Abstract
Background
Anti-CD19 chimeric antigen receptor (CAR) T cell therapy holds great promise in the treatment of patients with hematologic malignancies. A high occurrence of cardiac dysfunction has been noted in children treated with CAR T cell therapy.
Objectives
The aim of this study was to define the occurrence of major adverse cardiovascular events (MACE) in adult patients treated with CAR T cell therapy and assess the relationships among clinical factors, echocardiographic parameters, laboratory values, and cardiovascular outcomes.
Methods
Baseline clinical, laboratory, and echocardiographic parameters were collected in 145 adult patients undergoing CAR T cell therapy. MACE included cardiovascular death, symptomatic heart failure, acute coronary syndrome, ischemic stroke, and de novo cardiac arrhythmia. Baseline parameters associated with MACE were identified using Cox proportional cause-specific hazards regression analysis.
Results
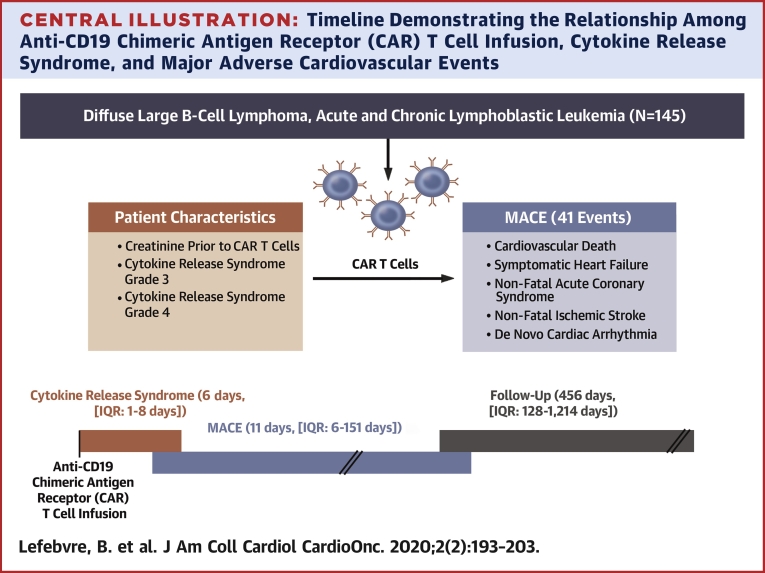
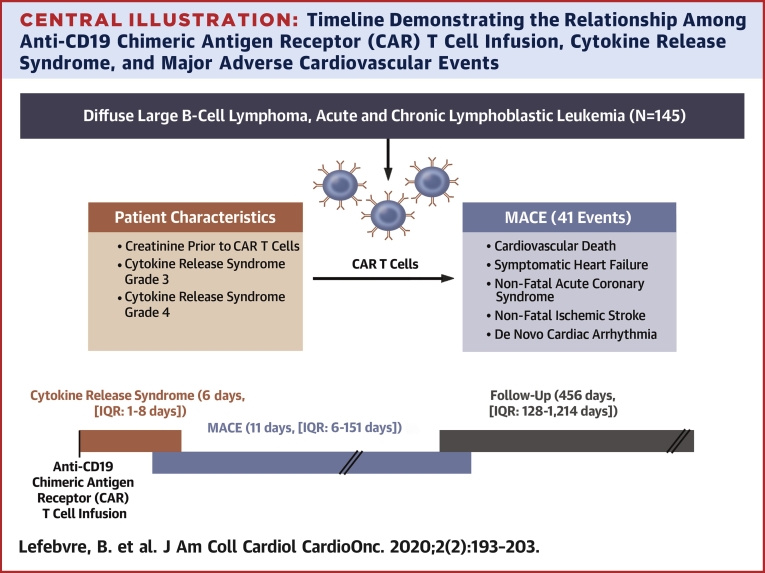
Thirty-one patients had MACE (41 events) at a median time of 11 days (interquartile range: 6 to 151 days) after CAR T cell infusion. The median follow-up period was 456 days (interquartile range: 128 to 1,214 days). Sixty-one patients died. Cytokine release syndrome (CRS) occurred 176 times in 104 patients; the median time to CRS was 6 days (interquartile range: 1 to 8 days). The Kaplan-Meier estimates for MACE and CRS at 30 days were 17% and 53%, respectively. The Kaplan-Meier estimates for survival at 1 year was 71%. Multivariable Cox proportional cause-specific hazards regression analysis determined that baseline creatinine and grade 3 or 4 CRS were independently associated with MACE.
Conclusions
Patients treated with CAR T cell therapy are at an increased risk for MACE and may benefit from cardiovascular surveillance. Further large prospective studies are needed to confirm the incidence and risk factors predictive of MACE.
Key Words: cardio-oncology, cardiovascular, CAR T cells
Abbreviations and Acronyms: ALL, acute lymphoblastic leukemia; CAD, Coronary artery disease; CAR, Chimeric antigen receptor; CHF, congestive heart failure; CI, confidence interval; CLL, chronic lymphocytic leukemia; CRS, cytokine release syndrome; DLBCL, diffuse large B-cell lymphoma; HR, hazard ratio; HUP, Hospital of the University of Pennsylvania; IQR, interquartile range; KM, Kaplan-Meier; LVEF, left ventricular ejection fraction; MACE, major adverse cardiovascular events
Central Illustration
Anti-CD19 chimeric antigen receptor (CAR) T cell therapy, first developed at the University of Pennsylvania, is a tremendous advance in the field of hematologic malignancies. The patient’s own T cells are engineered to target CD19, an antigen frequently and highly expressed in some B cell malignancies, notably acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL), and diffuse large B-cell lymphoma (DLBCL) (1,2). In clinical trials, CAR T cell therapy has shown a remarkable response rate (70% to 90%) and durability of remission; in a chemotherapy-resistant DLBCL population, 65% of patients were recurrence free at 1 year (3, 4, 5). To date, there are 2 U.S. Food and Drug Administration–approved CD19 immunotherapies for refractory B-cell ALL and large B-cell lymphoma, tisagenlecleucel (Kymriah, Novartis, Basel, Switzerland) and axicabtagene ciloleucel (Yescarta, Kite Pharma, Santa Monica, California).
A frequent and potentially lethal side effect of CAR T cell therapy is cytokine release syndrome (CRS), with symptoms including mild to high fever, severe hypotension, and hypoxia, occurring usually within days after CAR T cell therapy. In a recent large systematic review and meta-analysis, CRS was reported in more than one-half of the patients receiving CAR T cell therapy (6). CRS is a systemic inflammatory response syndrome triggered in particular by the release of large amounts of interleukin-6 (7). The release of cytokines induces fever, vascular leakage, and potentially direct myocardial injury.
Cardiovascular complications and potential cardiotoxicity of CAR T cell therapy are especially relevant concerns in this patient population, as they may have pre-existing cardiac dysfunction or risk factors (8) and/or received multiple previous cycles of cardiotoxic chemotherapy. Cardiovascular complications of CAR T cell therapy have been reported in 2 retrospective studies in the pediatric population (1,9) and recently in a third retrospective study in adults (10). In 39 children with acute leukemia, Fitzgerald et al. (9) reported that more than one-third of patients developed cardiovascular complications such as shock or cardiomyopathy. In the other study, by Burstein et al. (1), 24 patients among 98 children (24%) had hypotension requiring inotropic support. Of these 24 patients, 10 (50% of children who underwent echocardiography) demonstrated left ventricular systolic dysfunction. The cardiomyopathy was mostly reversible in these children. Left ventricular dysfunction in the pediatric heart, however, may be different in severity, reversibility, and progression compared with the adult heart.
In the present study, we defined the rate of occurrence and the natural history of cardiovascular events in all consecutive adult patients treated with CAR T cell therapy. Clinical, laboratory, and echocardiographic parameters were collected to investigate the association between these parameters and the cardiovascular outcomes of patients treated with CAR T cell therapy.
Methods
Identification of patients and endpoints
The study was conducted at the Hospital of the University of Pennsylvania (HUP) and was approved by the local Institutional Review Board. All consecutive adult patients (≥18 years of age) with CD19+ malignancy (DLBCL, ALL, or CLL) treated with experimental and commercial CAR T cell therapy at HUP between August 2010 and January 2019 were identified. Major adverse cardiovascular events (MACE) (11) were defined as cardiovascular death, symptomatic heart failure, nonfatal acute coronary syndrome, nonfatal ischemic stroke, and de novo cardiac arrhythmia. To identify the occurrence of MACE, each chart was reviewed individually (B.L.). All MACE were recorded and defined using the American College of Cardiology/American Heart Association outcome definitions outlined for clinical trials by Hicks et al. (11). Symptomatic heart failure was identified when 3 or more of the following 4 criteria were met: symptoms of heart failure, clinical signs of heart failure on physical examination, laboratory or imaging or radiographic findings of heart failure (B-type natriuretic peptide or N-terminal pro–B-type natriuretic peptide, Kerley B-lines or pulmonary edema, pleural effusion, decreased left ventricular ejection fraction [LVEF]), and initiation of new treatment for heart failure (pharmacological therapies such as diuretic agents and/or mechanical support). One or more symptoms of heart failure and 2 or more signs on physical examination were necessary for the diagnosis. Symptoms of heart failure were defined as dyspnea at rest or during exercise, decreased exercise capacity, and symptoms of volume overload. Clinical signs on physical examination were defined as peripheral edema, ascites in the absence of hepatic disease, pulmonary crackles or rales, increased jugular venous pressure, S3 gallop, and significant and rapid weight gain related to fluid retention. De novo cardiac arrhythmias such as atrial fibrillation were identified on electrocardiography. All cardiovascular events were adjudicated by 2 independent cardiologists blinded to all other clinical and quantitative echocardiographic data (B.L. and Y.K.). A third cardiologist (M.S.-C.) further adjudicated if any disagreement occurred between the 2 initial reviewers. Signs and symptoms of heart failure arising concurrently with sepsis were excluded from MACE.
Cardiovascular risk factors and pre-existing cardiovascular disease were extracted manually from each electronic medical record. All baseline characteristics were reported at the time of or most proximal to CAR T infusion. Cardiovascular disease was defined by pre-existing coronary artery disease, chronic heart failure, atrial fibrillation, or stroke. Cardiovascular risk factors such as hypertension, hypercholesterolemia, and diabetes were classified as present if both diagnosis and treatment were identified in the medical chart. Other cardiovascular risk factors, such as obesity and smoking history, were also identified. Noncardiac death was also reported and included death due to septic shock, multiple organ failure, or progression of cancer. To minimize loss of follow-up from death, every patient was also searched in a national necrology database. Vital signs and laboratory results were extracted from the HUP electronic medical record.
CRS events were reviewed and graded according to the latest American Society for Transplantation and Cellular Therapy consensus grading for CRS (12). CRS is defined into 4 grades according to symptoms and clinical status. Grades 3 and 4 CRS include temperature ≥38°C, hypotension requiring at least 1 vasopressor, and/or hypoxia with high-flow oxygen supplementation, positive pressure, or intubation.
Echocardiographic analysis
Echocardiographic images were extracted from the HUP database. Measurements were acquired by a single observer (B.L.) blinded to clinical data and the occurrence of MACE and reported from the average of 3 consecutive cardiac cycles using the recommendations from the American Society of Echocardiography. LVEF was calculated using the modified Simpson biplane method, and left atrial volume was calculated using the biplane method (13).
Statistical analysis
Categorical data are expressed as percentages and continuous data as mean ± SD or median (interquartile range [IQR]). Normality was determined using the Kolmogorov-Smirnov test. Differences among patients with ALL, CLL, and DLBCL were determined using 1-way analysis of variance or the Kruskal-Wallis test. Kaplan-Meier (KM) estimates at 30 days, 6 months, and 12 months were also performed for each cancer subtype and MACE, CRS, and survival rates. The cumulative incidence function was used to estimate the incidence of MACE and noncardiac death.
A Cox proportional cause-specific hazards regression analysis was used to determine the parameters associated with MACE in our population. All variables were tested using univariable analysis. Initially, clinically significant noncollinear variables with p values <0.10 by univariable analysis were entered into a multivariable Cox proportional cause-specific hazards regression. Multicollinearity was tested using the variance inflation factor. The decision was made to use a maximum of 4 variables (41 events, 1 parameter per 10 events) to avoid model instability. To confirm the results, multivariable Cox proportional cause-specific hazards regression was repeated with stepwise regression using the backward elimination method using all variables with p values <0.10 by univariable analysis. All variables were subsequently reentered individually into the final model, to assess for significance in the presence of the final model variables. Hazard ratios (HRs) are expressed with 95% confidence intervals (CIs). All CRS gradings were compared with CRS grade 0 as an indicator. Univariable and multivariable analysis were also confirmed using the Fine and Gray distribution hazard regression model in a competing risk regression model.
Data were analyzed using SPSS version 16.0 (SPSS, Chicago, Illinois) and R version ii386 3.5.0 (R Foundation for Statistical Computing, Vienna, Austria). A p value <0.05 was considered to indicate statistical significance.
Results
Characteristics of patients treated with CAR T Cell therapy
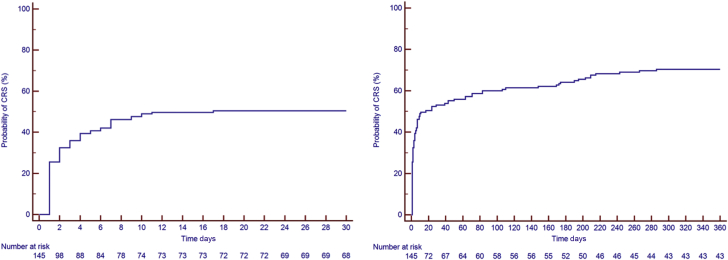
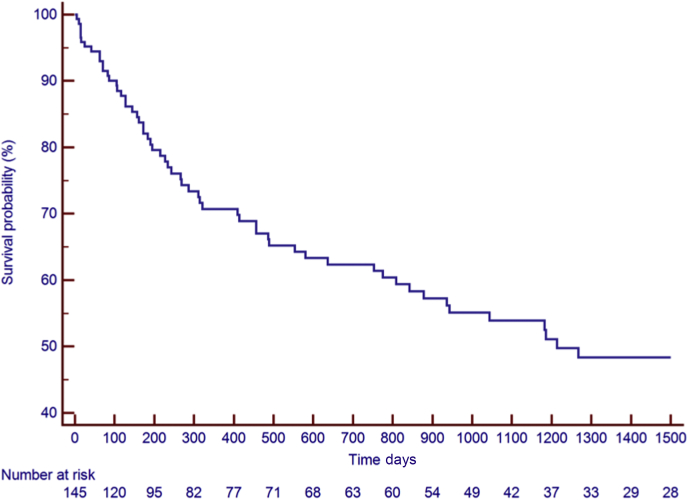
Between August 2010 and January 2019, a total of 145 patients were identified (median age 60 years [IQR: 50 to 66 years], 74% men). Thirty-six patients (25%) were diagnosed with ALL, 43 patients with DLBCL (30%), and 66 patients (46%) with CLL. Baseline clinical characteristics, reported at the time of or most proximal to CAR T cell infusion, medications, and laboratory results are presented in Table 1. The median follow-up period was 456 days (IQR: 128 to 1,214 days; range: 5 to 3,103 days). There were 176 occurrences of CRS in 104 patients (72%), with a median time to CRS of 6 days (IQR: 1 to 8 days; range: 0 to 175 days). The KM estimates for CRS rate were 53% at 30 days, 64% at 6 months, and 71% at 12 months (Figure 1). Sixty-one patients died, 59 of them of noncardiac causes. The KM estimates for overall survival was 95% at 30 days, 81% at 6 months, and 71% at 12 months (Figure 2). Six patients (4%) were lost to follow-up at a median time of 391 days.
Table 1.
Characteristics of 145 Patients Treated With CAR T Cell Therapy
| All Patients∗ (N = 145) | MACE (n = 31) | No MACE∗ (n = 114) | |
|---|---|---|---|
| Baseline demographic and cardiovascular characteristics | |||
| Median age at infusion (yrs) | 60 (50–66) | 50 (29–61) | 61 (54–67) |
| Male | 107 (74) | 26 (84) | 81 (71) |
| History of smoking (current or former) | 59 (41) | 16 (52) | 43 (38) |
| Hypertension | 52 (36) | 13 (42) | 39 (34) |
| Diabetes | 13 (9) | 4 (13) | 9 (8) |
| Hyperlipidemia | 44 (30) | 13 (42) | 31 (27) |
| Heart failure | 4 (3) | 1 (3) | 3 (3) |
| Coronary artery disease | 14 (10) | 5 (16) | 9 (8) |
| Atrial fibrillation | 12 (8) | 5 (16) | 7 (6) |
| Baseline LVEF (%) | 61 ± 9 (n = 124 [86%]) | 62 ± 7 (n = 28 [90%]) | 61 ± 9 (n = 96 [84%]) |
| Type of cancer | |||
| Acute lymphoblastic leukemia | 36 (25) | 8 (26) | 28 (25) |
| Chronic lymphocytic leukemia | 66 (46) | 18 (58) | 48 (42) |
| Diffuse large B-cell lymphoma | 43 (30) | 5 (16) | 38 (33) |
| Baseline treatment and medication | |||
| Previous anthracycline use | 87 (60) | 14 (45) | 73 (64) |
| Radiation | 33 (23) | 4 (13) | 29 (25) |
| Aspirin | 9 (6) | 4 (13) | 5 (4) |
| ACE inhibitors/ARBs | 24 (17) | 6 (19) | 18 (16) |
| Beta-blockers | 27 (19) | 7 (23) | 20 (18) |
| Statins | 31 (21) | 12 (39) | 19 (17) |
| Oral hyperglycemic agents | 11 (8) | 3 (10) | 8 (7) |
| Insulin | 5 (3) | 3 (10) | 2 (2) |
| Baseline blood tests, vital signs, and outcomes | |||
| Hgb (g/dl) | 11.3 ± 2.2 | 10.7 ± 2.3 | 11.5 ± 2.2 |
| Median WBC count (×103/μl) | 3.0 (1.2–6.3) | 2.1 (0.5–3.9) | 3.3 (1.6–6.9 |
| Platelet count (×103/μl) | 114 ± 66 | 94 ± 65 | 120 ± 65 |
| Creatinine (mg/dl) | 0.92 ± 0.27 | 1.03 ± 0.27 | 0.89 ± 0.26 |
| Median GFR (ml/min/1.73 m2) | 85.0 (67.6–111.7) | 112.0 (91.5–162.0) | 79.0 (65.5–95.5) |
| Systolic blood pressure at infusion (mm Hg) | 121 ± 16 | 118 ± 15 | 121 ± 16 |
| Diastolic blood pressure at infusion (mm Hg) | 70 ± 10 | 66 ± 8 | 71 ± 10 |
| Anti-interleukin-6 use | 36 (25) | 15 (48) | 21 (18) |
| Vasopressor use | 33 (23) | 17 (52) | 16 (14) |
Values are median (interquartile range), n (%), or mean ± SD.
ACE = angiotensin-converting enzyme; ARB = angiotensin II receptor blocker; CAR T cell = anti-CD19 chimeric antigen receptor T cell; GFR = glomerular filtration rate; Hgb = hemoglobin; LVEF = left ventricular ejection fraction; MACE = major adverse cardiovascular events; WBC = white blood cell.
Patients lost to follow-up and those with with noncardiac death prior to MACE are included.
Figure 1.
Kaplan-Meier Estimates of CRS
(Left) Kaplan-Meier curve at 30 days. (Right) Kaplan-Meier curve over 360 days. The Kaplan-Meier estimates for cytokine release syndrome (CRS) were 53% at 30 days, 64% at 6 months, and 71% at 12 months.
Figure 2.
Kaplan-Meier Estimates of Survival Rate
The Kaplan-Meier estimates for survival were 95% at 30 days, 81% at 6 months, and 71% at 12 months.
Occurrence and characteristics of MACE developed by patients treated with CAR T Cell therapy
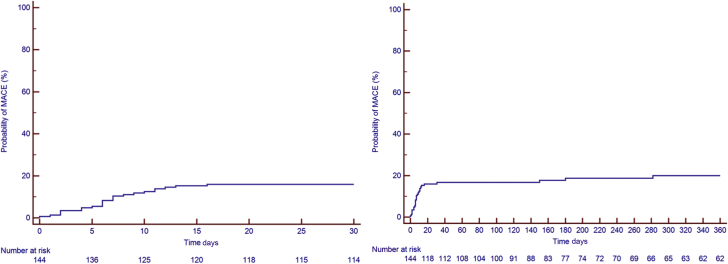
Thirty-one patients developed MACE, for a total of 41 events. All events were considered in the subsequent analyses. The median time to MACE was 11 days after CAR T cell infusion (IQR: 6 to 151 days; range 0 to 2,372 days). The KM estimates for MACE were 17% at 30 days, 19% at 6 months, and 21% at 12 months (Figure 3). The adjudication of all heart failure events is included in Supplemental Table 1. The median follow-up period of patients in the MACE group was 753 days (IQR: 215 to 1,714 days; range: 14 to 3,061 days). There were 22 heart failure events (1 of which was a stress-induced cardiomyopathy) in 21 patients (15%), 12 episodes of atrial fibrillation in 11 patients (7.5%), 2 events of other arrhythmias (supraventricular tachycardia, nonsustained ventricular tachycardia), 2 episodes of acute coronary syndrome, and 2 cardiac deaths. The cardiac deaths were a pulseless electric activity arrest and a massive pulmonary embolism leading to a ST-segment elevation myocardial infarction. Two patients had atrial fibrillation concurrent with the use of ibrutinib and thus were not included among those experiencing MACE. The cumulative incidence of MACE and noncardiac deaths in all patients is shown in Supplemental Figure 1.
Figure 3.
Kaplan-Meier Estimates of MACE
(Left) Kaplan-Meier curve at 30 days. (Right) Kaplan-Meier curve over 360 days. The Kaplan-Meier estimates for major adverse cardiovascular events (MACE) were 17% at 30 days, 19% at 6 months, and 21% at 12 months.
Echocardiographic characteristics of patients treated with CAR T Cell therapy
Baseline echocardiographic findings are listed in Table 2. LVEF obtained both from echocardiography and multiple-gated acquisition was available for 124 patients (86%) and averaged 61 ± 9%. Prior to CAR T cell infusion, 110 patients underwent echocardiography (78 of whom had full reports available that could be used for further analysis). The median time from echocardiography to CAR T cell infusion was 43 days (IQR: 21 to 80 days; range: 2 to 615 days) and was not associated with MACE. The mean baseline LVEF in patients who developed MACE was 62 ± 7% (echocardiography performed in 28 patients) and 61 ± 9% in patients who did not. Seventeen patients (55% of those with MACE) underwent repeat echocardiography when MACE occurred. The mean LVEF measured at the time of MACE was 49 ± 14%.
Table 2.
Echocardiographic Parameters at Baseline
| All Patients∗ (N = 78) | MACE (n = 16) | No MACE∗ (n = 62) | |
|---|---|---|---|
| Heart rate (beats/min) | 75 ± 17 | 72 ± 8 | 75 ± 17 |
| LVEDD (cm) | 4.65 ± 0.53 | 4.72 ± 0.17 | 4.62 ± 0.52 |
| LVESD (cm) | 3.15 ± 0.52 | 3.20 ± 0.32 | 3.14 ± 0.52 |
| IVS (cm) | 0.93 ± 0.18 | 0.97 ± 0.03 | 0.92 ± 0.18 |
| PWT (cm) | 0.93 ± 0.14 | 0.93 ± 0.01 | 0.93 ± 0.14 |
| RV base (cm) | 3.45 ± 0.53 | 3.45 ± 0.52 | 3.46 ± 0.54 |
| TAPSE (cm) | 2.3 ± 0.4 | 2.3 ± 0.5 | 2.3 ± 0.4 |
| RV S′ (mm/s) | 13.2 ± 3.1 | 13.5 ± 2.5 | 13.2 ± 2.7 |
| MV E | 67.8 (59.8–81.0) | 71.8 (63.2–83.1) | 67.6 (59.4–80.7) |
| MV a | 61.0 (52.9–82.9) | 56.4 (46.5–67.2) | 64.2 (54.8–87.4) |
| Mitral E/a ratio | 1.2 ± 0.5 | 1.3 ± 0.2 | 1.2 ± 0.5 |
| Mitral E/e′ | 8.7 ± 3.5 | 10.0 ± 0.1 | 8.3 ± 3.3 |
| PASP (mm Hg) | 27 ± 6 | 30 ± 7 | 26 ± 6 |
| LAVI (cm/m2) | 29.7 ± 11.0 | 35.1 ± 11.7 | 28.0 ± 10.1 |
| LVEF, Simpson method (%) | 62.1 ± 7.2 | 61.8 ± 7.5 | 62.3 ± 6.5 |
Values are mean ± SD or median (interquartile range).
IVS = interventricular septal thickness; LAVI = left atrial volume index; LVEDD = left ventricular end-diastolic diameter; LVESD = left ventricular end-systolic diameter, MV = mitral valve; PASP = pulmonary artery systolic pressure, PWT = posterior wall thickness, RV = right ventricular; TAPSE = tricuspid annular plane systolic excursion; other abbreviations as in Table 1.
Patients lost to follow-up and those with noncardiac death prior to MACE are included.
Clinical, laboratory, and echocardiographic parameters associated with MACE
The differences between patients with and without MACE are presented in Table 1 (and Supplemental Table 2) and the univariable Cox proportional cause-specific hazards regression model in Table 3 (and additional Fine and Gray analysis in Supplemental Table 4). The variables associated with MACE were prior atrial fibrillation (HR: 2.83; 95% CI: 1.08 to 7.43; p = 0.035), aspirin use (HR: 3.13; 95% CI: 1.09 to 8.99; p = 0.034), statin use (HR: 2.29; 95% CI: 1.11 to 4.73; p = 0.025), insulin use (HR: 5.70; 95% CI: 1.70 to 19.08; p = 0.005), baseline creatinine (HR: 4.30 for each 1 mg/dl increase; 95% CI: 1.19 to 15.59; p = 0.026), overall CRS grading (HR: 2.10; 95% CI: 1.47 to 2.98; p < 0.001), CRS grade 2 (HR: 0.39; 95% CI: 0.17 to 0.91; p = 0.029), CRS grade 3 (HR: 3.31; 95% CI: 1.55 to 7.09; p = 0.002), CRS grade 4 (HR: 9.79; 95% CI: 3.96 to 24.21; p < 0.001), diastolic blood pressure (HR: 0.95 for each 1 mm Hg increase; 95% CI: 0.91 to 0.99; p = 0.007), hemoglobin (HR: 0.83 for each 1 g/dl increase; 95% CI: 0.69 to 0.99; p = 0.035), and platelet count (HR: 0.99 for each 1,000/μl increase; 95% CI: 0.99 to 1.00; p = 0.027). There was a trend in the association between MACE with higher E/e′ ratio (HR: 1.15 for each 1-unit increase; 95% CI: 1.00 to 1.31; p = 0.046) and larger indexed left atrial volume (HR: 1.04 for each 1 ml/m2 increase; 95% CI: 1.00 to 1.08; p = 0.075). Analyses of diastolic function using the 2016 guidelines (14) indicated that 31 patients had normal diastolic function (40%), 22 had grade I diastolic dysfunction (28%), 1 had grade II dysfunction (1%), 3 had grade III dysfunction (4%), and 21 had indeterminate diastolic function (27%). There was no association between the presence of diastolic dysfunction and MACE (p = 0.866).
Table 3.
Univariable Cox Proportional Cause-Specific Hazards Regression
| Hazard Ratio (95% CI) | p Value | |
|---|---|---|
| Age | 1.01 (0.98–1.04) | 0.431 |
| Sex (male) | 1.73 (0.66–4.51) | 0.265 |
| Hypertension | 1.40 (0.68–2.89) | 0.362 |
| Diabetes mellitus | 1.70 (0.59–4.86) | 0.326 |
| Dyslipidemia | 1.58 (0.77–3.25) | 0.212 |
| Congestive heart failure | 1.17 (0.16–8.60) | 0.879 |
| Coronary artery disease | 2.07 (0.79–5.42) | 0.139 |
| Atrial fibrillation | 2.83 (1.08–7.43) | 0.035 |
| Stroke | 2.33 (0.55–9.84) | 0.250 |
| Chronic kidney disease | 2.08 (0.63–6.88) | 0.228 |
| Smoking | 1.35 (0.92–1.97) | 0.122 |
| Radiation | 0.66 (0.23–1.90) | 0.441 |
| Cancer subtypes | 0.75 (0.44–1.27) | 0.278 |
| Transplantation | 0.92 (0.35–2.43) | 0.872 |
| Aspirin | 3.13 (1.09–8.99) | 0.034 |
| ACE inhibitors/ARBs | 1.23 (0.50–3.01) | 0.648 |
| Beta-blockers | 1.35 (0.58–3.15) | 0.488 |
| Calcium channel blockers | 0.86 (0.26–2.84) | 0.806 |
| Statins | 2.29 (1.11–4.73) | 0.025 |
| Diuretic agents | 0.97 (0.23–4.06) | 0.964 |
| Oral anticoagulation | 0.88 (0.12–6.45) | 0.898 |
| Insulin | 5.70 (1.70–19.08) | 0.005 |
| Ferritin | 1.00 (1.00–1.00) | 0.634 |
| CRS grade | 2.10 (1.47–2.98) | <0.001 |
| 1 | 0.25 (0.03–1.86) | 0.176 |
| 2 | 0.39 (0.17–0.91) | 0.029 |
| 3 | 3.31 (1.55–7.09) | 0.002 |
| 4 | 9.79 (3.96–24.21) | <0.001 |
| LVEF | 1.00 (0.96–1.05) | 0.778 |
| Systolic blood pressure | 0.99 (0.97–1.01) | 0.346 |
| Diastolic blood pressure | 0.95 (0.91–0.99) | 0.007 |
| Heart rate | 1.00 (0.98–1.02) | 0.868 |
| Hgb | 0.83 (0.69–0.99) | 0.035 |
| WBC count | 1.00 (0.99–1.01) | 0.619 |
| Platelet count | 0.99 (0.99–1.00) | 0.027 |
| Creatinine | 4.30 (1.19–15.59) | 0.026 |
| GFR | 0.99 (0.98–1.00) | 0.181 |
| Mitral E/e′ | 1.15 (1.00–1.31) | 0.046 |
| PASP | 1.05 (0.93–1.18) | 0.150 |
| Diastolic dysfunction | 1.11 (0.32–3.85) | 0.866 |
| Left atrial volume index | 1.04 (1.00–1.08) | 0.075 |
| Interventricular septal thickness | 3.13 (0.19–50.91) | 0.422 |
Baseline creatinine, statin treatment, and CRS grading were chosen for the multivariable competing risk analysis according to their clinical relevance, effect size in univariable models, and limited to these variables to avoid multicollinearity. The multivariable competing risk regression analysis revealed that baseline creatinine (HR: 15.54 for each 1 mg/dl increase; 95% CI: 3.67 to 65.86; p < 0.001) and grade 3 CRS (HR: 8.42; 95% CI: 3.48 to 20.40; p < 0.001) or grade 4 CRS (HR: 29.86; 95% CI: 9.80 to 90.94; p < 0.001) were independently associated with MACE (Table 4). These results were confirmed with the stepwise regression using the backward elimination method and by individually adding variables to the final model. The same results were found with the Fine and Gray method (Supplemental Table 5). Statin use at baseline was not independently associated with MACE.
Table 4.
Association With MACE Determined Using Multivariable Cox Proportional Cause-Specific Hazards Regression Analyses
| Hazard Ratio (95% CI) | p Value | |
|---|---|---|
| Statin | 1.83 (0.88–3.81) | 0.105 |
| Creatinine | 15.54 (3.67–65.86) | <0.001 |
| CRS grade 1 | 0.49 (0.06–3.74) | 0.489 |
| CRS grade 2 | 0.95 (0.33–2.71) | 0.917 |
| CRS grade 3 | 8.42 (3.48–20.40) | <0.001 |
| CRS grade 4 | 29.86 (9.80–90.94) | <0.001 |
Abbreviations as in Table 3.
Discussion
The present study demonstrates that adult patients with CD19+ malignancy treated with CAR T cell therapy are at risk for MACE, mainly symptomatic heart failure, most of which occur within weeks of the infusion (Central Illustration). The variables independently associated with MACE in our study included baseline creatinine and grades 3 and 4 CRS.
Central Illustration.
Timeline Demonstrating the Relationship Among Anti-CD19 Chimeric Antigen Receptor (CAR) T Cell Infusion, Cytokine Release Syndrome, and Major Adverse Cardiovascular Events
As the median time to major adverse cardiovascular events (MACE) was 5 days later than the median time to cytokine release syndrome (CRS) onset, this suggests that CRS and its treatments can at least contribute to the occurrence of MACE in patients treated with anti-CD19 chimeric antigen receptor T cell therapy. IQR = interquartile range (25th to 75th quartile).
Between August 2010 and January 2019, a total of 145 patients were identified. The median age (60 years), male distribution (74%), and distribution of malignancies (25% ALL, 30% DLBCL, and 46% CLL) are representative of the current population receiving CAR T cell therapy (15,16). The KM estimates for overall survival were 95% at 30 days, 81% at 6 months, and 71% at 12 months (Figure 2). The mortality in our cohort is less than previously described, likely because of the improving expertise and the expanding applications to patients with less severe conditions and to patients who have received less intensive chemotherapy regimens (4,15,17).
In a recent publication from our group in 450 patients with acute myeloid leukemia or ALL, only 3% of patients with ALL had heart failure (18). Another study investigating patients with hematologic cancers treated with anthracyclines reported that 4% of patients with lymphoma developed symptomatic heart failure at a median time of 523 days after the initiation of anthracycline therapy (19). Our study, with a prevalence of 15% for symptomatic heart failure at a median time of 11 days, suggests a higher heart failure incidence than previously described, underlining the effect of CAR T cell treatment.
The cardiovascular profile of the patients treated with CAR T cell cells was slightly worse compared with that noted in the general population with respect to the baseline rates of heart failure, hypertension, and coronary artery disease (20, 21, 22, 23). According to age, <2% of persons 40 to 59 years of age in the general population are reported to have heart failure, compared with 3% in our study. The prevalence of coronary disease for those 40 to 59 years of age in the general population is about 6%, which is lower than in our study (10%) (22). The prevalence of hypertension is estimated at 32% in the general population, compared with 36% in our study (20,24). The prevalence of diagnosed diabetes mellitus is approximately 10%, slightly higher than in our study (9%) (21). The rate of current or previous smoking may appear elevated (41%) in our study. However, when taking into account current smokers only (12 of 145 patients [8%]), the prevalence of current smoking was less than the current rate of smoking in the US (14%) (25). The difference in proportion of previous heart failure or coronary disease could be attributed to prior cancer therapy (e.g., 60% anthracycline use, 23% radiation, 21% stem cell transplantation). Similarly, atrial fibrillation (8%) was more prevalent in our study than in the general population (<2% younger than 65 years) and may be explained by the use of ibrutinib (for CLL, diagnosed in 46% of our patients).
Cancer subtype does not seem to influence the risk for MACE (HR: 0.75; 95% CI: 0.44 to 1.28; p = 0.278), despite the different cardiovascular profiles of patients. Patients with CLL and DLBCL were older than patients with ALL and had increased cardiovascular risk, but their previous and current treatment may be less aggressive than that of patients with ALL. KM estimates for each cancer subtype and MACE, CRS, and survival were also performed at 30 days, 6 months, and 12 months (see Supplemental Figures 2 to 4).
In our study, 104 patients (72%) had at least 1 CRS episode, which is higher than the previously documented rate (55.3%) in a recent large systematic review and meta-analysis (6). We used the most current and broadest CRS consensus definition to identify the episodes (12), which may explain the increased detection of CRS.
The use of statins, insulin, and aspirin and higher baseline creatinine levels were each associated with MACE and likely reflect patients with a high cardiovascular risk profile and comorbidities pre-treatment. The analysis of baseline echocardiographic characteristics revealed that there was also a trend toward diastolic dysfunction and high left ventricular filling pressures and MACE. However, diastolic function categories as defined using the 2016 guidelines (14) were not associated with MACE; this result could be explained by the paucity of moderate or severe diastolic dysfunction and the relatively high frequency of indeterminate diastolic function.
Interestingly, baseline LVEF was not associated with MACE, underlining the importance of analyzing baseline diastolic function indexes (E/e′, left atrial volumes) in these patients. Although prior atrial fibrillation and the use of aspirin and insulin were associated with MACE, they were not included in further analysis, as the proportion of patients treated with aspirin and insulin or with previous atrial fibrillation was low.
The occurrence of grades 3 and 4 CRS was strongly associated with MACE. It is possible that CRS may result in depressed myocardial function, explaining the high rate of heart failure. It is also possible that patients with CRS receive large quantities of intravenous fluids that can worsen the volume overload state. The exact CRS treatment was left to the discretion of the treating physicians but usually comprises intensive fluid resuscitation followed by vasopressors. The median time to MACE was 5 days later than the median time to CRS onset, suggesting that CRS and its treatments may contribute to MACE in patients treated with CAR T cell therapy.
Study limitations
The data were obtained retrospectively. As with all retrospective studies, MACE could have been misclassified because of reporting errors or loss to follow-up. However, each medical chart was carefully reviewed, and signs and symptoms of MACE were individually adjudicated. Also, our overall loss to follow-up was small (6 patients [4%]). To decrease loss to follow-up, every patient was researched using a national necrology database. Thus, it is unlikely that deaths were missed. CIs for many of our risk estimates were also wide, and as such, these results need to be confirmed in additional studies.
Given that patients were referred from around the world to receive CAR T cell therapy, prior anthracycline use could have been underreported, and it was not possible to determine the total radiation or anthracycline dose previously received. It is widely acknowledged that total radiation or anthracycline dose is directly associated with MACE; the association of anthracycline dose or radiation with MACE could have been underestimated.
Clinical relevance
As CAR T cell therapy use becomes more available and accessible, it is crucial to understand not only the benefits but the possible side effects and the time frame in which they can occur. In contrast to radiation or other potentially cardiotoxic chemotherapies, no guidelines are currently available for screening or surveillance of cardiac function of patients treated with CAR T cell infusion. Understanding the incidence and the natural history of treatment-induced side effects will allow better screening and follow-up for these patients. To this effect, an extensive monitoring program in the United Kingdom is under way (26).
Conclusions
To our knowledge, this is the largest retrospective study conducted in adult patients treated with CAR T cell therapy evaluating the occurrence of and risk factors associated with adverse cardiovascular events. Using multivariable Cox proportional cause-specific hazards regression analysis, we determined that baseline creatinine and grade 3 or 4 CRS were independently associated with MACE. CAR T cell therapy has opened a new field in the treatment of hematologic malignancies and is now under investigation for other indications, including solid tumors, acute myeloid leukemia, and multiple myeloma (27, 28, 29). Given that CAR T cell use will only increase in the future and that no recommendations for monitoring and follow-up of left ventricular function and MACE currently exist (30), prospective studies are needed to ascertain the incidence and predictors of MACE.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Patients undergoing cancer treatment with CAR T cell infusion are at risk for MACE, mainly symptomatic heart failure. Patients at increased risk for MACE may benefit from cardiovascular monitoring.
TRANSLATIONAL OUTLOOK: The use of CAR T cell therapy will increase in the future. Currently, no recommendations for cardiovascular monitoring and follow-up exist. Prospective studies are needed to confirm the incidence and identify the predictors of MACE in CAR T cell-treated patients. This is important to inform the follow-up and management of these patients.
Acknowledgments
The authors thank Jesse Chittams, MS, and Ronald Kamusiime, MS, for assistance with statistical analysis.
Footnotes
This study was funded through National Heart, Lung, and Blood Institute grant 1R01HL130539-01 (to Dr. Scherrer-Crosbie). The authors have reported that they have no relationships relevant to the contents of this paper to disclose. This study was partially presented as an oral presentation at the American Heart Association Scientific Meeting, November 2019, Philadelphia. James Fang, MD, served Guest Associate Editor for this paper. Anju Nohria, MD, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: CardioOncologyauthor instructions page.
Appendix
For supplemental tables and figures, please see the online version of this paper.
Appendix
References
- 1.Burstein D.S., Maude S., Grupp S., Griffis H., Rossano J., Lin K. Cardiac profile of chimeric antigen receptor T cell therapy in children: a single-institution experience. Biol Blood Marrow Transplant. 2018;24:1590–1595. doi: 10.1016/j.bbmt.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 2.Shimabukuro-Vornhagen A., Gödel P., Subklewe M. Cytokine release syndrome. J Immunother Cancer. 2018;6:56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim W.A., June C.H. The principles of engineering immune cells to treat cancer. Cell. 2017 09;168:724–740. doi: 10.1016/j.cell.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porter D.L., Hwang W.-T., Frey N.V. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7:303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuster S.J., Bishop M.R., Tam C.S. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 6.Grigor E.J.M., Fergusson D., Kekre N. Risks and benefits of chimeric antigen receptor T-cell (CAR-T) therapy in cancer: a systematic review and meta-analysis. Transfus Med Rev. 2019;33:98–110. doi: 10.1016/j.tmrv.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Leick M.B., Maus M.V. Toxicities associated with immunotherapies for hematologic malignancies. Best Pract Res Clin Haematol. 2018;31:158–165. doi: 10.1016/j.beha.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Assuncao B.M.B.L., Handschumacher M.D., Brunner A.M. Acute leukemia is associated with cardiac alterations before chemotherapy. J Am Soc Echocardiogr. 2017;30:1111–1118. doi: 10.1016/j.echo.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald J.C., Weiss S.L., Maude S.L. Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit Care Med. 2017;45:e124–e131. doi: 10.1097/CCM.0000000000002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alvi R.M., Frigault M.J., Fradley M.G. Cardiovascular events among adults treated with chimeric antigen receptor T-cells (CAR-T) J Am Coll Cardiol. 2019;74:3099–3108. doi: 10.1016/j.jacc.2019.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hicks K.A., Tcheng J.E., Bozkurt B. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials. J Am Coll Cardiol. 2015;66:403–469. doi: 10.1016/j.jacc.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Lee D.W., Santomasso B.D., Locke F.L. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25:625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PubMed] [Google Scholar]
- 13.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Nagueh S.F., Smiseth O.A., Appleton C.P. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016 Apr;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Schuster S.J., Svoboda J., Chong E.A., Nasta S.D., Mato A.R., Anak Ö. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377:2545–2554. doi: 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maude S.L., Frey N., Shaw P.A. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neelapu S.S., Locke F.L., Bartlett N.L. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang Y., Assuncao B.L., Denduluri S. Symptomatic heart failure in acute leukemia patients treated with anthracyclines. J Am Coll Cardiol CardioOnc. 2019;1:208–217. doi: 10.1016/j.jaccao.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali M.T., Yucel E., Bouras S. Myocardial strain is associated with adverse clinical cardiac events in patients treated with anthracyclines. J Am Soc Echocardiogr. 2016;29:522–527.e3. doi: 10.1016/j.echo.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention Hypertension prevalence and control among adults: United States 2015–2016. NCHS Data Brief No. 289. https://www.cdc.gov/nchs/products/databriefs/db289.htm Available at: [PubMed]
- 21.Centers for Disease Control and Prevention National diabetes statistics report 2020: estimates of diabetes and its burden in the United States. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf Available at:
- 22.Virani S.S., Alonso A., Benjamin E.J. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 23.Fang J., Shaw K.M., Keenan N.L. Prevalence of coronary heart disease—United States 2006–2010. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6040a1.htm Available at:
- 24.Nwankwo T., Yoon S.S., Burt V.L. Hypertension among adults in the United States: National Health and Nutrition Examination Survey 2011–2012. NCHS Data Brief. 2013;(133):1–8. [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention Current cigarette smoking among adults in the United States. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm Available at:
- 26.Ghosh A.K., Chen D.H., Guha A., Mackenzie S., Walker J.M., Roddie C. CAR T cell therapy–related cardiovascular outcomes and management. J Am Coll Cardiol CardioOnc. 2020;2:97–109. doi: 10.1016/j.jaccao.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghosh A., Mailankody S., Giralt S.A., Landgren C.O., Smith E.L., Brentjens R.J. CAR T cell therapy for multiple myeloma: where are we now and where are we headed? Leuk Lymphoma. 2018;59:2056–2067. doi: 10.1080/10428194.2017.1393668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yong C.S.M., Dardalhon V., Devaud C., Taylor N., Darcy P.K., Kershaw M.H. CAR T-cell therapy of solid tumors. Immunol Cell Biol. 2017;95:356–363. doi: 10.1038/icb.2016.128. [DOI] [PubMed] [Google Scholar]
- 29.Rotiroti M.C., Arcangeli S., Casucci M. Acute myeloid leukemia targeting by chimeric antigen receptor T cells: bridging the gap from preclinical modeling to human studies. Hum Gene Ther. 2017;28:231–241. doi: 10.1089/hum.2016.092. [DOI] [PubMed] [Google Scholar]
- 30.Plana J.C., Galderisi M., Barac A. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–939. doi: 10.1016/j.echo.2014.07.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.