Abstract
Background
Coronavirus disease 2019 (COVID-19) has put a strain on regular healthcare worldwide. In the Netherlands, the national screening programs, including for breast cancer, were halted temporarily. This posed a challenge to breast cancer care, because ∼40% of cases are detected through national screening. Therefore, the aim of the present study was to evaluate the effects of the COVID-19 pandemic on the surgical care of patients with breast cancer in the Netherlands.
Materials and Methods
The present multicenter retrospective cohort study investigated the effects of COVID-19 on patients with breast cancer who had undergone surgery from March 9 to May 17, 2020. The primary endpoints were the number of surgical procedures performed during the study period, tumor characteristics, surgery type, and route of referral. The secondary endpoint was the incidence of postoperative complications during the study period.
Results
A total of 217 consecutive patients with breast cancer requiring surgery were included. We found an overall decrease in the number of patients with breast cancer who were undergoing surgery. The most significant decline was seen in surgery for T1-T2 and N0 tumors. A decline in the number of referrals from both the national screening program and general practitioners was observed. The incidence of postoperative complications remained stable during the study period.
Conclusions
The temporary halt of the national screening program for breast cancer resulted in fewer surgical procedures during the study period and a pronounced decrease in surgery of the lower tumor stages.
Keywords: Breast cancer, Complications, Coronavirus disease 2019, Pandemic, Surgery
Micro-Abstract
Coronavirus disease 2019 has had enormous effects on healthcare systems worldwide. The present multicenter observational study of 217 consecutive patients with breast cancer found a decrease in the number of patients undergoing surgery. Additionally, multivariate analysis found no changes in the incidence of postoperative complications during the 10-week study period.
Introduction
Coronavirus disease 2019 (COVID-19) is a highly infectious disease caused by acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and responsible for the ongoing pandemic.1 SARS-CoV-2 can mostly be detected with polymerase chain reaction from oropharyngeal swabs.2 At the beginning of July 2020, > 11,000,000 cases and > 530,000 deaths had been confirmed worldwide.3
The COVID-19 pandemic has posed a challenge to regular healthcare.4 Worldwide, measures to alleviate the burden on healthcare systems, such as the suspension of elective surgeries, were implemented.5 In the Netherlands, similar policies were implemented regarding oncologic care. If possible, elective surgeries were postponed, and the national screening programs for breast and colorectal cancer were temporarily halted starting March 16, 2020.4 , 6 This has led to a concerning decline in breast cancer diagnoses.4 , 7 Breast cancer is the most common cancer and the leading cause of cancer death in women worldwide.8 In the Netherlands, the incidence of breast cancer has been roughly 17,000 cases annually, with ∼40% detected through the national screening program.9 , 10 Surgical resection, with or without radiotherapy and/or systemic treatment, of the primary tumor is the treatment of choice for patients with new-onset breast cancer.11, 12, 13 The measures required to alleviate the burden on healthcare systems due to COVID-19 have strongly affected patients with breast cancer, especially the postponed surgeries and halting of national screening programs.4 , 6
Recently, the number of breast cancer diagnoses has decreased by ≤ 50%, which can be attributed to the temporary halting of national screening programs and/or fewer referrals from general practitioners (GPs).4 However, the consequences on surgical care for breast cancer are unknown. Therefore, the aim of the present study was to determine the effect of the COVID-19 pandemic on the surgical demand for breast cancer.
Materials and Methods
Patient Selection
The present retrospective multicenter cohort study included all consecutive surgical patients treated from March 9 to May 17, 2020 in 5 hospitals in the Netherlands: University Medical Centre Utrecht, Ziekenhuis Rivierenland, St Antonius Ziekenhuis, Diakonessenhuis, and Meander Medical Centre. Consecutive patients were included if they were aged > 18 years and had undergone breast cancer surgery. The ethics committee of all participating centers approved the present study and decided that patient informed consent was not required. The study is a part of the trial registered in the research registry (available at: www.researchregistry.com; unique identifying no., researchregistry5720).
COVID-19 Diagnosis
The included patients could have been tested for COVID-19 before or during their admission in the hospital. In addition, COVID-19–related symptoms were recorded. Reverse transcriptase polymerase chain reaction testing for SARS-CoV-2 was used at all the centers, in accordance with the European guidelines for analysis.2 The genes used for analysis included the RdRp, E, and N genes. The E gene assay was used first, followed by confirmatory testing using the RdRp gene assay. For the present study, COVID-19–related symptoms were defined as cough, fever, fatigue, chest pain, dyspnea, and other flu-like symptoms.
Endpoints and Definitions
The primary endpoints were the number of surgical procedures performed for breast cancer, TNM classification before surgery, type of surgical procedure, and initial referral (ie, the national screening program or the GP). The secondary endpoint was the incidence of postoperative complications. Additionally, we aimed to determine which factors influenced the risk of postoperative complications.
Surgical procedures were defined as breast-conserving therapy (BCT), mastectomy (with and without immediate reconstruction), and other procedures (ie, lymph node dissection, lymph node biopsy, repeat excision, scar excision).
The general condition of the patients before surgery was assessed using the American Society of Anesthesiologists classification.14 The severity of the complications was determined using to the Clavien-Dindo classification.15 Major complications were defined as Clavien-Dindo class ≥ III. The tumors were categorized using TNM classification system.16 Phyllodes tumors were not graded using the TNM classification.17 Radiotherapy, antihormonal therapy, immunotherapy, and chemotherapy were classified as adjuvant and/or neoadjuvant therapy. Patient comorbidities included a history of cardiovascular disease, pulmonary disease, renal disease, and diabetes.
Statistical Analysis
Descriptive statistics were used to describe the patient and treatment characteristics. Continuous data are reported as the mean ± standard deviation or median and interquartile range, depending on the distribution.
Multiple imputation by chained equations using the MICE package in R was performed to impute missing data. Missing data were compared with the available data to determine whether the data were missing at random. The imputation was repeated 10 times, followed by application of Rubin’s rule to combine parameter estimates and standard errors.18 , 19 The imputed data were compared later with the complete data to determine the validity of the imputation model. The imputed data were used in the analyses. Two-sided P values < .05 were considered statistically significant.
Multivariate logistic regression analyses were performed to study the risk of complications for patients who had undergone surgery. The odds ratio (OR) and 95% confidence intervals (CIs) were used to quantify the risk. Possible confounding factors and effect modifiers were age, body mass index, American Society of Anesthesiologists classification, type of breast surgery, number of comorbidities, TNM classification, week in which the surgery had been performed, symptoms associated with COVID-19, and testing for COVID-19.
All calculations were performed using RStudio, version 1.2.5001 (with R version, x64 3.6.3). Visualization of the plots was performed using the ggplot2 package.
Results
Patient Characteristics
A total of 217 consecutive patients with breast cancer with a mean age of 62 years were included in the present study. Most of the patients had had a diagnosis of stage T1-T2 breast cancer (81.7%) without lymph node involvement (N0; 71%). In addition, 10 patients had had a phyllodes tumor. None of the included patients had had metastatic disease. Of the 217 patients, 61 (28.1%) and 170 (78.3%) had received neoadjuvant and adjuvant treatment, respectively. The surgery was BCT for 139 patients (64.1%). Of the 217 patients, 21 had been tested for COVID-19 (9.7%), none of whom tested positive. Complications occurred in 18 patients, of whom 8 (3.7%) had developed major complications (Table 1 ).
Table 1.
Baseline Patient Characteristics (n = 217)
| Parameter | Value |
|---|---|
| Age, y | 62.2 ± 13.1 |
| BMI, kg/m2 | |
| Median | 23.3 |
| IQR | 23.0-30.4 |
| ASA classification | |
| I | 47 (21.7) |
| II | 130 (59.9) |
| III | 40 (18.4) |
| National screening program diagnosis | 55 (25.7) |
| T stage | |
| T0 | 28 (13.5) |
| T1 | 119 (57.5) |
| T2 | 50 (24.2) |
| T3 | 6 (2.9) |
| T4 | 4 (1.9) |
| Data missing | 10 (4.6) |
| N stage | |
| N0 | 147 (71.0) |
| N1 | 49 (23.7) |
| N2 | 7 (3.4) |
| N3 | 4 (1.9) |
| Data missing | 10 (4.6) |
| M stage | 0 (0.0) |
| Neoadjuvant therapy | 61 (28.1) |
| Adjuvant therapy | 170 (78.3) |
| Type of surgery | |
| BCT | 139 (64.1) |
| Mastectomy | 63 (29.3) |
| Other | 15 (6.9) |
| Patients tested for COVID-19 | 21 (9.7) |
| Complications | |
| None | 199 (91.7) |
| Minor | 10 (4.6) |
| Major | 8 (3.7) |
Data presented as mean ± standard deviation or n (%), unless noted otherwise.
Abbreviations: ASA = American Society of Anesthesiologists; BCT = breast conserving therapy; BMI = body mass index; COVID-19 = coronavirus disease 2019; IQR = interquartile range.
Breast Surgery Types During the Study Period
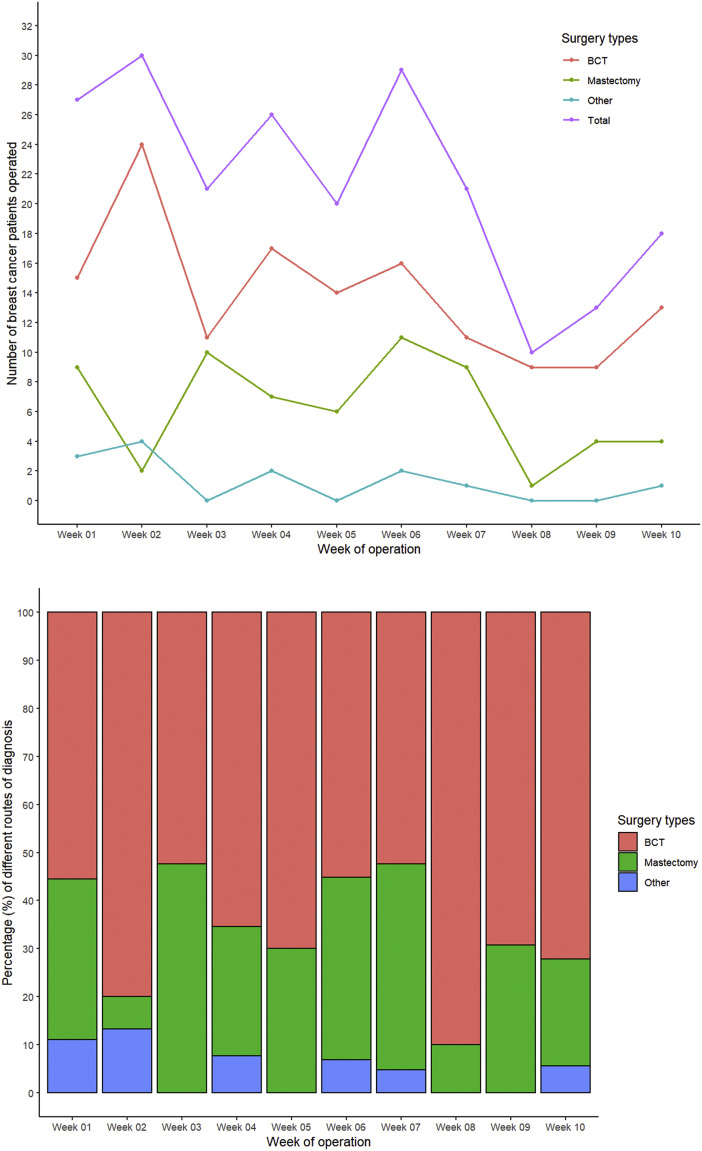
The number of the different types of breast surgery performed from March 9 to May 17 are presented Figure 1 . An overall decline occurred in the total number of surgical procedures performed that was most pronounced after week 6 of the study period. However, in the last 2 weeks (weeks 9 and 10), a slight increase occurred in the total number of breast cancer procedures performed. The number of BCT procedures had declined steady throughout, except for the slight increase in the last 2 weeks of the study period. However, the number of mastectomies and other types of breast surgery had remained stable over time. No significant differences were found in the proportion of surgical procedures performed when stratified by the study week (P = .173).
Figure 1.
Number of Breast Cancer Surgeries Presented by Type of Surgical Procedure
Abbreviation: BCT = breast-conserving therapy.
Initial Referral and T and N Stage
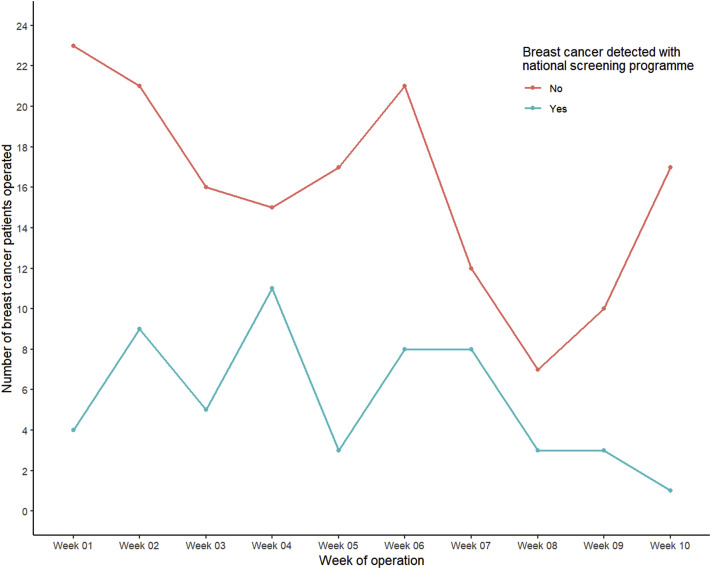
The route of referral, including the number of patients referred by GPs or the national screening program, is presented in Figure 2 . During the study period, an overall decrease was found in the number of patients with breast cancer requiring surgery who had originally been diagnosed through the national screening program. However, the number of patients who had been referred by their GP had declined sharply after week 6 of the study, although the number had increased again after 8 weeks.
Figure 2.
Number and Percentage of Patients With Breast Cancer Who Had Undergone Surgery From March 9 to May 17, 2020 With Referral From National Screening Program or General Practitioner
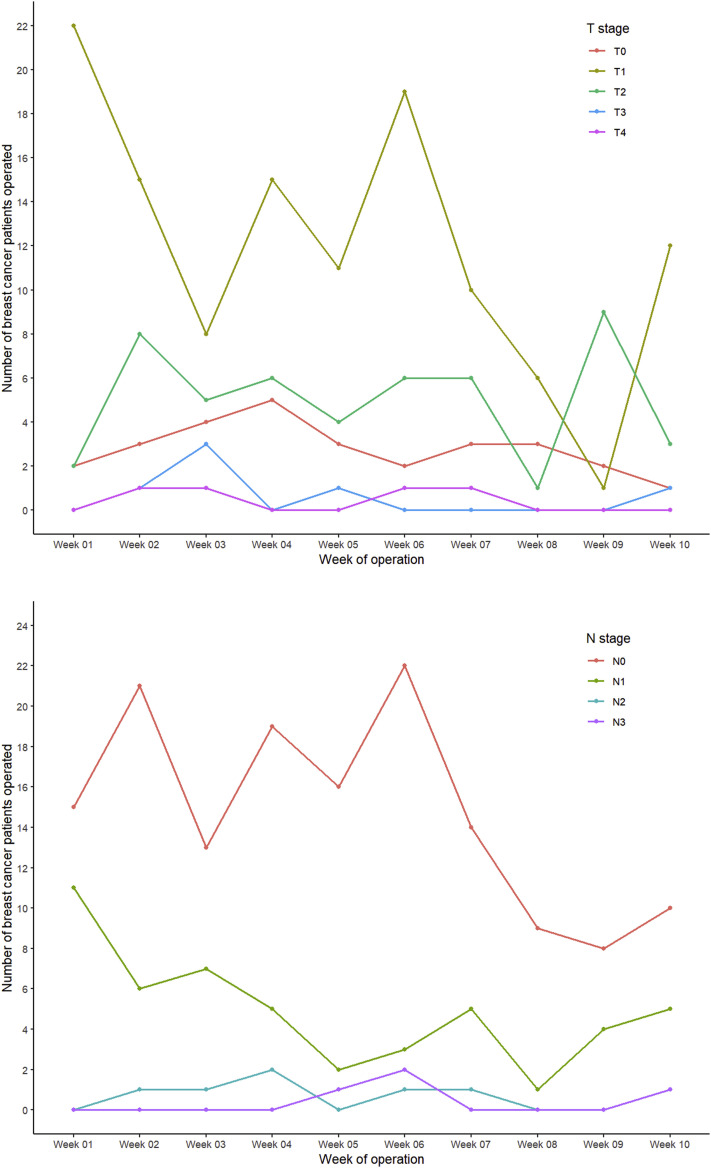
The number of breast cancer patients with T1 and N1 tumors had gradually declined during the study period. A decrease in the number of patients with stage N0 tumors was seen after week 6. The other T and N stages remained stable during the study period (Figure 3 ).
Figure 3.
Number of Surgical Procedures Stratified by T and N Stage
Complications
No increase in the number or severity of postoperative complications was found during the study period. Multivariate analysis demonstrated that only the number of comorbidities and surgery type contributed to the risk of postoperative complications in the patients requiring breast cancer surgery (Table 2 ). Patients undergoing mastectomy had a significantly greater risk of developing postoperative complications (OR, 3.73; 95% CI, 1.14-12.23; P = .030) compared with patients undergoing BCT. Likewise, the number of comorbidities increased the risk of postoperative complications (OR, 1.95; 95% CI, 1.05-3.45; P = .035). COVID-19–related symptoms and neoadjuvant therapy did not increase the risk of postoperative complications.
Table 2.
Multivariate Analysis Results of Risk of Postoperative Complications
| Parameter | Estimate | OR (95% CI) | SE | Z Value | P Value |
|---|---|---|---|---|---|
| Week of study | −0.143 | 0.87 (0.70-1.08) | 0.11 | −1.301 | .193 |
| Patient age | 0.038 | 1.04 (0.99-1.09) | 0.026 | 1.478 | .140 |
| BMI | −0.033 | 0.97 (0.89-1.05) | 0.042 | −0.801 | .423 |
| Comorbidities | 0.670 | 1.95 (1.05-3.65) | 0.318 | 2.104 | .035 |
| ASA classification | 0.405 | 1.50 (0.53-4.26) | 0.533 | 0.760 | .448 |
| T stage | −0.190 | 0.83 (0.40-1.70) | 0.369 | −0.516 | .606 |
| N stage | −0.310 | 0.73 (0.26-2.07) | 0.529 | −0.586 | .558 |
| Type of surgery | |||||
| BCT | NA | 1.00 (reference) | NA | NA | NA |
| Mastectomy | 1.318 | 3.73 (1.14-12.23) | 0.605 | 2.177 | .030 |
| Other | 0.583 | 1.79 (0.15-21.74) | 1.273 | 0.458 | .647 |
| Neoadjuvant therapy | 0.625 | 1.87 (0.50-7.02) | 0.675 | 0.926 | .355 |
| COVID-19 symptoms | 0.070 | 1.07 (0.29-3.90) | 0.659 | 0.106 | .916 |
Abbreviations: ASA = American Society of Anesthesiologists; BCT = breast-conserving therapy; BMI = body mass index; CI = confidence interval; COVID-19 = coronavirus disease 2019; OR = odds ratio, NA = not applicable; SE = standard error.
Discussion
The results from the present multicenter retrospective cohort study showed an overall decrease in the number of breast cancer surgeries performed during the COVID-19 pandemic in the Netherlands. Furthermore, the number of referrals from the national screening program and GPs had declined. Also, no increase in the number of postoperative complications occurred during the study period. Finally, the presence of COVID-19–related symptoms did not increase the risk of postoperative complications.
The COVID-19 pandemic has placed an enormous strain on healthcare systems worldwide. Many measures taken by hospitals in less affected areas, such as the reallocation of hospital resources and prioritizing care, were based on the experiences from the countries highly affected by the pandemic.20, 21, 22 For surgical procedures, the guidelines advised providing only the most essential (oncologic) care to accommodate the increased demand for the care of patients with COVID-19 in hospitals. In addition, this advice was given to reduce the risk of postoperative complications in patients with COVID-19 and to reduce the risk of spreading the disease.23 , 24 As in other countries, in the Netherlands, the authorities recommended only performing essential surgery.6 Furthermore, the Dutch national screening program for breast cancer was temporarily halted from mid-March 2020 to mid-June 2020 to allow for reallocation of healthcare workers.4 Before the COVID-19 pandemic, ∼40% of new-onset breast cancer cases were detected through the national screening program.9 , 10 The combination of the temporary halt of national screening and the recently reported decline in new breast cancer diagnoses is worrisome.9 , 10
The halt of the national screening programs affected the number of surgical procedures for breast cancer. The results from the present study demonstrated a sharp decrease in the number of breast cancer surgeries. This decrease occurred because surgical resection with or without radiotherapy and/or systemic treatment is the treatment of choice for patients with new-onset breast cancer.11, 12, 13 Our results have confirmed previous findings that, nationwide, fewer breast cancer cases have been diagnosed during the COVID-19 pandemic.4 Furthermore, this decrease was especially prominent 6 weeks after the temporary closure of the national screening program, equal to week 7 in our study period. In the Netherlands, treatment (neoadjuvant therapy, radiotherapy, and/or surgery) is normally required within 6 weeks after the initial cancer diagnosis.25 Therefore, a decline in the number of surgical procedures could be expected 6 weeks after halting national screening. Moreover, the present study found that, not only had a decrease occurred in those referred through the national screening program, but also a decreased had occurred in those referred by GPs. However, at the beginning of the pandemic, the Dutch government had discouraged patients from visiting their GP unless absolutely necessary. Although the number of patients referred by GPs had increased steadily in the last weeks of the study, the number of patients referred by national screening had decreased further. The increase of GP referrals might have resulted from awareness campaigns by the Dutch government later in the study period, which encouraged patients with symptoms to visit their GP.26
The present study demonstrated a decline in stage T1-T2 and N0 tumors, which can be attributed to the temporary closure of the Dutch national screening program. This was not surprising because most screening-detected breast cancer cases will be diagnosed at an early stage.27 However, we do not believe that the temporary halting of the breast cancer screening program will have a significant effect on long-term outcomes because most breast cancer discovered by the screening program will be early-stage breast cancer that will develop slowly. Thus, we do not believe an increase will occur in the number of higher stage breast cancer cases because the breast cancer screening program was stopped for only 4 months. Our findings have confirmed previous findings from the Netherlands Comprehensive Cancer Organisation, which found fewer breast cancer diagnoses nationwide during the COVID-19 pandemic.4 The number of cases with higher T and N stages appeared to remain relatively stable over time. The decrease in stage T1-T2 and N0 tumors also explains the decrease in BCT procedures performed, because BCT is preferred over mastectomy for these tumor stages.11, 12, 13 Despite the relatively small risk of postoperative complications from breast cancer surgery, many studies have recommended postponing or not performing these procedures during the pandemic.23 , 24 , 28 , 29 However, we found no increase in postoperative complications in the patients undergoing breast cancer surgery during the COVID-19 pandemic in our study. The multivariate analysis results showed that patients undergoing mastectomy and patients with multiple comorbidities had an increased risk of developing postoperative complications. However, these findings are in line with those from previous studies reporting a significantly greater complication rate for patients undergoing mastectomy and patients with more comorbidities.28 , 29
The present study had some limitations. First, the number of included patients was relatively small. Therefore, the number of stage T3-T4 tumors was low, making the recognition of patterns for this patient group more difficult. The relative low number of stage N2-N3 tumors posed similar challenges in pattern recognition over time. However, the findings from our study did show a clear decreasing pattern in the number of lower stage tumors during the study period. Second, only 21 patients were tested for COVID-19, and the tests results for all 21 were negative. No change occurred in the incidence of postoperative complications. However, more research is necessary to determine the direct risk of COVID-19 positivity compared with COVID-19 negativity on the development of postoperative complications for patients undergoing breast cancer surgery. Therefore, it was not possible to determine the direct risk of the presence of COVID-19 on the development of postoperative complications in patients with breast cancer. Third, significant fluctuations were found in the weekly number of patients undergoing surgical procedures, which most likely resulted from the relatively small number of patients. Finally, the present study had focused on surgical care, although nonsurgical care (ie, radiotherapy, chemotherapy, antihormonal therapy) has also likely to have been affected by the COVID-19 pandemic.30 , 31 Because nonsurgical care was not addressed in our study, we could not report the effects of the COVID-19 pandemic on breast cancer treatment in all its facets.
Conclusions
Considering these limitations, the present study has shown that the effect of the COVID-19 pandemic has dramatically decreased the number of breast cancer surgeries performed. This decrease resulted not only from the temporary closure of the national screening program but also from fewer referrals from GPs. We found no change in the number of postoperative complications. However, more research is necessary to determine the direct risk of developing postoperative complications for patients with COVID-19 undergoing breast cancer compared with the risk for patients without COVID-19. Furthermore, provided that the necessary precautions have been taken to avoid the spread of COVID-19, patients should be encouraged to visit their GP, and one should strive to restart national screening programs. However, in the event of a second wave, we would recommend temporary closure of the national screening program but the continuation of surgical procedures for patients referred by GPs.
Clinical Practice Points
-
•
COVID-19 has had enormous effect on regular healthcare worldwide.
-
•
The measures taken to reduce the spread of COVID-19 have included social distancing but also temporary closure of national screening programs.
-
•
The measures taken to reallocate hospital resources to COVID-19 care have included postponing surgical procedures (eg, oncologic surgery).
-
•
Another reason for postponing oncologic surgical procedures was the fear of an increased risk of postoperative complications.
-
•
The present multicenter observational study found a decrease in the number of surgical procedures performed to treat breast cancer.
-
•
This reduction resulted not only from suspension of the national screening program but also from fewer referrals from GPs.
-
•
The decrease in the weekly number of surgical procedures performed was most pronounced for lower stage breast cancer.
-
•
None of the patients with breast cancer undergoing surgery had tested positive for COVID-19; however, only patients with COVID-19–like symptoms (eg, fever, cough, dyspnea) were tested for COVID-19.
-
•
Nevertheless, the multivariate analysis results showed no increase in postoperative complications; thus, even if some of the untested patients had actually been positive for COVID-19, they had not had at an increased risk of developing postoperative complications, because the number of postoperative complications had remained stable during the study period.
Disclosure
The authors have stated that they have no conflict of interest.
References
- 1.Li H., Liu S.-M., Yu X.-H., Tang S.-L., Tang C.-K. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int J Antimicrob Agents. 2020;55:105951. doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corman V.M., Landt O., Kaiser M., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johns Hopkins Center for Systems Science and Engineering Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 Available at:
- 4.Dinmohamed A.G., Visser O., Verhoeven R.H.A., et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21:750–751. doi: 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burki T.K. Cancer guidelines during the COVID-19 pandemic. Lancet Oncol. 2020;21:629–630. doi: 10.1016/S1470-2045(20)30217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nederlanse Vereniging voor Heelkunde Handvat voor chirurgische ingrepen tijdens Corona-crisis 2020 [in Dutch] https://heelkunde.nl/themas/thema?dossierid=23855104&title=Berichten%252bvanuit%252bde%252bNVvH Available at: Accessed: June 1, 2020.
- 7.Integraal Kankercentrum Nederland COVID 19 en borstkanker [in Dutch] https://www.iknl.nl/covid-19 Available at: Accessed: May 29, 2020.
- 8.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 9.Vondeling G.T., Menezes G.L., Dvortsin E.P., et al. Burden of early, advanced and metastatic breast cancer in The Netherlands. BMC Cancer. 2018;18:262–263. doi: 10.1186/s12885-018-4158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koleva-Kolarova R.G., Daszczuk A.M., de Jonge C., et al. A modelling study to evaluate the costs and effects of lowering the starting age of population breast cancer screening. Maturitas. 2018;109:81–88. doi: 10.1016/j.maturitas.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal S., Pappas L., Neumayer L., Kokeny K., Agarwal J. Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg. 2014;149:267–274. doi: 10.1001/jamasurg.2013.3049. [DOI] [PubMed] [Google Scholar]
- 12.Hartmann-Johnsen O.J., Karesen R., Schlichting E., Nygard J.F. Survival is better after breast conserving therapy than mastectomy for early stage breast cancer: a registry-based follow-up study of Norwegian women primary operated between 1998 and 2008. Ann Surg Oncol. 2015;22:3836–3845. doi: 10.1245/s10434-015-4441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Maaren M.C., de Munck L., de Bock G.H., et al. 10 Year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncol. 2016;17:1158–1170. doi: 10.1016/S1470-2045(16)30067-5. [DOI] [PubMed] [Google Scholar]
- 14.Owens W.D. American Society of Anesthesiologists physical status classification system is not a risk classification system. Anesthesiology. 2001;94:378. doi: 10.1097/00000542-200102000-00042. [DOI] [PubMed] [Google Scholar]
- 15.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao A.A., Feneis J., Lalonde C., Ojeda-Fournier H. A pictorial review of changes in the BI-RADS fifth edition. Radiographics. 2016;36:623–639. doi: 10.1148/rg.2016150178. [DOI] [PubMed] [Google Scholar]
- 17.Tan B.Y., Acs G., Apple S.K., et al. Phyllodes tumours of the breast: a consensus review. Histopathology. 2016;68:5–21. doi: 10.1111/his.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beaulieu-Jones B.K., Lavage D.R., Snyder J.W., Moore J.H., Pendergrass S.A., Bauer C.R. Characterizing and managing missing structured data in electronic health records: data analysis. JMIR Med Inform. 2018;6:e11. doi: 10.2196/medinform.8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z. Multiple imputation with multivariate imputation by chained equation (MICE) package. Ann Transl Med. 2016;4:30. doi: 10.3978/j.issn.2305-5839.2015.12.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravikumar N., Nallasamy K., Bansal A., et al. Novel coronavirus 2019 (2019-nCoV) infection: part I—preparedness and management in the pediatric intensive care unit in resource-limited settings. Indian Pediatr. 2020;57:324–334. doi: 10.1007/s13312-020-1785-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guest J.L., Del Rio C., Sanchez T. The 3 steps needed to end the COVID-19 pandemic: bold public health leadership, rapid innovations, and courageous political will. JMIR Public Health Surveill. 2020;6:e19043. doi: 10.2196/19043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zangrillo A., Beretta L., Silvani P., et al. Fast reshaping of intensive care unit facilities in a large metropolitan hospital in Milan, Italy: facing the COVID-19 pandemic emergency. Crit Care Resusc. 2020;22:91–94. doi: 10.51893/2020.2.pov1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Jabir A., Kerwan A., Nicola M., et al. Impact of the coronavirus (COVID-19) pandemic on surgical practice—part 1. Int J Surg. 2020;79:168–179. doi: 10.1016/j.ijsu.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Jabir A., Kerwan A., Nicola M., et al. Impact of the coronavirus (COVID-19) pandemic on surgical practice—part 2 (surgical prioritisation) Int J Surg. 2020;79:233–248. doi: 10.1016/j.ijsu.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.SONCOS (Stitchting Oncologische Samenwerking) Dutch guidelines stating that all patients must have had the first treatment within 6 weeks after cancer diagnosis [in Dutch] https://www.nvog.nl/wp-content/uploads/2018/02/SONCOS-normeringsrapport-versie-5-2017.pdf Available at:
- 26.Dutch Ministry Campaign to encourage patients to visit the general practitioner during COVID-19 in order to keep up with cancer diagnoses [in Dutch] https://www.rijksoverheid.nl/documenten/mediateksten/2020/04/17/letterlijke-tekst-persconferentie-na-ministerraad-17-april-2020 Available at:
- 27.Elferink M.A.G., Toes-Zoutendijk E., Vink G.R., et al. [National population screening for colorectal carcinoma in the Netherlands: results of the first years since the implementation in 2014] Ned Tijdschr Geneeskd. 2018;162:D2283. [PubMed] [Google Scholar]
- 28.El-Tamer M.B., Ward B.M., Schifftner T., Neumayer L., Khuri S., Henderson W. Morbidity and mortality following breast cancer surgery in women. Ann Surg. 2007;245:665–671. doi: 10.1097/01.sla.0000245833.48399.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ten Wolde B., Kuiper M., de Wilt J.H.W., Strobbe L.J.A. Postoperative complications after breast cancer surgery are not related to age. Ann Surg Oncol. 2017;24:1861–1867. doi: 10.1245/s10434-016-5726-x. [DOI] [PubMed] [Google Scholar]
- 30.van Maaren M.C., Strobbe L.J.A., Koppert L.B., Poortmans P.M.P., Siesling S. Nationwide population-based study of trends and regional variation in breast-conserving treatment for breast cancer. Br J Surg. 2018;105:1768–1777. doi: 10.1002/bjs.10951. [DOI] [PubMed] [Google Scholar]
- 31.Kilsdonk M.J., van Dijk B.A., Otter R., van Harten W.H., Siesling S. Regional variation in breast cancer treatment in the Netherlands and the role of external peer review: a cohort study comprising 63,516 women. BMC Cancer. 2014;14:596. doi: 10.1186/1471-2407-14-596. [DOI] [PMC free article] [PubMed] [Google Scholar]