Abstract
Regulatory T (Treg) cells play a major role in immune suppression permitting tumors to evade immune surveillance. Depletion of intra-tumoral Treg cells can result in tumor regression. However, systemic depletion of Tregs may also induce autoimmune adverse events. Near-infrared photoimmunotherapy (NIR-PIT) is a newly developed cell-specific cancer therapy that locally kills specific cells in the tumor. Antibody-photoabsorber (IRDye700DX) conjugates (APC) are injected and bind to the tumor and subsequent administration of NIR light to the tumor results in rapid cell death only in targeted cells. CD25-targeted NIR-PIT has been shown to induce spatially selective depletion of tumor-associated Treg cells. In this study, we compared the efficacy of an antibody fragment, anti-CD25-F(ab’)2 and a full antibody, anti-CD25-IgG as agents for NIR-PIT. Tumor-bearing mice were divided into four groups: (1) no treatment; (2) anti-CD25-IgG-IR700 i.v. only; (3) anti-CD25-F(ab’)2-IR700 i.v. with NIR light exposure and (4) anti-CD25-IgG-IR700 i.v. with NIR light exposure. Although both anti-CD25-targeted NIR-PITs resulted in significant tumor growth inhibition, the anti-CD25-F(ab’)2-IR700 based NIR-PIT was superior to the anti-CD25-IgG-IR700 NIR-PIT. The anti-CD25-F(ab’)2-IR700 demonstrated faster clearance from the body than the anti-CD25-IgG-IR700. Sustained circulation of anti-CD25-IgG-IR700 may block IL-2 binding on activated effector T-cells decreasing immune response. In conclusion, anti-CD25-F(ab’)2 based NIR-PIT was more effective in reducing tumor growth than anti-CD25-IgG based NIR-PIT. Absence of the Fc portion of the APC leads to faster clearance and therefore promotes a superior activated T cell response in tumors.
Keywords: photoimmunotherapy, regulatory T cell, CD25, Fc
Introduction
Cancer immunotherapies, such as immune checkpoint inhibition therapy or chimeric antigen receptor (CAR) T cell therapy, have recently achieved clinical success in patients with diverse types of cancer. Such immune modulatory therapies have dramatically altered the therapy of many cancers.1,2 In addition to checkpoint inhibition, another pivotal strategy for cancer immunotherapy is to eliminate immune suppressor cells such as regulatory T cells.3 Regulatory T (Treg) cell lead to immunosuppression that plays a major role in cancer growth.4 In both humans and mice, a large number of Treg cells infiltrate cancers, and their presence correlates with poor prognosis.5 Therefore, Treg cells are good targets for cancer immunotherapy. There are various strategies to deplete or ablate Treg cells by targeting molecules on the surface of Treg cells. Among these, CD25 is a highly expressing target molecule, yet, antibodies targeting CD25 that are currently used in clinic have mainly been used to suppress the immune system so as to combat the effects of autoimmune disease or reduce transplant rejection. By blocking IL-2 binding to the receptor on activated effector cells, immune response is reduced and can therefore, be used to treat diseases defined by overly active immune responses.6 However, when targeting CD25, activated effector cells that also express CD25 are depleted in addition to Treg cells that could interfere with activation of effector cells.7 Furthermore, systemic blockade of immunosuppressive regulatory mechanisms run the risk of inducing autoimmune adverse events.8–10 Therefore, a method which induces rapid and selective depletion of intra-tumoral Treg cells should be theoretically desirable.
Near-infrared photoimmunotherapy (NIR-PIT) is a novel cancer treatment that uses an antibody-photoabsorber conjugate (APC).11 The photoabsorber, IRDye700DX (IR700), is a water-soluble silica-phthalocyanine dye that is readily conjugated to a targeting antibody. When injected, APCs bind to their cognate receptor on the cell. Subsequent exposure to NIR light causes highly selective and lethal damage only to the APC-bound cells, resulting in inducing rapid immunogenic cell death.12,13 A human clinical trial of NIR-PIT with an antibody targeting epidermal growth factor receptor (EGFR) conjugated to IR700 (RM1929/ASP1929) in patients with inoperable head and neck cancer is now under an FDA-designated fast-track global phase III clinical trial (https://clinicaltrials.gov/ct2/show/NCT03769506). Intra-tumoral Treg cells constitutively express CD25. Furthermore, in large proportion of tumors, most of the intra-tumoral CD8+ T cells and natural killer (NK) cells are not activated and do not express much CD25.14,15 Therefore, CD25-targed NIR-PIT, which selectively depletes Treg cells only at the tumor site but before CD8+ T and NK cells are activated, induces regression of treated murine tumors.15 However, several concerns remain about CD25-targed NIR-PIT. First, Fc-mediated antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) may occur resulting in depletion of activated CD25+ T cells. To avoid this, IR700-conjugated anti-CD25-F(ab’)2, which does not have an Fc region, was used in our previous study. Another concern is that the anti-CD25 APC could block the interleukin-2 (IL-2) receptor in effector T cells. IL-2 signal promotes proliferation and survival of newly activated T cells and effector cells. Therefore, binding of anti-CD25 to CD25 (IL-2 receptor) may interrupt IL-2 signaling in T cells. This problem could also be solved by utilizing an F(ab’)2 fragment of CD25 antibody which has a shorter half-life and thus, would have much less opportunity to block IL-2 receptor when effector cells activate and express CD25 after depletion of Treg cells. However, manufacturing of the anti-CD25-F(ab’)2 is laborious and costly especially for clinical therapy usage. Currently, two anti-CD25 monoclonal antibodies have been approved by the FDA, daclizumab and basiliximab; both of which block IL-2 binding and are used for immunosuppression in organ transplantation.16,17 If anti-CD25-IgG based NIR-PIT is effective despite of these concerns, it would be relatively straightforward to translate to the oncology clinic. The aim of this study, therefore, was to evaluate the efficacy of anti-CD25-IgG compared to anti-CD25-F(ab’)2 in NIR-PIT.
Results
Results of APC synthesis of IgG-IR700 and F(ab’)2-IR700
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) showed anti-CD25-IgG-IR700 and non-conjugated anti-CD25-IgG had approximately the same molecular weight. IR700 fluorescence of anti-CD25-IgG-IR700 was confirmed (Figure. 1A). Flow cytometry showed CD25 expressing HT-2-A5E cells had fluorescent signals after they were incubated with anti-CD25-IgG-IR700 (Figure. 1B). These fluorescence signals were completely blocked with excess non-conjugated anti-CD25-IgG. Similar results were obtained with F(ab’)2-IR700.
Figure 1. Conjugation of IR700 to anti-CD25-IgG, and evaluation of in vitro NIR-PIT.
A. Evaluation of anti-CD25-IgG-IR700 by SDS-PAGE (left: Colloidal Blue staining, right: 700 nm fluorescence). Diluted anti-CD25-IgG was used as a control. B. HT-2-A5E cells showed enhanced fluorescence signal after incubation with anti-CD25-IgG-IR700. C. Microscopic NIR-PIT with anti-CD25-IgG-IR700. NIR light exposure induced immediate necrotic cell death. D. In vitro NIR-PIT. NIR light exposure alone did not induce cell death. E. Anti-CD25-F(ab’)2-PIT induced more cell death than anti-CD25-IgG-PIT (n = 3, *p < 0.01, unpaired t-test).
In vitro NIR-PIT with anti-CD25-F(ab’)2-PIT induces more efficient target cell destruction
In order to assess the efficacy of target cell destruction, HT-2-A5E cells were incubated with anti-CD25-IgG-IR700 then exposed to NIR light. Anti-CD25-IgG-IR700-bound HT-2-A5E cells showed immediate cellular swelling, bleb formation, and rupture of cell membranes after NIR light exposure (Figure. 1C). The efficacy of NIR-PIT was measured quantitatively by flow cytometry as the frequency of cell death detected by Propidium Iodide (PI) staining. First, we checked therapeutic effect against MC38-luc cells. The percentage of dead cells did not increase after either type of anti-CD25 NIR-PIT (Figure S1). Second, we investigated CD25 expressing HT-2-A5E cells. Without APC binding, there was no increase in cell death. This result confirmed the absence of cytotoxicity from NIR light exposure alone (Figure 1D). Next, we compared efficacies of in vitro NIR-PITs with anti-CD25-F(ab’)2-IR700 and anti-CD25-IgG-IR700. In both treatments, the percentage of dead cells increased in a light dose dependent manner. When the light dose was higher than 4 J/cm2, NIR-PIT with anti-CD25-F(ab’)2-IR700 killed target cells more effectively than treatment with anti-CD25-IgG-IR700 (Figure 1E). We suspected this difference was caused by the difference in the number of IR700 molecules conjugated to anti-CD25-F(ab’)2 and IgG. On average four IR700 molecules were conjugated to anti-CD25-F(ab’)2, while on average only three IR700s were conjugated to anti-CD25-IgG. To normalize for the effect of the number of conjugated IR700 molecules, the efficacy of anti-CD25-F(ab’)2 conjugated with three-IR700 was also evaluated. No significant difference between the efficacy of NIR-PIT with three-IR700-conjugated anti-CD25-F(ab’)2 and that of anti-CD25-IgG-IR700 was observed after 64 J/cm2 of NIR light exposure (Figure S2A). This result suggested that efficacy of target cell destruction may be related to the number of IR700 conjugated to anti-CD25 antibodies.
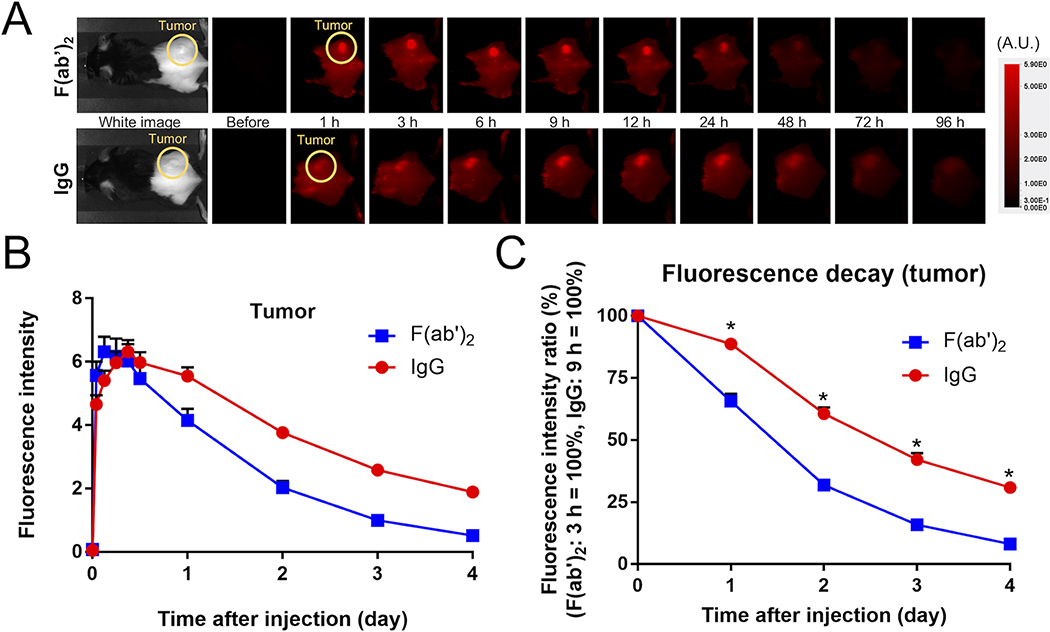
In vivo fluorescence imaging showed slower clearance of anti-CD25-IgG-IR700
To compare the clearances of the anti-CD25-F(ab’)2-IR700 and the anti-CD25-IgG-IR700, each APC was injected into tumor bearing mice and serial fluorescence images were obtained. Both APCs showed rapid accumulation within tumors. Groups of mice exposed to anti-CD25-F(ab’)2-IR700 and anti-CD25-IgG-IR700 exhibited peak average fluorescence at 3 h and 9 h after injection, respectively (Figure 2A). Fluorescence of anti-CD25-IgG-IR700 decreased more slowly than that of anti-CD25-F(ab’)2-IR700 indicating slower clearance (Figure 2B). Fluorescence decay curves of tumor sites verified the more rapid clearance of anti-CD25-F(ab’)2-IR700 (Figure 2C).
Figure 2. In vivo fluorescence imaging.
A. Serial 700 nm fluorescence images were acquired. Fluorescence intensities were evaluated at the tumor. B. Fluorescence intensity of tumor. Anti-CD25-F(ab’)2-IR700 showed its peak at 3 h after injection, whereas anti-CD25-IgG-IR700 had its peak at 9 h after injection. C. Fluorescence decay curve at tumor site. Fluorescence intensities of tumor sites after 1 day were divided by the peak values. Anti-CD25-F(ab’)2-IR700 showed faster decay compared with anti-CD25-IgG-IR700 (n = 10, *p < 0.0001, unpaired t-test).
Anti-CD25-IgG-PIT is less effective than anti-CD25-F(ab’)2-PIT in a unilateral tumor murine model
The treatment efficacy of anti-CD25-F(ab’)2-PIT and anti-CD25-IgG-PIT were compared in vivo in a unilateral tumor model. The treatment and imaging regimen are shown in Figure 3A. Accumulation of APCs was confirmed with fluorescence imaging (Figure 3B). After exposure to 50 J/cm2 of NIR light, IR700 fluorescence signals of tumors decreased in both NIR-PIT groups consistent with photobleaching indicating adequate light exposure. The early treatment effect was evaluated with luciferase activity (Figure 3C). No significant difference was shown between the two treatments by bioluminescence imaging (BLI) within the first 5 days after NIR-PIT (Figure 3D). However, tumor growth was significantly inhibited in both NIR-PIT groups after 5 days, while anti-CD25-IgG-IR700 without NIR exposure did not affect tumor growth (p < 0.05 (IgG-PIT group), p < 0.01 (F(ab’)2-PIT group), vs. Control group; Figure 3E). Conversely, the treatment effect of the F(ab’)2-PIT group was significantly greater than that of the IgG-PIT group (p < 0.05; Figure 3E) and the F(ab’)2-PIT group achieved significantly prolonged survival compared to the IgG-PIT group (Figure 3F). Intratumoral Treg depletion was validated with flow cytometry. After 24 hours of NIR light exposure, both NIR-PIT groups successfully and comparably depleted intratumoral Tregs whereas injection of anti-CD25-IgG-IR700 without light exposure did not affect Treg population (Figure 3G). In order to test whether the difference of tumor suppression efficacy was due to the difference in the number of conjugated IR700 molecules, we compared treatment efficacy between anti-CD25-IgG-PIT and anti-CD25-F(ab’)2-PIT both of which were conjugated with three IR700s. Even with an equal number of conjugated IR700 molecules, anti-CD25-F(ab’)2-PIT suppressed tumor growth more effectively than IgG-PIT (Figure S2B). This result suggested that the factor contributing to the higher tumor suppression with anti-CD25-F(ab’)2-PIT was not the number of the conjugated IR700s but is related to differences in clearance. We hypothesized that the remaining anti-CD25-IgG-IR700 may block the binding of IL-2 to IL-2 receptors on the activated effector cells. To assess if IL-15, an alternative T cell stimulator, could reduce the negative effect of blockade of IL-2 receptors by remaining anti-CD25-IgG-IR700, the efficacy of combination therapy of anti-CD25-IgG-PIT and IL-15 was evaluated. The combination therapy showed a superior effect to either single therapy group (p <0.01, vs. other groups; Figure S3).
Figure 3. In vivo effect of NIR-PIT in unilateral tumor model.
A. NIR-PIT regimen and imaging schedule are shown. B. Fluorescence images. NIR-light-exposed tumors showed immediate fluorescence decay. C. Bioluminescence images were obtained serially. D. Quantitative luciferase activity showed no significant difference between any two groups in 5 days after NIR-PIT (n = 9–10, one-way ANOVA). E. Both PIT groups suppressed tumor growth (n = 9–10, *p < 0.05, **p < 0.01, vs. Control group, one-way ANOVA followed by Tukey’s test). Further, F(ab’)2-PIT group suppressed tumor growth more strongly than IgG-PIT group (n = 10, ***p < 0.05, unpaired t-test). F. F(ab’)2-PIT group achieved significantly prolonged survival, compared with IgG-PIT group (n = 10, *p < 0.05, log-rank test). G. Evaluation of intratumoral Treg depletion one day after light exposure. Both NIR-PIT groups successfully and comparably depleted intratumoral Tregs (n = 3, *p < 0.05, one-way ANOVA followed by Tukey’s test).
Anti-CD25 F(ab’)2-PIT is more effective than anti-CD25-IgG-PIT in inducing responses in unexposed contralateral tumors in a bilateral tumor model
One of the advantages of CD25-targeted NIR-PIT is that the therapeutic effect is not limited to the immediate site of PIT. This is because newly activated T cells not only attack tumors near the site of NIR-PIT, but also migrate throughout the body and attack tumors of similar type elsewhere. In order to compare the therapeutic efficacy of anti-CD25-F(ab’)2-PIT and anti-CD25-IgG-PIT on tumors not receiving light, NIR-PIT was performed in a bilateral tumor model in which one tumor was exposed to NIR light and the other was not.
The treatment and imaging regimen are shown in Figure 4A. Local NIR light exposure was administered only to the right-sided tumor (NIR (+) tumor). The left-sided tumor was covered with aluminum foil during the NIR-light exposure (NIR (−) tumor). After exposure to 50 J/cm2 of NIR light, the fluorescence signal of the NIR (+) tumor was decreased in both NIR-PIT groups (Figure 4B) while the fluorescence in the contralateral tumor was unchanged. Luciferase activity and tumor growth were compared in 4 tumor groups; the NIR (+)/(−) tumors of the F(ab’)2-PIT/IgG-PIT groups (Figure 4C). No significant difference was shown between any tumor groups by BLI within 5 days of NIR-PIT (Figure 4D). However, tumor growth was significantly inhibited in the F(ab’)2-PIT group compared with the IgG-PIT group for both NIR (+) tumors but also for NIR (−) tumors (p < 0.01 (both side), vs. IgG-PIT group; Figure 4E). No significant difference was observed between the NIR (+) and NIR (−) tumor in each same group. Also, significantly prolonged survival of mice was achieved in the F(ab’)2-PIT group (p < 0.01, vs. IgG-PIT group; Figure 4F).
Figure 4. In vivo effect of NIR-PIT in bilateral tumor model.
A. NIR-PIT regimen and imaging schedule are shown. B. Fluorescence images. NIR light was exposed to only right tumor. Left tumor was covered with aluminum foil. After NIR light exposure, only right tumor showed fluorescence decay, while fluorescence of left tumor was remained. C. Bioluminescence images were obtained serially. D. Quantitative luciferase activity showed no significant difference between any two groups in 5 days after NIR-PIT (n = 10, one-way ANOVA). E. Tumor growths of the both sides were significantly suppressed in the F(ab’)2-PIT group compared with IgG-PIT group (n = 10, *p < 0.01, vs. same side of IgG-PIT group, one-way ANOVA followed by Tukey’s test). F. F(ab’)2-PIT group achieved significantly prolonged survival compared with IgG-PIT group (n = 10, *p < 0.01, log-rank test).
Post NIR-PIT histology reveals increased number of tumor infiltrating lymphocytes (TILs) with anti-CD25-F(ab’)2-PIT
Tumor infiltrating lymphocytes (TILs) are cytotoxic effector T cells and their density is correlated with effective tumor killing. The presence of TILs was compared for animals receiving anti-CD25-F(ab’)2-PIT or anti-CD25-IgG-PIT with multiplex immunohistochemical analysis.
Seven days after NIR light exposure, tumors from each PIT group were extracted. H&E staining showed more hematopoietic cells inside tumor tissue in the F(ab’)2-PIT group than the IgG-PIT group suggesting more TILs (Figure 5A). Increased infiltration of TIL was verified with multiplex immunohistochemical staining (Figure 5B). More lymphocytes infiltrated the tumor in the F(ab’)2-PIT group than the IgG-PIT group. Quantitative assessment was also performed (Figure 5C, S4). Tissue was characterized as stroma, tumor, and necrosis. CD8+ cells, CD4+Foxp3- cells, and CD4+Foxp3+ Treg cells were counted in each tissue component. F(ab’)2-PIT accumulated more CD8+ cells within the tumor than IgG-PIT (p <0.05; Figure 5C).
Figure 5. Histological analysis.
Tumors were extracted 7 days after NIR-PITs. A. H&E staining. The scale bars represent 100 μm. In F(ab’)2-PIT group, more cells with hematopoietic cell like morphology were seen inside tumor tissue. B. Lymphocyte distributions were assessed with Opal multiplex IHC. CD8, CD4 and Foxp3 were shown in magenta, green and yellow respectively. Anti-pan-cytokeratin (CK; shown in cyan) was used to mark tumor tissue. C. Tumor infiltrating lymphocytes (TILs) were counted within stroma and tumor. More CD8+ cells infiltrated tumor in the F(ab’)2-PIT group than in the other groups (n = 3, *p < 0.05, vs. other groups, one-way ANOVA followed by Tukey’s test).
Discussion
IL-2, a 15.5 kDa cytokine, is primarily produced by CD4+ T cells following antigen stimulation but is also produced, to a lesser extent, by other cells.18 IL-2 promotes proliferation of T cells and is also a key growth factor for NK cells.19 IL-2 interacts with three types of IL-2 receptor; low-, intermediate-, and high-affinity receptors.20 CD25, also known as IL-2 receptor α-chain (IL-2Rα), is a low-affinity IL-2 receptor but forms a high-affinity IL-2 receptor with CD122 (IL-2Rβ) and CD132 (IL-2Rγ).21 The intermediate-affinity IL-2 receptor is made up of CD122 and CD132 but lacks CD25. Intermediate-affinity IL-2 receptors are present on resting CD8+ T cells and NK cells, whereas low- and high-affinity IL-2 receptors are expressed on activated lymphocytes.18,21 Importantly, Treg cells constitutively express high-affinity IL-2 receptors.22 Therefore, prior to PIT, CD25-targeted conjugates preferentially bind to intra-tumoral Treg cells and subsequent NIR-PIT results in their selective depletion in the treated tumor bed. However, the effect of removing Treg cells is to activate CD8+ cells whereupon they will express high affinity receptors for CD25. Remaining circulating anti-CD25-mAb-IR700 could block these receptors hindering T cell expansion.
This study demonstrates that anti-CD25-F(ab’)2-PIT was more effective than anti-CD25-IgG-PIT in killing tumors and inducing abscopal effects in vivo. Although anti-CD25-F(ab’)2-PIT induced cell death more effectively in the initial in vitro study, NIR-PITs with anti-CD25-F(ab’)2-IR700 and anti-CD25-IgG-IR700 showed comparable in vitro effects after equalizing the number of conjugated IR700s to three. Even when the number of IR700 molecules per antibody was the same, in vivo results favored the conjugate based on F(ab’)2. Accumulation of both APCs within tumors was confirmed with in vivo fluorescence suggesting that sufficient numbers of Treg cells existed within the tumors to act as a target. Anti-CD25-F(ab’)2-IR700 showed faster fluorescence decay at tumor sites than anti-CD25-IgG-IR700 because it has a shorter biologic half-life than IgG.23,24 In the current study, the tumoral fluorescence of anti-CD25-F(ab’)2-IR700 and anti-CD25-IgG-IR700 decreased to 65.6% and 88.7% respectively, on average, within 24 h after injection.
The faster clearance of anti-CD25-F(ab’)2-IR700 and the absence of ADCC or CDC might be considered a disadvantage in treating tumors. However, anti-CD25-F(ab’)2-PIT proved superior to anti-CD25-IgG-PIT in vivo. Although NIR-PIT with either APC showed efficacy in tumor treatment compared with the control group, the efficacy of tumor treatment with anti-CD25-IgG-PIT was lower than that of anti-CD25-F(ab’)2-PIT. Interestingly, flow cytometry revealed that these two therapies deplete the intratumoral Treg cells approximately equally at one day after light exposure. Moreover, BLI demonstrated that there was no significant difference between the two PIT groups in the first 5 days after PIT. However, after 5 days the differences between the two agents became more striking. This timing corresponds to the known maximal activation of T cells in the tumor microenvironment. After several days, effector T cells start expressing CD25. The shorter half-life of anti-CD25-F(ab’)2-IR700 is advantageous because less antibody remains in the circulation resulted in less blocking of IL-2 receptor on activated T cells. Furthermore, anti-CD25-IgG-IR700 may induce ADCC/CDC on effector T cells after they express CD25 further muting the immune response. These factors favor anti-CD25-F(ab’)2 based NIR-PIT over the full antibody. Histological findings also supported this story. In the anti-CD25-F(ab’)2-PIT group, greater numbers CD8+ TILs infiltrated tumor compared to the IgG-PIT group 7 days after NIR-PIT. Anti-CD25-IgG-IR700 could inhibit proliferating effector cells that were newly primed in the local tumor beds or peripheral lymphoid organs. We evaluated IL-15, an alternative T cell activator which is unrelated to CD25. When IL-15 was administrated after anti-CD25-IgG-PIT, tumor growth was significantly more suppressed compared to anti-CD25 IgG-PIT alone. Although further research is needed to elucidate the mechanism of action of those therapies, the disadvantage of the anti-CD25-IgG-PIT could be reduced by administration of IL-15.
Abscopal effect of F(ab’)2-PIT was also superior to that of IgG-PIT, which was confirmed in the bilateral tumor model. This is quite reasonable because F(ab’)2-PIT recruited much more CD8+ T cells than IgG-PIT, as was shown in multiplex immunohistochemical study; proliferated CD8+ T cells should attack the distant tumor cells. Our previous study demonstrated that CD25-targeted NIR-PIT induced shrinkage of shaded distant tumor without depletion of the Treg cells within that non-treated tumor by showing immunological analysis.15 Although the possibility of the light leakage to the contralateral tumor could be not denied completely, it is unlikely because the IR700 fluorescence of the contralateral tumors did not change after the light exposure.
The intravenous injection of anti-CD25-IgG-IR700 by itself did not show anti-tumor effects. The anti-CD25 antibody clone PC61 (rat IgG1), which is used in this study, depletes Treg cells in peripheral blood, spleen, and lymph nodes in the mouse model.25 Although this antibody is effective in tumor suppression when it is administered before or soon after tumor inoculation, it failed to delay growth or prolong survival against established tumors,26–29 which is consistent with our results. This is because the Fc fragment of the rat IgG1 fails to induce sufficient ADCC within the murine tumor environment. PC61 successfully depletes intra-tumoral Treg cells and shows anti-tumor effect when its Fc fragment is changed to that of the murine IgG2a.14 In other words, depletion of Treg cells at tumor sites induces an anti-tumor effect. PC61 is used only for recognizing and binding to the surface of the target cell; ADCC/CDC is not necessary. Therefore, anti-CD25 (PC61-clone) based NIR-PIT was effective without modifying the Fc fragment of the antibody.
In the current study, some mice, in both the unilateral and bilateral tumor models, showed a complete response after NIR-PIT. These results surpass that of a previous report,15 even though lower doses of light (50 J/cm2 vs. 100 J/cm2) were used in the present study. The major difference between these two studies was the NIR-light source; Light emitting diodes (LEDs) were used as the NIR light source in the previous study, whereas laser light was used for this study. Laser light reportedly induces stronger NIR-PIT effects than LEDs as the light spectrum is more narrowly matched to that of IR700 absorbance whereas LED light does not have sharp a peak at 700nm.30 Shorter exposure time is also an advantage of the laser system. In this research, NIR light exposure of 50 J/cm2 took only 5.5 min with the laser system, approximately one third of the time for the same dose by LEDs. This rapid depletion of Treg happens faster than the ability of the tumor to restore Treg from the circulation resulting in superior immune-activation. Thus, laser systems will be the best light source for NIR-PIT for human trials.
In preclinical settings, NIR-PIT has been shown to be effective with a variety of different antibodies.11,31–34 However, most of these treatments targeted the cancer cells themselves rather than cells in the tumor microenvironment. CD25-targeted NIR-PIT, which rapidly and locally destroys CD25 expressing Treg cells, will be applicable to many different tumor types and could, therefore, become a versatile cancer treatment. Moreover, combination therapy of tumor-cell-targeted NIR-PIT and CD25-targeted therapy would be ideal to enhance therapeutic efficacy.
Conclusion
Anti-CD25-F(ab’)2-IR700 demonstrated superior killing efficacy to anti-CD25-IgG-IR700 based NIR-PIT in vivo. The faster clearance of the F(ab’)2 fragment contributed to the therapeutic efficacy of CD25-targeted NIR-PIT by minimizing circulating APCs with their ability to suppress activated T cells by blocking the IL-2 receptor. This method holds promise, not only for local therapies but also for inducing systemic responses by stimulating T cell immunity and will be even more effective when combined with tumor-directed NIR-PIT.
Materials and methods
Reagents
A water soluble, silica-phthalocyanine derivative IRDye 700DX NHS ester was obtained from LI-COR Biosciences (Lincoln, NE, USA). Anti-mouse CD25 antibody (PC-61.5.3) was purchased from Bio X Cell (West Lebanon, NH, USA). All other chemicals were of reagent grade.
Creation of anti-CD25-F(ab’)2
Anti-CD25-F(ab’)2 was generated from anti-CD25-IgG (PC-61.5.3), as previously described.15
Synthesis of IR700-conjugated anti-CD25-IgG/F(ab’)2
Anti-CD25-IgG (1 mg, 6.8 nmol) or anti-CD25-F(ab’)2 (733 μg, 6.8 nmol) was incubated with IR700 NHS ester (66.8 μg, 34.2 nmol) in phosphate buffer (pH 8.5) at room temperature for 1 h. The mixture was purified with a Sephadex G25 column (PD-10; GE Healthcare, Piscataway, NJ, USA). The concentration of IR700 was determined with absorption at 689 nm using UV-Vis (8453 Value System; Agilent Technologies, Santa Clara, CA, USA). The protein concentration was confirmed with Coomassie Plus protein assay kit (Thermo Fisher Scientific, Rockford, IL, USA) by measuring the absorption at 595 nm. The number of IR700 per IgG/F(ab’)2 was calculated from division of those values. With this method, an average of three IR700 molecules were bound to one anti-CD25-IgG, whereas four molecules were bound to a single anti-CD25-F(ab’)2. For some studies, three-IR700-conjugated anti-CD25-F(ab’)2 was also made. Success of SDS-PAGE with a 4–20% gradient polyacrylamide gel (Life Technologies, Gaithersburg, MD, USA). Non-conjugated antibody and ultrapure water were used for positive and negative controls, respectively. After electrophoresis at 80 V for 2.5 h, the gel was observed with a Pearl Imager (LI-COR Biosciences) using the 700 nm fluorescence channel. The gel was then stained with Colloidal Blue to compare the molecular weight of the conjugate to that of non-conjugated antibody.
Cell culture
Luciferase-expressing MC38 (murine colon cancer, MC38-luc) cells were established as previously described.15 CD25-expressing murine T lymphocyte HT-2 clone A5E (HT-2-A5E) cells were obtained from ATCC (Manassas, VA, USA). MC38-luc cells were cultured in RPMI 1640 medium (Thermo Fisher Scientific) supplemented with 10% fetal bovine serum, 100 IU/mL penicillin, and 100 μg/mL streptomycin (Thermo Fisher Scientific). For HT-2-A5E cells, 0.05 mM 2-mercaptoethanol and 0.1 nM human IL-2 were also added. Cells were cultured in a humidified incubator at 37°C in an atmosphere of 95% air and 5% CO2.
Cell-specific binding analysis
To verify specific binding of anti-CD25-IgG-IR700 to HT-2-A5E cells, fluorescence from the cells was evaluated with a flow cytometer (FACSCalibur, BD Biosciences, San Jose, CA, USA) and CellQuest software (BD Biosciences). HT-2-A5E cells (1 × 105) were incubated with 10 μg/mL of anti-CD25-IgG-IR700 for 1 h at 37°C. After washing with phosphate buffered saline (PBS), fluorescence of the cells was analyzed. To confirm the specific binding of the anti-CD25-IgG-IR700, 10 times the amount of non-conjugated anti-CD25-IgG was added to some samples 1 hour prior to the administration of the APC.
Fluorescence microscopy
The effect of anti-CD25-IgG-IR700 NIR-PIT was evaluated with a fluorescence microscope (IX81; Olympus America, Melville, NY, USA). Anti-CD25-IgG-IR700 (10 μg/mL) was incubated with 400,000 HT-2-A5E cells for 1 h at 37°C. After washing with PBS, the cells were observed with a microscope and transmitted light differential interference contrast (DIC) images were taken. IR700 fluorescence was detected with a 590–650 nm excitation filter and a 665–740 nm band pass emission filter. The cells were then exposed to NIR light (690 nm, 150 mW/cm2, 50 J/cm2) using ML7710 laser system (Modulight, Tampere, Finland). DIC images were acquired again after light exposure.
In vitro NIR-PIT
The cytotoxic effects of anti-CD25-IgG-IR700 and anti-CD25-F(ab’)2-IR700 were quantitatively evaluated with flow cytometry. Anti-CD25-IgG-IR700 (64 pmol/mL, 10 μg/mL) or anti-CD25-F(ab’)2-IR700 (64 pmol/mL, 7.6 μg/mL) was incubated with 400,000 HT-2-A5E cells for 1 h at 37°C. After washing with PBS, phenol-red-free medium was added. NIR light (150 mW/cm2) was applied at 0, 1, 4, 16, and 64 J/cm2. One hour after treatment, cells were stained with 1 μg/mL PI. The percentage of PI-stained cells was analyzed by flow cytometry.
Animals and tumor models
All in vivo procedures were conducted in compliance with the Guide for the Care and Use of Laboratory Animal Resources (1996), US National Research Council, and approved by the local Animal Care and Use Committee. Six- to eight-week-old female C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Six million MC38-luc cells were inoculated into the dorsum of the mice. Bilateral dorsal tumors were established in an experiment. The hair overlying the tumor site was removed for NIR-light exposure and imaging studies. For determination of tumor volume, the greatest longitudinal diameter (length) and the greatest transverse diameter (width) were measured with a caliper. Tumor volume was calculated as follow; tumor volume = length × width2 × 0.5. Tumor volumes were measured three times a week until the length reached 2 cm, whereupon the mice were euthanized with CO2.
In vivo fluorescence imaging
Anti-CD25-IgG-IR700 (0.64 nmol, 100 μg) or anti-CD25-F(ab’)2-IR700 (0.64nmol, 76 μg) was injected into a lateral tail vein 5 days after inoculation of tumor. Serial dorsal fluorescence images of IR700 were obtained with the 700 nm fluorescence channel of the Pearl Imager (LI-COR Biosciences). The images were taken before and 1, 3, 6, 9, 12, 24, 48, 72, 96 hours after intravenous injection of the conjugate. Pearl Cam Software (LI-COR Biosciences) was used for analyzing fluorescence. Regions of interest (ROIs) were placed on the tumor.
In vivo NIR-PIT
To evaluate the efficacy of the treatments, tumor-bearing mice were randomized into 4 groups as follows: (1) no treatment (Control); (2) 0.64 nmol (100 μg) of anti-CD25-IgG-IR700 i.v., no NIR light exposure (IgG i.v.); (3) 0.64 nmol (76 μg) of anti-CD25-F(ab’)2-IR700 i.v., NIR light (690 nm, 150 mW/cm2, 50 J/cm2) was administered on day 1 (F(ab’)2-PIT); (4) 0.64nmol (100 μg) of anti-CD25-IgG-IR700 i.v., NIR light (690 nm, 150 mW/cm2, 50 J/cm2) was administered on day 1 (IgG-PIT). Injections of APCs were performed 5 days after tumor inoculation for mice bearing one tumor, or 4 days for mice bearing two tumors. Acute effects of the treatments were evaluated with BLI). For BLI, 200 μL of 15 mg/mL D-luciferin (Gold Biotechnology, St. Louis, MO, USA) was injected intraperitoneally, and mice were analyzed with a BLI system (Photon Imager; Biospace Lab, Paris, France) and M3 Vision Software (Biospace Lab) for luciferase activity.35 ROIs were set on the entire tumors.
Flow-cytometric analysis of tumor-infiltrating Treg after each treatment
Single-cell suspensions of tumors were prepared for Flow cytometry. Tumors of each treatment group were harvested 24 hours after NIR light exposure then digested with collagenase type IV (1 mg/mL, Thermo Fisher Scientific) and DNase I (20 μg/mL, Millipore Sigma, Burlington, MA, USA). Tumors were then gently dissociated and filtered with 70 μm cell strainer (Corning, Corning, NY, USA). The cells were stained with antibodies against CD3e (145–2C11; BioLegend, San Diego, CA, USA) and CD4 (RM4–5; Thermo Fisher), followed by fixation and permeabilization with Intracellular Fixation & Permeabilization Buffer Set (Thermo Fisher Scientific) and incubation with anti-Foxp3 antibody (FJK-16s; Thermo Fisher). The stained cells were evaluated with a flow cytometer and the data were analyzed with the FlowJo software (FlowJo LLC, Ashland, OR, USA).
Histological analysis
Histological changes after NIR-PIT were evaluated. Tumors were extracted 7 days after each NIR-PIT. Extracted tumors were then fixed with 10% formalin, embedded in paraffin, thinly sliced, stained with Hematoxylin and Eosin (H&E) staining.
The multiplex IHC is performed with Opal 7-Color Automation IHC Kit (Akoya Bioscience, Hopkinton, MA, USA) and BOND Rxm automated stainer (Leica Biosystems; Wetzlar, Germany) using the following antibodies; anti-pan-cytokeratin (CK) (rabbit poly; Bioss, Woburn, MA, USA), anti-CD4 (EPR19514; Abcam, Cambridge, UK), anti-CD8α (EPR20305; Abcam) and anti-Foxp3 (1054C; Novus Biologicals, Centennial, CO).
The slides were imaged in the Mantra Quantitative Pathology Workstation (PerkinElmer, Waltham, MA) and analyzed with inForm Cell Analysis software (PerkinElmer).
Statistical analysis
Data are expressed as means ± s.e.m., unless otherwise indicated. Statistical analysis was performed with GraphPad Prism (GraphPad Software, La Jolla, CA, USA). For two-group comparison, an unpaired t-test was used. For multiple-group comparison, a one-way analysis of variance (ANOVA) followed by Tukey’s test was used. The cumulative probability of survival based on tumor length (2 cm) was estimated with the Kaplan-Meier survival curve analysis, and the results were compared with log-rank test. p-value of less than 0.05 was considered significant.
Supplementary Material
Acknowledgements
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research (ZIA BC 011513). FI was also supported with a grant from National Center for Global Health and Medicine Research Institute, Tokyo, Japan.
Abbreviations
- ADCC
antibody-dependent cellular cytotoxicity
- APC
antibody-photoabsorber conjugate
- BLI
bioluminescence imaging
- CDC
complement-dependent cytotoxicity
- IR700
IRDye700DX
- NIR-PIT
near-infrared photoimmunotherapy
- PI
Propidium Iodide
- SDS-PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TIL
tumor infiltrating lymphocytes
- Treg
regulatory T
Footnotes
Conflict of interest
There is no conflict of interest on this work to be disclosed.
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI:
References
- (1).Chen DS, and Mellman I (2013) Oncology meets immunology: the cancer-immunity cycle. Immunity 39, 1–10. [DOI] [PubMed] [Google Scholar]
- (2).Yang Y (2015) Cancer immunotherapy: harnessing the immune system to battle cancer. The Journal of clinical investigation 125, 3335–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Smyth MJ, Ngiow SF, Ribas A, and Teng MW (2016) Combination cancer immunotherapies tailored to the tumour microenvironment. Nature reviews. Clinical oncology 13, 143–58. [DOI] [PubMed] [Google Scholar]
- (4).Nishikawa H, and Sakaguchi S (2014) Regulatory T cells in cancer immunotherapy. Current opinion in immunology 27, 1–7. [DOI] [PubMed] [Google Scholar]
- (5).Tanaka A, and Sakaguchi S (2017) Regulatory T cells in cancer immunotherapy. Cell research 27, 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Sharabi A, Tsokos MG, Ding Y, Malek TR, Klatzmann D, and Tsokos GC (2018) Regulatory T cells in the treatment of disease. Nature reviews. Drug discovery. [DOI] [PubMed] [Google Scholar]
- (7).Takeuchi Y, and Nishikawa H (2016) Roles of regulatory T cells in cancer immunity. International immunology 28, 401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Taguchi O, and Takahashi T (1996) Administration of anti-interleukin-2 receptor alpha antibody in vivo induces localized autoimmune disease. European journal of immunology 26, 1608–12. [DOI] [PubMed] [Google Scholar]
- (9).Facciabene A, Motz GT, and Coukos G (2012) T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer research 72, 2162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Caspi RR (2008) Immunotherapy of autoimmunity and cancer: the penalty for success. Nature reviews. Immunology 8, 970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, and Kobayashi H (2011) Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nature medicine 17, 1685–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Sato K, Ando K, Okuyama S, Moriguchi S, Ogura T, Totoki S, Hanaoka H, Nagaya T, Kokawa R, Takakura H, et al. (2018) Photoinduced Ligand Release from a Silicon Phthalocyanine Dye Conjugated with Monoclonal Antibodies: A Mechanism of Cancer Cell Cytotoxicity after Near-Infrared Photoimmunotherapy. ACS Central Science 4, 1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Ogawa M, Tomita Y, Nakamura Y, Lee MJ, Lee S, Tomita S, Nagaya T, Sato K, Yamauchi T, Iwai H, et al. (2017) Immunogenic cancer cell death selectively induced by near infrared photoimmunotherapy initiates host tumor immunity. Oncotarget 8, 10425–10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Arce Vargas F, Furness AJS, Solomon I, Joshi K, Mekkaoui L, Lesko MH, Miranda Rota E, Dahan R, Georgiou A, Sledzinska A, et al. (2017) Fc-Optimized Anti-CD25 Depletes Tumor-Infiltrating Regulatory T Cells and Synergizes with PD-1 Blockade to Eradicate Established Tumors. Immunity 46, 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Sato K, Sato N, Xu B, Nakamura Y, Nagaya T, Choyke PL, Hasegawa Y, and Kobayashi H (2016) Spatially selective depletion of tumor-associated regulatory T cells with near-infrared photoimmunotherapy. Science translational medicine 8, 352ra110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Nashan B, Moore R, Amlot P, Schmidt AG, Abeywickrama K, and Soulillou JP (1997) Randomised trial of basiliximab versus placebo for control of acute cellular rejection in renal allograft recipients. CHIB 201 International Study Group. Lancet (London, England) 350, 1193–8. [DOI] [PubMed] [Google Scholar]
- (17).Vincenti F, Kirkman R, Light S, Bumgardner G, Pescovitz M, Halloran P, Neylan J, Wilkinson A, Ekberg H, Gaston R, et al. (1998) Interleukin-2-receptor blockade with daclizumab to prevent acute rejection in renal transplantation. Daclizumab Triple Therapy Study Group. The New England journal of medicine 338, 161–5. [DOI] [PubMed] [Google Scholar]
- (18).Liao W, Lin JX, and Leonard WJ (2013) Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 38, 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Gaffen SL, and Liu KD (2004) Overview of interleukin-2 function, production and clinical applications. Cytokine 28, 109–23. [DOI] [PubMed] [Google Scholar]
- (20).Waldmann TA (1993) The IL-2/IL-2 receptor system: a target for rational immune intervention. Trends in pharmacological sciences 14, 159–64. [DOI] [PubMed] [Google Scholar]
- (21).Spolski R, Li P, and Leonard WJ (2018) Biology and regulation of IL-2: from molecular mechanisms to human therapy. Nature reviews. Immunology 18, 648–659. [DOI] [PubMed] [Google Scholar]
- (22).Sakaguchi S, Sakaguchi N, Asano M, Itoh M, and Toda M (1995) Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. Journal of immunology (Baltimore, Md. : 1950) 155, 1151–64. [PubMed] [Google Scholar]
- (23).Saxena A, and Wu D (2016) Advances in Therapeutic Fc Engineering - Modulation of IgG-Associated Effector Functions and Serum Half-life. Frontiers in immunology 7, 580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Wahl RL, Parker CW, and Philpott GW (1983) Improved radioimaging and tumor localization with monoclonal F(ab’)2. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 24, 316–25. [PubMed] [Google Scholar]
- (25).Setiady YY, Coccia JA, and Park PU (2010) In vivo depletion of CD4+FOXP3+ Treg cells by the PC61 anti-CD25 monoclonal antibody is mediated by FcgammaRIII+ phagocytes. European journal of immunology 40, 780–6. [DOI] [PubMed] [Google Scholar]
- (26).Golgher D, Jones E, Powrie F, Elliott T, and Gallimore A (2002) Depletion of CD25+ regulatory cells uncovers immune responses to shared murine tumor rejection antigens. European journal of immunology 32, 3267–75. [DOI] [PubMed] [Google Scholar]
- (27).Jones E, Dahm-Vicker M, Simon AK, Green A, Powrie F, Cerundolo V, and Gallimore A (2002) Depletion of CD25+ regulatory cells results in suppression of melanoma growth and induction of autoreactivity in mice. Cancer immunity 2, 1. [PubMed] [Google Scholar]
- (28).Shimizu J, Yamazaki S, and Sakaguchi S (1999) Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. Journal of immunology (Baltimore, Md. : 1950) 163, 5211–8. [PubMed] [Google Scholar]
- (29).Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, and Nakayama E (1999) Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer research 59, 3128–33. [PubMed] [Google Scholar]
- (30).Sato K, Watanabe R, Hanaoka H, Nakajima T, Choyke PL, and Kobayashi H (2016) Comparative effectiveness of light emitting diodes (LEDs) and Lasers in near infrared photoimmunotherapy. Oncotarget 7, 14324–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Nagaya T, Nakamura Y, Okuyama S, Ogata F, Maruoka Y, Choyke PL, Allen C, and Kobayashi H (2017) Syngeneic Mouse Models of Oral Cancer Are Effectively Targeted by Anti-CD44-Based NIR-PIT Molecular cancer research: MCR 15, 1667–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Nagaya T, Nakamura Y, Sato K, Zhang YF, Ni M, Choyke PL, Ho M, and Kobayashi H (2016) Near infrared photoimmunotherapy with an anti-mesothelin antibody. Oncotarget 7, 23361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Nagaya T, Nakamura Y, Sato K, Harada T, Choyke PL, and Kobayashi H (2016) Near infrared photoimmunotherapy of B-cell lymphoma. Molecular oncology 10, 1404–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Watanabe R, Hanaoka H, Sato K, Nagaya T, Harada T, Mitsunaga M, Kim I, Paik CH, Wu AM, Choyke PL, et al. (2015) Photoimmunotherapy targeting prostate-specific membrane antigen: are antibody fragments as effective as antibodies? Journal of nuclear medicine : official publication, Society of Nuclear Medicine 56, 140–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Maruoka Y, Nagaya T, Nakamura Y, Sato K, Ogata F, Okuyama S, Choyke PL, and Kobayashi H (2017) Evaluation of Early Therapeutic Effects after Near-Infrared Photoimmunotherapy (NIR-PIT) Using Luciferase-Luciferin Photon-Counting and Fluorescence Imaging. Molecular pharmaceutics 14, 4628–4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.