Abstract
Purpose
The COVID-19 pandemic presents serious challenges for brachytherapists, and in the time-sensitive case of locally advanced cervical cancer, the need for curative brachytherapy (BT) is critical for survival. Given the high-volume of locally advanced cervical cancer in our safety-net hospital, we developed a strategy in close collaboration with our gynecology oncology and anesthesia colleagues to allow for completely clinic-based intracavitary brachytherapy (ICBT).
Methods and Materials
This technical report will highlight our experience with the use of paracervical blocks (PCBs) and oral multimodal analgesia (MMA) for appropriately selected cervical ICBT cases, allowing for completely clinic-based treatment.
Results
18 of 19 (95%) screened patients were eligible for in-clinic ICBT. The excluded patient had significant vaginal fibrosis. 38 of 39 intracavitary implants were successfully transitioned for entirely in-clinic treatment utilizing PCBs and oral MMA (97% success rate). One case was aborted due to inadequate analgesia secondary to a significantly delayed case start time (PO medication effect diminished). 95% of patients reported no pain at the conclusion of the procedure. The median (IQR) D2cc for rectum and bladder were 64.8 (58.6–70.2) Gy and 84.1 (70.9–89.4) Gy, respectively. Median (IQR) CTV high-risk D90 was 88.0 (85.6–89.8) Gy.
Conclusions
In a multidisciplinary effort, we have successfully transitioned many ICBT cases to the clinic with the use of PCB local anesthesia and oral multimodality therapy in direct response to the current pandemic, thereby mitigating exposure risk to patients and staff as well as reducing overall health care burden.
Keywords: Locally advanced cervical cancer (LACC), Intracavitary brachytherapy (ICBT), COVID-19, Paracervical block (PCB), Multimodal anesthesia
Introduction
For cervical gynecological malignancies, overall treatment time ≤7 weeks (including brachytherapy [BT] boost) is necessary for optimal local control (1). During COVID-19, many procedures are being postponed. Substitution of BT in cervical cancer is associated with a worse overall survival (2). Furthermore, the American Brachytherapy Society have issued guidance that BT boost for cervical cancer should not be delayed under any circumstance for a patient not displaying COVID-19 symptoms (3). Each operative encounter puts our immunocompromised patients at nosocomial risk, especially if general anesthesia is required wherein the generation of aerosols during intubation increases risk to staff and physicians (4). Given that hospital resources, operative time slots, anesthesia, nursing personnel, and personal protective equipment may be limited, a physician must employ judicious use of these precious resources. Therefore, we must balance timely BT with reducing high-risk events. This technical report will highlight a strategy developed in close collaboration with our gynecology and anesthesia colleagues to utilize paracervical blocks (PCBs) with multimodal oral analgesia for appropriately selected BT cases, thus allowing for treatment to be completely ambulatory.
Materials and methods
Transitioning from the operating room to the clinic
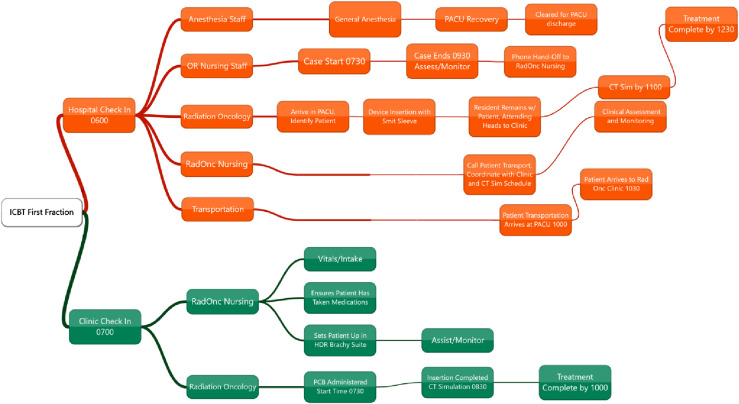
Before COVID-19 pandemic, the workflow for intracavitary brachytherapy (ICBT) insertions within our system was as follows: the patient checks in at the hospital around 6 am; the patient goes to the preoperative area, consent is checked, undergoes anesthesia evaluation, and the brachytherapists greet the patient confirming the procedure and site. The patient is then brought to the operating room (OR) around 7:30 am, and a typical case will conclude around 9:30 am. The patient is then brought to the PACU where they await clearance for transport by anesthesia. Once cleared, the patient is transported to our clinic usually by 11 am. They have CT simulation; contouring of targets and OAR, and then 3D planning utilizing Eclipse with MRI fusions is completed. The prescription and fractionation is ∼24 Gy in 3 fractions (as per Rao et al.) (5) with CT-based planning using parameters from the EMBRACE II protocol. Treatment is then delivered, and the implant removed. The patient leaves the clinic with a responsible adult around 12:30 pm if there have been no delays [Fig. 1 ].
Fig. 1.
Workflow of operating room intracavitary brachytherapy insertion (top, orange) and ambulatory intracavitary brachytherapy insertion (bottom, green). (Top, orange) The patient checks in at the hospital around 6 am, goes to the preoperative area, anesthesia evaluation, and the brachytherapists greet the patient confirming the procedure and site. The patient is then brought to the operating room around 7:30 am, and a typical case will conclude around 9:30 am. The patient is then brought to the PACU where they await clearance for transport by anesthesia. Once cleared, the patient is transported to our clinic usually by 11 am. They have CT simulation; contouring of targets and OAR, and then 3D planning utilizing Eclipse with MRI fusions is completed. Fraction is administered, the device removed, and the patient discharged with a responsible adult around 12:30 pm. (Bottom, green) The patient arrives to the clinic around 7 am, they are brought back to the brachytherapy suite by nursing staff, the nurse confirms the patient has taken PO medications. The patient is placed in stirrups and generally the case begins around 7:30 am with examination, PCB, and then the device insertion and packing. The patient is then taken to CT simulation around 8:15 am. Contouring and treatment planning then occurs. The treatment fraction is administered, the device pulled, and the patient discharged with a responsible adult as early as 10 am. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Benefits of utilizing general anesthesia and an operating suite for the first ICBT insertion include a good physical examination, and the ability to dilate the cervix and place a Smit sleeve to make future tandem insertions swift and tolerable. Although one way to decrease this risk is spinal anesthesia, as was mentioned in a recent article (6) discussing proposed changes in the COVID-19 era, an anesthesiologist or CNA is still required, which may be a difficult and limited request in the setting of pandemic or in a clinic not associated with a hospital. In addition, results from the study by Lim et al. revealed that general anesthesia causes more complications than local or topical anesthesia (7). At our institution, an attractive alternative was proposed for PO multimodal analgesia with PCB. Multimodal analgesia utilizes a combination of analgesics acting on different targets of the pain pathway (i.e. NSAIDs, acetaminophen, gabapentinoids, NMDA receptor antagonists, etc.) to create synergistic analgesia (8). This technique reduces opioid requirements and their side effects (9). Multimodal anesthesia is accomplished using two or more drugs of different classes, followed by neuraxial analgesia, regional block, or local infiltration (10). With the help of our anesthesia faculty, we therefore developed a PO regimen suitable for clinic use [Table 1 ].
Table 1.
Multimodal oral analgesia regimen
| Medication | Dose | Rationale |
|---|---|---|
| Hydromorphone PO | 4 mg | Pain |
| Ativan PO | 1 mg | Anxiolytic |
| Gabapentin PO | 300 mg | Adjuvant pain |
| Ibuprofen PO | 800 mg | Reduce cervical spasms |
| Promethazine PO | 25 mg | Antiemetic |
The method presented here allows the patient to arrive at our clinic around 7 am. They are then brought back to BT suite by nursing staff who also confirm that the patient has taken PO medications (and we encourage patients to bring medications with them so there is certainty). The patient is placed in stirrups and generally the case begins around 7:30 am with examination, PCB, and then device insertion and packing. The patient is then taken to CT simulation around 8:15 am. Contouring and treatment planning then occurs (3D planning as before) between 8:30 and 9:30 am. The treatment fraction is administered, the device pulled, and the patient discharged with a responsible adult as early as 10 am. Therefore, in-clinic insertion with PCB takes 3 h for a typical procedure, whereas OR-based technique care delivery requires approximately 6 h for most cases [Fig. 1].
Evidence for paracervical block
The literature demonstrating benefit of PCB for pain reduction during cervical dilation is consistent. Subjectively, women reported pain of 1.5/10 with PCB vs 6.5/10 with saline during hysteroscopy and endometrial biopsy in one randomized trial (11). Another randomized trial of PCB evaluating 120 patients found that pain during cervical dilation decreased from 80 to 40 of 100 mm measured using a visual analog scale (12). A Cochrane review in 2013 of PCB found a significant reduction of pain with cervical dilation, with a lesser effect on uterine manipulation (13). A randomized trial applicable to BT found a significant pain reduction (13 mm vs 54 mm on pain visual analog scale) in patients undergoing laminaria placement, a procedure similar to dilation for BT, in which only mild uterine manipulation is anticipated (14). Despite the successful implementation of PCBs in several BT clinics internationally, adoption of brachytherapist-directed local anesthesia in the US has been limited ([15], [16], [17]).
Development and implementation
Initially, we invited gynecologic oncology faculty to host a departmental in-service discussing the evidence for and technical aspects of performing PCB. Our faculty, residents, nurses, and other members of clinic leadership were in attendance. For the first case, we began with a patient also undergoing general anesthesia and were fortunate to be accompanied by our gynecologic oncologist to the OR, who provided expert instruction and supervision. To allow for increased comfort with technique, our brachytherapists performed several cases in the OR using PCB with PO multimodal analgesia and anesthesia on standby as monitored anesthesia care. For these cases, we attempted to mimic in-clinic conditions; therefore, no Smit sleeve was placed; this has the added benefit of preventing uterine dilation and the associated discomfort. Initially, the faculty performed the PCB with the residents present to observe and then proceeded with device insertion under supervision, as per standard practices of our residency program. Once the faculty were comfortable with the PCB, and residents had observed at least one case, the PCB was then performed by the resident with radiation oncology faculty oversight. Approximately 3 weeks passed between the first insertion in the OR with gynecologic oncologist instruction and the first procedure performed independently in-clinic with clinic leadership and other BT attendings in attendance as a proof of concept. These procedures were well-tolerated and at this time, were able to be completed by a resident with direct attending supervision throughout. The general sentiment among faculty and residents was that the technique was quite simple and similar to the placement of a shallow fiducial marker with an interstitial needle.
Patient selection
Candidates for PO multimodal anesthesia with PCB undergo a BT planning MRI to assess response to external beam and ensure normal uterine geometry (i.e. no significant retroversion). Radiologic evidence of a patent cervical os and the ability to directly visualize the cervix (which we assessed during a patient's final weekly encounter) are both reassuring. However, patients with significant fibrosis of the vaginal canal or who poorly tolerate pelvic examination may not be good candidates. The uterine angle and length are measured on the MRI to avoid use of the uterine sound. Shared decision-making is initiated with the patient at the time of BT planning to evaluate the patient's goals and willingness to proceed with the procedure in the clinic setting. In general, we have found that multiparous individuals with a history of vaginal delivery often tolerate the procedure well. Patients with severe psychiatric comorbidities, history of PTSD, or sexual trauma survivors might not be ideal candidates for an in-clinic procedure. Finally, American Society of Anesthesiologists physical status classification should be II or less (mild diseases only without substantive functional limitations). Examples include (but not limited to) current smoker, social alcohol drinker, obesity, well-controlled DM/HTN, mild lung disease (18).
Technique
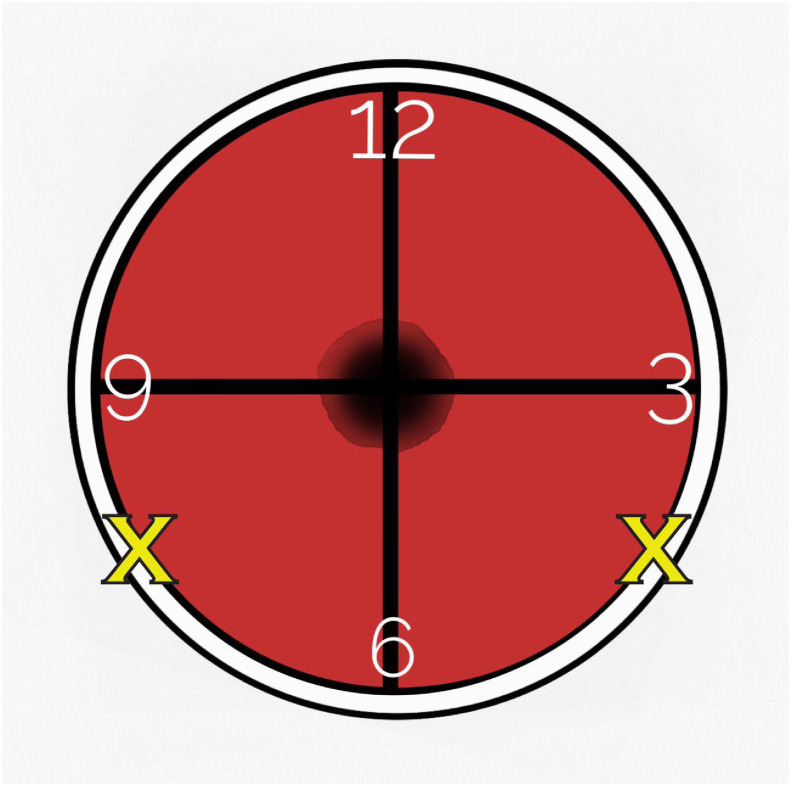
The patient is instructed to take 800 mg ibuprofen, 4 mg hydromorphone, 2 mg lorazepam, 25 mg promethazine, and 300 mg gabapentin orally, 60 min before the procedure. Of note, these doses are approximate and may be tailored to a patient's weight and history of narcotic use, allergies, anxiety level, and symptoms during their external beam course. Ensure the patient has 1:1 nursing coverage throughout the procedure for conversational support. Vital monitoring, including heart rate, blood pressure, respiratory rate, and pulse oximetry should be checked every 15 minutes during the procedure. Our clinic uses 10 mL of 1% lidocaine without epinephrine (although some protocols use up to 20 mL) (12) injected in equal amounts (5 mL) at 4 and 8 o'clock at the cervical–vaginal junction (fornix) at about 2 cm depth [Fig. 2 ]. A critical step is to gently aspirate before injection to ensure the needle has not pierced a vessel. If the patient reacts readily to the insertion of the needle, an attempt is made to inject slightly posteriorly to avoid direct contact on the utero-sacral ligament. Care should always be taken to avoid the 3 and 9 o'clock locations to avoid injury to the uterine artery. Inject slowly over a 30 s period (take more time if larger volume of lidocaine is used) while gently withdrawing using a 20- or 22-gauge spinal needle of 3.5–5 cm length. A spinal needle is preferable for this procedure because of the lower piercing effect of the pencil point tip compared with standard needles. Allow at least 3 min for the lidocaine to provide full anesthetic effect (12, 19). In some cases, we applied up to 10 mL of 2% lidocaine gel to the vaginal canal before PCB. This diminished the discomfort of administering the PCB, and theoretically helps with discomfort during packing. In the case of minor bleeding from the injection site, apply gentle pressure with gauze attached to end of ring forceps to achieve hemostasis.
Fig. 2.
Paracervical block locations in reference to the cervical os. Five mL of 1% lidocaine without epinephrine is injected at the cervicovaginal junction (fornix) represented at 4 o'clock and 8 o'clock with an X over a 30-s period at ∼2-cm depth. Spinal needle is aspirated before injection to ensure no blood return.
Safety/toxicity
PCB is a low-risk procedure and the dose of lidocaine is well below the lethal dose (4.5 mg/kg without epinephrine). Important considerations in the choice between lidocaine and bupivacaine are onset and duration of action. Lidocaine has an onset of about 2–3 min and duration of 1–2 h, whereas bupivacaine has a significantly longer duration of action of 2–4 h, but slower onset of >5 min (20). The optional addition of epinephrine constricts the local vasculature, thereby keeping the anesthetic contained for a longer period and decreasing the systemic absorption, as well as extending the duration of action. Our center generally uses lidocaine to take advantage of quick onset; however, in our experience, bupivacaine decreases postoperative opioid requirements and this is supported in the literature (21). Although some centers have tried mixing solutions of lidocaine with bupivacaine to take advantage both properties, the clinical benefit seems to be narrow (22).
Adverse effects of lidocaine toxicity include circumoral numbness, tinnitus, altered mental status, seizure, and cardiac arrhythmias; whereas adverse effects of epinephrine include tachycardia and other sympathetic phenomena (23). Clinics should have a protocol in place for the rare case of emergency. This should include emergency transport services to the hospital, a crash cart available, and at least one person performing or supervising the procedure to be Advanced Cardiac Life Support certified and familiar with the treatment of local anesthetic systemic toxicity.
Results and discussion
Given the high volume of gynecologic BT cases at our institution and anticipated lack of OR resources amidst the COVID-19 pandemic, we were fortunate to be able to develop and implement a method of analgesia, which allows our patients to access curative BT without the need for general anesthesia, gynecologists, or support staff other than a single nurse. In our clinic, utilization of multimodality oral medications and PCB has now become standard for patients who are good candidates.
At selection, 5% of patients were excluded from in-clinic PCB insertion for excessive vaginal canal fibrosis. Thus far, 38 insertions have been performed on 18 different patients, and only 1 case was aborted (97% success rate). The aborted case was complicated by a delay to beginning the insertion, which resulted in patient's PO analgesia waning. She had history of narcotic abuse and likely was a fast metabolizer of narcotics. According to with nursing assessments, there was “no pain reported” at the conclusion of the procedure in 95% of cases. We did not formally assess a pain scale during the procedure.
The median (interquartile range [IQR]) D 2cc for rectum and bladder were 64.8 (58.6–70.2) Gy and 84.1 (70.9–89.4) Gy, respectively (in line with EMBRACE II aims of 65 Gy for rectum and 80 Gy for bladder, and limits of <75 Gy for rectum and <90 Gy for bladder). Median vaginal wall max point dose was 120.0 (110.3–130.3) Gy, consistent with aims of 120–140 Gy. Median (IQR) CTV high risk D 90 was 88.0 (85.6–89.8) Gy. Therefore, our plans adequately met the aims for treatment targets and OARs. We did not experience systematic difficulty placing optimal ovoid size or packing the vaginal canal while using the PCB technique.
Generally, the impact on residency training was positive; most of these cases were completed entirely by a resident under direct faculty supervision. Furthermore, this has decreased the burden on our trainees who, under the old protocol, spent valuable time attending to patients in the postanesthesia care unit before transport. In addition, with BT cases taking less time, busy trainees may allot their time to other tasks. Residents have become increasingly comfortable with the use of PCBs, and we will continue to use the technique beyond the pandemic in the appropriately selected patient. Not only does it decrease risk to the patient (compared with general or spinal anesthesia), reduce recovery time, and decrease health care cost but also it allows for the entire BT case to be completed in a shorter calendar period, as the brachytherapist is not limited by the availability of other services, OR slots, or hospital transport.
Future direction/implications
This report describes intracavitary device placement without any interstitial needles. Future directions involve determining the feasibility of PCB and multimodal PO anesthesia for cases in which a hybrid intercavitary/interstitial device is used. Furthermore, we are interested to eventually implement pudendal block which may be ideal for interstitial gynecologic and prostate cases (24). Finally, this technique could be pursued with a global health mission; and while we perform 3D-based planning, we see no reason why PCB with multimodal oral anesthesia could not also be adapted for conventional point A/B prescription for use in low-resource regions, making curative BT more accessible globally.
Conclusion
In a multidisciplinary effort, we have successfully transitioned many ICBT cases to the clinic with the use of PCB local anesthesia and oral multimodality therapy in direct response to the current pandemic, thereby mitigating exposure risk to patients and staff as well as reducing overall health care burden.
Footnotes
Disclosure: The author(s) received no specific funding for this work. The authors report no proprietary or commercial interest in any product or concept discussed in this article.
References
- 1.Tanderup K., Fokdal L.U., Sturdza A. Effect of tumor dose, volume and overall treatment time on local control after radiochemotherapy including MRI guided brachytherapy of locally advanced cervical cancer. Radiother Oncol. 2016;120:441–446. doi: 10.1016/j.radonc.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 2.Karlsson J., Dreifaldt A.C., Mordhorst L.B. Differences in outcome for cervical cancer patients treated with or without brachytherapy. Brachytherapy. 2017;16:133–140. doi: 10.1016/j.brachy.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 3.https://www.americanbrachytherapy.org/consensus-statements/ Available at:
- 4.Tran K., Cimon K., Severn M. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao B.S., Das P., Subramanian B.V. A comparative analysis of two different dose fractionation regimens of high dose rate intracavitary brachytherapy in treatment of carcinoma of uterine cervix: a prospective randomized study. J Clin Diagn Res. 2017;11:XC06. doi: 10.7860/JCDR/2017/22489.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohindra P., Beriwal S., Kamrava M. Proposed brachytherapy recommendations (practical implementation, indications, and dose fractionation) during COVID-19 pandemic. Brachytherapy. 2020;19:390–400. doi: 10.1016/j.brachy.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim K.H., Lu J.J., Wynne C.J. A study of complications arising from different methods of anesthesia used in high-dose-rate brachytherapy for cervical cancer. Am J Clin Oncol. 2004;27:449–451. doi: 10.1097/01.coc.0000128723.00352.ad. [DOI] [PubMed] [Google Scholar]
- 8.Murphy A.M., Haykal S., Lalonde D.H. Contemporary approaches to postoperative pain management. Plast Reconstr Surg. 2019;144(6) doi: 10.1097/PRS.0000000000006268. 1080e-94e. [DOI] [PubMed] [Google Scholar]
- 9.Elvir-Lazo O.L., White P.F. Postoperative pain management after ambulatory surgery: role of multimodal analgesia. Anesthesiol Clin. 2010;28:217–224. doi: 10.1016/j.anclin.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Rosero E.B., Joshi G.P. Preemptive, preventive, multimodal analgesia: what do they really mean? Plast Reconstr Surg. 2014;134:85S–93S. doi: 10.1097/PRS.0000000000000671. [DOI] [PubMed] [Google Scholar]
- 11.Cicinelli E., Didonna T., Schonauer L.M. Paracervical anesthesia for hysteroscopy and endometrial biopsy in postmenopausal women. A randomized, double-blind, placebo-controlled study. J Reprod Med. 1998;43:1014–1018. [PubMed] [Google Scholar]
- 12.Renner R.M., Edelman A.B., Nichols M.D. Refining paracervical block techniques for pain control in first trimester surgical abortion: a randomized controlled noninferiority trial. Contraception. 2016;94:461–466. doi: 10.1016/j.contraception.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Tangsiriwatthana T., Sangkomkamhang U.S., Lumbiganon P. Paracervical local anaesthesia for cervical dilatation and uterine intervention. Cochrane Database Syst Rev. 2013:CD005056. doi: 10.1002/14651858.CD005056.pub3. [DOI] [PubMed] [Google Scholar]
- 14.Soon R.1, Tschann M., Salcedo J. Paracervical block for laminaria insertion before second-trimester abortion: a randomized controlled trial. Obstet Gynecol. 2017;130:387–392. doi: 10.1097/AOG.0000000000002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leong Y.H., Tan K.H., Choo B.A. Novel anesthetic technique for combined intracavitary and interstitial brachytherapy for cervix cancer in an outpatient setting. J Contemp Brachytherapy. 2017;9:236. doi: 10.5114/jcb.2017.68469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan P.W., Koh V.Y., Tang J.I. Outpatient combined intracavitary and interstitial cervical brachytherapy: barriers and solutions to implementation of a successful programme–a single institutional experience. J Contemp Brachytherapy. 2015;7:259. doi: 10.5114/jcb.2015.52625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petric P., Hudej R., Hanuna O. MRI-assisted cervix cancer brachytherapy pre-planning, based on application in paracervical anaesthesia. Radiol Oncol. 2014;48:293–300. doi: 10.2478/raon-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abouleish A.E., Leib M.L., Cohen N.H. ASA provides examples to each ASA physical status class. ASA Monitor. 2015;79:38–39. [Google Scholar]
- 19.Glantz J.C., Shomento S. Comparison of paracervical block techniques during first trimester pregnancy termination. Int J Gynecol Obstet. 2001;72:171–178. doi: 10.1016/s0020-7292(00)00292-7. [DOI] [PubMed] [Google Scholar]
- 20.Cousins M.J., Bridenbaugh P.O., editors. Neural blockade in clinical anesthesia and management of pain. Lippincott Williams & Wilkins; Philadelphia, PA: 1998. [Google Scholar]
- 21.Partridge B.L., Stabile B.E. The effects of incisional bupivacaine on postoperative narcotic requirements, oxygen saturation and length of stay in the post-anesthesia care unit. Acta Anaesthesiol Scand. 1990;34:486–491. doi: 10.1111/j.1399-6576.1990.tb03129.x. [DOI] [PubMed] [Google Scholar]
- 22.Collins J.B., Song J., Mahabir R.C. Onset and duration of intradermal mixtures of bupivacaine and lidocaine with epinephrine. Can J Plast Surg. 2013;21:51–53. doi: 10.1177/229255031302100112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torp K.D., Simon L.V. Lidocaine toxicity: StatPearls. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK482479 Available at: [PubMed]
- 24.Schenck M., Schenck C., Rübben H. Pudendal nerve block in HDR-brachytherapy patients: do we really need general or regional anesthesia? World J Urol. 2013;31:417–421. doi: 10.1007/s00345-012-0987-x. [DOI] [PubMed] [Google Scholar]