Editor—Coronavirus disease 2019 (COVID-19) results in a broad spectrum of clinical presentations, including viral pneumonia and acute respiratory distress syndrome (ARDS).1 Increasingly compelling evidence suggests that the underlying pathophysiology of severe COVID-19 pneumonia is microvascular thrombosis.2 , 3 Although the pulmonary vasculature can be seen in exquisite detail with computed tomography pulmonary angiography (CTPA), routine CTPA is neither feasible nor recommended in COVID-19 patients.4 Therefore, tools enabling bedside evaluation of lung perfusion, including monitoring the response to therapeutic interventions such as anticoagulation, are increasingly important during this pandemic.
Electrical impedance tomography (EIT) is a noninvasive, bedside, radiation-free technology that allows clinicians to monitor and optimise ventilation strategies in real time.5 , 6 More recently, EIT has been used to assess regional lung perfusion in critically ill patients.6, 7, 8 In a swine study, EIT was equivalent to high-sensitivity positron emission tomography in detecting alterations in lung perfusion.9 An observational study of 68 patients with acute respiratory failure showed that the evaluation of dead space with EIT (with a cut-off of 30.4%) results in a sensitivity of 90.9% and a specificity of 98.6% for diagnosis of pulmonary embolism.7
An available EIT method for generating lung perfusion maps is a first-pass kinetic approach performed by rapid injection of 10 ml hypertonic sodium chloride as a contrast agent through a central venous catheter during an end-expiratory hold manoeuvre on the mechanical ventilator.6 It is ideally suited to study the causes of refractory hypoxaemia, and to monitor disease progression and the response to intervention in this patient population.10 Here, we describe the use of EIT to diagnose a significant lung perfusion defect and to monitor response to anticoagulation over time until normalisation of lung perfusion. Confirmation of pulmonary thrombosis and its resolution was obtained using CTPA.
A 66-yr-old man, who consented to this correspondence, presented to the emergency department with progressively worsening shortness of breath, cough, and fever suggestive for COVID-19 pneumonia. He was severely dyspnoeic, requiring 6 L min−1 of oxygen by nasal prongs (?) to maintain an oxygen saturation of 92–93%. His D-dimer was increased (7473 ng ml−1), consistent with the acute inflammatory process of COVID-19, and suspicion for pulmonary embolism was low. Deterioration of the patient and increasing oxygen requirement resulted in tracheal intubation for respiratory failure (arterial partial pressure of oxygen [Pao 2]/fraction of inspired oxygen [FiO2] ratio, 247 mm Hg), and he was admitted to the ICU. Real-time reverse transcriptase–polymerase chain reaction testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was positive. On hospital day 6, because of progressive deterioration in gas exchange (Pao 2/FiO2=120 mm Hg) despite lung-protective measures including pronation and the initiation of inhaled nitric oxide (iNO), EIT was used to evaluate regional ventilation and pulmonary blood flow distribution.
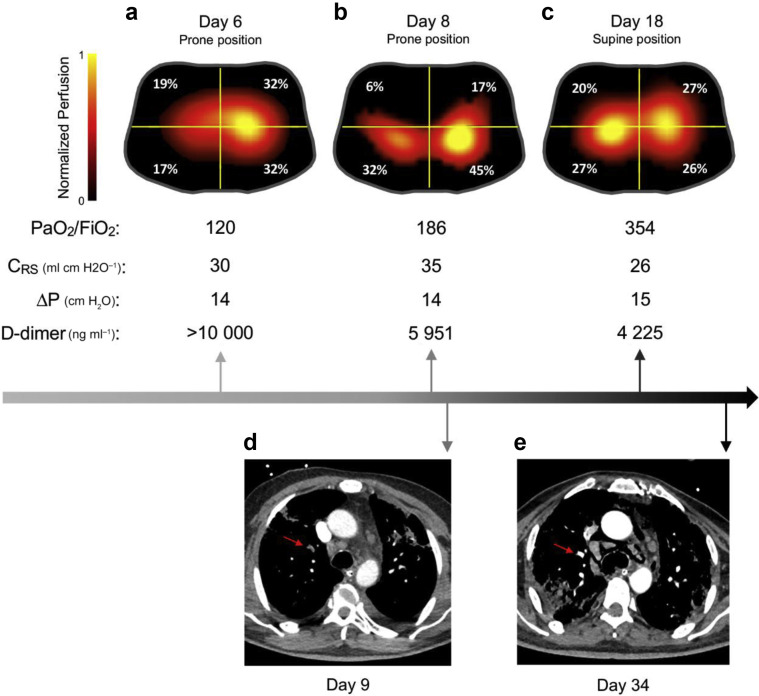
The patient was evaluated in the prone position while receiving 40 ppm iNO and lung-protective mechanical ventilation with PEEP of 14 cm H2O. EIT showed homogenous ventilation, with dead space estimated at 66% of the tidal volume, and imbalanced perfusion distribution, with a significant discrepancy between the left (64%) and right (36%) lungs (Fig. 1 a). Our findings were consistent with a major perfusion impairment, likely caused by pulmonary thrombosis (D-dimer >10 000 ng ml−1). Echocardiography revealed an increased right ventricular systolic pressure (>35 mm Hg) and signs of right ventricular strain despite iNO administration, suggesting the presence of pulmonary hypertension. A bubble test excluded an intracardiac shunt. As a result, the ICU team started therapeutic anticoagulation with continuous infusion of unfractionated heparin i.v. A second EIT (Fig. 1b) was performed on day 8 and was followed by CTPA (Fig. 1d) that confirmed segmental and subsegmental right upper lobe pulmonary perfusion defects, matching the location of the deficit indicated by EIT. Over the subsequent 10 days, the patient improved clinically, with a reduction in D-dimer and an improvement in oxygenation (Pao 2/FiO2=354 mm Hg). A tracheostomy was performed on day 17, and repeat EIT (Fig. 1c) showed homogenous lung perfusion and subsequent CTPA on day 34 (Fig. 1e) confirmed resolution of the pulmonary artery filling defects. The D-dimer continued to decrease to 3222 ng ml−1. Ventilatory settings and respiratory mechanics remained virtually unchanged throughout. At hospital day 58, the tracheostomy was closed with oxygen therapy via nasal cannula (1–3 L min−1), with discharge to a pulmonary rehabilitation centre after 68 days of hospitalisation.
Fig 1.
Serial lung perfusion evaluation in a COVID-19 patient. (a–c) Electrical impedance tomography (EIT) perfusion images obtained at days 6, 8, and 18. Images were generated with Enlight 1800 (Timpel SA, São Paulo, Brazil) using the first-pass kinetics method. The lung is divided into four quadrants (regions of interest). Colour scale was adjusted by linear normalisation. (d) Axial CT pulmonary angiography image shows segmental and subsegmental pulmonary emboli in the right upper lobe (red arrow). (e) Axial CT pulmonary angiography image shows resolution of the pulmonary artery filling defect (red arrow) in comparison with the previous image. Day, day from admission to emergency department; CRS, respiratory system compliance; ΔP, driving pressure.
Recent radiological, physiological, and pathological studies suggest a possible central role for pulmonary vascular alterations in the pathophysiology of COVID-related hypoxaemia.2 , 3 We found that EIT detected imbalance in lung perfusion despite even distribution of ventilation in a patient who was later confirmed to have pulmonary thrombosis. EIT was also effective in showing resolution of the perfusion defects following treatment. Follow-up CTPA provided evidence for resolution of clots, confirming what was observed with the EIT.
EIT is a radiation-free, noninvasive bedside technique that allows individualised ventilation setting,6 identification of perfusion impairment that might require CTPA,7 and multiple assessments of pulmonary perfusion over time to determine response to therapies.
Declarations of interest
LB is supported by US NIH/NHLBI grant K23HL128882, receives devices and equipment from Praxair Inc. and Masimo Corp., and has a grant from iNO Therapeutics LLC. This study was supported by the Reginald Jenney Endowment Chair at Harvard Medical School to LB, by LB Sundry Funds at Massachusetts General Hospital, and by laboratory funds of the Anesthesia Center for Critical Care Research of the Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital. RMK is a consultant for Medtronic, Orange Med, and has received research grants from Medtronic and Venner Medical (Dänischenhagen, Germany). All other authors have no conflicts to declare.
References
- 1.Guan W.-J., Ni Z.-Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;80:656–665. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackermann M., Verleden S.E., Kuehnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. Covid-19 does not lead to a ‘typical’ acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ACR Recommendations for the use of chest radiography and computed tomography (CT) for suspected COVID-19 infection. Available from: https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection (accessed 12 May 2020).
- 5.Girrbach F., Petroff D., Schulz S. Individualised positive end-expiratory pressure guided by electrical impedance tomography for robot-assisted laparoscopic radical prostatectomy: a prospective, randomised controlled clinical trial. Br J Anaesth. 2020 doi: 10.1016/j.bja.2020.05.041. http://www.sciencedirect.com/science/article/pii/S0007091220304232 Available from: [DOI] [PubMed] [Google Scholar]
- 6.Bachmann M.C., Morais C., Bugedo G. Electrical impedance tomography in acute respiratory distress syndrome. Crit Care. 2018;22:263. doi: 10.1186/s13054-018-2195-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He H., Chi Y., Long Y. Bedside evaluation of pulmonary embolism by saline contrast electrical impedance tomography method: a prospective observational study. Am J Respir Crit Care Med Am Thorac Soc – AJRCCM. 2020 doi: 10.1164/rccm.202005-1780LE. https://www.atsjournals.org/doi/10.1164/rccm.202005-1780LE Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grassi L.G., Santiago R., Florio G., Berra L. Bedside evaluation of pulmonary embolism by electrical impedance tomography. Anesthesiology. 2020;132:896. doi: 10.1097/ALN.0000000000003059. [DOI] [PubMed] [Google Scholar]
- 9.Bluth T., Kiss T., Kircher M. Measurement of relative lung perfusion with electrical impedance and positron emission tomography: an experimental comparative study in pigs. Br J Anaesth. 2019;123:246–254. doi: 10.1016/j.bja.2019.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarantonello F., Andreatta G., Sella N., Navalesi P. Prone position and lung ventilation/perfusion matching in acute respiratory failure due to COVID-19. Am J Respir Crit Care Med. 2020;202:278–279. doi: 10.1164/rccm.202003-0775IM. [DOI] [PMC free article] [PubMed] [Google Scholar]