognition as a potential regulator of lipid metabolism. Previously, we confirmed the interaction of fatty acids and acylcarnitines with Oxy-Myoglobin, using both molecular dynamic simulations and Isothermal Titration Calorimetry studies. However, those studies were limited to testing only the binding sites derived from homology to fatty acid binding proteins and predictions using automated docking. To explore the entry mechanisms of the lipid ligands into myoglobin, we conducted molecular dynamic simulations of murine Oxy- and Deoxy-Mb structures with palmitate or palmitoylcarnitine starting at different positions near the protein surface. The simulations indicated that both ligands readily (under ~10-20 ns) enter the Oxy-Mb structure through a dynamic area (“portal region”) near heme, known to be the entry point for small molecule gaseous ligands like O2, CO and NO. The entry is not observed with Deoxy-Mb where lipid ligands move away from protein surface, due to a compaction of the entry portal and the heme-containing crevice in the Mb protein upon O2 removal. The results suggest quick spontaneous binding of lipids to Mb driven by hydrophobic interactions, strongly enhanced by oxygenation, and consistent with the emergent role of Mb in lipid metabolism.
Keywords: Myoglobin, Fatty acid, Acylcarnitines
INTRODUCTION
Traditionally, myoglobin (Mb) has been considered only as an oxygen (O2) storage/transport system which enables “red muscle” (Type 1 fibers) and heart cells to maintain the physiological function of the cell by supporting oxidative metabolism of fuels. Five different Mb isoforms have been reported in humans, where Mb-I (~75-80% of total Mb in cardiomyocytes and skeletal muscles) and Mb-II (~15-20%) differ by only single residue (K54 and E54, respectively); Mb III, IV and V together constitute the remaining 5% of total Mb [1-6]. Higher concentration of muscle Mb is expressed in high-altitude populations (hypoxic environment), where Mb II isoform forms the major constituent [7, 8]. Studies conducted on myoglobin knockout mice (myo−/−) also revealed the relevance of Mb in NO homeostasis in relation to left ventricular dysfunction [9-11].
There is a growing body of evidence that points to oxygenated-myoglobin (Oxy-Mb) as a protein involved in the binding of fatty acids (FAs), in addition to intramyocellular fatty acid binding proteins (FABPs). In 1977, Gloster et al. reported that rat Mb interacts with oleic acid, but with an affinity 40 times lower compared to oleic acid-albumin binding [12]. Despite the lower affinity, the large abundance of Mb in muscle and cardiomyocytes makes a potential contribution of Mb to intracellular fatty acid binding physiologically important. Similar results on binding affinity were observed using chicken gizzard Mb interacting with different long-chain fatty acids [13]. The results from these studies have shown that unsaturated FAs have higher binding affinity toward Mb compared to the saturated FAs, and demonstrated that freshly prepared Mb has a higher binding than commercially available Mb. Freshly prepared myoglobin is rich in the ferrous (Fe2+) form which favors lipid binding to Oxy-Mb, whereas commercial myoglobin is a mixture of all the three forms of Oxy-, Deoxy- and met-myoglobins (met-myoglobin is in the ferric form (Fe3+))form which does not favor lipid binding. To achieve a greater enrichment of Oxy-Mb when using commercial myoglobin, addition of sodium dithionite and oxygen purge into the solution is recommended [14, 15]. More recently, experimental studies involving commercially available horse myoglobin with NMR measurements revealed that long-chain FAs bind to Mb only in the oxygenated form, and do not show significant specific or non-specific interaction with the Deoxy-Mb [16-19]. Our previous studies predicted the fatty acid binding pocket in Mb and identified critical amino acid residues involved in the formation of Oxy-Mb-FA complex, which revealed a strong interaction of long-chain FAs (palmitic & oleic acid) only with Oxy-Mb, and not Deoxy-Mb [20]. Further, using commercially available horse myoglobin, isothermal titration calorimetry studies combined with molecular dynamic (MD) simulations demonstrated that like long-chain FAs, long-chain acylcarnitines also interact preferentially with Oxy-Mb with higher affinity than their respective fatty acids, and a minimum chain length (≥C12) for FAs and acylcarnitines is required for stable binding with Oxy-Mb [14]. Studies conducted by Jue et al. have also shown Mb knockout in mice affects the overall triglyceride metabolism and in other studies involving NMR measurements, the interaction of different chain lengths fatty acids to Mb were similar to our isothermal titration calorimetry studies [14, 19, 21].
In considering all potential binding and entry sites for FAs and acylcarnitines on the Mb protein, it is relevant to consider the behavior of other ligands. Entry migration of gases such as O2 has been extensively studied in both myoglobin and hemoglobin structures [22-29]. Site-directed mutagenesis, Xenon binding kinetics and implicit ligand sampling methods revealed various diffusion pathways across globin family proteins [29-31]. Substitution of amino acid residues using random mutagenesis on sperm whale Mb highlighted a significant affect in the gas ligand-binding kinetics using a series of precise physical measurements, rather than by classical genetic or biochemical selection method [32]. Several studies including spectroscopy, crystallography, and mutagenesis mapping experiments [33, 34] which were accompanied by molecular dynamic simulations [35] interrogated the pathways and mechanism of movement of small molecule ligands within the cavities and tunnels of proteins. In most cases, experiments have identified the functional roles of heme pocket amino acid residues at the E7 channel (gated by His64); the region near distal histidine forms the major route for ligand escape [27, 29, 30, 33]. The residues (Leu29, Phe33, Phe43, Phe46, His64 & Val68) that line the distal histidine pathway are well-conserved in Mb, and the size of this pocket is the major determinant of the rate of the ligand entry [31]. The outward rotation of the distal histidine of the E7 channel forms the main route for ≥ 75% of ligand entry, with the remainder of O2 migration through the interior of Mb [29, 31]. In both hemoglobin and myoglobin, mutational analyses involving the filling of the E7 channel with large indole rings provide evidence that ligands predominantly enter the protein near the portal region with the outward rotation of the distal histidine [27].
Our previous computational results pointed to the same Mb binding pocket for both fatty acid and acylcarnitine, located in the hydrophobic cavity of the Oxy-Mb structure in direct contact with the heme, and wrapping around the bound oxygen. The specificity of this binding and the critical residues involved remain to be fully evaluated. The co-crystallization of the Oxy-Mb/fatty acid or Oxy-Mb/acylcarnitine complex might be challenging to obtain, as the high hydrophobicity of the alkyl tail of fatty acid/acylcarnitine may impede the nucleation process. Here, to address the mechanisms and specificity of fatty acid and acylcarnitine binding to Mb, we employed an alternative approach to simulate both Oxy- and Deoxy-Mb with the ligands positioned at various starting places and different conformations in explicit water and at physiological ionic strength, thereby providing an insight into the penetration events of the ligand and also the potential for alternative binding/entry sites.
The question remains whether the traditional pathway for small ligands entry is similar to the entry route for the fatty acids and acylcarnitines. Our previous MD simulation results involving C6-C10 fatty acids/acylcarnitines with horse Oxy-Mb indicated the same portal region as the exit pathway and the lipid trajectory analysis revealed that the ligand starts to move out of the binding site as the tail is too short to have stable hydrophobic interactions with the surrounding residues [14]. The NMR results from Jue et al. also confirmed that, in the presence of selective chain lengths of fatty acids with equine Mb, there is a noticeable change in the heme group signal intensity affecting 5-methyl and 8-methyl propionates of heme and that group did not observe any signal intensity with shorter fatty acid chain lengths [16-19]. Considering these outcomes, in the current experiments, we have chosen the E7 channel as the main portal region for exploring both fatty acid and acylcarnitine entrance. In other experiments to find alternative entry routes, we have chosen different starting points around the myoglobin structure. To transition to a system that is more amenable to genetic manipulations in future experimental testing of Mb in vivo, in the current report we focus on FA/acylcarnitine binding events with a mouse Mb structural model.
METHODS
Preparing the starting conformations for different protein-ligand simulations
In this study, the mouse myoglobin structures were modelled from our previous modelled horse structures [14, 20]. MODELLER (v9.17) was used [36, 37] to generate the mouse model structure using pairwise sequence alignment (Fig. 1). In order to understand the mechanism of ligand entry (PLM and PLC) into the Oxy- and Deoxy-Mb structures, we aimed at unbiased search of ligand entry using two different types of starting conformations. For the simulations involving Deoxy-Mb structures with ligands, we used the same starting coordinates as Oxy-Mb, except oxygen molecule coordinates are removed and re-hydrated every system to run the MD simulations in a view to observe the structural changes within the Deoxy-Mb when the O2 molecule is removed along with lipid entry/exit. Upon removal of O2 molecule we have used the default heme parameters present in CHARMM36 force field, whereas for the oxygenated Mb we have adjusted the CHARMM force filed parameters to be optimized specifically for Oxy-Mb (see details in MD parameters section) [38-40].
Fig. 1.
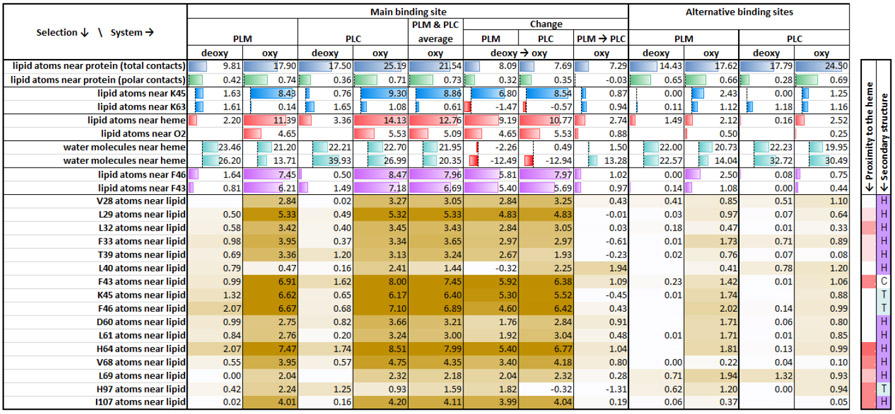
Pairwise alignment of horse and mouse sequence. The numbers of the residues of interest are highlighted with yellow (top row). In the row of amino acid (AA) change, black color block represents the strongest change in AA. In the Secondary structure row, “C” represents “Coil”, “H” - “Helix” and “T” - “Turn”. In the rows of proximity to heme and contacts with PLM/PLC, colored blocks represent the closest contacts (Light color - larger distance and Dark color - close proximity of AA to the heme; notably, none of the significant AA changes occur in the frequent heme and lipid contact positions). The contacts of AA with PLM/PLC lipids are color-coded in a similar way in the shades of tan.
In the first case, the ligand is positioned at a distance of approximately 7 to 9 Å away from the binding site and in close proximity with its carboxylate group near select lysine residues (K45 & K63). To select 10 different starting conformations of the ligand, we ran MD simulations of the ligand in vacuum generating 300 different ligand poses while keeping the Mb structure in the same conformation. A clustering algorithm was applied on these 300 random ligand poses and placed them in 10 different clusters, and the top ligand from each cluster was chosen as the starting point for protein-ligand simulation. Following this technique, we were able to generate different ligand conformations which differ from the original starting point both with respect to its orientation near the lysine residues and also in attaining different shape conformation at the entry site (Fig. 2).
Fig. 2.
Comparison of the starting structures of PLM in mouse Oxy-Mb from M-1 to M-10 which are placed outside the main binding pocket of the heme (the location based on our previous work on horse Oxy-Mb wherein the simulated PLM got stabilized in the putative binding pocket). The protein backbone is depicted as cartoon, whereas PLM (yellow), heme (cyan), Lys45 and Lys63 (cyan) are displayed as sticks and oxygen molecule is represented in ball shape (red). In all the frames, water molecules and ions are excluded for clarity.
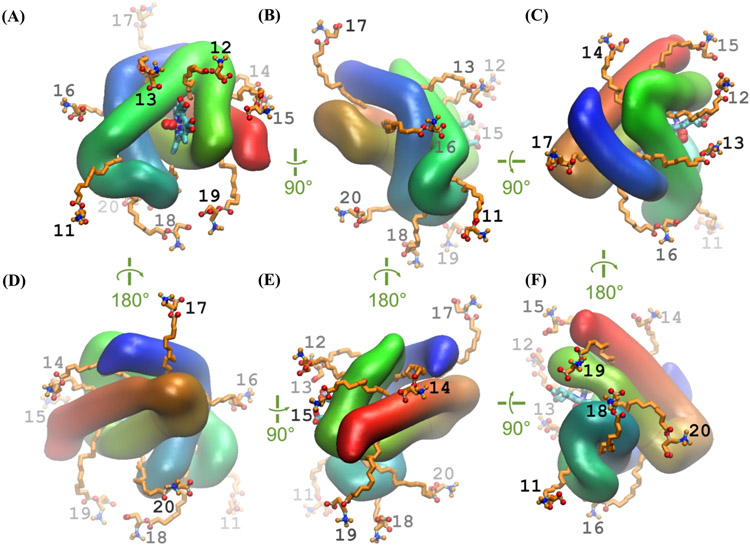
In the second case, we have prepared 10 starting conformations for the “alternative” lipid entry locations based on the hydrophobicity scale described by William et. al., which is calculated by the free energy for transfer of amino acids from water to n-octanol, a whole-residue hydrophobicity scale [41]. In Fig. 8, the protein is represented as a smooth surface and the helices are colored from N- (blue) to C-terminus (red). The fatty acids are placed in the hydrophobic crevice between the helices with immersion of only the terminal end of fatty acid tail (up to 2 carbons) to initialize the penetration process under the push of hydrophobic interactions. Even though there are possibly some more spots to consider exploring, we manually picked these top 10 spots after visual inspection as deemed the most promising for lipid binding - hydrophobic patches on the surface leading to a crevice of sufficient width and depth to accommodate most of the lipid tail. For starting arrangement of the lipids for each one of these alternative locations, we used the same conformation of the PLC (the one closest to the time-average of our thermal randomization trajectory) and only varied spatial position and orientation relative to the protein to form a contact favorable for lipid entry. In all the locations, we have positioned the lipid tail to immerse (1-2 carbons) into the target greasy spot, while the rest of the lipid was hovering over the nearest hydrophobic region of the protein surface – arguably a favorable starting point for entry into the spot. In total, we generated 80 different protein-ligand complexes for MD simulations “(Case I - Common starting point with different ligand conformations: 10 for Oxy-Mb with PLM, 10 for Deoxy-Mb with PLM, 10 for Oxy-Mb with PLC, and 10 for Deoxy-Mb with PLC. Case II - Alternate starting point with different ligand conformations: 10 for Oxy-Mb with PLM, 10 for Deoxy-Mb with PLM, 10 for Oxy-Mb with PLC and 10 for Deoxy-Mb with PLC)”.
Fig. 8.
Structure of the mouse Oxy-Mb with PLC placed at 10 different locations around the protein. Individual lipids labelled 11 through 20 are placed near the protein surface with their tails (~1-2 carbons) placed inside the hydrophobic crevices. The protein backbone is depicted as tube and colored from N- (blue) to C-terminus (red), whereas PLC (orange), heme (cyan) are displayed as sticks and oxygen in ball shape (red). Six orthogonal projections are presented on panels A-F. In all the panels, water molecules and ions are excluded for clarity.
Molecular dynamic (MD) simulations
NAMD package [42] developed by the Theoretical and Computational Biophysics Group at University of Illinois at Urbana-Champaign was used to conduct molecular dynamics simulations on all the 80 different protein-lipid complexes. Visual molecular dynamics (VMD), a molecular graphics program, was used extensively for the display, analysis and interpretation for all the protein-ligand complexes [43]. All the calculations were carried out using the CHARMM36 force field parameters [39, 40] with the isothermal-isobaric (NPT) ensemble for both protein and lipid structures. NPT ensemble corresponds more closely to the laboratory conditions which are performed at constant temperature and pressure. For oxygen-bound myoglobin, we used the updated partial atomic charges of the heme prosthetic group and oxygen molecule from Daigle et al. study, in which the parameters were calculated and optimized using standard Ab initio quantum mechanical (QM) calculations [38]. Each of the protein (either Oxy- or Deoxy-Mb), and the lipid (either PLM or PLC), were embedded in a box containing TIP3 water model [44]. These minimizations use periodic boundary conditions, where protein-lipid complex is solvated in a rectangular 3D periodic box that is extended to at least 10 Å in every direction of which the dimensions were chosen to be larger than the protein-lipid complex. To maintain electro-neutrality for the each system, a sufficient amount of Na+ & Cl− ions was added up to equivalent of 150 mM salt concentration to each protein-lipid complex (Deoxy-Mb/PLM, Deoxy-Mb/PLM, Oxy-Mb/PLC & Deoxy-Mb/PLC) by replacing the water molecules at random positions in the water box. Throughout the simulations run, a constant pressure (1 atm) and temperature regulation (1°K to 300°K) with a collision frequency of 1.0 is maintained using Langevin Dynamics [45, 46]. Both VDW and electrostatic forces were treated using a 12 Å cutoff, with the switching distance 1.5 Å (i.e. the last 1.5 Å before the cutoff distance, where all the non-bonded interactions are linearly tapered down to zero). Long-range electrostatic interactions were treated using particle mesh Ewald (PME) method [47]. Prior to the MD simulations, internal constraints were relaxed by energy minimization for all the complexes and a three step protocol was employed for the simulation run. In the first step, energy minimization was performed only on the solvent molecules keeping the protein fixed using the steepest descent in the first 3000 steps to avoid clashes between conflicting contacts. In the second step, we kept the heavy atoms of the Oxy- / Deoxy-Mb fixed a conjugate gradient method for 3000 steps, while both the solvent and hydrogen atoms in the Oxy- / Deoxy-Mb were allowed to relax. In the concluding step, all the solvent molecules and the protein atoms were allowed to relax for the subsequent 3000 steps during optimization. To attain equilibrium, each system was subjected to steady heating until it reached 300°K at 1 atm and the coordinates of each system were saved for every 1 picosecond (ps).
The contacts between the non-hydrogen atoms of the lipid, water, and Mb were estimated using a distance cutoff criterion. The distance threshold was assigned separately for each combination of atomic group types (e.g. water oxygen contacts with the heme non-hydrogen atoms) and it was set such that it would cover the first contact shell. That distance was estimated based on the radial distribution function (time-averaged probability distribution of the pairwise distances between the atoms of the contacting groups plotted vs. the pairwise distance value). The cutoff distance was defined as the distance of the first minimum of the radial distribution function (i.e. covering completely the most likely position of the atoms in the first contact shell as represented by the first maximum). Free Energy Perturbation (FEP) estimates for the bound and the unbound conformations of PLM and PLC with Mb were performed using FEP module of NAMD [42]. The output from the FEP simulations were integrated using the SOS estimator of the FEP analysis plugin in VMD [43, 48]. A detailed methodology is presented in the supplementary methods section (Data in Brief document).
RESULTS
Sequence homology of murine and equine myoglobin.
Previous studies of Mb-lipid binding leveraged the published results on equine Mb [14, 20]. To facilitate functional studies of Mb physiology in vivo and in cell culture, it will be helpful to consider the mouse model. To develop a murine Mb structure with which to conduct binding simulations, amino acid homology was determined by comparing equine and murine Mb (Fig. 1). Both of the sequences displayed a high degree of similarity (82.3% sequence identity) in amino acid sequence and overall structure, where none of the significant amino acids differences occur in the frequent heme and lipid contact positions.
Observation of palmitate and palmitoylcarnitine entry into the murine Oxymyoglobin structure.
Initial work on lipid binding with myoglobin (NMR study) was mainly performed using palmitate [16, 18], and this fatty acid is routinely used in the majority of studies examining muscle fatty acid oxidation and biochemistry. Thus, the findings may be placed in a historical framework of knowledge related to regulation of fat metabolism. Additionally, we have previously shown using different chain lengths of fatty acids and acylcarnitines interacting with Mb, and confirmed that binding is most robust with long-chain molecules such as palmitate [14]. Finally, palmitate is one of the most abundant fatty acids in the body and hence the findings here should have direct physiological relevance.
First, we considered how robust the long chain fatty acid entry into the “portal region” remains when the ligand’s presentation to the site is modified. For each separate MD run, different thermally randomized starting conformations and orientations of PLM or PLC were placed near (~7Å) the entrance “portal region,” while keeping the Oxy-Mb structure in the same conformation; the two lipids differed only in the head group structure for these simulations. For Oxy-Mb, a total of twenty MD runs (10 for PLM and 10 for PLC) were performed (Table 1A). Importantly, in all the above cases the initial location of the PLM and PLC was in the bulk solution near myoglobin (Fig. 2), not embedded inside as we had done previously for equine Mb studies (see Fig. 2 inset for illustration of starting PLM placement in equine Mb). Taking into account all the simulation runs, the ligand exhibited a random walk in some cases, whereas in others the carboxyl group of the ligand maintains its close proximity with the amino group of lysine residue, K45 (see Fig. 3 and Video -1 & 2).
Table 1: Summary of MD simulations using murine Mb.
(A) Ten different models of PLM and PLC involved placement at the same location (~7Å) away from the heme pocket in each of the simulations comprising either Oxy- or Deoxy-Mb structure, except model-10 where the simulation starts with its hydrophobic tail half immersed into the binding pocket. “E” represents entry of ligand into the main hydrophobic pocket, where the respective ligands find their path and occupy the binding site, while “N” represents “no entry,” where the ligand is unable to penetrate into the groove and eventually diffuses into the surrounding hydrophilic environment. (B) Top ten models (selected from randomized models after visual inspection) of PLM and PLC placed at 10 different locations around Mb structure with lipid hydrophobic tail immersed (maximum of ~2 carbons) into the hydrophobic patch on either Oxy- or Deoxy-Mb. Apart from the main entry site, there are two separate alternate entry points “A1” and “A2” that can be observed both in the Oxy- and Deoxy-Mb structures with respect to either PLM or PLC.
| (A): Case – I. The same starting location of the “lipid circa the portal region,” but with different ligand conformation and orientation. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MODELS- | M1 | M2 | M3 | M4 | M5 | M6 | M7 | M8 | M9 | M10 |
| OXY-MB + PLM | E | E | E | E | E | E | E | E | E | E |
| DEOXY-MB + PLM | N | N | N | N | N | N | N | N | N | E |
| OXY-MB + PLC | E | E | E | E | E | E | E | E | E | E |
| DEOXY-MB + PLC | N | N | N | N | N | N | N | N | N | E |
| (B): Case – II. Different starting locations around the Mb, with similar ligand conformation | ||||||||||
| MODELS- | M11 | M12 | M13 | M14 | M15 | M16 | M17 | M18 | M19 | M20 |
| OXY-MB + PLM | E | N | N | A1 | N | A2 | N | N | N | N |
| DEOXY-MB + PLM | N | N | N | A1 | N | A2 | N | N | N | E |
| OXY-MB + PLC | E | N | N | A1 | N | A2 | N | N | N | N |
| DEOXY-MB + PLC | N | N | N | A1 | N | A2 | N | N | N | N |
Fig. 3.
Consecutive snapshots taken during the entry of PLM (yellow in Panels A-D) and PLC (orange in Panels E-H) into the Oxy-Mb structure. The upper panel frames A-D were taken from model-6 of Oxy-Mb interacting with PLM and the lower panel frames E-H were taken from model-3 of Oxy-Mb interacting with PLC at different consecutive time intervals during lipid entry process. In both examples, the ligands initially enter the Oxy-Mb with hydrophobic tail pointing into the hydrophobic Mb pocket, then attain a characteristic “U” shape structure by the final frame.
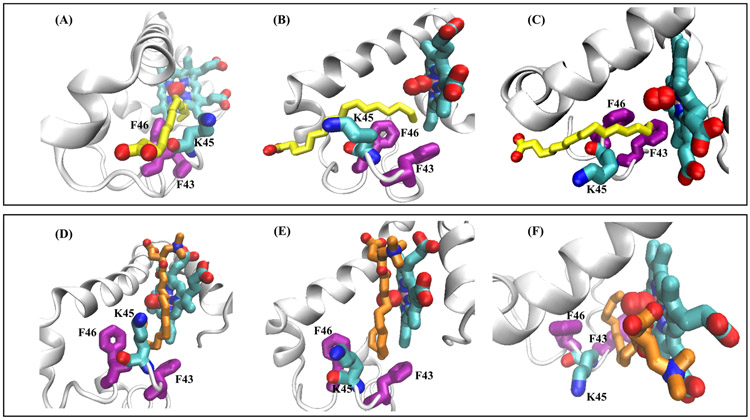
In the simulations, the alkyl tail ”wiggles” near the portal region, driven by thermal fluctuations and hydrophobic exclusion from water that typically ended up with the tail stabilized in a hydrophobic crevice of the entry portal. Similar features were observed for almost all the simulations, which we will discuss in detail for two sample structures depicted in Fig. 3: one from PLM (model-6) and another from PLC (model-3) sets. The upper frames in Fig. 3A-3D represent sequence snapshots of the PLM entry, whereas the lower frames (Fig. 3E-3H) represent PLC entry into murine Oxy-Mb. For the PLM entry simulation, initial contact between the carboxyl group and amino group of the lysine (K45) is lost (Fig. 3B) and the alkyl tail slowly diffuses over the nearby hydrophilic area. After a short period, the ligand is adsorbed onto the protein’s surface at the vicinity of the non-polar portal region, with its tail positioned (Fig. 3C and see Video -1 & 2) to enter into the hydrophobic heme region core. Comparing Fig. 3A vs Fig. 3C, the PLM ligand takes almost a 180° turn to re-orient before the initialization of the entry process, while the carboxyl moiety retains full contact with the surrounding water molecules. During the entry process, the PLM ligand interacts with two amino acids phenylalanine (F46) and leucine (L49). The PLM alkyl tail first comes in contact with the L49 and is slowly pushed into the hydrophobic groove after it gains contact with the F46 amino acid side chain. The PLM tail penetrates in the gap between K45 and F46 (Fig. 4A-4C) and shows a slight tilt which occurs because of widening of the loop region (amino acids from 43 to 51).
Fig. 4.
Descriptive snapshots of PLM and PLC initial entry process into the Oxy-Mb structure. A&D shows the side view, B&E shows the center view and C&F shows the top view of the ligand entry. The upper panel (A-C) shows the PLM entry through Lys45 and Phe46, whereas PLC enters through Phe43 and Lys45 (D-F). The protein backbone is depicted as cartoon, whereas PLM (yellow), PLC (orange), heme and Lys45 (cyan) and Phe43 and Phe46 (purple) are displayed as sticks and oxygen molecule is represented in ball shape (red). In all the panels, water molecules and ions are excluded for clarity.
The penetration of the PLM ligand occurs rapidly after this step when the alkyl tail comes in contact with two other amino acids (L29 & F43). Soon after, the PLM ligand is further pushed deep inside while contacting L32, which in turn helps the carboxyl head group to re-gain the contact with the amino group of K45. After a 10 ns simulation run, the ligand is stabilized and repositions deeper into the cavity. In the entire process, the amino acids K45, F46, L49, D54, K63 and H64 are the major residues mediating the entrance of PLM in this route, whereas L29, L32, F33 and F43 are the major contacts during repositioning the alkyl tail to the deeper position. After the stabilization is achieved, additional contacts of L107, V28 and V68 with the alkyl tail of the PLM are observed. During the entire process of PLM entry and stabilization, the distal histidine (H64 -which is involved in the reversible binding of the O2) did not lose its hydrogen bonding with the oxygen molecule. The water surrounding the PLM ligand (nearly ~50 molecules) at the starting point diminishes to ~6 to 10 molecules, interacting occasionally with the carboxylate head group, whereas the hydrophobic alkyl tail is completely occupying the hydrophobic groove of Oxy-Mb. This highlights the likely importance of the hydrophobic push from water as the one of the main contributors driving PLM entry. The time course of permeation (Fig. 5) varies widely (from 1 to ~20 ns); however, most of the lipids enter the crevice within 5 ns, as can be judged by the decreasing number of water molecules contacting the lipids and the growing number of contacts between the lipid and the heme.
Fig. 5.
Timeline of the lipid entry into the Oxy-Mb pocket. The entry process of PLM (A, C) and PLC (B, D) is reflected in the water loss near the lipid (A, B) and increase in the number of the lipid-heme contacts (C, D). Each colored line corresponds to a separate simulation with randomized starting position near the entry portal (magenta M-1, red M-2, orange M-3, yellow M-4, lime M-5, green M-6, cyan M-7, light-blue M-8, navy-blue M-9, purple M-10). The dotted black line approximates an average of all 10 traces and indicates that entry typically occurs within the first few ns. The similarity of the final number of water levels indicates that there is a consistent dehydration of lipid driving the entry. A higher variation of the final number of contacts with heme reflects some variability in conformations of the bound lipids.
In comparison with PLM, the PLC ligand also exhibits a similar pattern in the penetration events. However, there is a certain variation in the entry route of PLC (model-3) involving other amino acids at the entry site. At the start of the MD run (Fig. 3E-H, and see Video −3 & 4), the presence of the carnitine moiety in the PLC interacts with the surrounding water molecules and the carboxylate group of the carnitine maintains its close proximity with the amino group of K45. For a considerable amount of time (~20 ns), the PLC alkyl tail wiggles in the surrounding environment and then penetrates into the groove (Fig. 4D-4F) when it gains contact with phenylalanine (F43). The F43 side chain moves slightly aside which allows the PLC tail into the pocket. When the tail gains additional contact with L32, the alkyl tail penetrates faster into the hydrophobic groove. For PLM entry, the alkyl tail passed near K45 and F46, whereas in PLC the alkyl tail moves in between K45 and F43. This difference between the PLM and PLC routes can be explained because of the initial placement of the ligand and their different conformation and head group contacts at the start of each MD run. The carnitine head group adds both hydrophobic (two CH2 and one CH) and hydrophilic (carboxyl and trimethylammonium) groups. Using different starting models, PLC alkyl tail also exhibits additional hydrophobic contacts with the protein (time-averaged 25.2 direct contacts between heavy atoms of lipid and the protein for PLC vs. 17.9 contacts for PLM), with the main contributions coming from contacts with L40, F43, F46, D60, H64, and V68, as well as K45 and K63, while showing similar number of hydrophilic contacts as PLM (Table 2 & Table S1). PLC also shows an increase in the lipid atoms contacting the heme and the oxygen molecule (14.1 heme contacts and 5.5 oxygen contacts for PLC, vs.11.4 and 4.7, respectively, for PLM). In the simulations involving model-10 for both the ligands (PLM and PLC with Oxy-Mb), where half of the alkyl tail is already placed in the hydrophobic pocket, the movement of both these ligands happened at a faster pace comparing to the other starting points, and quickly attained a “U” shape structure.
Table 2. Statistics of most frequent contacts between murine Mb, water and the bound lipids.
The values in the table represent time-averaged number of non-hydrogen atoms (see descriptions in the first column) that are within the first minimum of the pairwise radial distribution function to each other (details in Methods). The summary numbers have background histograms in the table. The values per specific Mb residue are color coded from white to tan color, with darker shades corresponding to higher contact frequencies. The rightmost columns with proximity of the residue to heme and the secondary structure are coded the same way as on Fig. 1. See whole-protein table of contacts for specific residues in the supplemental table S1.
 |
Bound conformation similar to our previous predictions
Across the globin superfamily members, all the proteins share a common 3-Dimensional (3D) fold with the exception of some proteins having additional extensions at their termini. The sequences that form the globin fold can have as low as 16% sequence identity even though the fold of the globin superfamily is highly evolutionarily conserved [49-52]. In our previous study, we explored the phenomenon of binding of different chain lengths (starting from C6 to C18) of fatty acids and acylcarnitines based on the molecular docking experiments and MD simulations of horse myoglobin [14, 20]. In the present work, to examine the structural differences of the lipid binding pattern in an Mb homolog from another species, a binding site comparison was made between horse vs mouse Oxy-Mb bound to either PLM or PLC after the complexes attain stability, which resulted in some useful conclusions. On the completion of 100 ns MD simulation run, the average protein-ligand structure (from the last 20 ns run) is extracted from both the horse and mouse complexes and superimposed on each other using their backbone using root mean square deviation (RMSD) function from VMD. Fig. 6 represents the two sets of overlaid structures of ligands with respect to the horse (blue) vs mouse (PLM-yellow / PLC-orange). In a binding site comparison analysis, the PLM from the horse Oxy-Mb+PLM complex (blue) shows somewhat deeper penetration (~4 carbons) compared to PLM in the mouse model (yellow) (Fig. 6A). In contrast, PLC molecules in both the horse and mouse models align similarly and show equivalent penetration depth of the alkyl tail into the hydrophobic pocket (Fig. 6B).
Fig. 6.
Structural comparison of the horse Oxy-Mb with PLM and PLC complexes with mouse Oxy-Mb with PLM and PLC complexes aligned by the protein backbone. Zoom-in panels of the binding pocket show good alignment of both PLM and PLC structures in horse and mouse oxy-Mb structures. The entire complex of horse Oxy-Mb is colored blue, whereas mouse Oxy-Mb with PLM is colored yellow and Oxy-Mb with PLC is colored orange both exhibiting a “U” shaped conformation.
Accordingly, both the PLM and PLC ligands showed similar characteristic “U” shape structure surrounding the O2 molecule without disturbing the hydrogen bond between the O2 molecule and distal histidine (His64). Interestingly, the residues that line the fatty acid binding site are well matched between the two Mb homologs and show high degree of conservation. On the other hand, the non-conserved binding site residues (abbreviated here as an amino acid change from horse to mouse: R31G, T34K, G35T, E41D, H48N, T51S, A53E, T66C and V67T), in the vicinity of the hydrophobic groove, have respective side chains facing toward the hydrophilic environment and do not show any interaction with PLM and PLC either in the entry or after the ligand stabilization. In both the examples, not only similar side chains are lining the pockets, but also the backbone structure locally adopts similar conformation to form structurally similar binding pose within the same protein fold.
Structural changes caused by deoxygenation prevent binding of both palmitate and palmitoylcarnitine to murine Mb
It was shown experimentally [16-19] that deoxygenated equine Mb does not bind FAs, which is also supported by the docking studies and simulations of the long-chain FAs or long-chain acylcarnitines placed in the putative binding site [14, 20]. In the present study, we have tested whether the PLM and PLC placed near the binding crevice would enter Deoxy-Mb. The starting coordinates of lipids were exactly the same as in Oxy-Mb simulations, the only difference was the removal of oxygen. Neither PLM nor PLC penetrated the hydrophobic core of the E7 channel region in myoglobin in all the starting conformations (Fig. S1), except for model-10. In model-10, unlike other models, the tails of the lipids were partially immersed into the Mb core at the start: this allowed for the immersed hydrophobic tails to penetrate somewhat, despite the more limited access to the hydrophobic core upon deoxygenation. We should note that the final position of the lipid tails in model-10 (see Video-5 & 6) was somewhat different than the typical U-shaped conformations in the Oxy-Mb. Instead of going around the oxygen, the tails in the two Deoxy-Mb permeations were straightened up and positioned next to the edge of the heme, away from the oxygen binding location.
In the rest of the Deoxy-Mb simulations, deoxygenation prevented lipid entry due to relocation of the heme closer to the opposite wall of the hydrophobic crevice, so that the cavity for the lipid compacted by ~3.5 Å (Fig. 7, heme colored in blue); thus, it became more difficult for the PLM or PLC tail to penetrate (see Video −7, 8, 9 & 10). Moreover, upon compaction the very entrance to the crevice (the region between the loop carrying F43 on one side and the loop close to the heme near I99) narrowed, so that the backbone atoms of these opposing loops approached one another by ~3 Å, while the side chains started to form more frequent and tighter contacts, thus hindering the entrance. Additionally, in Deoxy-Mb, K45 residue showed a tendency to form a salt bridge with the nearest carboxyl of the heme, securing the narrower entry gap. The compaction occurred very quickly, on the scale of a few nanoseconds. Two supplemental movies illustrate the structural differences between the Deoxy-Mb with no lipid bound, and two Oxy-Mb structures - one with PLC bound at the main site near heme (Video 11), and another with PLC located outside the binding pocket, away at the surface of the protein (Video 12).
Fig. 7.
Structural changes in Deoxy-Mb that prohibit binding of lipids. The structures of Oxy-Mb with bound PLC (colored red) and Deoxy-Mb without lipid (blue) were aligned by the backbone. The snapshots illustrate that in the absence of oxygen the crevice adopts more compact conformation as the heme moves closer to the opposite side by ~3.5 Å, decreasing the space available for a lipid tail. The entrance to the crevice gets narrower as well, hindering entrance of the lipid. The snapshots were taken close to the end of the simulations, however most of the structural changes occur in the very first few nanoseconds.
In contrast to the effect of deoxygenation, the entry of lipid did not lead to significant expansion of the crevice, apparently due to a sufficient volume and flexibility in the crevice. With the bound lipid, we did not see a significant distortion neither to the protein side chains lining the crevice, nor the heme structure, including the geometry of the oxygen position relative to the heme. While PLM ad PLC readily bind Oxy-Mb through the main entry portal, they do slightly alter the Oxy-Mb structure by widening the entry region. Deep in the main biding crevice, the change caused by the very tail of the lipids is fairly small, the buried edge of the heme moves outward by ~0.3 Å, while the crevice expands in the direction orthogonal to the heme plane by ~0.65 Å (a relatively small change comparing to the scale of thermal fluctuations of the protein backbone in our simulations, 1.2 Å). However, the widening is more pronounced closer to the water-exposed side of the crevice, so that the entrance widens by ~2.5 Å (as measured between the alpha-carbons of residues F43 and H97). The helix covalently bound to the heme moves outward by 0.8 Å and allows the exposed edge of the heme to tilt toward it by 1.95 Å. The general trend in these changes follows the transformations caused by oxygenation of the heme (see below). More detailed spatial measurements can be found in the Data in Brief section (Fig. S2 and Table S2).
Alternate binding sites (not affected by deoxygenation)
To examine whether the lipids can enter into myoglobin through sites other than the main entry portal described above and in our previous publications [14, 20], ligands were positioned on 10 different locations of the Mb structure (Fig. 8). At the beginning of each simulation at each of the locations, PLM or PLC were placed in the same thermally randomized conformation at each of the locations (visually picked 10 out of the library of 300 randomized structures to represent several distinct conformations and orientations of the lipid relative to the pore vestibule observed during the randomization: e.g., with the tail, headgroup or the middle pointed to the entrance, with the headgroup contacting different polar residues near the vestibule etc.).
Out of all the 40 simulations started at the alternative locations, we observed 11 entry events in total (Table 1B). In three of them (PLM and PLC started at model-11, and PLM at model-13, all with Oxy-Mb) the lipid ended up entering the aforementioned main entry portal in the presence of bound oxygen. As shown in Fig. 8, model-11 structures of both PLM and PLC are close to the entry portal region, similar to the 10 different conformations of Fig. 2, just positioned at the remote corner of the portal. Thus, both PLM and PLC made an entry through immersing its alkyl tail into the previously-described hydrophobic pocket, whereas in deoxy-Mb structures with the same starting alternative positions, both the ligands moved away from the binding pocket into the hydrophilic environment. The third simulation (model-13) that resulted in lipid binding to the same main portal region of Oxy-Mb, have started with PLM molecule away from the main portal, in a different hydrophobic groove. That remote starting position was separated from the main portal by a hydrophilic “ridge” formed by an alpha-helix (Fig. 8 shows PLC in the same starting position (model-13)). Nevertheless, the PLM ligand, while exhibiting a random walk, reaches the portal region where the head carboxyl group is interacting with the amino group of K45, the hydrophobic interactions with the alkyl tail initiates a partial penetration and later slowly enters into the hydrophobic pocket (see Video-13).
In addition to the main entry portal, we have observed entry of lipid ligand into another hydrophobic crevice of Mb, from two alternative starting locations: model-14 and model-16 for both the ligands with both Oxy-Mb and Deoxy-Mb (Total observations: 8 different binding events). As shown in Fig. 9, when PLM or PLC are placed near the greasy spot at the protein surface, the terminal two carbons of the alkyl tail are in close contact with the hydrophobic residues. During the course of the MD simulation, the ligands moved into the hydrophobic groove between the two helices and attained a linear shape. The starting points for model-14 and model-16 are exactly opposite to each other in the myoglobin structure, whereas the final conformation of these two models occupy the same binding crevice with the lipid head groups pointing toward the hydrophilic environment on one or the other side. Illustrated in Fig. 9 (also see Video −14, 15, 16 & 17) are examples of the PLC-Mb structure starting from the initial position of PLC (colored green) and ending up inside the groove (colored orange) of both Oxy-Mb (Fig. 9A & 9B) and Deoxy-Mb (Fig. 9C & 9D) after a 100 ns MD run. The entry of both ligands (either PLM or PLC) in both the Oxy- & and Deoxy-Mb structures is very similar in movement pattern. Residues that are commonly encountered by the ligand entry within 4 Å distance (W7, W14, K16, H24, V28, L69, I75, L76, H82, I86, L115, R118, H119, S120, M131, L135, L137) did not show large side chain movements. While this entry leads to a stable adsorption of the lipid, the bound ligand is at a large distance from the heme and does not seem to influence protein, heme or oxygen conformation and the interactions do not depend on the Mb oxygenation state.
Fig. 9.
Depiction of the alternative lipid binding pocket identified for model-14 and model-16 for both Oxy- (A & B) and Deoxy-Mb (C & D) structures with PLC (similar pocket was observed for PLC). Green color (displayed as sticks) indicates the starting conformation, whereas orange color (displayed as sticks and embedded in the transparent orange surface) represents the final binding conformation. A1 and A2 represents two different modes of entry into the Mb protein, similar for both of the oxygenation states.
DISCUSSION
The current molecular dynamic simulations performed on both forms of mouse myoglobin (Oxy- and Deoxy-) with either PLM or PLC extend our previous studies using equine Mb [14, 20], demonstrating similar lipid/Oxy-Mb binding near the heme pocket “entry portal” and unmasking an additional potential binding groove for PLM and PLC. Interestingly, both the PLM and PLC follow a similar route as O2 entry into the Mb (near the heme propionates), but only when Mb is in the oxygenated form. The initial step of the entry is the combination of (a) thermal fluctuation that propels the end of the fatty acid chain into the crevice, and (b) persistent hydrophobic exclusion of the hydrophobic atoms by water molecules, ultimately driving the penetration of the alkyl tail of both the ligands into the hydrophobic crevice of the Oxy-Mb. This pattern of entry mechanism was observed in all the simulations (10 each of Oxy-Mb with PLM & Oxy-Mb with PLC, Table -1A). While the timescale of permeation varied among different starting positions, the spontaneous nature of the binding and short average time for entry (5 ns) suggests a strong driving force and an absence of any major structural obstacles in the pathway. We have recently published experimentally measured estimates for the dissociation constant and free energy of binding of PLM (29.1 μM, 6.43 kcal/mol) and PLC (9.76 μM, 7.11 kcal/mol) to the highly homologous horse myoglobin using Isothermal Titration Calorimetry [14]. The known lipid-binding proteins typically have their binding energy in the range of −7.5 kcal/mol to −12.2 kcal/mol [53], which places Mb rather on the weaker side of the spectrum; however, it is comparable to proteins like CRBPII, while Mb Kd fits into physiological range of the cellular levels of the fatty acids. The results of the free energy estimations at different binding locations are presented in the Data in Brief section (see Table S3).
An additional striking feature for both PLM and PLC binding is the absence of entry into the myoglobin’s heme pocket when it is in the deoxygenated state. This supports our previous results and studies conducted by other groups that demonstrated that Deoxy-Mb shows limited interaction with long-chain fatty acid, and we have demonstrated the same for long-chain acylcarnitine ligands. Failure of entry of these ligands into the portal region of Deoxy-Mb can be best explained due to the compaction of the heme-containing hydrophobic crevice in the myoglobin protein, which happens on the scale of a few nanoseconds upon deoxygenation.
Despite some binding at some of the alternative locations, the portal region near the heme can be considered the main and specific binding pocked for lipids. This view is formed from the consensus between the published results from Automated Docking approaches, MD simulations of the retention of the docked lipids, and the current simulations of fast spontaneous oxygenation-dependent entry in the vast majority of simulations started from essentially any lipid orientation near the portal region. In contrast, in simulations started at most of alternative locations the lipids failed to bind to both Oxy-Mb and Deoxy-Mb, despite the pre-arrangement in an optimized position with partial immersion of the hydrophobic tail to facilitate binding. This suggests that if some binding at an alternative location does exist, it is likely to be weaker, much less specific, and unrelated to Mb oxygenation. Nevertheless, two of the alternative binding locations (leading to the same crevice) seem to show a consistent, oxygenation-independent binding. Some non-specific binding of long-chain FAs to Mb has been observed experimentally [54]; however, that finding needs to be confirmed by other experimental approaches and the methods employed a very high concentration of FAs.
CONCLUSIONS
In this study, we have observed for the first time a complete process of spontaneous penetration of lipid molecules into the myoglobin core, on the timescale of a few nanoseconds. The entry was reproducibly observed for Oxy-Mb when PLM or PLC molecules were placed in the bulk phase in the vicinity of Oxy-Mb near the heme-binding region, with varying starting conformations and orientations of the lipids. All the simulations exhibited a similar entry route near the distal cavity portal region of the Oxy-Mb, which also forms the major entry pathway for small gaseous molecule ligands. The bound conformations displayed common features in all the complexes, with the lipid tail typically around the heme, in contact with the oxygen molecule.
Since Mb is very abundant in type 1 oxidative muscle fibers and cardiomyocytes that rely heavily on fat oxidation and trafficking, its binding to PLM and PLC may have important physiological relevance to support normal muscle trafficking and sequestration of these bioactive lipids. Also, as oxygen forms the major constituent for mitochondrial oxidative phosphorylation, our new findings raise the possibility that a major increase in the cellular concentration of either long-chain fatty acids or long-chain acylcarnitines could directly affect the oxygen release as both these lipids hinder the region near oxygen exit pathway.
Supplementary Material
Acknowledgements:
The investigators acknowledge funding support from USDA-Agricultural Research Service Project 6026-51000-010-05S. Original studies stemmed from research conducted under NIH-NIDDK R01DK078328-01 (SHA). The investigators acknowledge support in part by the National Science Foundation under Grant CRI CNS-0855248, Grant EPS-0701890, Grant EPS-0918970, Grant MRI CNS-0619069, and OISE-0729792. AA was supported by the Grant NIH-NIGMS R01GM107652. This project was also supported by the Arkansas INBRE program, with a grant from the National Institute of General Medical Sciences, (NIGMS), P20 GM103429 from the National Institutes of Health. We would also like to thank Drs. Srinivas Jayanthi and Suresh Thallapuranam for ongoing collaborations and Albert Everett for his support in running jobs on HPCC server.
Abbreviations:
- FA
fatty acid
- AC
acylcarnitine
- Oxy-Mb
oxygenated myoglobin
- Deoxy-Mb
deoxygenated myoglobin
- Mb
myoglobin
- PLM
palmitate
- PLC
palmitoylcarnitine
- FABP
fatty acid binding protein
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interests with the contents of this article.
REFERENCES
- [1].Boulton FE, Huntsman RG, Yawson GI, Romero Herrera AE, Lorkin PA, Lehmann H, The second variant of human myoglobin; 138(H16) arginine leads to glutamine, Br J Haematol 20(1) (1971) 69–74. [DOI] [PubMed] [Google Scholar]
- [2].Boulton FE, Huntsman RG, Romero Herrera AE, Lorkin PA, Lehmann H, A human myoglobin variant 133 (H-10)lysine--asparagine, Biochimica et biophysica acta 229(3) (1971) 871–6. [DOI] [PubMed] [Google Scholar]
- [3].Boulton FE, Huntsman RG, Romero Herrera A, Lorkin PA, Lehmann H, The third variant of human myoglobin showing an unusual amino acid substitution: 138(H16)arginine--tryptophan, Biochimica et biophysica acta 229(3) (1971) 716–9. [DOI] [PubMed] [Google Scholar]
- [4].Boulton FE, Huntsman RG, Lorkin PA, Lehmann H, Abnormal human myoglobin: 53 (D4) glutamic acid--lysine, Nature 223(5208) (1969) 832–3. [DOI] [PubMed] [Google Scholar]
- [5].Antonini E, Rossi-Fanelli A, Heterogeneity of human myoglobin, Arch Biochem Biophys 65(2) (1956) 587–90. [DOI] [PubMed] [Google Scholar]
- [6].Perkoff GT, Hill RL, Brown DM, Tyler FH, The characterization of adult human myoglobin, J Biol Chem 237 (1962) 2820–7. [PubMed] [Google Scholar]
- [7].Gelfi C, De Palma S, Ripamonti M, Eberini I, Wait R, Bajracharya A, Marconi C, Schneider A, Hoppeler H, Cerretelli P, New aspects of altitude adaptation in Tibetans: a proteomic approach, FASEB journal : official publication of the Federation of American Societies for Experimental Biology 18(3) (2004) 612–4. [DOI] [PubMed] [Google Scholar]
- [8].Reynafarje B, Myoglobin content and enzymatic activity of muscle and altitude adaptation, J Appl Physiol 17 (1962) 301–5. [DOI] [PubMed] [Google Scholar]
- [9].Flogel U, Fago A, Rassaf T, Keeping the heart in balance: the functional interactions of myoglobin with nitrogen oxides, J Exp Biol 213(Pt 16) (2010) 2726–33. [DOI] [PubMed] [Google Scholar]
- [10].Schlieper G, Kim JH, Molojavyi A, Jacoby C, Laussmann T, Flogel U, Godecke A, Schrader J, Adaptation of the myoglobin knockout mouse to hypoxic stress, Am J Physiol Regul Integr Comp Physiol 286(4) (2004) R786–92. [DOI] [PubMed] [Google Scholar]
- [11].Flogel U, Godecke A, Klotz LO, Schrader J, Role of myoglobin in the antioxidant defense of the heart, FASEB journal : official publication of the Federation of American Societies for Experimental Biology 18(10) (2004) 1156–8. [DOI] [PubMed] [Google Scholar]
- [12].Gloster J, Harris P, Fatty acid binding to cytoplasmic proteins of myocardium and red and white skeletal muscle in the rat. A possible new role for myoglobin, Biochemical and biophysical research communications 74(2) (1977) 506–13. [DOI] [PubMed] [Google Scholar]
- [13].Gotz FM, Hertel M, Groschel-Stewart U, Fatty acid binding of myoglobin depends on its oxygenation, Biological chemistry Hoppe-Seyler 375(6) (1994) 387–92. [DOI] [PubMed] [Google Scholar]
- [14].Chintapalli SV, Jayanthi S, Mallipeddi PL, Gundampati R, Suresh Kumar TK, van Rossum DB, Anishkin A, Adams SH, Novel Molecular Interactions of Acylcarnitines and Fatty Acids with Myoglobin, J Biol Chem 291(48) (2016) 25133–25143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schenkman KA, Marble DR, Burns DH, Feigl EO, Myoglobin oxygen dissociation by multiwavelength spectroscopy, Journal of applied physiology 82(1) (1997) 86–92. [DOI] [PubMed] [Google Scholar]
- [16].Sriram R, Kreutzer U, Shih L, Jue T, Interaction of fatty acid with myoglobin, FEBS letters 582(25-26) (2008) 3643–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shih L, Chung Y, Sriram R, Jue T, Interaction of myoglobin with oleic acid, Chem Phys Lipids 191 (2015) 115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shih L, Chung Y, Sriram R, Jue T, Palmitate interaction with physiological states of myoglobin, Biochimica et biophysica acta 1840(1) (2014) 656–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jue T, Shih L, Chung Y, Differential Interaction of Myoglobin with Select Fatty Acids of Carbon Chain Lengths C8 to C16, Lipids 52(8) (2017) 711–727. [DOI] [PubMed] [Google Scholar]
- [20].Chintapalli SV, Bhardwaj G, Patel R, Shah N, Patterson RL, van Rossum DB, Anishkin A, Adams SH, Molecular dynamic simulations reveal the structural determinants of Fatty Acid binding to oxy-myoglobin, PLoS One 10(6) (2015) e0128496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jue T, Simond G, Wright TJ, Shih L, Chung Y, Sriram R, Kreutzer U, Davis RW, Effect of fatty acid interaction on myoglobin oxygen affinity and triglyceride metabolism, J Physiol Biochem 73(3) (2016) 359–370. [DOI] [PubMed] [Google Scholar]
- [22].Olson JS, Soman J, Phillips GN Jr., Ligand pathways in myoglobin: a review of Trp cavity mutations, IUBMB Life 59(8-9) (2007) 552–62. [DOI] [PubMed] [Google Scholar]
- [23].Morikis D, Champion PM, Springer BA, Sligar SG, Resonance raman investigations of site-directed mutants of myoglobin: effects of distal histidine replacement, Biochemistry 28(11) (1989) 4791–800. [DOI] [PubMed] [Google Scholar]
- [24].Nienhaus K, Knapp JE, Palladino P, Royer WE Jr., Nienhaus GU, Ligand migration and binding in the dimeric hemoglobin of Scapharca inaequivalvis, Biochemistry 46(49) (2007) 14018–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Knapp JE, Pahl R, Cohen J, Nichols JC, Schulten K, Gibson QH, Srajer V, Royer WE Jr., Ligand migration and cavities within Scapharca Dimeric HbI: studies by time-resolved crystallo-graphy, Xe binding, and computational analysis, Structure 17(11) (2009) 1494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Blouin GC, Schweers RL, Olson JS, Alkyl isocyanides serve as transition state analogues for ligand entry and exit in myoglobin, Biochemistry 49(24) (2010) 4987–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Birukou I, Soman J, Olson JS, Blocking the gate to ligand entry in human hemoglobin, J Biol Chem 286(12) (2011) 10515–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Birukou I, Schweers RL, Olson JS, Distal histidine stabilizes bound O2 and acts as a gate for ligand entry in both subunits of adult human hemoglobin, J Biol Chem 285(12) (2010) 8840–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Scott EE, Gibson QH, Olson JS, Mapping the pathways for O2 entry into and exit from myoglobin, J Biol Chem 276(7) (2001) 5177–88. [DOI] [PubMed] [Google Scholar]
- [30].Dantsker D, Roche C, Samuni U, Blouin G, Olson JS, Friedman JM, The position 68(E11) side chain in myoglobin regulates ligand capture, bond formation with heme iron, and internal movement into the xenon cavities, J Biol Chem 280(46) (2005) 38740–55. [DOI] [PubMed] [Google Scholar]
- [31].Cohen J, Arkhipov A, Braun R, Schulten K, Imaging the migration pathways for O2, CO, NO, and Xe inside myoglobin, Biophys J 91(5) (2006) 1844–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Huang X, Boxer SG, Discovery of new ligand binding pathways in myoglobin by random mutagenesis, Nat Struct Biol 1(4) (1994) 226–9. [DOI] [PubMed] [Google Scholar]
- [33].Salter MD, Blouin GC, Soman J, Singleton EW, Dewilde S, Moens L, Pesce A, Nardini M, Bolognesi M, Olson JS, Determination of ligand pathways in globins: apolar tunnels versus polar gates, J Biol Chem 287(40) (2012) 33163–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tomita A, Sato T, Ichiyanagi K, Nozawa S, Ichikawa H, Chollet M, Kawai F, Park SY, Tsuduki T, Yamato T, Koshihara SY, Adachi S, Visualizing breathing motion of internal cavities in concert with ligand migration in myoglobin, Proc Natl Acad Sci U S A 106(8) (2009) 2612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Paramo T, East A, Garzon D, Ulmschneider MB, Bond PJ, Efficient Characterization of Protein Cavities within Molecular Simulation Trajectories: trj_cavity, J Chem Theory Comput 10(5) (2014) 2151–64. [DOI] [PubMed] [Google Scholar]
- [36].Fiser A, Do RK, Sali A, Modeling of loops in protein structures, Protein Sci 9(9) (2000) 1753–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sali A, Blundell TL, Comparative protein modelling by satisfaction of spatial restraints, Journal of molecular biology 234(3) (1993) 779–815. [DOI] [PubMed] [Google Scholar]
- [38].Daigle R, Guertin M, Lague P, Structural characterization of the tunnels of Mycobacterium tuberculosis truncated hemoglobin N from molecular dynamics simulations, Proteins 75(3) (2009) 735–47. [DOI] [PubMed] [Google Scholar]
- [39].Klauda JB, Venable RM, Freites JA, O'Connor JW, Tobias DJ, Mondragon-Ramirez C, Vorobyov I, MacKerell AD Jr., Pastor RW, Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types, The journal of physical chemistry. B 114(23) (2010) 7830–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].MacKerell AD, Bashford D Jr , M. , Dunbrack RL Jr., Evanseck JD, Field MJ, Fischer S, Gao J, Guo H, Ha S, Joseph-McCarthy D, Kuchnir L, Kuczera K, Lau FTK, Mattos C, Michnick S, Ngo T, Nguyen DT, Prodhom B, Reiher WE, Roux B III, Schlenkrich M, Smith JC,Stote R, Straub J, Watanabe M, Wiórkiewicz-Kuczera J, Yin D, and Karplus M , All-Atom Empirical Potential for Molecular Modeling and Dynamics Studies of Proteins, The Journal of Physical Chemistry B 102(18) (1998) 3568–3616. [DOI] [PubMed] [Google Scholar]
- [41].Wimley WC, Creamer TP, White SH, Solvation energies of amino acid side chains and backbone in a family of host-guest pentapeptides, Biochemistry 35(16) (1996) 5109–24. [DOI] [PubMed] [Google Scholar]
- [42].Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kale L, Schulten K, Scalable molecular dynamics with NAMD, Journal of computational chemistry 26(16) (2005) 1781–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Humphrey W, Dalke A, Schulten K, VMD: visual molecular dynamics, Journal of molecular graphics 14(1) (1996) 33–8, 27-8. [DOI] [PubMed] [Google Scholar]
- [44].J.C. Jorgensen William L., Madura Jeffry D., Impey Roger W. and Klein Michael L., Comparison of simple potential functions for simulating liquid water, The Journal of Chemical Physics 79(2) (1983) 926–935. [Google Scholar]
- [45].D.J.T.a.M.L.K. Martyna Glenn J., Constant pressure molecular dynamics algorithms, The Journal of Chemical Physics 101(5) (1994) 4177–4189. [Google Scholar]
- [46].Y.Z. Feller Scott E., Pastor Richard W. and Brooks Bernard R., Constant pressure molecular dynamics simulation: The Langevin piston method, The Journal of Chemical Physics 103(11) (1995) 4613–4621. [Google Scholar]
- [47].Darden TA, York D, Pedersen LG, Particle mesh Ewald: An N·log(N) method for Ewald sums in large systems, J Chem Phys 98(12) (1993) 10089–10092. [Google Scholar]
- [48].Pohorille A, Jarzynski C, Chipot C, Good practices in free-energy calculations, The journal of physical chemistry. B 114(32) (2010) 10235–53. [DOI] [PubMed] [Google Scholar]
- [49].Perutz MF, Rossmann MG, Cullis AF, Muirhead H, Will G, North AC, Structure of haemoglobin: a three-dimensional Fourier synthesis at 5.5-A. resolution, obtained by X-ray analysis, Nature 185(4711) (1960) 416–22. [DOI] [PubMed] [Google Scholar]
- [50].Perutz MF, Muirhead H, Cox JM, Goaman LC, Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 A resolution: the atomic model, Nature 219(5150) (1968) 131–9. [DOI] [PubMed] [Google Scholar]
- [51].Kendrew JC, Dickerson RE, Strandberg BE, Hart RG, Davies DR, Phillips DC, Shore VC, Structure of myoglobin: A three-dimensional Fourier synthesis at 2 A. resolution, Nature 185(4711) (1960) 422–7. [DOI] [PubMed] [Google Scholar]
- [52].Kendrew JC, Bodo G, Dintzis HM, Parrish RG, Wyckoff H, Phillips DC, A three-dimensional model of the myoglobin molecule obtained by x-ray analysis, Nature 181(4610) (1958) 662–6. [DOI] [PubMed] [Google Scholar]
- [53].Richieri GV, Ogata RT, Zimmerman AW, Veerkamp JH, Kleinfeld AM, Fatty acid binding proteins from different tissues show distinct patterns of fatty acid interactions, Biochemistry 39(24) (2000) 7197–204. [DOI] [PubMed] [Google Scholar]
- [54].Hendgen-Cotta UB, Esfeld S, Coman C, Ahrends R, Klein-Hitpass L, Flogel U, Rassaf T, Totzeck M, A novel physiological role for cardiac myoglobin in lipid metabolism, Sci Rep 7 (2017) 43219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.