Highlights
-
•
Platypnea-orthodeoxia syndrome (POS) is observed in COVID-19 acute respiratory distress syndrome (ARDS) survivors.
-
•
POS is associated with older age, lower body mass index and varying degrees of dyspnea.
-
•
Arterial to end-tidal carbon dioxide and alveolar to arterial oxygen partial pressure differences were persistently elevated.
-
•
POS is likely a gravitational exacerbation of intrapulmonary shunt in ARDS due to COVID-19 specific changes.
-
•
POS may cause alarm and requires adjustment in the rehabilitation approach during the recovery period.
Keywords: Coronavirus, Critical care, Pneumonia, Respiratory physiology, Rehabilitation
Abstract
Platypnea-orthodeoxia syndrome (POS) is a rare clinical syndrome characterized by orthostatic oxygen desaturation and positional dyspnea from supine to an upright position. We observed POS in 5 of 20 cases of severe 2019 novel coronavirus (COVID-19) pneumonia, which demonstrated persistently elevated shunt fraction even after liberation from mechanical ventilation. POS was first observed during physiotherapy sessions; median oxygen desaturation was 8 % (range: 8–12 %). Affected individuals were older (median 64 vs 53 years old, p = 0.05) and had lower body mass index (median 24.7 vs 27.6 kg/m2, p = 0.03) compared to those without POS. While POS caused alarm and reduced tolerance to therapy, this phenomenon resolved over a median of 17 days with improvement of parenchymal disease. The mechanisms of POS are likely due to gravitational redistribution of pulmonary blood flow resulting in increased basal physiological shunting and upper zone dead space ventilation due to the predominantly basal distribution of consolidative change and reported vasculoplegia and microthrombi in severe COVID-19 disease.
1. Introduction
Platypnea-orthodeoxia syndrome (POS) is a rare clinical syndrome characterized by orthostatic dyspnea and a measurable drop in arterial oxygen saturation of >5 % or a PaO2 >4 mmHg (Agrawal et al., 2017). At our centre, we observed the novel finding of POS in patients with severe 2019 novel coronavirus disease (COVID-19), and, therefore aim to describe the clinical characteristics and outcome of these affected individuals.
2. Methods
We retrospectively reviewed medical records of all severe COVID-19 cases admitted to the intensive care unit (ICU) at the National Centre for Infectious Diseases (NCID), Singapore over a 4-month period (29/01/2020 to 29/05/2020). Only individuals with POS who survived the ICU stay were included for analysis. Patients who did not require invasive mechanical ventilation (IMV) or admitted to the ICU for non-respiratory complications were excluded. The baseline characteristics, physiology, ventilator settings and the outcomes of ICU stay are described and compared to cases without POS. Informed consent and institutional ethics board review were waived under the provision of the Infectious Disease Act, Singapore.
3. Results
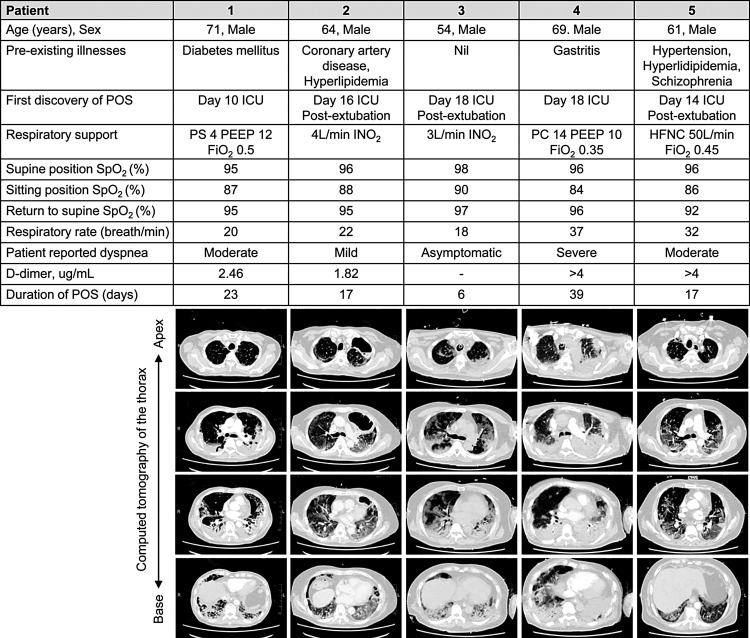
Thirty-five patients received IMV for respiratory failure during the study period: 20 survived, 8 remained in the ICU and 7 died. POS was observed in 5 of 20 survivors; median age (and range) was 64 (54–71) years, body mass index was 24.7 (21.1–26.5) kg/m2 and ICU presentation occurred on Day 7 (Day 1-8) of COVID-19 illness. All cases received lung protective ventilation for acute respiratory distress syndrome (ARDS) and were responsive to incremental positive end-expiratory pressure (PEEP). Baseline characteristics, investigations, ventilator settings, and respiratory physiology are described in Table 1 . CT thorax showed changes of multi-lobar mixed ground glass opacities and consolidation particularly in the posterior lung segments and lower lobes (Fig. 1 ). Other findings were interstitial thickening and early fibrotic change (Patient 1 and 5), cavitary pneumonia (Patient 2; no positive microbiology) and a small segmental pulmonary embolism (Patient 5). Patients 1–3 received lopinavir/ritonavir and interferon-1B therapy while Patients 4 and 5 received remdesivir. Two patients had microbiologically confirmed ventilator-associated pneumonia (Patient 3: Klebsiella pneumoniae and, Patient 4: Enterobacter aerogenes).
Table 1.
Characteristics of COVID-19 ARDS survivors with and without POS.
| Characteristics | POS (n = 5) | No POS (n = 15) | All (n = 20) | p-value |
|---|---|---|---|---|
| Age, years | 64 (54–71) | 53 (35–68) | 61 (35–71) | 0.05 |
| Male, n (%) | 5 (100) | 12 (80) | 17 (85) | 0.54 |
| Body mass index, kg/m2 | 24.7 (21.1–26.5) | 27.6 (13.5–40.2) | 26.4 (13.5–40.2) | 0.03 |
| CCI | 2 (1–4) | 2 (0–7) | 2 (0–7) | 0.82 |
| Day of COVID-19 illness on ICU admission | 7 (1–8) | 6 (2–15) | 7 (1–15) | 0.85 |
| PaO2:FiO2 ratio, mmHg | 100 (49–145) | 114 (50–198) | 110 (49–198) | 0.25 |
| PEEP, cmH2O | 10 (8–12) | 12 (8–14) | 11 (8–14) | 0.12 |
| VTe, mLs/kg IBW | 6.9 (5.8–8.1) | 6.3 (5.3–9.8) | 6.5 (5.3–9.8) | 0.31 |
| NM blockage, n (%) | 3 (60) | 9 (60) | 12 (60) | 0.69 |
| Prone positioning, n (%) | 3 (60) | 7 (47) | 10 (50) | 0.50 |
| Baseline investigation | ||||
| C-reactive protein, mg/L | 249 (135–336) | 137 (56–291) | 141 (56–336) | 0.15 |
| Procalcitonin*, ug/L | 0.39 (0.14-0.57) | 0.28 (0.08–9.79) | 0.29 (0.08–9.79) | 0.85 |
| AST, U/L | 72 (43–146) | 49 (17–161) | 54 (17–161) | 0.22 |
| ALT, U/L | 72 (40–89) | 36 (19–135) | 49 (19–135) | 0.07 |
| LDH, U/L | 752 (632–1981) | 696 (263–1460) | 708 (263–1981) | 0.39 |
| Respiratory physiology | ||||
| Compliance, mL/cmH2O | ||||
| Day 1 of IMV | 42 (31–52) | 37 (23–60) | 38 (23–60) | 0.59 |
| Last day of IMV | 71 (34–127) | 92 (31–169) | 81 (31–169) | 0.76 |
| PaCO2-PETCO2, mmHg | ||||
| Day 1 of IMV | 8 (1–25) | 7 (0–18) | 6.5 (-7.0–25.0) | 0.57 |
| Last day of IMV | 8 (3–11) | 0 (0–6) | 3.0 (-8.0–11.0) | <0.01 |
| PAO2-PaO2, mmHg | ||||
| Before intubation | 522 (272–623) | 469 (158–614) | 506 (158–623) | 0.40 |
| Day 1 of IMV | 146 (99–303) | 202 (32–570) | 196 (32–570) | 0.36 |
| Last day of IMV | 120 (67–132) | 84 (11–158) | 92 (11–158) | 0.12 |
| Following extubation | 155 (131–243) | 54 (26–99) | 66 (26–243) | <0.01 |
| Duration of IMV, days, | 16.0 (6.0–23.0) | 6.0 (3.0–66.0) | 7.5 (3.0–66.0) | 0.10 |
| ICU stay, days | 18.0 (16.0–29.0) | 8.0 (4.0–68.0) | 13.5 (4.0–68.0) | 0.07 |
Data is described as median (range) and count (percentage) for continuous and categorial variables respectively. Groups were compared by the Mann-Whitney U test or Fisher’s exact test where applicable. P-value of < 0.05 was considered statistically significant.
*Procalcitonin was measured on ICU presentation in 3 (60 %) and 14 (93 %) of individuals with POS and without POS respectively.
COVID-19: disease due to 2019 novel coronavirus infection, ARDS: acute respiratory distress syndrome, POS: platypnea-orthodeoxia syndrome, CCI: Charlson comorbidity index, PaO2: arterial partial pressure of oxygen, FiO2: fraction of inspired oxygen, PEEP: positive end-expiratory pressure, VTe: exhaled tidal volume, IBW: ideal body weight, NM: neuromuscular, AST: aspartate transaminase, ALT: alanine transaminase, LDH: lactate dehydrogenase, PaCO2-PETCO2: carbon dioxide partial pressure difference between arterial blood and end-tidal gas, PAO2-PaO2: oxygen partial pressure difference between alveolar gas and arterial blood, IMV: invasive mechanical ventilation, ICU: intensive care unit.
Fig. 1.
Clinical details and CT thorax findings of individuals with platypnea-orthodeoxia syndrome.
POS: platypnea-orthodeoxia syndrome, SpO2: oxygen saturation by pulse oximetry, IMV: invasive mechanical ventilation, ICU: intensive care unit, PSV: pressure-support in cmH2O, PEEP: positive end-expiratory pressure in cmH2O, FiO2: fraction of inspired oxygen, INO2: intranasal supplemental oxygen, PC: pressure-control in cmH2O, HFNC: high flow nasal cannula, ‘-’: not measured.
POS was discovered during physiotherapy when affected individuals were sat up from a recumbent position. Oxygen desaturation of 8–12 % was associated with varying degrees of dyspnea and tachypnea (Fig. 1). Repeated orthostatic desaturation was present in all patients. In patient 1, re-intubation was considered during an episode of persistent orthostatic desaturation requiring 100 % high flow oxygen following extubation but later resolved upon lying supine. A modified physiotherapy approach was instituted: bed exercises, pre-emptive increases in supplemental oxygen in anticipation of movement and/or exercise, and interval training with multiple breaks. POS resolved over a median (range) of 17 (6–39) days. Compared to ICU survivors without POS, patients with POS were older and had lower body mass index, elevated arterial-alveolar carbon dioxide partial pressure gradient on the last day of IMV and elevated alveolar-arterial oxygen gradient following extubation (Table 1).
4. Discussion
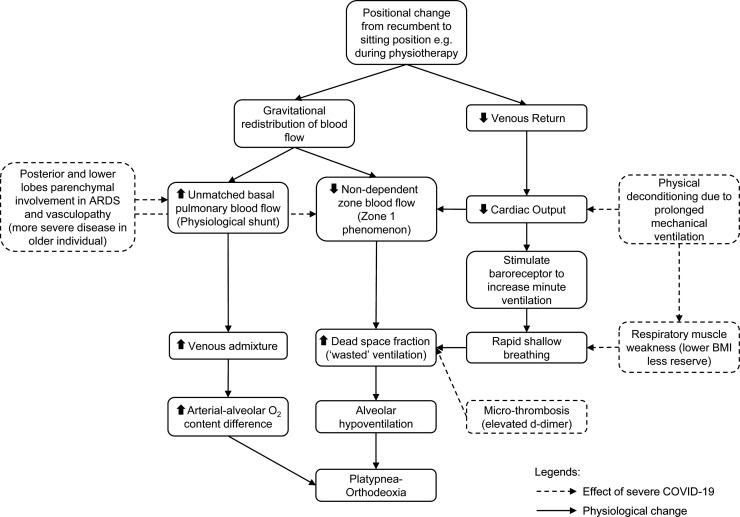
To the best of our knowledge, we report the first observations of POS following severe COVID-19 ARDS. Although often attributed to right-to-left intracardiac shunting, POS occurs in settings of increased venous admixture secondary to intrapulmonary anatomical (e.g. pulmonary arteriovenous malformation) or rarer physiological pulmonary shunts, due to basal-predominant parenchymal diseases (Agrawal et al., 2017). Our patients fulfilled criteria for moderate-severe ARDS. Venous admixture or intrapulmonary shunting is a recognized pathophysiological mechanism of hypoxemia in ARDS (Radermacher et al., 2017). However, the underlying mechanisms of POS, particularly in the context of severe COVID-19 illness are likely more complex. Alveolar hypoventilation has a role, for instance by increasing the proportion of ‘wasted’ ventilation (zone 1 phenomenon) which is compounded by reduced cardiac output, and an imbalanced load-capacity conferred by reduced lung compliance and critical illness myopathy (Fig. 2 ) (Radermacher et al., 2017; Lumb, 2010; Hert and Albert, 1994; McGregor et al., 1961). Zone 1 phenomenon is also exaggerated in the presence of microthrombi, and microangiopathy observed in severe COVID-19 disease (Fox et al., 2020). This could explain the disparity in severity of symptoms where individuals with the worst dyspnea had highest D-dimer levels (Fig. 1). Therefore, we hypothesize that POS is a gravitational exacerbation of intrapulmonary shunt in ARDS due to COVID-19 related vasculopathy and increased wasted ventilation.
Fig. 2.
Proposed pathophysiological mechanism of platypnea-orthodeoxia syndrome in severe COVID-19 illness.
The main limitations of our clinical findings are the lack of definitive exclusion of intra-cardiac anatomical shunting, e.g. by contrast-enhanced echocardiography or scintigraphy with macroaggregated albumin. In our institution, these tests present logistical challenges in a negative pressure isolation setting. While present in one-fifth of ARDS patients, we believe that the likelihood of patent foramen ovale is low due to: 1) the absence of paradoxical hypoxemia despite incremental PEEP, 2) no obvious chamber enlargement on bedside echocardiography, and 3) eventual resolution of POS over time (Dessap et al., 2010). Hepatopulmonary shunting was also unlikely with normal liver function.
As of 29th May 2020, Singapore has seen 33,860 COVID-19 cases (Ministry of Health, Singapore, 2020). Our centre managed 42 of 113 (37 %) patients who required ICU care. POS is notably a shared feature in a quarter of our COVID-19 ICU survivors, and may be a feature of older patients with more severe COVID-19 disease and/or inflammation. The reported finding of peripheral and dependent consolidation in more severely ill patients on sequential CT scans is also observed in our patients (Shi et al., 2020). POS is rarely reported post-ARDS, illustrating a potential key observation in severe COVID-19 (Hert and Albert, 1994). Of note, however, exercise-related oxygen desaturation, which shares some key pathophysiological mechanisms to orthodeoxia is described post-ARDS, and, POS may represent a more severe form of exertional desaturation (Hert and Albert, 1994; Herridge et al., 2003). In our experience, detecting POS in the late or post-ICU setting following severe COVID-19 illness likely reflects the increased physiotherapy-related intervention from prolonged hospitalisation, thereby ‘unmasking’ intrapulmonary shunting during postural change. Another reason is alveolar de-recruitment as evident by the increased in alveolar-arterial oxygen gradient following extubation. Furthermore, increased detection of POS is likely due to the close remote surveillance monitoring using continuous wearable devices, routine practice at our centre in post-ICU care.
5. Conclusion
POS is an under-recognized clinical feature in severe COVID-19 ARDS. Healthcare personnel should be made aware of the possibility for POS in severe COVID-19 ICU care. In relatively resource-constrained or overwhelmed healthcare settings, we propose direct clinical observation with bedside SpO2 monitoring during positional change focusing on patients who are older and with more severe disease. Recognising POS can reduce morbidity, unnecessary alarm, improve patient safety and allow for adjustments in the rehabilitation process. Our report calls for further studies on the intermediate and long-term clinical outcomes of post-ICU COVID-19 survivors, including those with, and without POS.
Funding
This work was funded in part by the Singapore National Medical Research Council through the COVID-19 Research Fund (Ref: COVID19RF-001). Author Dr. Po Ying Chia is supported by the Singapore National Medical Research Council (NMRC) Research Training Fellowship (NMRC/Fellowship/0056/2018).
CRediT authorship contribution statement
Geak Poh Tan: Conceptualization, Methodology, Data curation, Formal analysis, Writing - original draft. Sharlene Ho: Conceptualization, Methodology, Data curation, Formal analysis, Writing - original draft, Writing - review & editing. Bingwen Eugene Fan: Conceptualization, Data curation, Writing - original draft. Sanjay H. Chotirmall: Conceptualization, Methodology, Writing - original draft, Writing - review & editing. Cher Heng Tan: Conceptualization, Methodology. Sennen Jin Wen Lew: Data curation, Writing - original draft, Writing - review & editing. Po Ying Chia: Writing - original draft, Writing - review & editing. Barnaby E. Young: Writing - original draft, Writing - review & editing. John Arputhan Abisheganaden: Conceptualization, Methodology, Writing - review & editing, Supervision. Ser Hon Puah: Conceptualization, Methodology, Data curation, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
The authors would like to thank Dr. Gin Tsen Chai for assistance in proofreading the manuscript and all healthcare workers involved in the care of COVID-19 patients.
References
- Agrawal A., Palkar A., Talwar A. The multiple dimensions of Platypnea Orthodeoxia syndrome: a review. Respir Med. 2017;129:31–38. doi: 10.1016/j.rmed.2017.05.016. [DOI] [PubMed] [Google Scholar]
- Dessap A.M., Boissier F., Leon R., Carreira S., Campo F.R., Lemaire F., Brochard L. Prevalence and prognosis of shunting across patent foramen ovale during acute respiratory distress syndrome. Crit. Care Med. 2010;38:1786–1792. doi: 10.1097/CCM.0b013e3181eaa9c8. [DOI] [PubMed] [Google Scholar]
- Fox S.E., Akmatbekov A., Harbert J.L., Li G., Brown J.Q., Heide R.S.V. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series rom New Orleans. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herridge M.S., Cheung A.M., Tansey C.M., Matte-Martyn A., Diaz-Granados N., Al-Saidi F., Cooper A.B., Guest C.B., Mazer C.D., Mehta S., Stewart T.E., Barr A., Cook D., Slutsky A.S., Canadian Critical Care Trials Group One-year outcomes in survivors of the acute respiratory distress syndrome. N. Engl. J. Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- Hert R., Albert R.K. Sequelae of the adult respiratory distress syndrome. Thorax. 1994;49:8–13. doi: 10.1136/thx.49.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumb A.B. 6th ed. Elsevier; 2010. Nunn’S Applied Respiratory Physiology. [Google Scholar]
- McGregor M., Adam W., Sekelj P. Influence of posture on cardiac output and minute ventilation during exercise. Circ. Res. 1961;9:1089–1092. doi: 10.1161/01.RES.9.5.1089. [DOI] [Google Scholar]
- Ministry of Health, Singapore . 2020. Situation Report – 29 May 2020 Coronavirus Disease (COVID-19) [Internet]https://www.moh.gov.sg/docs/librariesprovider5/2019-ncov/situation-report---29-may-2020.pdf [Accessed on 1 June 2020] Available from: [Google Scholar]
- Radermacher P., Maggiore S.M., Mercat A. Fifty years of research in ARDS. Gas exchange in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2017;196:964–984. doi: 10.1164/rccm.201610-2156SO. [DOI] [PubMed] [Google Scholar]
- Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J., Fan Y., Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;4:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]