Abstract
Introduction
Coronavirus disease-19 (COVID-19) pandemic continues to grow all over the world. Several studies have been performed, focusing on understanding the acute respiratory syndrome and treatment strategies. However, there is growing evidence indicating neurological manifestations occur in patients with COVID-19. Similarly, the other coronaviruses (CoV) epidemics; severe acute respiratory syndrome (SARS-CoV-1) and Middle East respiratory syndrome (MERS-CoV) have been associated with neurological complications.
Methods
This systematic review serves to summarize available information regarding the potential effects of different types of CoV on the nervous system and describes the range of clinical neurological complications that have been reported thus far in COVID-19.
Results
Two hundred and twenty-five studies on CoV infections associated neurological manifestations in human were reviewed. Of those, 208 articles were pertinent to COVID-19. The most common neurological complaints in COVID-19 were anosmia, ageusia, and headache, but more serious complications, such as stroke, impairment of consciousness, seizures, and encephalopathy, have also been reported.
Conclusion
There are several similarities between neurological complications after SARS-CoV-1, MERS-CoV and COVID-19, however, the scope of the epidemics and number of patients are very different. Reports on the neurological complications after and during COVID-19 are growing on a daily basis. Accordingly, comprehensive knowledge of these complications will help health care providers to be attentive to these complications and diagnose and treat them timely.
Key Words: COVID-19, SARS-CoV-1, MERS-CoV, Neurological manifestations, Coronavirus
1. Introduction
Coronavirus disease-19 (COVID-19) pandemic continues to grow all over the world. [1] Currently, several research studies have been performed, focusing on the understanding of the acute respiratory syndrome and treatment strategies. [1] However, there is growing evidence of the neurological manifestations in patients with COVID-19. Similarly, the other coronaviruses (CoV) epidemics; severe acute respiratory syndrome (SARS-CoV-1) and Middle East respiratory syndrome (MERS-CoV) have been associated with neurological complications. CoV neurotropism, direct invasion of the virus to the central nervous system (CNS) and post infection neurological complications were suggested as the cause of these presentations. [1]
1.1. History
Viral respiratory infection pandemic is not a new event in medical history. Reports of respiratory infection outbreaks back to 1173 AD. The first confirmed pandemic of respiratory infections, occurred in 1580. [2]. More recently, in the 20th and 21st centuries, there have been several reposts of such pandemics and outbreaks, including the Spanish Flu pandemic of the early 20th century, SARS-CoV-1 epidemic in 2003 and MERS-CoV outbreak in 2012. [1,2] Neuropsychiatric complications during and after these pandemics have been noticed by many scientists. One of the first neurological presentations, which was reported after the 1580 pandemic was encephalitis. [3]
The Spanish Flu Pandemic in 1918 was the first respiratory infection pandemic in the 20th century. It infected over 500 million people worldwide. [3] Several investigations were performed during and after the pandemic on neuropsychiatric symptoms, treatments, and delayed complications. It was reported that many patients who recovered from the respiratory symptoms of the disease had very pronounced nervous system sequelae, such as depression, neurasthenia, acute post-flu psychosis, and neuritis, some persisting until one year after the illness. [4]
Besides these neurological presentations, which were considered to be caused directly by the infection, scientists believe that an autoimmune response might have caused delayed neurological complications in these patients. [5] As an example, Encephalitis lethargica, a neurological syndrome which widely coincided with, and lasted for a decade after the Spanish Flu pandemic, was believed to be in relation to the influenza infection. [5] Moreover, despite controversies, it was shown that Parkinson's disease was two to three times more prevalent among individuals who were born between 1888 and 1924. This group was born or were young at the time of the Spanish Flu pandemic. [5]
With respect to the current pandemic, we reviewed the neurological complications of CoV infection in human.
1.2. CoV
CoV are large, enveloped, positive-sense RNA viruses. They infect humans and several groups of the animal species. They generally cause upper and lower respiratory tract and gastrointestinal infections, hepatitis or neurological manifestations. Human coronaviruses (HCoV) which causes human infections were first discovered in 1965. [6] Until now seven types of CoV have been discovered: SARS-CoV-2, SARS-CoV-1, and MERS-CoV which are associated with the three epidemics and caused severe disease in humans, HCoV-OC43, HCoV-229E, HCoV-NL63 and HCoV-HKU1. [7] CoV may invade the CNS, disseminate, and participate in induction of neurological diseases. Before SARS-CoV-2, three other types including: SARS-CoV-1, HCoV-229E and HCoV-OC43 had been shown to cause CNS infection. [7]
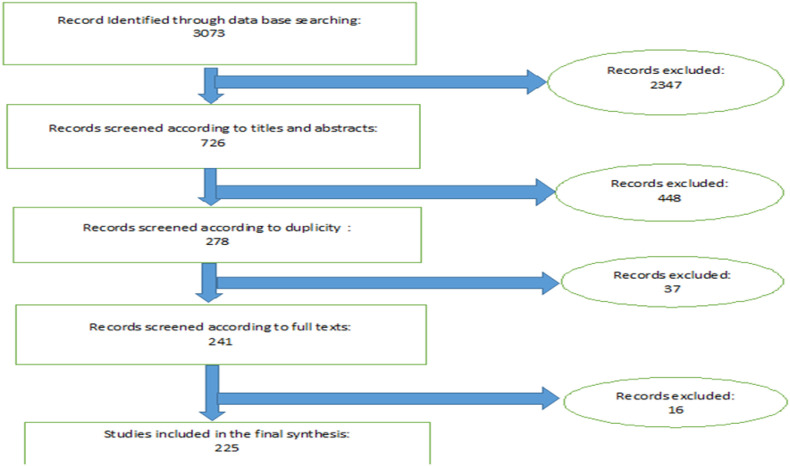
2. Methods
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Figure 1 ) statement. [8] We searched PubMed till July 7, 2020 for HCoV-229E and HCoV-OC43 (Part A, B), from Jan 1, 2000, to July 7, 2020, for SARS-CoV-1 (part C), from Jan 1, 2010, to July 7, 2020, for MERS-CoV (Part D) and from Jan 1, 2020, to July 7, 2020, for Covid-19 (Part E). These Keywords were used:
Fig. 1.
PRISMA algorithm of this study
Part A: “HCoV-229E” AND “Neuro” OR “Brain”
Part B:” HCoV-OC43” AND “Neuro” OR “Brain”
Part C "Severe acute respiratory syndrome of Coronavirus" OR "SARS" AND "Neuro" OR "Brain"
Part D: "Middle East respiratory syndrome coronavirus" OR "MERS" AND "Neuro" OR "Brain"
Part E: “Coronavirus” OR “COVID” AND “Neuro” OR “Brain”
Articles written in English were included. The authors evaluated the titles and abstracts of each article for screening and inclusion. Articles evaluating CoV infections with respect to the neurological complications in human (original articles and reports of the cases (case series, case reports, letters, correspondence, or short communications which presented at least 1 case)) were reviewed in full text by the authors and included. (In COVID-19 we just included the studies which described neurological symptoms in adults) Studies not related to the CoV infections or their neurological manifestations were excluded from the review. Duplicated results were removed. We also reviewed relevant references in each article. Data from each article was extracted into the Microsoft Excel software.
3. Results and discussion
3.1. HCoV-OC43 and HCoV-229E
CNS damages caused by HCoV was suggested in the 1980s. In 1980 Burks JS et al. [10] isolated CoV from the brains of two MS patients. Subsequently the hypothesis concerning the relationship between CoV infection and demyelinating diseases in humans CNS was studied several times. [10,11,12]
Arbour N et al showed that human CNS cells including oligodendrocytes, astrocytes, microglia, and neurons are susceptible to acute infection with HCoV-OC43 during in vitro cultures, and other than microglia, the rest have a potential of persistent infection. [13] In animal studies, direct invasion of the virus via nasal canal caused a rapid CNS infection. [14]. Cristallo A et al [11] reported presence of HCoV-OC43 RNA in the Cerebrospinal fluid (CSF) of MS patients; However, Dessau RB et al. [12] did not find any evidence of chronic 229E or OC43 infection in brain tissue of MS patients. In 1992, Fazzini E et al showed higher levels of HCoV-OC43, and HCoV-229E antibodies in CSF of Parkinson disease patients compared to controls. [9]
Two cases of fatal encephalitis with HCoV-OC43 infection were reported in 2 immunosuppressed infants (9-month old infant on chemotherapy for leukemia and 11-month boy with severe combined immunodeficiency). [15,16] Moreover, acute disseminated encephalomyelitis(ADEM) was reported with HCoV-OC43 infection in a 15-year-old boy. In this case, CSF was positive for the virus. [17] In 2015, acute flaccid paralysis was reported in a 3-year-old girl after infection with HCoV 229E and OC43 [18] In another study by Li Y et al. [19] from China, CoV was suggested as an important cause of acute encephalitis-like syndrome in children.
3.2. SARS-CoV-1
The outbreak of SARS-CoV-1 was in 2002- 2003 and affected more than 8,000 people worldwide. [20] Clinical presentations in most of the patients were a simple common cold. However, in patients with an impaired immune system, it caused respiratory distress, pneumonia, and even death. [20] In some of these patients, neurological complications were reported. (Table 1 ) [[21], [22], [23], [24], [25], [26], [27], [28]]. Cerebrovascular pathologies and ischemic strokes were reported in 5 patients. Most of these patients had severe infection and several comorbidities, which might have made them more susceptible to stroke. [21] In these cases, hypo-perfusion as result of septic shock, utilization of intravenous immunoglobulin as a part of the treatment regimen, hypercoagulability state, cardiogenic shock, and vasculitis might represent the potential underlying mechanisms for the cerebrovascular events. [21]
Table 1.
Neurological Complications Reported During and After SARS-CoV-1 Infection.
| No. |
Neurological Symptom |
Ref. No. | No. of patients | Mean Age of the patients(Range) | Notes |
|---|---|---|---|---|---|
| Symptoms related to CNS | |||||
| 1 | Ischemic stroke | [21] | 5 Patients 3F 2M |
57.6(39–68) | Large artery ischemic stroke especially in critically ill patients. |
| 2 | Headache | [22] | F | 62 | |
| 3 | Seizure | [23] | F | 32 | CSF positive for SARS-CoV-1 |
| 4 | Encephalitis | [24] | M | 39 | Autopsy tissue from the patient revealed neuronal necrosis, glial cell hyperplasia, and infiltration of monocytes and T cells. |
| Symptoms related to peripheral nervous system (PNS) | |||||
| 5 | Guillain-Barré syndrome (GBS) | [25] | 3F | 47(42–51) | Both acute inflammatory demyelinating polyneuropathy (AIDP) and acute motor axonal neuropathy (AMAN) |
| 6 | Critical illness polyneuropathy | [26] | F | 51 | |
| Smell impairment | [27] | F | 27 | ||
| Symptoms related Skeletal Muscle injury | |||||
| 7 | Myopathy | [28] [25], | 5 M | 54.8(31–81) | Leung TW et al. [28] study was a post-mortem study, steroid-induced myopathy suggested for these patients.(4 cases) |
Abbreviation: M: Male, F: Female
Encephalitis was reported in a 39-year-old patient. In his autopsy, SARS-CoV-1 RNA was isolated from the specimen and virions were visualized in neurons on electron microscopy. [24] Other studies on brain tissue specimens of autopsy donors detected SARS-CoV-1 in the cytoplasm of neurons in the cortex and hypothalamus. [29]
Neuropathy and myopathy were seen in 9 patients. Tsai LK, et al. [25] reported patients with different types of Guillain-Barré syndrome (GBS) (acute inflammatory demyelinating polyneuropathy(AIDP) and acute motor axonal neuropathy (AMAN)). Chao CC et al. [26] described a patient with critical illness polyneuropathy. Myopathy was reported in 5 patients. [25,28] (Table 1)
Despite many suggested that those complications were caused by the direct attack of the virus to the nerves and muscles, critical illness neuropathy and myopathy should be considered as another potential underlying cause. In the case of myopathy, many of these patients were treated with corticosteroids for the primary illness and steroid-induced myopathy might have been a differential diagnosis in such patients. [25,28]
3.3. MERS-CoV outbreak
MERS or Camel Flu caused by the MERS-CoV, was first identified in 2012 in Saudi Arabia and caused outbreaks in South Korea and Saudi Arabia. Symptoms varied from none or mild common cold such as fever, myalgia, and cough to acute respiratory distress syndrome (ARDS), sepsis, multi-organ failure, and even death. [30] About 2500 confirmed cases were detected worldwide, and the fatality rate was reported as 34.3%. [31].
Prevalence of neurological complications were high among patients with MERS-CoV infection. In a study by Saad M et al. [32] 29 out of 70 patients with MERS-CoV infection had neurological manifestations. Serious neurological complications such as ischemic stroke and encephalitis were also reported in these patients. (Table 2 ) Some of these complications did not coexist with the respiratory symptoms and were delayed for approximately 2–3 weeks later. [36] Intracranial hemorrhage (ICH) was reported in 2 patients; in one, an abnormal coagulation panel and disseminated intravascular coagulation (DIC) were noted leading to severe multiple organ failure, but the other patient had a normal coagulation panel and platelet count. [34,35] Both patients were women and relatively young.
Table 2.
Neurological Complications Reported During and After MERS-CoV Infection.
| No. |
Neurological Symptom |
Ref. | No. of patients | Mean Age of the patients(Range) | Notes |
|---|---|---|---|---|---|
| Symptoms related to CNS | |||||
| 1 | Ischemic stroke | [33] | 2M | 65.6(57–74) | |
| 2 | Intracranial hemorrhage (ICH) | [34], [35] | 2F | 38(34–42) | ICH in one of the patients was related to DIC and thrombocytopenia but the other patient had normal coagulation at the time of ICH. |
| 3 | Headache | [32] | 9 (12.9%) | NR | |
| 4 | Seizure | [32] | 6(8.6%) | NR | |
| 5 | Confusion | [32] | 18 (25.7) | NR | |
| 6 | Encephalitis | [33] | M | 45 | CSF: Not significant |
| 7 | Bickerstaff's encephalitis overlapping with GBS | [36] | M | 55 | |
| Symptoms related to Peripheral nervous system (PNS) | |||||
| 8 | Guillain-Barré syndrome (GBS) | [34], [36] | 4 patients 2M 2F |
38.7(28–46) | |
Abbreviation: M: Male, F: Female
Contrarily to the fact that CNS infection were confirmed in animal models after intranasal inoculation with MERS-CoV, the virus has never been detected in the CNS of humans. [37]
3.4. COVID 19 pandemic
To date there are several reports on neurological complications of COVID-19. We divided the neurological symptoms in such patients into three different groups: symptoms related to CNS involvement [[38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [114], [115], [116], [117], [118], [119], [120], [121], [122], [123], [124], [125], [126], [127], [128], [129], [130], [131], [132], [133], [134], [135], [136], [137], [138], [139], [140], [141], [142], [143], [144], [145], [146], [147], [148], [149], [150], [151], [152], [153], [154], [155], [156], [157], [158], [159], [160], [161], [162], [163], [164], [165], [166], [167], [168], [169], [170], [171], [172], [173], [174], [175], [176], [177], [178], [179], [180], [181], [182], [183], [184], [185], [186], [187], [188], [189], [190], [191], [192], [193], [194], [195], [196], [197], [198], [199], [200], [201], [202], [203], [204], [205], [206], [207], [208], [209], [210], [211], [212], [213], [214]], symptoms related to the peripheral nervous system (PNS) involvement, and symptoms related to skeletal muscle injury or neuromuscular junction (NMJ) disorder. We also emphasize important clinical points in each case. (Table 3, Table 4, Table 5 )
Table 3.
CNS Complications Reported During and After SARS-CoV-2 Infection.
| No. |
Neurological Symptom |
Ref. No. | No. of patients | Mean Age of the patients(Range) | Notes |
|---|---|---|---|---|---|
| Headache and dizziness | |||||
| 1 | Headache 34 Articles |
[38] [39] [40], [41], [42], [43], [44], [45], [46], [47], [48], [49],. [50], [51], [52], [53], [54], [55], 56), [57], [58], [59], [60] [61],[62], [63] [64] [65] [66], [67], [68], [69] [70], [178] | 2073 | NR in all articles. | |
| 2 | Dizziness 11 Articles |
[39], [43], [47], [48], [57], [60], [62], [69], [70], [71], [72] | 173 | NR in all articles. | Kong Z et al. [72]reported a 53 Y/O F with dizziness as the first presentation of COVID-19. |
| Cerebrovascular events | |||||
| 3 | Ischemic Stroke 37 Articles |
[39], [57], [58], [60], [63], [73], [74], [75], [76] [77], [78], [79], [80],. [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [178], [192] | 363 | NR in all articles. | In large population studies ischemic stroke was reported in 1% [77] to 2.5% [84] of patients. Young adult patients without any past medical history [80] and patients with large-vessel stroke were reported. [80,85,95] One 38 Y/O F patient with stroke and CADASIL was reported. [89] A 67 Y/O M simultaneous cerebral infarcts in multiple arterial territories were reported. [91] |
| 4 | TIA 3 Articles |
[58], [75], [94] | 7 | NR in all articles. | |
| 5 | Cerebral Hemorrhage 19 Articles |
[39], [57], [58], [60], [63], [75] [86]), [92], [98], [99], [100], [101], [106], [107], [108], [109], [110], [111], [112], | 61 | NR in all articles. | Muhammad S et al. [107] reported a 60 Y/O F with aneurysm and ICH. Ghosh R, et al. [111], reported a 19 Y/O F with Moyamoya Angiopathy and ICH. |
|
6 |
Cerebral Venous Sinus Thrombosis 9 Articles |
[99], [101], [102], [114], [115], [116], [117], [118], [192] | 13 | NR in all articles. | Malentacchi M et al. [102] reported 81 Y/O M with both arterial and venous thrombosis. |
| Acute demyelination | |||||
| 7 | Acute disseminated encephalomyelitis (ADEM) 4 Articles |
[120], [121], [122], [123] | 4 2M 2F |
61(51–71) | Reichard RR et al. [122], study reported post-mortem examinations of a 71 Y/O M which revealed ADEM-like appearance at the brain biopsy. |
| 8 | Acute Myelitis 5 Articles |
[125], [126], [127], [128], [129] | 5 3M 2F |
55.6(22–69) | In one of these patients [125] CSF or MRI exam was not performed. Sotoca J et al. [127], reported 69 y/o F acute necrotizing myelitis. Giorgianni A, et al. [128] reported a 22 Y/O F with transient acute-onset tetra paresis with normal MRI and CSF exam. |
| 9 | Optic Neuritis 1 Article |
[60] | 1 | NR | |
| 10 | Acute encephalomyelitis 4 Articles |
[132], 179) [187] [196] | 4 2M 2F |
21-54 | Zoghi A et al. [132], reported 21 Y/O M suspicious to ADEM or neuromyelitis-optica spectrum disorder. Zanin L, et al. [179] reported a 54 Y/O F with seizure and brain and spine demyelinating lesions. Brun G et al. [187] reported a 54 Y/O F with Multiple supra-tentorial punctiform and tumefactive lesions involving the white matter bilaterally. Demirci Otluoglu G et al. [196] reported a 48 Y/O M with Acute encephalomyelitis and positive CSF for SARS-CoV2 |
| Impaired consciousness, encephalopathy and encephalitis | |||||
|
11 |
Decreased level of consciousness And Encephalopathy 22 Articles |
[39], [40], [57], [58] [60], [62] [88], [96], [98], [100], [101], [133], [135], [136] [137], [138], [139] [140], [141] [178] [184] [189] [195] [197] | 4,54 | NR in all articles. | Yin R et al. [133] reported a 64 Y/O M with altered consciousness and Psychiatric symptoms, his brain CT scan was Normal. Farhadian S et al. [138], reported a 78 Y/O F with acute encephalopathy and elevated CSF inflammatory markers. Hosseini AA et al. [139], reported 2 patients (46 Y/O, 79 Y/O) F with Delirium as a presenting feature in COVID-19, neuro-invasion or autoimmune encephalopathy was suggested as the cause. Kulick-Soper CV, et al. [189] reported a 54 Y/O F with bilateral globus pallidus lesions and possibility of hypoxic brain damage. |
|
12 |
Leukoencephalopathy 3Articles |
[144], [145] [188] | 18 | NR in all articles. | Radmanesh A et al. [145] reported 4 patients with leukoencephalopathy one patient with micro-hemorrhages and six patients with both presentations. |
| 13 | Acute Necrotizing Encephalopathy(ANE) 6 Articles |
[146] [147], [148], [149] [178] [193] | 8 | NR in all articles. | Virhammar J, et al. [146], reported 55 Y/O F with ANE with abnormal CSF. Radmanesh A et al. [147], reported 50 Y/O M delayed post-hypoxic necrotizing leukoencephalopathy. Dixon L et al. [149] reported ANE in a 59 Y/O F with history of aplastic anemia who died despite steroid therapy. |
|
14 |
Encephalitis 13 Articles |
[60], [98], [100], [150], [151], [151], [152], [153], [154], [155], [156], [157], [158] | 22 | NR in all articles. | Wong PF et al. [151], reported a 40 Y/O M Rhombencephalitis. Pilotto A et a.l [152], a 60 y/o patient with steroid responsive encephalitis. Efe IE et al. [157], reported a 35 Y/O F with encephalitis mimicking glial tumor. |
| 15 | Mild encephalitis/encephalopathy with a Reversible Splenial Lesion(MERS) 1 Article |
[160] | 1 M |
75 | |
|
16 |
Posterior reversible encephalopathy syndrome (PRES). 8 Articles |
[58], [101], [161], [162], [163] [164],[165], [190] | 10 | NR in all articles. | Franceschi AM et al. [161], reported 2 patients with hemorrhagic PRES. Kaya Y et al. [162], reported a 38 M with cortical blindness PRES like syndrome. Coolen T et al. [190]performed early postmortem brain MRI in patients who died from COVID-19 complications. |
| Seizure | |||||
|
17 |
Seizure 20 Articles |
[39], [58], [60], [63], [101], [150], [166], [167], [168], [169], [170], [171], [172], [173], [174], [175] [176] [177], [178], [179] | 48 | NR in all articles. | Somani S et al. [166], Balloy G, et al. [167] and), Le Guennec L et al. [172] reported 4 patients with status epilepticus. Logmin K et al. [171], reported a 70 Y/O patient with non-epileptic seizures. Elgamasy S et al. [174] reported 73 Y/O F with focal epilepsy. Vollono C et al. [175]reported a case of focal status epilepticus from left fronto-centro-temporal area(MRI showed extensive gliosis and atrophy at that site due to previous herpes encephalitis.) Scullen T et al. [178],reported a case of non-convulsive status epilepticus. |
| Movement disorders | |||||
| 18 | Generalized Myoclonus, Hypokinetic-rigid syndrome 2 Articles |
[182], [183] | 4 3M 1F |
71.25(58–88) | |
| CNS Vasculitis | |||||
| 19 | CNS Vasculitis 2 Articles |
[98] [185], | 2 | NR in all articles. | |
| Cranial Nerve abnormalities | |||||
| 20 | Anosmia 28 Articles |
[39], [56], [57], [58], [62], [69], [101], [182], [183], [198], [199], [200], [201], [202], [203], [204], [205], [206], [61], [207], [208], [209], [210], [211], [212], [213] [214], |
3730 | NR in all articles. | Mermelstein S [202] a 27 Y/O neurology registrar, reported her symptoms and anosmia after COVID-19. |
| 21 | Ageusia 14 Articles |
[39], [56], [57], [58], [61], [62], [69], [200], [201] [203] [204], [205], [206], [214] | 2590 | NR in all articles. | |
| 22 | Impaired Vision 3 Articles |
[39], [57], [204] | 12 | NR in all articles. | |
Abbreviation: M: Male, F: Female, NR: Not reported.
Table 4.
PNS Complications Reported During and After SARS-CoV-2 Infection.
| No. | Neurological Symptom |
Ref. No. | No. of patients | Mean Age of the patients(Range) | Notes |
|---|---|---|---|---|---|
| Cranial Nerve abnormalities | |||||
| 1 | Impaired Eye movement 4 Articles |
[57], [58], [194], [215] | 12 | NR in all articles. | Pascual-Goñi E et al. [194], reported a 36 Y/O F and bilateral sixth nerve palsy with impression of Wernicke encephalopathy. Dinkin M et al. [215], reported a 36 Y/O M and third nerve palsy and impression of Miller-Fisher syndrome. |
| 2 | Trigeminal neuropathy 2 Articles |
[57], [216] | 9 | NR in all articles. | |
| 3 | Facial nerve palsy 2 Articles |
[58], [217] | 4 | NR in all articles. | |
| 4 | Auditory Impairment 2 Articles |
[62] [57] | 5 | NR in all articles. | |
| 5 | Glossopharyngeal neuralgia 1 Article |
[57] | 9 | NR | |
| GBS and other Neuropathies | |||||
| 6 | GBS and GBS variants 36 Articles |
[60], [63], [101], [191], [215], [218], [219], [220], [221], [222], [223], [224], [225], [226], [227], [228], [229], [230] [231] [232] [233] [234] [235] [236], [237], [238], [239], [240] [241] [242], [243], [244] [245] [246], [247], [248] | 52 | NR in all articles. | Su XW et al [226], reoorted a patient GBS with dysautonomia. Juliao Caamaño DS et al. [230], reported a patient with Facial diplegia an Atypical Variant of GBS Pfefferkorn T, et al. [247] reported a 51 Y/O M acute polyradiculoneuritis. |
Abbreviation: M: Male, F: Female, NR: Not reported
Table 5.
Complications related to Skeletal Muscles and Neuromuscular Junction (NMJ) Reported During and After SARS-CoV-2 Infection.
| No. | Neurological Symptom |
Ref. No. | No. of patients | Mean Age of the patients(Range) | Notes |
|---|---|---|---|---|---|
| Symptoms related to Skeletal Muscles and Neuromuscular Junction (NMJ) | |||||
| 1 | Skeletal muscles injury and Rhabdomyolysis 3 Articles |
[39], [58], [60] | 38 | NR in all articles. | |
| 2 | Myopathy | [60], [250], [251] | 28 | NR in all articles. | |
| 3 | Myositis | [252] | 1 | 58 Y/O F | 58 Y/O F with muscle biopsy suggestive of Myositis. |
| 4 | Myasthenic crisis | [253] | 1 | 56 Y/O F | With history of myasthenia gravis |
| 5 | Neuroleptic Malignant Syndrome | [254] | 1 | Middle age man | In patient with past medical history of psychiatric disorders. |
Abbreviation: M: Male, F: Female, NR: Not reported
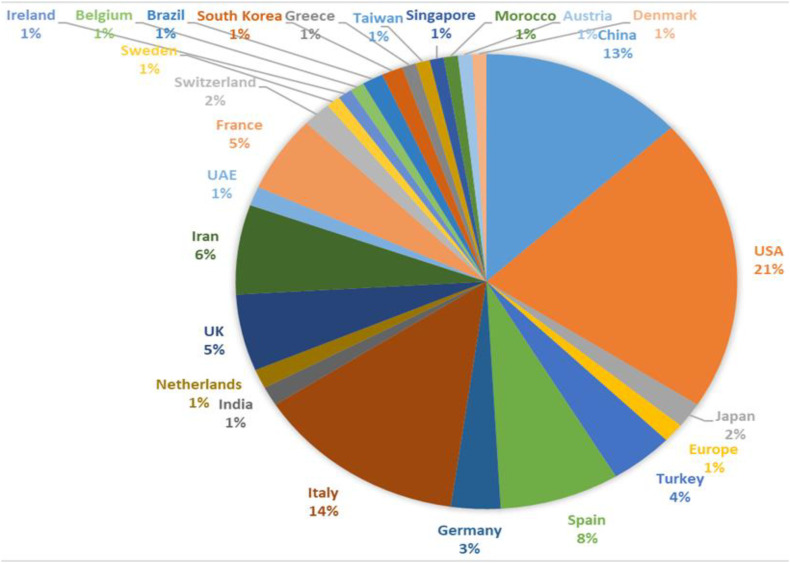
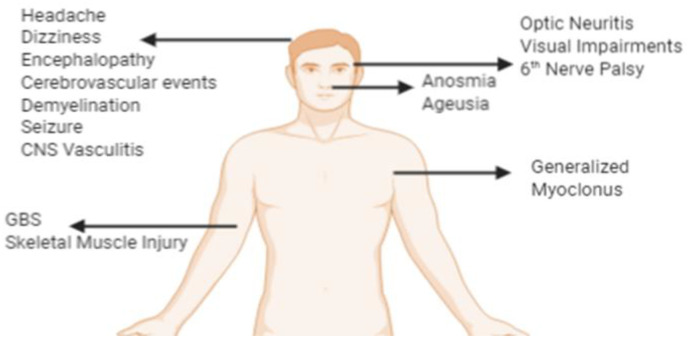
Studies were performed in 25 different countries; the United States with 46 publications had the highest number of publications on this topic. (Figure 2 ) The most common neurological symptoms in COVID-19 are headache, dizziness, anosmia, and ageusia. More severe neurological findings include stroke, impairment of consciousness, coma, seizures, neuropathy, and encephalopathy. (Figure 3 )
Fig. 2.
Pie chart of the rate of published articles according to the country of origin
Fig. 3.
Neurological Symptoms in COVID-19 (Designed with BioRender.com)
3.4.1. Symptoms related to CNS
3.4.1.1. Headache and dizziness
Headache and dizziness have been reported as two of the most common initial presentations in many patients with COVID-19. These two are very common symptoms in many neurological pathologies such as meningitis, encephalitis and vasculitis. It was shown that they can also occur in temporal association with a systemic viral infection. [38,39,40]
In COVID-19, headache has been reported in 2073 patients in 34 studies. (Table 3) The severity of headache was reported to be moderate to severe. Headaches were reported to have tension-type quality, [69] pain was reported to be bilateral with exacerbation by bending over, and mostly located in the temporo-parietal region or sometimes more anteriorly toward the forehead. [38] In most of these patients, headaches occurrence was associated with a past medical history of headaches. [69]
Several potential underlying pathophysiological mechanisms were suggested, particularly for headaches in the forehead and periorbital regions [38]; notably, it could be due to a direct invasion of SARS-CoV-2 to the trigeminal nerve endings in the nasal cavity. The other proposed underlying mechanism is trigemino-vascular activation due to involvement of the endothelial cells of the vessel walls with high expression of angiotensin-converting enzyme 2 (ACE2). A third proposed mechanism, the release of the pro-inflammatory mediators and cytokines during COVID-19 might stimulate the perivascular trigeminal nerve endings and cause headache. [38] We summarized the studies which reported Headache and dizziness in patients with COVID-19 in Table 3.
Eleven studies reported dizziness as one of the presenting symptoms of COVID-19 in 173 patients. (Table 3) Kong Z et al. [72] reported a 53-year-old woman with dizziness as the initial symptom of COVID-19.
3.4.1.2. Cerebrovascular events
Acute Ischemic stroke(AIS) has been reported in approximately 1-3% of patients with COVID-19 [39,77,84]; this is similar with other CoV infections (SARS-CoV-1 and MERS-CoV). During the current pandemic, 370 patients with SARS-CoV-2 infection out of 37 studies (Table 3) were reported to suffer from AIS or transient ischemic attack (TIA). Most of these patients had several underlying co-morbidities which made them more susceptible to thromboembolic events. [21,77].
However, there are reports on AIS occurring in young adults with SARS-Cov-2 infection and without any past medical history or cardiovascular risk factors. [80,85,95] (Figure. 4 ). Systemic effects of SARS-Cov-2 might be the underlying mechanism in these cases. Coagulation abnormalities have been shown in critically ill COVID 19 patients. This was characterized by rise in procoagulant factors, including serum levels of fibrinogen (94%), platelet (62%), interleukin-6 (IL-6) and D-dimer (100%) which subsequently may contribute to elevated rate of thromboembolic events and higher rate of mortality and morbidity. [103]. SARS-CoV-2 may cause an inflammatory response in the body. Elevated levels of C-reactive protein (CRP), interleukin-7 (IL-7), IL-6 and other inflammatory markers makes the existing atherosclerotic plaque more susceptible to rupture [84]. Cardiac manifestations and arrhythmic complications of COVID-19 can be another potential mechanism contributing to higher rate of ischemic events in these patients. [104]. The other proposed mechanism involves ACE2. It was shown that SARS-CoV-2 virus binds to ACE2 which is located in the lung, small intestinal and brain vessel endothelial cells. Depletion of ACE2 by SARS-CoV-2 virus may cause imbalance of the renin angiotensin system (RAS) which might result in endothelial dysfunction and subsequently ischemic events. [105]
Fig 4.
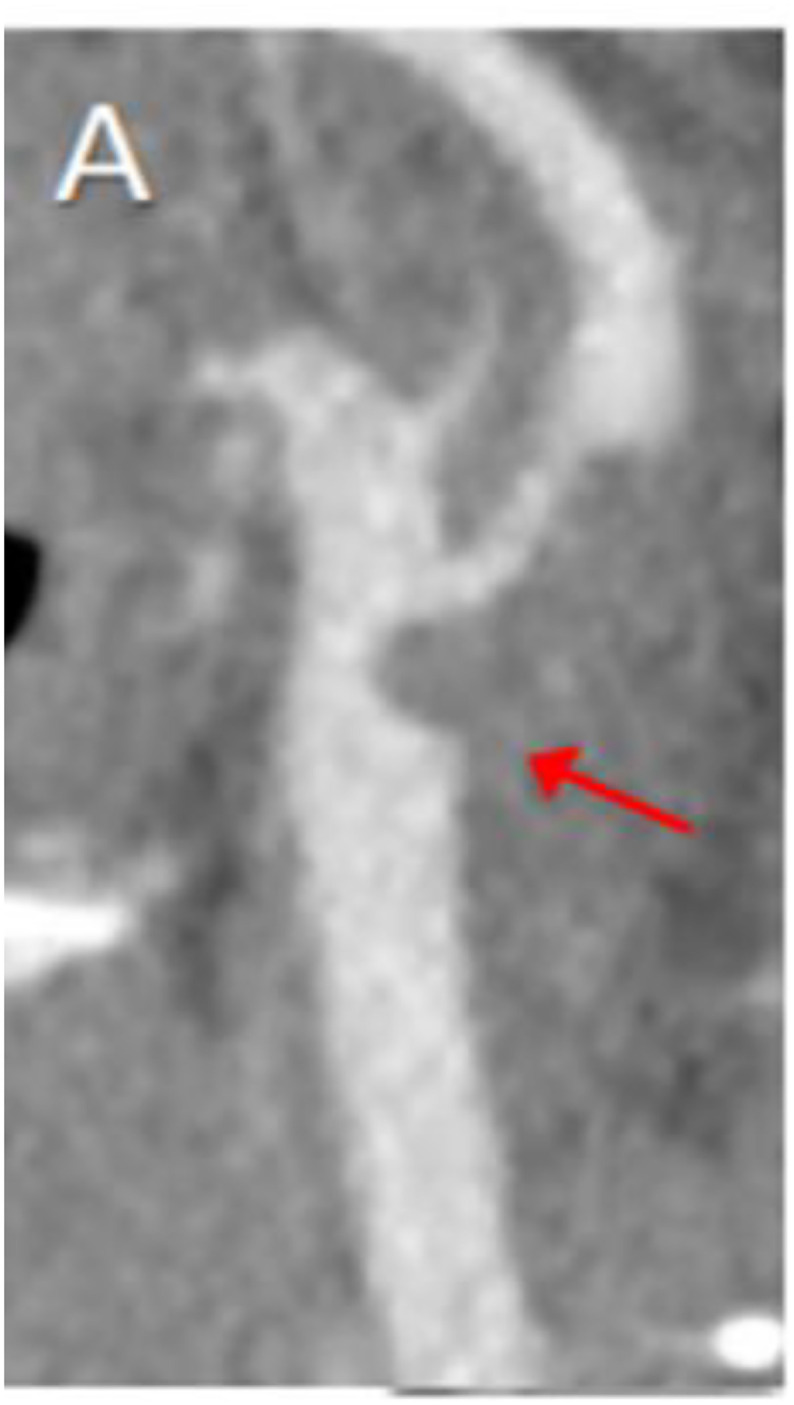
CT Angiography of the neck shows macro thrombus within the Common Carotid artery bifurcation extending into the Internal Carotid artery in a previously healthy 33-Year-Old woman. (From Fara MG et al. [95])
Intracranial Hemorrhage (ICH) was seen in about 0.5% of the patients with COVID-19 in large population studies. [39,60] Overall 61 patients in 19 studies were reportedly presented with ICH. (Table 3) In the past CoV pandemic, ICH was reported as a neurological complication with MERS-CoV infection [34,35]. In critically ill patients, COVID-19 has been associated with coagulopathies such as DIC, thrombocytopenia, elevated D-dimer, and prolonged prothrombin time which can result in hemorrhage. [113] Another potential mechanism is the effect of SARS-CoV-2 on ACE2. As mentioned earlier, SARS-CoV-2 has been shown to use the ACE 2 receptor for cell entry. ACE2, the SARS-CoV-2 binding site, is a critical component of the counter-regulatory pathway of the RAS, which is one of the most important regulators of blood pressure. SARS-CoV-2–induced ACE2 downregulation may lead to vasoconstriction and dysfunction of cerebral autoregulation and subsequently blood pressure spikes which eventually can causes arterial wall rupture and hemorrhage. [106]. There are reports that suggested even neurosurgical interventions were accompanied with more hemorrhagic complications in patients with COVID-19. [113]
Cerebral Venous Sinus Thrombosis was reported in 13 patients out of 9 studies. (Table 3). Overall it was shown that venous and arterial thromboembolic complications are seen in 5–15% of patients with severe COVID-19. [119] Combination of low-grade DIC and a localized pulmonary thrombotic micro-angiopathy might be the cause. The COVID-19 coagulopathy is characterized by a significant increase in D-dimers, high fibrinogen levels, mild prolonged prothrombin time, and a modest thrombocytopenia. [114,119] Besides, in patients with COVID-19 a transient raise of antiphospholipid antibodies is seen which may play a role in pathophysiology of thrombosis. [114,119] Cytokine storm particularly in critically ill COVID-19 patients is the other possible mechanism. It suppresses the anticoagulant pathways and release von Willebrand factor which might lead to thrombosis in such patients. [119]
3.4.1.3. Demyelinating diseases
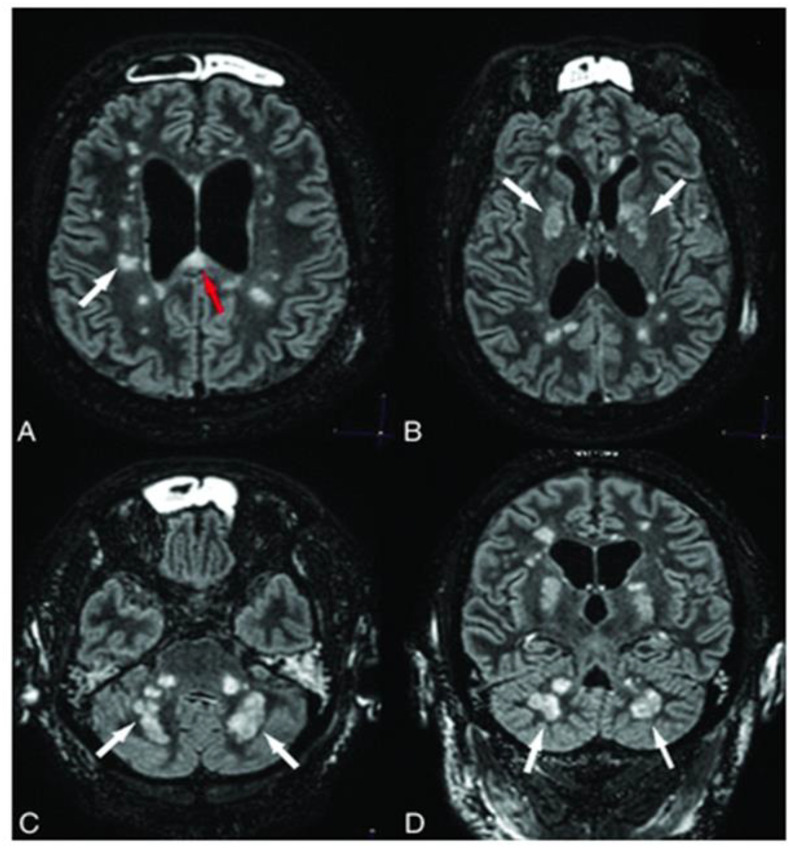
As of now, five patients with ADEM have been reported after CoV infection; one adolescent with HCoV-OC43 [17] and 4 adults after SARS-CoV-2. [[120], [121], [122], [123]] relation between CoV infections and demyelinating diseases of CNS has been suggested in last decades. [12,124] Despite the fact of ADEM usually occurring in children [17], in the current pandemic all reported ADEM patients were older than 50-year-old. This finding might be due to higher prevalence of COVID-19 in adults. From the reported patients, two recovered with methylprednisolone and intravenous immunoglobulins [120,121] and two of them died [122,123], one was a 71-year-old-man with underlying comorbidities and ADEM was diagnosed in post-mortem biopsy. [122] The other one was a 58-year-old man who was treated with dexamethasone and died as consequence of status epilepticus. [123] (Figure. 5 )
Fig. 5.
Fluid-attenuated inversion recovery (FLAIR) mages show diffuse confluent white matter hyperintensity particularly at the left-side (A-D) without significant enhancement on T1-weighted brain MRI (C, F). Involvement of (black arrow), deep gray matter (black arrowhead), and dorsal midbrain (white arrow) is evident. From Abdi S et al. [123]
MS Exacerbation: Early insights about COVID-19 in MS patients suggest that the risk of infection and associated morbidity in this population is not significantly different from the non-COVID19 patients [130] Moreover, worsening outcome with regards to with disease-modifying therapies has not been reported. [131]
Acute encephalomyelitis in COVID-19, were reported in 4 patients. (Table 3) Zoghi et al. reported a 21-year-old-man with encephalomyelitis following SARS-CoV-2 infection. The patient reported having upper respiratory infection symptoms for 2 weeks prior to this presentation. [132] Based on the clinical and brain MRI findings, there were suspicion of ADEM or neuromyelitis optica spectrum disorder(NMOSD) following COVID-19. In another study, Demirci Otluoglu G et al. [196] reported a 48-year-old man with acute encephalomyelitis and positive CSF for SARS-CoV-2.
Acute Myelitis was reported in 5 patients after COVID-19. [[125], [126], [127], [128], [129]] It was shown that 30–60% of idiopathic transverse myelitis cases are associated with an antecedent respiratory, gastrointestinal, or systemic illness. [126] In the recent reported patients, post-infectious etiology in terms of secondary immunogenic overreaction was proposed as the underlying mechanism for myelitis after COVID-19. [125] Sotoca J et al. [127], reported a 69-year-old woman with acute necrotizing myelitis.
Optic Neuritis after COVID-19 was reported in only one patient. [60]
3.4.1.4. Impaired consciousness
Decreased level of consciousness and Encephalopathy were reported in 7.5% [39] to 31% [98] of the patients with COVID-19 in 22 articles encompassing 454 patients. (Table 3) Altered consciousness is a general term with several underlying mechanisms. In COVID-19 patients, possible mechanisms include infections, parenchymal damages, electrolyte imbalance, hypoxic, toxic and metabolic encephalopathies and non-convulsive status epilepticus. [58,100,134,135]
Leukoencephalopathy after COVID-19 was reported in 18 patients out of 3 studies. Radmanesh A et al. [145] reported diffuse white matter T2 hyperintensity plus restricted diffusion in 11 critically ill COVID-19 patients. Despite the fact that these findings are nonspecific and that their exact etiology is not certain; they attributed such findings to delayed post-hypoxic leukoencephalopathy. This pattern has been described in patients approximately 10-14 days after a hypoxic insult such as carbon monoxide poisoning, drug overdose, and cardiopulmonary arrests [145] and It is believed to relate to oligodendroglial cell death and subsequently demyelination. Other potential etiologies can be direct cerebral infection, sepsis-associated encephalopathy, post-infectious demyelination, and posterior reversible encephalopathy syndrome (PRES). [145]
Acute Necrotizing Encephalopathy(ANE) which was reported in 8 patients (Table 3) with COVID-19 is a distinct entity defined as rapid onset of neurological symptoms often secondary to a viral infection such as herpes viruses and influenza. Despite the association with viral infection, ANE is usually not considered as an inflammatory encephalitis. The cytokine storm which has been described with SARS-CoV-2 and some other viral infections can cause ANE, particularly in critically ill patients. In the absence of CSF pleocytosis, an intense surge of pro-inflammatory cytokines causes focal damage to the blood-brain barrier and induces edema and subsequent necrosis. [146,147] (Figure. 6 )
Fig. 6.
Fluid-attenuated inversion recovery (FLAIR) image shows hyper intensities within the bilateral thalami and medial temporal lobes (arrows) and also evidence of hemorrhage on C, G, hypo intense signal (arrows) on susceptibility-weighted images(SWI) and rim enhancements in D, H. From Poyiadji N. et al. [148]
Encephalitis following COVID-19 was reported in 22 patients out of 13 studies. (Table 3) Recently, SARS-CoV-2 was detected in brain tissues and capillary endothelial cells at autopsy and viral infection of CNS was confirmed. [159] Two different potential route of virus entry were suggested; First possible route is through the trigeminal and olfactory nerve endings. Infiltration through the olfactory system could explain the increased FLAIR signal in the medial temporal lobe. Moreover, signal changes which is seen in the brainstem and thalamus may represent a central infiltration through the trigeminal system. [146] The second possible mechanism of viral invasion may be increased permeability of the Blood Brain Barrier (BBB) due to high levels of pro-inflammatory cytokines in the CSF. [148]
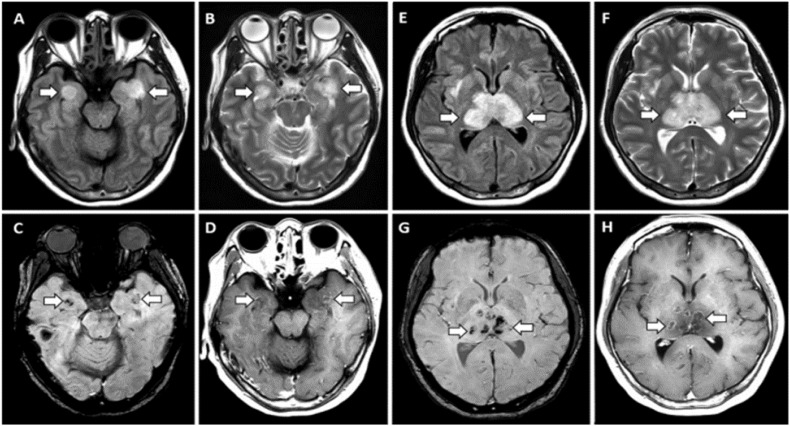
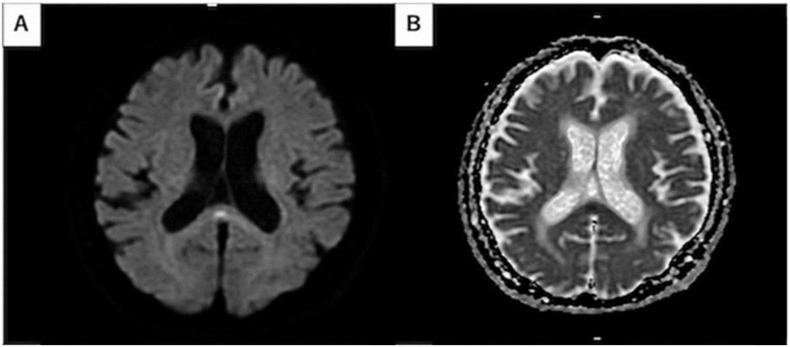
Mild Encephalitis/Encephalopathy with a Reversible Splenial Lesion(MERS) is suggested to be in relation with various viral infection, electrolyte imbalance disorders, organ failure, and administration of a certain medications. [160] In relation with COVID-19, a 75-year-old man with MERS was reported who presented with transient cerebellar ataxia and alteration of consciousness. [160] (Figure 7 )
Fig. 7.
Diffusion weighted image (DWI) shows high signal intensity in the splenium of corpus callosum. Apparent diffusion coefficient (ADC) map demonstrates correlated slight decrease in ADC. From Hayashi M et al. [160]
Posterior reversible encephalopathy syndrome (PRES) usually presents with acute impairment in level of consciousness, headache, visual disturbance and seizures. It is usually associated with cortical or subcortical vasogenic edema, involving predominantly the parietal and occipital regions bilaterally. [163] This condition is commonly associated with fluctuation in blood pressure, renal failure, autoimmune conditions, infections and sepsis, preeclampsia or eclampsia and certain type of immunosuppressive-cytotoxic drugs. [163] PRES was reported in ten COVID-19 patients out of 8 studies. (Table 3) Definitive etiology is not fully understood but there are various proposed underlying mechanisms. In COVID-19, endothelial dysfunction related to SARS-CoV-2 in combination with hemodynamic instability and immunological activation with release of cytokines may increase the vascular permeability in the brain tissue. Furthermore, disruption of BBB in these cases may cause vasogenic edema and PRES. [[162], [163]] Kishfy L et al. [165] suggested that COVID-19 patients may be at higher risk of consequences of uncontrolled hypertension such as hypertensive encephalopathy and PRES due to endothelial dysfunction. For this reason, tight blood pressure control was suggested, particularly in ventilated COVID-19 patients.
3.4.1.5. Seizure
Seizure has been reported as a neurological manifestation in patients infected with SARS-CoV-1 [23], MERS-CoV [32] and SARS-CoV-2. In COVID-19, 48 patients out of 20 studies were reported to have seizures. (Table 3). CNS viral infections and subsequent activation of neuro-inflammatory pathways are known to lower the threshold for seizures and potentially facilitate epileptogenesis in certain individuals. [174] As an example, in a COVID-19 patient with prior structural brain damage, focal seizures originating from the lesion site was reported. [175] Moreover, the accumulation of inflammatory markers associated with SARS-CoV-2 infection, may cause a local cortical irritation that precipitates seizures [177]. In addition, viral encephalitis and direct invasion of the virus to the CNS may cause seizure in the affected patients. [177,178] In critically ill COVID-19 patients, metabolic and electrolyte imbalances, ongoing hypoxia and inflammatory/infectious processes may also contribute to seizure or abnormal EEG background. [134]
In a recent study on electroencephalography (EEG) findings of COVID-19 patients by Galanopoulou AS et al. [180], sporadic epileptiform activity, predominantly in the form of frontal sharp waves, were detected in 40.9% of patients with altered mental status. [180]. “Generalized background slowing” particularly in patients with decreased level of consciousness was reported in several investigations. [178,180,181] It was also shown that previous diagnosis of epilepsy can be a potential risk factor for COVID-19-associated seizure. [181]
3.4.1.6. Movement disorders
There are few reports about movement disorders in COVID-19. Rábano-Suárez P et al. [182] reported 3 patients with generalized myoclonus with both positive and negative jerks, with predominantly involved the facial, sternocleidomastoid, trapezius, and upper extremities muscles. In those patients, myoclonus occurred spontaneously and were extremely sensitive to multisensory stimuli (auditive and tactile) or voluntary movements. After Immunotherapy, all 3 patients improved, at least partially. [182]
Another study reported [183] a previously healthy 58-year-old man with COVID-19 who developed generalized myoclonus, fluctuating level of consciousness, opsoclonus, right-side dominant hypokinetic-rigid syndrome, and frank hypomimia. The patient also had brief conjugated, multidirectional, and chaotic saccadic ocular movements. DaT-SPECT confirmed bilateral decreased presynaptic dopamine uptake asymmetrically involving bilateral putamen. Parkinsonian symptoms in this patient improved spontaneously without specific treatment. Association between viral infection and Parkinson’s disease was suggested decades ago, namely since the Spanish Flu Pandemic [5]. As mentioned earlier, Fazzini E et al revealed elevated levels of HCoV-OC43 and HCoV-229E antibodies in the CSF of patients with Parkinson’s disease [9] Given scanty available about such association, no definite conclusion can be drawn.
3.4.1.7. CNS Vasculitis
Hanafi R, et al. [185] reported a 65-year-old man with COVID-19 who had extensive diffuse subcortical ischemic lesions in the brain resembling cerebral vasculitis. (Figure 8 ) He also developed a characteristic lower extremity skin rash. SARS-CoV-2 infects the host through its CoV spike glycoprotein, which binds to the ACE2 receptor. ACE2 receptor has higher expression in neurons and cerebral vessel endothelial cells which can cause high level of CNS invasion. [185,186] Histologic evidence of COVID-19–induced vasculitis has been reported in several other organs including lung, kidney, liver and skin. [186] Similarly, virus-related endothelial injury and endotheliitis might cause CNS vasculitis.
Fig. 8.
Hyperintense lesions within the periventricular white matter, cerebellar peduncles and basal ganglia (white arrows), and the corpus callosum (red arrow) indicative of diffuse ischemic lesions on the FLAIR image. From Hanafi R, et al. [185]
3.4.1.8. Cranial Nerves abnormalities
Anosmia and Ageusia, the prevalence of anosmia and ageusia ranges widely in different studies from 5% in a study from China [39] to about 88% in an Italian study [56]. Smell impairment was also reported in SARS-CoV-1 and influenza infections. [27] Anosmia was reported in 3730 patients out of 28 studies in the case of SARS-CoV-2. (Table 3) In COVID-19, anosmia is typically not accompanied by nasal swelling or rhinitis and based the findings of several studies it is an important common early clinical presentation even in the absence of other respiratory symptoms. [209] several studies reported that anosmia was more common among females, younger and non-hospitalized patients. [211,214] In most patients, anosmia went away on its own within 3 weeks. [211]
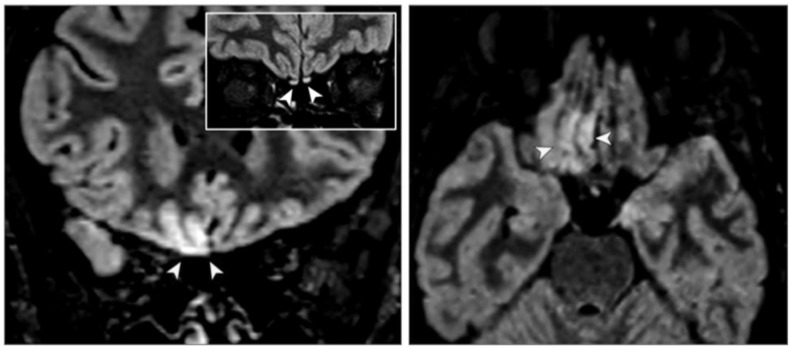
MRI abnormalities of the olfactory bulb of COVID-19 patients have been reported in several studies. [207] Politi SL et al. [209], reported a 25-year-old female with anosmia and subtle hyper-intensity signal in the olfactory bulbs and also cortical hyper-intensity in the right gyrus rectus. In follow/up MRI after 28 days, the olfactory bulbs were thinner and slightly less hyper-intense and the signal alteration in the cortex had completely disappeared. (Figure 9 ) The olfactory system is also considered as a potential route of virus entry. Various studies suggested that CoV may have CNS direct invasion via olfactory bulb. [146,159]
Fig 9.
FLAIR image shows cortical hyperintensity in the right gyrus (yellow arrowheads) in both axial and coronal sections and subtle hyperintensity in the bilateral olfactory bulbs (white arrowheads) in the coronal section. From Politi SL et al. [209]
Visual Impairments after COVID-19 was reported in 12 patients out of 3 studies. Selvaraj V et al. [204] reported a middle aged woman with COVID-19 who presented with sudden onset painless right eye monocular visual blurriness. Brain and orbit MRIs were unremarkable and eye examinations were normal. posterior ischemic optic neuropathy was considered as the cause. Thromboembolic events, systematic inflammation associated with COVID-19 and invasion of CoV to the CNS through the hematogenous route or direct invasion through the cribriform plate or conjunctiva were considered the potential underlying mechanisms. [204]
3.4.2. Symptoms Associated with Peripheral Nervous System (PNS)
3.4.2.1. Cranial Nerve abnormalities
Impaired Eye movement associated with COVID-19 was described in 12 patients out of 4 studies. (Table 4) Pascual-Goñi E et al. [194], reported a 60-year-old woman with right abducens nerve palsy. In brain MRI examination, FLAIR hyper-intensity of the pontine tegmentum and right sixth cranial nerve nucleus was noted.
Trigeminal neuropathy was reported in 9 patients out of 2 studies. (Table 4) de Freitas Ferreira ACA et al [216] reported a 39-year-old man with trigeminal neuropathy associated with SARS-Cov-2 and herpes zoster co-infection.
3.4.2.2. Guillain-Barré Syndrome (GBS)
GBS can occur post gastrointestinal or respiratory illness. The suggested mechanism is molecular mimicry in which the pathogen likely share epitopes similar to the components of the peripheral nerves. The antibodies produced by the host immune system to fight the virus, cross-react and bind to the peripheral nerves causing neuronal dysfunction. [249] AIDP and AMAN variants have been reported after SARS-CoV-1 and MERS-CoV infections. [25,34,46] Concerning COVID-19, 52 patients out of 36 studies were described to have different variants of GBS. (Table 4) Miller Fisher Syndrome was also reported in several cases. [101,228,237,239,246]
3.4.3. Symptoms associated with Skeletal Muscle and Neuromuscular Junction (NMJ)
Skeletal muscle injury and myopathy have been reported in COVID-19 patients. (Table 5) In the severe COVID-19 patients, critical illness myopathy has been reported. Major proposed risk factors for this type of myopathy are severe respiratory distress, systemic inflammatory response and sepsis. [250,251] Moreover direct invasion of the muscle by the virus is the other potential mechanism for myopathy. Similar to SARS-CoV-1, SARS-CoV-2 have the ability to penetrate the cells that express ACE2 receptors. As ACE2 is expressed in the muscle cells, the possibility of invasion of the muscles by the virus entering the cells via the ACE2 receptors should also be considered. In addition, hyper-inflammation and cytokine storms in the advanced phase of COVID-19 could cause immune-mediated muscle damage. [250] Considering the growing number of patients with COVID-19, myopathy should be considered as a major cause of long-term physical disability [251]
4. Similarities between neurological manifestations of CoV infections
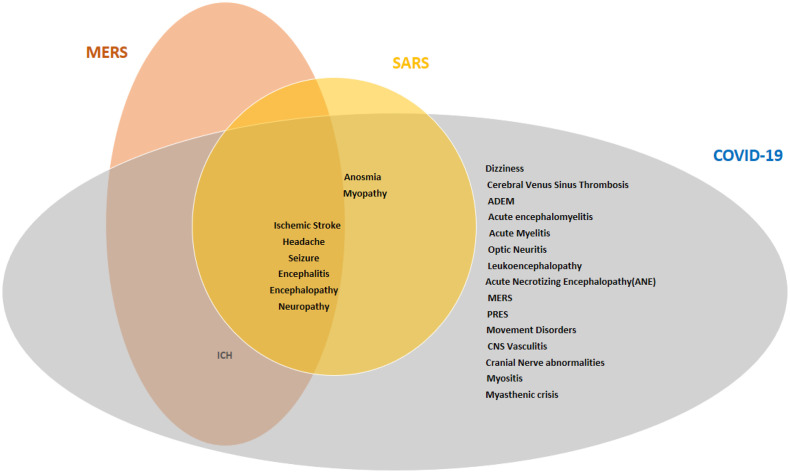
There are several similarities between neurological manifestations of different CoV infections. encephalitis was reported in HCoV-OC43, SARS-CoV-1, MERS-CoV and SARS-CoV-2 infections. [15,16,24,33,60,98,100,150,151,151,152,153,154,155,156,157,158] ADEM was reported after HCoV-OC43 and SARS-CoV-2 infection. [17,120,121,122,123] Headache, ischemic stroke, encephalitis and encephalopathy, seizure and neuropathy were reported in all the pandemics associated with CoV (SARS-CoV-1, MERS-CoV and SARS-CoV-2). ICH was reported in MERS-CoV and COVID-19 and myopathy and anosmia were reported in SARS-CoV-1 and COVID-19. (Figure 10 )
Fig. 10.
Venn diagram of neurological presentations in different CoV infections
Limitations
In this review, we tried to gather and summarize the results of all the studies reporting neurological disorders observed in patients with CoV infections. However, in some of the reported patients, the neurological manifestations might not be associated with the CoV infections and just coincidentally occurred due to the patient’s underlying comorbidities. Moreover, in patients with severe CoV infections, the associated sepsis and organs failure my lead to different neurological presentations which can be seen in any of critical conditions. In addition, in several studies, particularly in the case of COVID-19, sufficient investigations have not been performed and hard to believe that the neurological manifestation was related to CoV infection. Finally, yet importantly, the neurological symptoms in some of these patients might be medications side effects given CoV infected patients have been treated with different classes of medications, which have side effects, not necessarily reported in the studies.
5. Conclusion
There are similarities between the neurological complications associated with SARS-CoV-1, MERS-CoV and COVID-19. However, the scope of the pandemics and number of patients involved in each are different. Thus far, SARS-CoV-2 has infected millions of people worldwide. Reports on the neurological complications after and during COVID-19 are growing on a daily basis. Better understanding of the potential associated neurological complications will help health care providers to be more attentive to these complications and a more timely diagnosis and management.
Sources of funding
None.
Declaration of Competing Interest
Authors have no relevant financial disclosure to report.
References
- 1.Troyer E.A., Kohn J.N., Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020 Jul;87:34–39. doi: 10.1016/j.bbi.2020.04.027. S0889-1591(20)30489-X.vcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin P., Martin-Granel E. 2,500-year evolution of the term epidemic. Emerg Infect Dis. 2006;12(6):976–980. doi: 10.3201/eid1206.051263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunha B.A. Influenza: historical aspects of epidemics and pandemics. Infect Dis Clin North Am. 2004 Mar;18(1):141–155. doi: 10.1016/S0891-5520(03)00095-3. [DOI] [PubMed] [Google Scholar]
- 4.Turner E.B. Discussion on Influenza. Proc R S Med. 1919;12:76–78. [PMC free article] [PubMed] [Google Scholar]
- 5.Jang H., Boltz D.A., Webster R.G., Smeyne R.J. Viral parkinsonism. Biochim Biophys Acta. 2009 Jul;1792(7):714–721. doi: 10.1016/j.bbadis.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahn J.S., McIntosh K. “History and recent advances in coronavirus discovery”. The Pediatric Infectious Disease Journal. Pediatr Infect Dis J. November 2005;24(11 Suppl) doi: 10.1097/01.inf.0000188166.17324.60. S223-7, discussion S226. [DOI] [PubMed] [Google Scholar]
- 7.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutton B., Salanti G., Caldwell D.M. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern. Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 9.Fazzini E., Fleming J., Fahn S. Cerebrospinal fluid antibodies to coronavirus in patients with Parkinson’s disease. Mov Disord. 1992;7(2):153–158. doi: 10.1002/mds.870070210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burks J.S., DeVald B.L., Jankovsky L.D., Gerdes J.C. Two coronaviruses isolated from central nervous system tissue of two multiple sclerosis patients. Science. 1980 Aug 22;209(4459):933–934. doi: 10.1126/science.7403860. [DOI] [PubMed] [Google Scholar]
- 11.Cristallo A., Gambaro F., Biamonti G., Ferrante P., Battaglia M., Cereda P.M. Human coronavirus polyadenylated RNA sequences in cerebrospinal fluid from multiple sclerosis patients. New Microbiol. 1997 Apr;20(2):105–114. [PubMed] [Google Scholar]
- 12.Dessau R.B., Lisby G., Frederiksen J.L. Coronaviruses in brain tissue from patients with multiple sclerosis. Acta Neuropathol. 2001 Jun;101(6):601–604. doi: 10.1007/s004010000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arbour N., Côté G., Lachance C., Tardieu M., Cashman N.R., Talbot P.J. Acute and persistent infection of human neural cell lines by human coronavirus OC43. J Virol. 1999;73(4):3338–3350. doi: 10.1128/jvi.73.4.3338-3350.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubé M., Le Coupanec A., Wong A.H.M., Rini J.M., Desforges M., Talbot P.J. Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. J Virol. 2018;92(17):e00404–e00418. doi: 10.1128/JVI.00404-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nilsson A., Edner N., Albert J., Ternhag A. Fatal encephalitis associated with coronavirus OC43 in an immunocompromised child. Infect Dis (Lond). 2020;52(6):419–422. doi: 10.1080/23744235.2020.1729403. [DOI] [PubMed] [Google Scholar]
- 16.Morfopoulou S., Brown J.R., Davies E.G., Anderson G., Virasami A., Qasim W., et al. Human coronavirus OC43 associated with fatal encephalitis. N Engl J Med. 2016;375(5):497–498. doi: 10.1056/NEJMc1509458. [DOI] [PubMed] [Google Scholar]
- 17.Yeh E.A., Collins A., Cohen M.E., Duffner P.K., Faden H. Detection of Coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics. 2004 Jan;113(1):e73–e76. doi: 10.1542/peds.113.1.e73. Pt 1. [DOI] [PubMed] [Google Scholar]
- 18.Turgay C., Emine T., Ozlem K., Muhammet S.P., Haydar A.T. A rare cause of acute flaccid paralysis: Human coronaviruses. J Pediatr Neurosci. 2015 Jul-Sep;10(3):280–281. doi: 10.4103/1817-1745.165716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y., Li H., Fan R., Wen B., Zhang J., Cao X., et al. Coronavirus Infections in the Central Nervous System and Respiratory Tract Show Distinct Features in Hospitalized Children. Intervirology. 2016;59(3):163–169. doi: 10.1159/000453066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sørensen M.D., Sørensen B., Gonzalez-Dosal R., Melchjorsen C.J., Weibel J., Wang J., et al. Severe acute respiratory syndrome (SARS): development of diagnostics and antivirals. Annals of the New York Academy of Sciences. 2006;1067(1):500–505. doi: 10.1196/annals.1354.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Umapathi T., Kor A.C., Venketasubramanian N., Lim C.C., Pang B.C., Yeo T.T., et al. Large artery ischemic stroke in severe acute respiratory syndrome (SARS) J Neurol. 2004 Oct;251(10):1227–1231. doi: 10.1007/s00415-004-0519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding Y., Wang H., Shen H., Li Z., Geng J., Han H., et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003 Jul;200(3):282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lau K.K., Yu W.C., Chu C.M., Lau S.T., Sheng B., Yuen K.Y. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis. 2004 Feb;10(2):342–344. doi: 10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu J., Zhong S., Liu J., Li L., Li Y., Wu X., et al. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis. 2005 Oct 15;41(8):1089–1096. doi: 10.1086/444461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai L.K., Hsieh S.T., Chao C.C., Chen Y.C., Lin Y.H., Chang S.C., Chang Y.C. Neuromuscular disorders in severe acute respiratory syndrome. Arch Neurol. 2004 Nov;61(11):1669–1673. doi: 10.1001/archneur.61.11.1669. [DOI] [PubMed] [Google Scholar]
- 26.Chao C.C., Tsai L.K., Chiou Y.H., Tseng M.T., Hsieh S.T., Chang S.C., et al. Peripheral nerve disease in SARS: report of a case. Neurology. 2003 Dec 23;61(12):1820–1821. doi: 10.1212/01.wnl.0000099171.26943.d0. [DOI] [PubMed] [Google Scholar]
- 27.Hwang C.S. Olfactory neuropathy in severe acute respiratory syndrome: report of A case. Acta Neurol Taiwan. 2006 Mar;15(1):26–28. [PubMed] [Google Scholar]
- 28.Leung T.W., Wong K.S., Hui A.C., To KF, Lai S.T., Ng W.F., et al. Myopathic changes associated with severe acute respiratory syndrome: a post-mortem case series. Arch Neurol. 2005 Jul;62(7):1113–1117. doi: 10.1001/archneur.62.7.1113. [DOI] [PubMed] [Google Scholar]
- 29.Gu J., Gong E., Zhang B., Zheng J., Gao Z., Zhong Y., et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202(3):415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zumla A., Hui D.S., Perlman S. Middle East respiratory syndrome. Lancet. 2015;386(9997):995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.situation update M.E.R.S. World Health Organization. January. January 2020:2020. [Google Scholar]
- 32.Saad M., Omrani A.S., Baig K., Bahloul A., Elzein F., Matin M.A., et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014 Dec;29:301–306. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arabi Y.M., Harthi A., Hussein J., Bouchama A., Johani S., Hajeer A.H., et al. Severe neurologic syndrome associated with Middle East respiratory syndrome coronavirus (MERS-CoV) Infection. 2015;43:495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Algahtani H., Subahi A., Shirah B. Neurological Complications of Middle East Respiratory Syndrome Coronavirus: A Report of Two Cases and Review of the Literature. Case Rep Neurol Med. 2016;2016 doi: 10.1155/2016/3502683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Hameed F.M. Spontaneous intracranial hemorrhage in a patient with Middle East respiratory syndrome coronavirus. Saudi Med J. 2017 Feb;38(2):196–200. doi: 10.15537/smj.2017.2.16255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim J.E., Heo J.H., Kim H.O., Song S.H., Park S.S., Park T.H., et al. Neurological Complications during Treatment of Middle East Respiratory Syndrome. J Clin Neurol. 2017 Jul;13(3):227–233. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li K., Wohlford-Lenane C., Perlman S., Zhao J., Jewell A., Reznikov L., et al. Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J Infect Dis. 2016;213(5):712–722. doi: 10.1093/infdis/jiv499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bolay H., Gül A., Baykan B. COVID-19 is a Real Headache! Headache. 2020 May 15 doi: 10.1111/head.13856. PMID: 32412101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020 Apr 10;77(6):1–9. doi: 10.1001/jamaneurol.2020.1127. e201127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020 May;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020 Mar 17;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng Y., Liu W., Liu K., Fang Y., Shang J., Zhou L., et al. Clinical Characteristics of Fatal and Recovered Cases of Coronavirus Disease 2019 (COVID-19) in Wuhan, China: A Retrospective Study. Chin Med J (Engl) 2020 Jun 5;133(11):1261–1267. doi: 10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guan W.J., Ni Z.Y., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of 2019 novel coronavirus infection in China. N Engl J Med. 2020 Apr 30;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. 2002.2006.20020974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang D., Lin M., Wei L., Wang Z., Liang Y., Huang T., et al. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA. 2020;323(11):1092–1093. doi: 10.1001/jama.2020.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang G., Nie S., Zhang Z., Zhang Z. Longitudinal Change of SARS-Cov-2 Antibodies in Patients with COVID-19. J Infect Dis. 2020 Jun 29;222(2):183–188. doi: 10.1093/infdis/jiaa229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun L., Shen L., Fan J., Gu F., Hu M., An Y., et al. Clinical Features of Patients with Coronavirus Disease 2019 (COVID-19) from a Designated Hospital in Beijing, China. J Med Virol. 2020 May 5:1–12. doi: 10.1002/jmv.25966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020 Jun 6;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang R., Zhu L., Xue L., Liu L., Yan X., Wang J., et al. Clinical findings of patients with coronavirus disease 2019 in Jiangsu province, China: A retrospective, multicenter study. PLoS Negl Trop Dis. 2020 May 8;14(5) doi: 10.1371/journal.pntd.0008280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kato H., Shimizu H., Shibue Y., Hosoda T., Iwabuchi K., Nagamine K., et al. Clinical course of 2019 novel coronavirus disease (COVID-19) in individuals present during the outbreak on the Diamond Princess cruise ship. J Infect Chemother. 2020 Aug;26(8):865–869. doi: 10.1016/j.jiac.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang F., Yang Y., Dong K., Yan Y., Zhang S., Ren H., et al. CLINICAL CHARACTERISTICS OF 28 PATIENTS WITH DIABETES AND COVID-19 IN WUHAN, CHINA. Endocr Pract. 2020 Jun 2;26(6):668–674. doi: 10.4158/EP-2020-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lechien J.R., Chiesa-Estomba C.M., Place S., Van Laethem Y., Cabaraux P., Mat Q., et al. Clinical and Epidemiological Characteristics of 1,420 European Patients with mild-to-moderate Coronavirus Disease 2019. J Intern Med. 2020 Apr 30 doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu K., Fang Y.Y., Deng Y., Liu W., Wang M.F., Ma J.P., et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl). 2020 May 5;133(9):1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wan S., Xiang Y., Fang W., Zheng Y., Li B., Hu Y. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020, Mar 21 doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lechien J.R., Chiesa-Estomba C.M., Cabaraux P., Mat Q., Huet K., Harmegnies B., et al. Features of Mild-to-Moderate COVID-19 Patients With Dysphonia. J Voice. 2020 Jun 4 doi: 10.1016/j.jvoice.2020.05.012. S0892-1997(20)30183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karadaş Ö., Öztürk B., Sonkaya A.R. A prospective clinical study of detailed neurological manifestations in patients with COVID-19. Neurol Sci. 2020 Jun;25:1–5. doi: 10.1007/s10072-020-04547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pinna P., Grewal P., Hall J.P., Tavarez T., Dafer R.M., Garg R., et al. Neurological manifestations and COVID-19: Experiences from a tertiary care center at the Frontline. J Neurol Sci. 2020 Jun 3;415 doi: 10.1016/j.jns.2020.116969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singh J., Ali A. Headache as the Presenting Symptom in 2 Patients with COVID-19 and a History of Migraine: 2 Case Reports. Headache. 2020 Jun 10:10. doi: 10.1111/head.13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E., Sánchez-Larsen Á., Layos-Romero A., García-García J., et al. Neurologic Manifestations in Hospitalized Patients With COVID-19: The ALBACOVID Registry. Neurology. 2020 Jun 1 doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hornuss D., Lange B., Schröter N., Rieg S., Kern W.V., Wagner D. Anosmia in COVID-19 patients. Clin Microbiol Infect. 2020 May 22 doi: 10.1016/j.cmi.2020.05.017. S1198-743X(20)30294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liguori C., Pierantozzi M., Spanetta M., Sarmati L., Cesta N., Iannetta M., et al. Subjective neurological symptoms frequently occur in patients with SARS-CoV2 infection. Brain Behav Immun. 2020 Aug;88:11–16. doi: 10.1016/j.bbi.2020.05.037. S0889-1591(20)30876-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pons-Escoda A., Naval-Baudín P., Majós C., Camins A., Cardona P., Cos M., et al. Neurologic Involvement in COVID-19: Cause or Coincidence? A Neuroimaging Perspective. AJNR Am J Neuroradiol. 2020 Jun 11 doi: 10.3174/ajnr.A6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tian S., Hu N., Lou J., Chen K., Kag X., Xiang Z., et al. Characteristics of COVID-19 infection in Beijing. J Infect. 2020;80:401–406. doi: 10.1016/j.jinf.2020.02.018. Epub 2020 Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jin X., Lian J.S., Hu J.H., Gao J., Zheng L., Zhang Y., et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gupta N., Agrawal S., Ish P., Mishra S., Gaind R., Usha G., et al. Clinical and epidemiologic profile of the initial COVID-19 patients at a tertiary care centre in India. Monaldi Arch Chest Dis. 2020;90 doi: 10.4081/monaldi.2020.1294. [DOI] [PubMed] [Google Scholar]
- 67.Xu X.W., Wu X.X., Jiang X.G., Xu K., Ying L., Ma C., et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: Retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tostmann A., Bradley J., Bousema T., et al. Strong associations and moderate predictive value of early symptoms for SARS-CoV-2 test positivity among healthcare workers, the Netherlands, March 2020. Euro Surveill. 2020;25(2000508) doi: 10.2807/1560-7917.ES.2020.25.16.2000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vacchiano V., Riguzzi P., Volpi L., Tappatà M., Avoni P., Rizzo G., et al. Early neurological manifestations of hospitalized COVID-19 patients. Neurol Sci. 2020 Jul 2:1–3. doi: 10.1007/s10072-020-04525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhong Z.F., Huang J., Yang X., Peng J.L., Zhang X.Y., Hu Y., et al. Epidemiological and clinical characteristics of COVID-19 patients in Hengyang, Hunan Province, China. World J Clin Cases. 2020 Jun 26;8(12):2554–2565. doi: 10.12998/wjcc.v8.i12.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China. Clin Infect Dis. 2020; Mar 16:ciaa272. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kong Z., Wang J., Li T., Zhang Z., Jian J. 2019 novel coronavirus pneumonia with onset of dizziness: a case report. Ann Transl Med. 2020 Apr;8(7):506. doi: 10.21037/atm.2020.03.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Avula A., Nalleballe K., Narula N., Sapozhnikov S., Dandu V., Toom S., et al. COVID-19 presenting as stroke. Brain Behav Immun. 2020 Jul;87:115–119. doi: 10.1016/j.bbi.2020.04.077. S0889-1591(20)30685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beyrouti R., Adams M.E., Benjamin L., Cohen H., Farmer S.F., Goh Y.Y., et al. Characteristics of ischemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020 Aug;91(8):889–891. doi: 10.1136/jnnp-2020-323586. jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Benussi A., Pilotto A., Premi E., Libri I., Giunta M., Agosti C., et al. Clinical characteristics and outcomes of inpatients with neurologic disease and COVID-19 in Brescia, Lombardy, Italy. Neurology. 2020 May 22 doi: 10.1212/WNL.0000000000009848. [DOI] [PubMed] [Google Scholar]
- 76.Tunç A., Ünlübaş Y., Alemdar M., Akyüz E. Coexistence of COVID-19 and acute ischemic stroke report of four cases. J Clin Neurosci. 2020 Jul;77:227–229. doi: 10.1016/j.jocn.2020.05.018. S0967-5868(20)31081-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yaghi S., Ishida K., Torres J., Mac Grory B., Raz E., Humbert K., et al. SARS2-CoV-2 and Stroke in a New York Healthcare System. Stroke. 2020 Jul;51(7):2002–2011. doi: 10.1161/STROKEAHA.120.030335. STROKEAHA120030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Deliwala S., Abdulhamid S., Abusalih M.F., Al-Qasmi M.M., Bachuwa G. Encephalopathy as the Sentinel Sign of a Cortical Stroke in a Patient Infected With Coronavirus Disease-19 (COVID-19) Cureus. 2020 May 14;12(5) doi: 10.7759/cureus.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morassi M., Bagatto D., Cobelli M., D’Agostini S., Gigli G.L., Bnà C., et al. Stroke in patients with SARS-CoV-2 infection: case series. J Neurol. 2020 Aug;267(8):2185–2192. doi: 10.1007/s00415-020-09885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oxley T., Mocco J., Majidi S., Kellner C., Shoirah H., Singh P., et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N Engl J Med. 2020 May 14;382(20) doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goldberg M.F., Goldberg M.F., Cerejo R., Tayal A.H. Cerebrovascular Disease in COVID-19. AJNR Am J Neuroradiol. 2020 Jul;41(7):1170–1172. doi: 10.3174/ajnr.A6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moshayedi P., Ryan T.E., Mejia L.L.P., Nour M., Liebeskind D.S. Triage of Acute Ischemic Stroke in Confirmed COVID-19: Large Vessel Occlusion Associated with Coronavirus Infection. Front Neurol. 2020 Apr 21;11:353. doi: 10.3389/fneur.2020.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Klok F., Kruip M., van der Meer N., Arbuos M., Gommers D., Kant K. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020 Jul;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lodigiani C., Lapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sierra-Hidalgo F., Muñoz-Rivas N., Torres Rubio P., Chao K., Villanova Martínez M., Arranz García P., et al. Large artery ischemic stroke in severe COVID-19. J Neurol. 2020 Jun 27:1–3. doi: 10.1007/s00415-020-09967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Immovilli P., Terracciano C., Zaino D., Marchesi E., Morelli N., Terlizzi E., et al. Stroke in COVID-19 patients-A case series from Italy. Int J Stroke. 2020 Aug;15(6):701–702. doi: 10.1177/1747493020938294. 1747493020938294. [DOI] [PubMed] [Google Scholar]
- 87.Cavallieri F., Marti A., Fasano A., Salda A.D., Ghirarduzzi A., Moratti C., et al. Prothrombotic state induced by COVID-19 infection as trigger for stroke in young patients: A dangerous association. eNeurologicalSci. 2020 Jun 6;20 doi: 10.1016/j.ensci.2020.100247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xiong W., Mu J., Guo J., Lu L., Liu D., Luo J., et al. New onset neurologic events in people with COVID-19 infection in three regions in China. Neurology. 2020 Jun 17 doi: 10.1212/WNL. [DOI] [PubMed] [Google Scholar]
- 89.Williams O.H., Mohideen S., Sen A., Martinovic O., Hart J., Brex P.A., et al. Multiple internal border zone infarcts in a patient with COVID-19 and CADASIL. J Neurol Sci. 2020 Jun 9;416 doi: 10.1016/j.jns.2020.116980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sharifi-Razavi A., Karimi N., Zarvani A., Cheraghmakani H., Baghbanian S.M. Ischemic Stroke Associated With Novel Coronavirus 2019: A Report of Three Cases. Int J Neurosci. 2020 Jun 17:1–5. doi: 10.1080/00207454.2020.1782902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guillan M., Villacieros-Alvarez J., Bellido S., Perez-Jorge Peremarch C., Suarez-Vega V.M., Aragones-Garcia M., et al. Unusual simultaneous cerebral infarcts in multiple arterial territories in a COVID-19 patient. Thromb Res. 2020 Jun 9;193:107–109. doi: 10.1016/j.thromres.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Franceschi A.M., Arora R., Wilson R., Giliberto L., Libman R.B., Castillo M. Neurovascular Complications in COVID-19 Infection: Case Series. AJNR Am J Neuroradiol. 2020 Jun 11 doi: 10.3174/ajnr.A6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Khan M., Ibrahim R.H., Siddiqi S.A., Kerolos Y., Al-Kaylani M.M., AlRukn S.A., et al. COVID-19 and Acute Ischemic Stroke - A Case Series From Dubai, UAE. Int J Stroke. 2020 Aug;15(6):699–700. doi: 10.1177/1747493020938285. 1747493020938285. [DOI] [PubMed] [Google Scholar]
- 94.Cantador E., Núñez A., Sobrino P., Espejo V., Fabia L., Vela L., et al. Incidence and consequences of systemic arterial thrombotic events in COVID-19 patients. J Thromb Thrombolysis. 2020 Jun 9:1–5. doi: 10.1007/s11239-020-02176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fara M.G., Stein L.K., Skliut M., Morgello S., Fifi J.T., Dhamoon M.S. Macrothrombosis and stroke in patients with mild Covid-19 infection. J Thromb Haemost. 2020 May 28 doi: 10.1111/jth.14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., et al. Neurologic Features in Severe SARS-CoV-2 Infection. N Engl J Med. 2020 Jun 4;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Viguier A., Delamarre L., Duplantier J., Olivot J.M., Bonneville F. Acute ischemic stroke complicating common carotid artery thrombosis during a severe COVID-19 infection. J Neuroradiol. 2020 May 4 doi: 10.1016/j.neurad.2020.04.003. S0150-9861(20)30159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Varatharaj A., Thomas N., Ellul M.A., Davies N.W.S., Pollak T.A., Tenorio E.L., et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020 Jun 25 doi: 10.1016/S2215-0366(20)30287-X. S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li Y., Li M., Wang M., Zhou Y., Chang J., Xian Y., et al. Acute Cerebrovascular Disease Following COVID-19: A Single Center, Retrospective, Observational Study. Stroke Vasc Neurol. 2020 Jul 2 doi: 10.1136/svn-2020-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jain R., Young M., Dogra S., Kennedy H., Nguyen V., Jones S., et al. COVID-19 related neuroimaging findings: A signal of thromboembolic complications and a strong prognostic marker of poor patient outcome. J Neurol Sci. 2020 May 19;414 doi: 10.1016/j.jns.2020.116923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mahammedi A., Saba L., Vagal A., Leali M., Rossi A., Gaskill M., et al. Imaging in Neurological Disease of Hospitalized COVID-19 Patients: An Italian Multicenter Retrospective Observational Study. Radiology. 2020 May 21 doi: 10.1148/radiol.2020201933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Malentacchi M., Gned D., Angelino V., Demichelis S., Perboni A., Veltri A., et al. Concomitant Brain Arterial and Venous Thrombosis in a COVID-19 Patient. Eur J Neurol. 2020 Jun 5 doi: 10.1111/ene.14380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ranucci M., Ballotta A., Di Dedda U., Bayshnikova E., Dei Poli M., Resta M., et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020 Jul;18(7):1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kochi A.N., Tagliari A.P., Forleo G.B., Fassini G.M., Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. Journal of Cardiovascular Electrophysiology. 2020;31(5):1003–1008. doi: 10.1111/jce.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hess D.C., Eldahshan W., Rutkowski E. COVID-19-related stroke. Translational Stroke Research. 2020;1 doi: 10.1007/s12975-020-00818-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sharifi-Razavi A., Karimi N., Rouhani N. COVID 19, and intracerebral hemorrhage: causative or coincidental. New Microbes New Infect. 2020 Mar;27 doi: 10.1016/j.nmni.2020.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Muhammad S., Petridis A., Cornelius J.F., Hänggi D. Letter to editor: Severe brain haemorrhage and concomitant COVID-19 Infection: A neurovascular complication of COVID-19. Brain Behav Immun. 2020 May;5 doi: 10.1016/j.bbi.2020.05.015. S0889-1591(20)30802-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Agarwal A., Vishnu V.Y., Vibha D., Bhatia R., Gupta A., Das A., Srivastava M.V.P. Intracerebral Hemorrhage and SARS-CoV-2: Association or Causation. Ann Indian Acad Neurol. 2020 May-Jun;23(3):261–264. doi: 10.4103/aian.AIAN_362_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Benger M., Williams O., Siddiqui J., Sztriha L. Intracerebral haemorrhage and COVID-19: Clinical characteristics from a case series. Brain Behav Immun. 2020 Aug;88:940–944. doi: 10.1016/j.bbi.2020.06.005. (S0889-1591(20)31097-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li J., Long X., Zhu C., Hu S., Lin Z., Li J., Xiong N. A case of COVID-19 pneumonia with cerebral hemorrhage. Thromb Res. 2020 May 30;193:22–24. doi: 10.1016/j.thromres.2020.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ghosh R., Dubey S., Kanti Ray B., Chatterjee S., Benito-León J. COVID-19 Presenting With Thalamic Hemorrhage Unmasking Moyamoya Angiopathy. Can J Neurol Sci. 2020 Jun 4:1–3. doi: 10.1017/cjn.2020.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Carroll E., Lewis A. Catastrophic Intracranial Hemorrhage in Two Critically Ill Patients with COVID-19. Neurocrit Care. 2020 May;26:1–5. doi: 10.1007/s12028-020-00993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Degeneffe A., Bruneau M., Spitaels J., Gilis N., De Witte O., Lubansu A. Acute hemorrhage after intra-cerebral biopsy in COVID-19 patients: a report of 3 cases. World Neurosurg. 2020 Jun 8;141:157–161. doi: 10.1016/j.wneu.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chougar L., Mathon B., Weiss N., Degos V., Shor N. Atypical Deep Cerebral Vein Thrombosis with Hemorrhagic Venous Infarction in a Patient Positive for COVID-19. AJNR Am J Neuroradiol. 2020 Jun 18 doi: 10.3174/ajnr.A6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Poillon G., Obadia M., Perrin M., Savatovsky J., Lecler A. Cerebral Venous Thrombosis associated with COVID-19 infection: causality or coincidence? J Neuroradiol. 2020 May 11 doi: 10.1016/j.neurad.2020.05.003. S0150-9861(20)30167-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hughes C., Nichols T., Pike M., Subbe C., Elghenzai S. Cerebral Venous Sinus Thrombosis as a Presentation of COVID-19. Eur J Case Rep Intern Med. 2020 Apr 29;7(5) doi: 10.12890/2020_001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hemasian H., Ansari B. First case of Covid-19 presented with cerebral venous thrombosis: A rare and dreaded case. Rev Neurol (Paris). 2020 Jun;176(6):521–523. doi: 10.1016/j.neurol.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cavalcanti DD, Raz E, Shapiro M, Dehkharghani S, Yaghi S, Lillemoe K, et al. Cerebral Venous Thrombosis Associated with COVID-19. AJNR Am J Neuroradiol. 2020 Jun 18. [DOI] [PMC free article] [PubMed]
- 119.Speeckaert M.M., Speeckaert R., Delanghe J.R. Potential underlying mechanisms of cerebral venous thrombosis associated with COVID-19. J Neuroradiol. 2020 Jun 26 doi: 10.1016/j.neurad.2020.06.009. S0150-9861(20)30206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Novi G., Rossi T., Pedemonte E., Saitta L., Rolla C., Roccatagliata L., et al. Acute disseminated encephalomyelitis after SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm. 2020 Jun 1;7(5):e797. doi: 10.1212/NXI.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Parsons T., Banks S., Bae C., Gelber J., Alahmadi H., Tichauer M. COVID-19-associated acute disseminated encephalomyelitis (ADEM) J Neurol. 2020 May 30:1–4. doi: 10.1007/s00415-020-09951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Reichard R.R., Kashani K.B., Boire N.A., Constantopoulos E., Guo Y., Lucchinetti C.F. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020 Jul;140(1):1–6. doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Abdi S., Ghorbani A., Fatehi F. The association of SARS-CoV-2 infection and acute disseminated encephalomyelitis without prominent clinical pulmonary symptoms. J Neurol Sci. 2020 Jun 18;416 doi: 10.1016/j.jns.2020.117001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stewart J.N., Mounir S., Talbot P.J. Human coronavirus gene expression in the brains of multiple sclerosis patients. Virology. 1992 Nov;191(1):502–505. doi: 10.1016/0042-6822(92)90220-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhao K., Huang J., Dai D., Feng Y., Liu L., Nie S. Acute myelitis after SARS-CoV-2 infection: a case report. medRxiv. 2020 Jan 1 doi: 10.1101/2020.03.16.20035105. [DOI] [Google Scholar]
- 126.Zachariadis A., Tulbu A., Strambo D., Dumoulin A., Di Virgilio G. Transverse myelitis related to COVID-19 infection. J Neurol. 2020 Jun 29:1–3. doi: 10.1007/s00415-020-09997-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sotoca J., Rodríguez-Álvarez Y. COVID-19-associated acute necrotizing myelitis. Neurol Neuroimmunol Neuroinflamm. 2020 Jun 10;7(5):e803. doi: 10.1212/NXI.0000000000000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Giorgianni A., Vinacci G., Agosti E., Cariddi L.P., Mauri M., Baruzzi F., et al. Transient acute-onset tetraparesis in a COVID-19 patient. Spinal Cord. 2020 Jun 2:1–3. doi: 10.1038/s41393-020-0493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Munz M., Wessendorf S., Koretsis G., Tewald F., Baegi R., Krämer S., et al. Acute transverse myelitis after COVID-19 pneumonia. J Neurol. 2020 May;26:1–2. doi: 10.1007/s00415-020-09934-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Berger J.R., Brandstadter R., Bar-Or A. COVID-19 and MS disease-modifying therapies. Neurol Neuroimmunol Neuroinflamm. 2020 May 15;7(4):e761. doi: 10.1212/NXI.0000000000000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Louapre C., Collongues N., Stankoff B., Giannesini C., Papeix C., Bensa C., et al. Clinical Characteristics and Outcomes in Patients with Coronavirus Disease 2019 and Multiple Sclerosis. JAMA Neurol. 2020 Jun 26 doi: 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zoghi A., Ramezani M., Roozbeh M., Darazam I.A., Sahraian M.A. A case of possible atypical demyelinating event of the central nervous system following COVID-19. Mult Scler Relat Disord. 2020 Jun 24;44 doi: 10.1016/j.msard.2020.102324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yin R., Feng W., Wang T., Chen G., Wu T., Chen D., et al. Concomitant neurological symptoms observed in a patient diagnosed with coronavirus disease 2019. J Med Virol. 2020 Apr 15 doi: 10.1002/jmv.25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Asadi-Pooya A.A. Seizures associated with coronavirus infections. Seizure. 2020 May 11;79:49–52. doi: 10.1016/j.seizure.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Filatov A., Sharma P., Hindi F., Espinosa P.S. Neurological Complications of Coronavirus Disease (COVID-19): Encephalopathy. Cureus. 2020 Mar 21;12(3) doi: 10.7759/cureus.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lu L., Xiong W., Liu D., Liu J., Yang D., Li N., et al. New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: A retrospective multicenter study. Epilepsia. 2020 Jun;61(6):e49–e53. doi: 10.1111/epi.16524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Farhadian S., Glick L.R., Vogels C.B.F., Thomas J., Chiarella J., Casanovas-Massana A., et al. Acute Encephalopathy With Elevated CSF Inflammatory Markers as the Initial Presentation of COVID-19. Version 2. BMC Neurol. 2020 Jun 18;20(1):248. doi: 10.1186/s12883-020-01812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hosseini A.A., Shetty A.K., Sprigg N., Auer D.P., Constantinescu C.S. Delirium as a presenting feature in COVID-19: Neuroinvasive infection or autoimmune encephalopathy? Brain Behav Immun. 2020 Aug;88:68–70. doi: 10.1016/j.bbi.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Andriuta D., Roger P.A., Thibault W., Toublanc B., Sauzay C., Castelain S., et al. COVID-19 Encephalopathy: Detection of Antibodies Against SARS-CoV-2 in CSF. J Neurol. 2020 Jun 11:1–2. doi: 10.1007/s00415-020-09975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Espinosa P.S., Rizvi Z., Sharma P., Hindi F., Filatov A. Neurological Complications of Coronavirus Disease (COVID-19): Encephalopathy, MRI Brain and Cerebrospinal Fluid Findings: Case 2. Cureus. 2020 May 2;12(5):e7930. doi: 10.7759/cureus.7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ahmed S., Leurent B., Sampson E.L. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326–333. doi: 10.1093/ageing/afu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sachs J.R., Gibbs K.W., Swor D.E., Sweeney A.P., Williams D.W., Burdette J.H., et al. COVID-19-Associated Leukoencephalopathy. Radiology. 2020 May 14 doi: 10.1148/radiol.2020201753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Radmanesh A., Derman A., Lui Y.W., Raz E., Loh J.P., Hagiwara M., et al. COVID-19 -associated Diffuse Leukoencephalopathy and Microhemorrhages. Radiology. 2020 May 21 doi: 10.1148/radiol.2020202040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Virhammar J., Kumlien E., Fällmar D., Frithiof R., Jackmann S., Sköld M.K., et al. Acute necrotizing encephalopathy with SARS-CoV-2 RNA confirmed in cerebrospinal fluid. Neurology. 2020 Jun 25 doi: 10.1212/WNL.0000000000010250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Radmanesh A., Derman A., Ishida K. COVID-19-associated delayed posthypoxic necrotizing leukoencephalopathy. J Neurol Sci. 2020 May 27;415 doi: 10.1016/j.jns.2020.116945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: Imaging Features. Radiology. 2020 Aug;296(2):E119–E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dixon L., Varley J., Gontsarova A., Mallon D., Tona F., Muir D., et al. COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol Neuroimmunol Neuroinflamm. 2020 May 26;7(5):e789. doi: 10.1212/NXI.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020 May;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dogan L., Kaya D., Sarikaya T., Zengin R., Dincer A., Akinci I.O., et al. Plasmapheresis treatment in COVID-19-related autoimmune meningoencephalitis: Case series. Brain Behav Immun. 2020 Jul;87:155–158. doi: 10.1016/j.bbi.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pilotto A., Odolini S., Stefano Masciocchi S., Comelli A., Volonghi I., et al. Steroid-responsive encephalitis in Covid-19 disease. Ann Neurol. 2020 May 17 doi: 10.1002/ana.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Al-Olama M., Rashid A., Garozzo D. COVID-19-associated meningoencephalitis complicated with intracranial hemorrhage: a case report. Acta Neurochir (Wien). 2020 Jul;162(7):1495–1499. doi: 10.1007/s00701-020-04402-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ye M., Ren Y., Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav Immun. 2020 Apr;10 doi: 10.1016/j.bbi.2020.04.017. S0889-1591(20)30465-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Huang Y.H., Jiang D., Huang J.T. SARS-CoV-2 Detected in Cerebrospinal Fluid by PCR in a Case of COVID-19 Encephalitis. Brain Behav Immun. 2020 May;6 doi: 10.1016/j.bbi.2020.05.012. S0889-1591(20)30770-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Duong L., Xu P., Liu A. Meningoencephalitis without respiratory failure in a young female patient with COVID-19 infection in Downtown Los Angeles, early April 2020. Brain Behav Immun. 2020 Apr;17 doi: 10.1016/j.bbi.2020.04.024. S0889-1591(20)30509-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Efe I.E., Aydin O.U., Alabulut A., Celik O., Aydin K. COVID-19-Associated Encephalitis Mimicking Glial Tumor. World Neurosurg. 2020 May 29;140:46–48. doi: 10.1016/j.wneu.2020.05.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Afshar H., Yassin Z., Kalantari S., Aloosh O., Lotfi T., Moghaddasi M., et al. Evolution and resolution of brain involvement associated with SARS- CoV2 infection: A close Clinical - Paraclinical follow up study of a case. Mult Scler Relat Disord. 2020 May 21;43 doi: 10.1016/j.msard.2020.102216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Paniz-Mondolfi A., Bryce C., Grimes Z., Gordon R.E., Reidy J., Lednicky J., et al. Central Nervous System Involvement by Severe Acute Respiratory Syndrome coronavirus-2 (SARS-CoV-2) J Med Virol. 2020 Jul;92(7):699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hayashi M., Sahashi Y., Baba Y., Okura H., Shimohata T. COVID-19-associated Mild encephalitis/encephalopathy With a Reversible Splenial Lesion. J Neurol Sci. 2020 May 27;415 doi: 10.1016/j.jns.2020.116941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Franceschi A.M., Ahmed O., Giliberto L., Castillo M. Hemorrhagic Posterior Reversible Encephalopathy Syndrome as a Manifestation of COVID-19 Infection. AJNR Am J Neuroradiol. 2020 Jul;41(7):1173–1176. doi: 10.3174/ajnr.A6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kaya Y., Kara S., Akinci C., Kocaman A.S. Transient cortical blindness in COVID-19 pneumonia; a PRES-like syndrome: Case report. J Neurol Sci. 2020 Apr 28;413 doi: 10.1016/j.jns.2020.116858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Princiotta Cariddi L., Tabaee Damavandi P., Carimati F., Banfi P., Clemenzi A., Marelli M., et al. Reversible Encephalopathy Syndrome (PRES) in a COVID-19 Patient. J Neurol. 2020 Jun 24:1–4. doi: 10.1007/s00415-020-10001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gómez-Enjuto S., Hernando-Requejo V., Lapeña-Motilva J., Ogando-Durán G., Fouz-Ruiz D., Domingo-García J., et al. Verapamil as treatment for refractory status epilepticus secondary to PRES syndrome on a SARS-Cov-2 infected patient. Seizure. 2020 Jun 6;80:157–158. doi: 10.1016/j.seizure.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kishfy L., Casasola M., Banankhah P., Parvez A., Jan Y.J., Shenoy A.M., et al. Posterior reversible encephalopathy syndrome (PRES) as a neurological association in severe Covid-19. J Neurol Sci. 2020 Jul 15;414 doi: 10.1016/j.jns.2020.116943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Somani S., Pati S., Gaston T., Chitlangia A., Agnihotri S. De Novo Status Epilepticus in patients with COVID-19. Ann Clin Transl Neurol. 2020 Jul;7(7):1240–1244. doi: 10.1002/acn3.51071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Balloy G., Leclair-Visonneau L., Péréon Y., Magot A., Peyre A., Mahé P.J., et al. Non-lesional status epilepticus in a patient with coronavirus disease 2019. Clin Neurophysiol. 2020 Aug;131(8):2059–2061. doi: 10.1016/j.clinph.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lyons S., O’Kelly B., Woods S., Rowan C., Brady D., Sheehan G., et al. Seizure with CSF lymphocytosis as a presenting feature of COVID-19 in an otherwise healthy young man. Seizure. 2020 Jun 8;80:113–114. doi: 10.1016/j.seizure.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kadono Y., Nakamura Y., Ogawa Y., Yamamoto S., Kajikawa R., Nakajima Y., et al. A case of COVID-19 infection presenting with a seizure following severe brain edema. Seizure. 2020 Jun 9;80:53–55. doi: 10.1016/j.seizure.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Logmin K., Karam M., Schichel T., Harmel J., Wojtecki L. Non-epileptic seizures in autonomic dysfunction as the initial symptom of COVID-19. J Neurol. 2020 May;26:1–2. doi: 10.1007/s00415-020-09904-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Le Guennec L., Devianne J., Jalin L., Cao A., Galanaud D., Navarro V., et al. Orbitofrontal involvement in a neuroCOVID-19 patient. Epilepsia. 2020 Jun 26 doi: 10.1111/epi.16612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.De Stefano P., Nencha U., De Stefano L., Mégevand P., Seeck M. Focal EEG changes indicating critical illness associated cerebral microbleeds in a Covid-19 patient. Version 2. Clin Neurophysiol Pract. 2020 Jun 10;5:125–129. doi: 10.1016/j.cnp.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Karimi N., Sharifi Razavi A., Rouhani N. Frequent convulsive seizures in an adult patient with COVID-19: a case report. Iran Red Crescent Med J. 2020;22(3) [Google Scholar]
- 174.Elgamasy S., Kamel M.G., Ghozy S., Khalil A., Morra M.E., Islam S.M.S. First case of focal epilepsy associated with SARS-coronavirus-2. J Med Virol. 2020 Jun 2 doi: 10.1002/jmv.26113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Vollono C., Rollo E., Romozzi M., Frisullo G., Servidei S., Borghetti A., et al. Focal Status Epilepticus as Unique Clinical Feature of COVID-19: A Case Report. Seizure. 2020 May;78:109–112. doi: 10.1016/j.seizure.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Haddad S., Tayyar R., Risch L., Churchill G., Fares E., Choe M., et al. Encephalopathy and seizure activity in a COVID-19 well controlled HIV patient. IDCases. 2020 May 16;21 doi: 10.1016/j.idcr.2020.e00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hepburn M., Mullaguri N., George P., Hantus S., Punia V., Bhimraj A., et al. Acute Symptomatic Seizures in Critically Ill Patients with COVID-19: Is There an Association? Neurocrit Care. 2020 May;28:1–5. doi: 10.1007/s12028-020-01006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Scullen T., Keen J., Mathkour M., Dumont A.S., Coronavirus Kahn L. (COVID-19)-Associated Encephalopathies and Cerebrovascular Disease: The New Orleans Experience. World Neurosurg. 2020 May 28 doi: 10.1016/j.wneu.2020.05.192. S1878-8750(20)31163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zanin L., Saraceno G., Panciani P.P., Renisi G., Signorini L., Migliorati K., et al. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir (Wien). 2020 Jul;162(7):1491–1494. doi: 10.1007/s00701-020-04374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Galanopoulou A.S., Ferastraoaru V., Correa D.J., Cherian K., Duberstein S., Gursky J., et al. EEG Findings in Acutely Ill Patients Investigated for SARS-CoV-2/COVID-19: A Small Case Series Preliminary Report. Version 2. Epilepsia Open. 2020 May 17;5(2):314–324. doi: 10.1002/epi4.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pilato M.S., Urban A., Alkawadri R., Barot N.V., Castellano J.F., Rajasekaran V., et al. EEG Findings in Coronavirus Disease. J Clin Neurophysiol. 2020 Jul 1 doi: 10.1097/WNP.0000000000000752. [DOI] [PubMed] [Google Scholar]
- 182.Rábano-Suárez P., Bermejo-Guerrero L., Méndez-Guerrero A., Parra-Serrano J., Toledo-Alfocea D., Sánchez-Tejerina D., et al. Generalized myoclonus in COVID-19. Neurology. 2020 May 21 doi: 10.1212/WNL.0000000000009829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Méndez-Guerrero A., Laespada-García M., Gómez-Grande A., Ruiz-Ortiz M., Blanco-Palmero V.A., Azcarate-Diaz V.J., et al. Acute Hypokinetic-Rigid Syndrome Following SARS-CoV-2 Infection. Neurology. 2020 Jul 8:10. doi: 10.1212/WNL.0000000000010282. [DOI] [PubMed] [Google Scholar]
- 184.Balestrino R., Rizzone M., Zibetti M., Romagnolo A., Artusi C.A., Montanaro E., et al. Onset of Covid-19 with impaired consciousness and ataxia: a case report. J Neurol. 2020 May;27:1–2. doi: 10.1007/s00415-020-09879-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hanafi R., Roger P.A., Perin B., Kuchcinski G., Deleval N., Dallery F., et al. COVID-19 Neurologic Complication with CNS Vasculitis-Like Pattern. AJNR Am J Neuroradiol. 2020 Jun 18 doi: 10.3174/ajnr.A6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Brun G., Hak J.F., Coze S., Kaphan E., Carvelli J., Girard N., et al. COVID-19-White matter and globus pallidum lesions: Demyelination or small-vessel vasculitis? Neurol Neuroimmunol Neuroinflamm. 2020 May 22;7(4) doi: 10.1212/NXI.0000000000000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lang M., Buch K., Li M.D., Mehan W.A., Jr., Lang A.L., Leslie-Mazwi T.M., et al. Leukoencephalopathy Associated with Severe COVID-19 Infection: Sequela of Hypoxemia? AJNR Am J Neuroradiol. 2020 Jun 25 doi: 10.3174/ajnr.A6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kulick-Soper C.V., McKee J.L., Wolf R.L., Mohan S., Stein J.M., Masur J.H. Pearls & Oy-sters: Bilateral globus pallidus lesions in a patient with COVID-19. Neurology. 2020 Jun 25 doi: 10.1212/WNL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Coolen T., Lolli V., Sadeghi N., Rovaï A., Trotta N., Taccone F.S., et al. Early postmortem brain MRI findings in COVID-19 non-survivors. Neurology. 2020 Jun 16 doi: 10.1212/WNL. [DOI] [PubMed] [Google Scholar]
- 191.Sancho-Saldaña A., Lambea-Gil Á., Liesa J.L.C., Caballo M.R.B., Garay M.H., Celada D.R., et al. Guillain-Barré syndrome associated with leptomeningeal enhancement following SARS-CoV-2 infection. Clin Med (Lond). 2020 Jul;20(4):e93–e94. doi: 10.7861/clinmed.2020-0213. clinmed.2020-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kandemirli S.G., Dogan L., Sarikaya Z.T., Kara S., Akinci C., Kaya D., et al. Brain MRI Findings in Patients in the Intensive Care Unit with COVID-19 Infection. Radiology. 2020 May 8 doi: 10.1148/radiol.2020201697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kremer S., Lersy F., de Sèze J., Ferré J.C., Maamar A., Carsin-Nicol B., et al. Brain MRI Findings in Severe COVID-19: A Retrospective Observational Study. Radiology. 2020 Jun 16 doi: 10.1148/radiol.2020202222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Pascual-Goñi E., Fortea J., Martínez-Domeño A., Rabella N., Tecame M., Gómez-Oliva C., et al. COVID-19-associated ophthalmoparesis and hypothalamic involvement. Neurol Neuroimmunol Neuroinflamm. 2020 Jun 25;7(5):e823. doi: 10.1212/NXI.0000000000000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Anzalone N., Castellano A., Scotti R., Scandroglio A.M., Filippi M., Ciceri F., et al. Multifocal Laminar Cortical Brain Lesions: A Consistent MRI Finding in neuro-COVID-19 Patients. J Neurol. 2020 Jun 6:1–4. doi: 10.1007/s00415-020-09966-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Demirci Otluoglu G., Yener U., Demir M.K., Yilmaz B. Encephalomyelitis associated with Covid-19 infection: case report. r J Neurosurg. 2020 Jul 7:1–3. doi: 10.1080/02688697.2020.1787342. [DOI] [PubMed] [Google Scholar]
- 197.Radmanesh A., Raz E., Zan E., Derman A., Kaminetzky M. Brain Imaging Use and Findings in COVID-19: A Single Academic Center Experience in the Epicenter of Disease in the United States. AJNR Am J Neuroradiol. 2020 Jul;41(7):1179–1183. doi: 10.3174/ajnr.A6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Moein S.T., Hashemian S.M.R., Mansourafshar B., Khorram-Tousi A., Tabarsi P., Doty R.L. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 2020 Aug;10(8):944–950. doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gane S., Kelly C., Hopkins C. Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinology. 2020 Jun 1;58(3):299–301. doi: 10.4193/Rhin20.114. [DOI] [PubMed] [Google Scholar]
- 200.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., Horoi M., Le Bon S.D., Rodriguez A., et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020 Apr;6:1–11. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Beltrán-Corbellini Á., Chico-García J.L., Martínez-Poles J., Rodríguez-Jorge F., Natera-Villalba E., Gómez-Corral J., et al. Acute-onset smell and taste disorders in the context of COVID-19: a pilot multicentre polymerase chain reaction based case-control study. Eur J Neurol. 2020 Apr 22 doi: 10.1111/ene.14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mermelstein S. Acute anosmia from COVID-19 infection. Pract Neurol. 2020 Aug;20(4):343–344. doi: 10.1136/practneurol-2020-002583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gómez-Iglesias P., Porta-Etessam J., Montalvo T., Valls-Carbó A., Gajate V., Matías-Guiu J.A., et al. An Online Observational Study of Patients With Olfactory and Gustory Alterations Secondary to SARS-CoV-2 Infection. Front Public Health. 2020 May 29;8:243. doi: 10.3389/fpubh.2020.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Selvaraj V., Sacchetti D., Finn A., Dapaah-Afriyie K. Acute Vision Loss in a Patient with COVID-19. R I Med J. 2013;103(6):37–38. 2020 Jun 10. [PubMed] [Google Scholar]
- 205.Lee J.M., Lee S.J. Olfactory and Gustatory Dysfunction in a COVID-19 Patient with Ankylosing Spondylitis Treated with Etanercept: Case Report. J Korean Med Sci. 2020 Jun 1;35(21) doi: 10.3346/jkms.2020.35.e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tsivgoulis G., Fragkou P.C., Delides A., Karofylakis E., Dimopoulou D., Sfikakis P.P., et al. Quantitative evaluation of olfactory dysfunction in hospitalized patients with Coronavirus [2] (COVID-19) J Neurol. 2020 May 25:1–3. doi: 10.1007/s00415-020-09935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Laurendon T., Radulesco T., Mugnier J., Gérault M., Chagnaud C., El Ahmadi A.A., et al. Bilateral transient olfactory bulbs edema during COVID-19-related anosmia. Neurology. 2020 May 22 doi: 10.1212/WNL.0000000000009850. [DOI] [PubMed] [Google Scholar]
- 208.Li CW, Syue LS, Tsai YS, Li MC, Lo CL, Tsai CS, et al. Anosmia and olfactory tract neuropathy in a case of COVID-19. J Microbiol Immunol Infect. 2020 Jun 20:S1684-1182(20)30144-4. [DOI] [PMC free article] [PubMed]
- 209.Politi S.L., Salsano E., Grimaldi M. Magnetic Resonance Imaging Alteration of the Brain in a Patient With Coronavirus Disease 2019 (COVID-19) and Anosmia. JAMA Neurol. 2020 May 29 doi: 10.1001/jamaneurol.2020.2125. [DOI] [PubMed] [Google Scholar]
- 210.Galougahi M.K., Ghorbani J., Bakhshayeshkaram M., Naeini A.S., Haseli S. Olfactory Bulb Magnetic Resonance Imaging in SARS-CoV-2-Induced Anosmia: The First Report. Acad Radiol. 2020 Jun;27(6):892–893. doi: 10.1016/j.acra.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lee Y., Min P., Lee S., Kim S.W. Prevalence and Duration of Acute Loss of Smell or Taste in COVID-19 Patients. J Korean Med Sci. 2020 May 11;35(18):e174. doi: 10.3346/jkms.2020.35.e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Vaira L.A., Salzano G., Deiana G., De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020 Jul;130(7):1787. doi: 10.1002/lary.28692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Naeini A.S., Karimi-Galougahi M., Raad N., Ghorbani J., Taraghi A., Haseli S., et al. Paranasal sinuses computed tomography findings in anosmia of COVID-19. Am J Otolaryngol. 2020 Jul 3;41(6) doi: 10.1016/j.amjoto.2020.102636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Izquierdo-Domínguez A., Rojas-Lechuga M.J., Chiesa-Estomba C., Calvo-Henríquez C., Ninchritz-Becerra E., Soriano-Reixach M., et al. Smell and taste dysfunctions in COVID-19 are associated with younger age in ambulatory settings - a multicenter cross-sectional study. J Investig Allergol Clin Immunol. 2020 Jun 17:0. doi: 10.18176/jiaci.0595. [DOI] [PubMed] [Google Scholar]
- 215.Dinkin M., Gao V., Kahan J., Bobker S., Simonetto M., Wechsler P., et al. COVID-19 presenting with ophthalmoparesis from cranial nerve palsy. Neurology. 2020 May 1 doi: 10.1212/WNL.0000000000009700. [DOI] [PubMed] [Google Scholar]
- 216.de Freitas Ferreira A.C.A., Romão T.T., SIlva Macedo Y., Pupe C., Nascimento O.J. COVID-19 and herpes zoster co-infection presenting with trigeminal neuropathy. Eur J Neurol. 2020 May 24 doi: 10.1111/ene.14361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Goh Y., Beh D.L.L., Makmur A., Somani J., Chan A.C.Y. Pearls and Oy-sters: Facial nerve palsy as a neurological manifestation of Covid-19 infection. Neurology. 2020 May 21 doi: 10.1212/WNL.0000000000009863. [DOI] [PubMed] [Google Scholar]
- 218.Sedaghat Z., Karimi N. Guillain Barre syndrome associated with COVID-19 infection: A case report. J Clin Neurosci. 2020 Apr;15 doi: 10.1016/j.jocn.2020.04.062. S0967-5868(20)30882-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ottaviani D., Boso F., Tranquillini E., Gapeni I., Pedrotti G., Cozzio S., et al. Early Guillain-Barré syndrome in coronavirus disease 2019 (COVID-19): a case report from an Italian COVID-hospital. Neurol Sci. 2020 May 12:1–4. doi: 10.1007/s10072-020-04449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Camdessanche J.P., Morel J., Pozzetto B., Paul S., Tholance Y., Botelho-Nevers E. COVID-19 may induce Guillain-Barré syndrome. Rev Neurol (Paris) 2020 Jun;176(6):516–518. doi: 10.1016/j.neurol.2020.04.003. S0035-3787(20)30522-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Alberti P., Beretta S., Piatti M., Karantzoulis A., Piatti M.L., Santoro P., et al. Guillain-Barré syndrome related to COVID-19 infection. Neurol Neuroimmunol Neuroinflamm. 2020 Apr 29;7(4):e741. doi: 10.1212/NXI.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhao H., Shen D., Zhou H., Liu J., Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020 May;19(5):383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.El Otmani H., El Moutawakil B., Rafai M.A., El Benna N., El Kettani C., Soussi M., et al. Covid-19 and Guillain-Barré syndrome: More than a coincidence! Rev Neurol (Paris) 2020 Jun;176(6):518–519. doi: 10.1016/j.neurol.2020.04.007. S0035-3787(20)30543-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Scheidl E., Canseco D.D., Hadji-Naumov A., Bereznai B. Guillain-Barre syndrome during SARS-CoV-2 pandemic: a case report and review of recent literature. J Peripher Nerv Syst. 2020 Jun;25(2):204–207. doi: 10.1111/jns.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Toscano G., Palmerini F., Ravaglia S., Ruiz L., Invernizzi P., Cuzzoni M.G., et al. Guillain-Barré Syndrome Associated with SARS-CoV-2. N Engl J Med. 2020 Jun 25;382(26):2574–2576. doi: 10.1056/NEJMc2009191. NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Su X.W., Palka S.V., Rao R.R., Chen F.S., Brackney C.R., Cambi F. SARS-CoV-2 Associated Guillain-Barre Syndrome with Dysautonomia. Muscle Nerve. 2020 Aug;62(2):E48–E49. doi: 10.1002/mus.26988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Virani A., Rabold E., Hanson T., Haag A., Elrufay R., Cheema T., et al. Guillain-Barré Syndrome associated with SARS-CoV-2 infection. IDCases. 2020 Apr 18;20 doi: 10.1016/j.idcr.2020.e00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Gutiérrez-Ortiz C., Méndez A., Rodrigo-Rey S., San Pedro-Murillo E., Bermejo-Guerrero L., Gordo-Mañas R., et al. Miller Fisher Syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020 Apr 17 doi: 10.1212/WN. [DOI] [PubMed] [Google Scholar]
- 229.Coen M., Jeanson G., Alejandro Culebras Almeida L., Hübers A., Stierlin F., Najjar I., et al. Guillain-Barré Syndrome as a Complication of SARS-CoV-2 Infection. Brain Behav Immun. 2020 Jul;87:111–112. doi: 10.1016/j.bbi.2020.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Juliao Caamaño D.S., Alonso Beato R. Facial diplegia, a possible atypical variant of Guillain-Barré Syndrome as a rare neurological complication of SARS-CoV-2. J Clin Neurosci. 2020 Jul;77:230–232. doi: 10.1016/j.jocn.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Farzi M.A., Ayromlou H., Jahanbakhsh N., Bavil P.H., Janzadeh A., Shayan F.K. Guillain-Barré syndrome in a patient infected with SARS-CoV-2, a case report. J Neuroimmunol. 2020 Jun 20;346 doi: 10.1016/j.jneuroim.2020.577294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Bracaglia M., Naldi I., Govoni A., Brillanti Ventura D., De Massis P. Acute inflammatory demyelinating polyneuritis in association with an asymptomatic infection by SARS-CoV-2. J Neurol. 2020 Jun 25:1–3. doi: 10.1007/s00415-020-10014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Hutchins K.L., Jansen J.H., Comer A.D., Scheer R.V., Zahn G.S., Capps A.E., et al. COVID-19-Associated Bifacial Weakness with Paresthesia Subtype of Guillain-Barré Syndrome. AJNR Am J Neuroradiol. 2020 Jun 25 doi: 10.3174/ajnr.A6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Webb S., Wallace V.C., Martin-Lopez D., Yogarajah M. Guillain-Barré syndrome following COVID-19: a newly emerging post-infectious complication. BMJ Case Rep. 2020 Jun 14;13(6) doi: 10.1136/bcr-2020-236182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kilinc D., van de Pasch S., Doets A.Y., Jacobs B.C., van Vliet J., Garssen M.P.J. Guillain-Barré syndrome after SARS-CoV-2 infection. Eur J Neurol. 2020 Jun 13 doi: 10.1111/ene. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Helbok R., Beer R., Löscher W., Boesch S., Reindl M., Hornung R., et al. Guillain-Barré Syndrome in a Patient With Antibodies Against SARS-COV-2. Eur J Neurol. 2020 Jun 12 doi: 10.1111/ene.14388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Manganotti P., Pesavento V., Buoite Stella A., Bonzi L., Campagnolo E., Bellavita G., et al. Miller Fisher Syndrome Diagnosis and Treatment in a Patient With SARS-CoV-2. J Neurovirol. 2020 Jun 11:1–2. doi: 10.1007/s13365-020-00858-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Assini A., Benedetti L., Di Maio S., Schirinzi E., Del Sette M. Correction to: New clinical manifestation of COVID-19 related Guillain-Barrè syndrome highly responsive to intravenous immunoglobulins: two Italian cases. Neurol Sci. 2020 Jun 8:1. doi: 10.1007/s10072-020-04484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Reyes-Bueno J.A., García-Trujillo L., Urbaneja P., Ciano-Petersen N.L., Postigo-Pozo M.J., Martínez-Tomás C., et al. Miller-Fisher syndrome after SARS-CoV-2 infection. Eur J Neurol. 2020 Jun 5 doi: 10.1111/ene.14383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Oguz-Akarsu E., Ozpar R., Mirzayev H., Acet-Ozturk N.A., Hakyemez B., Ediger D., et al. Guillain-Barré Syndrome in a Patient With Minimal Symptoms of COVID-19 Infection. Muscle Nerve. 2020 Jun 4 doi: 10.1002/mus.26992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lascano A.M., Epiney J.B., Coen M., Serratrice J., Bernard-Valnet R., Lalive P.H., et al. SARS-CoV-2 and Guillain-Barré syndrome: AIDP variant with favorable outcome. Eur J Neurol. 2020 Jun 1 doi: 10.1111/ene.14368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Rana S., Lima A.A., Chandra R., Valeriano J., Desai T., Freiberg W., et al. Novel Coronavirus (COVID-19)-Associated Guillain-Barré Syndrome: Case Report. J Clin Neuromuscul Dis. 2020 Jun;21(4):240–242. doi: 10.1097/CND.0000000000000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Gigli G.L., Bax F., Marini A., Pellitteri G., Scalise A., Surcinelli A., et al. Guillain-Barré syndrome in the COVID-19 era: just an occasional cluster? J Neurol. 2020 May 19:1–3. doi: 10.1007/s00415-020-09911-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Padroni M., Mastrangelo V., Asioli G.M., Pavolucci L., Abu-Rumeileh S., Piscaglia M.G., et al. Guillain-Barré syndrome following COVID-19: new infection, old complication? J Neurol. 2020 Jul;267(7):1877–1879. doi: 10.1007/s00415-020-09849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Paybast S., Gorji R., Mavandadi S. Guillain-Barré Syndrome as a Neurological Complication of Novel COVID-19 Infection: A Case Report and Review of the Literature. Neurologist. 2020 Jul;25(4):101–103. doi: 10.1097/NRL.0000000000000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lantos J.E., Strauss S.B., Lin E. COVID-19-Associated Miller Fisher Syndrome: MRI Findings. AJNR Am J Neuroradiol. 2020 Jul;41(7):1184–1186. doi: 10.3174/ajnr.A6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Pfefferkorn T., Dabitz R., von Wernitz-Keibel T., Aufenanger J., Nowak-Machen M., Janssen H. Acute polyradiculoneuritis with locked-in syndrome in a patient with Covid-19. J Neurol. 2020 May 12:1–2. doi: 10.1007/s00415-020-09897-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Abdelnour L., Abdalla M., Babiker S. COVID 19 infection presenting as motor peripheral neuropathy. J Formos Med Assoc. 2020 Jun;119(6):1119–1120. doi: 10.1016/j.jfma.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zubair A.S., McAlpine L.S., Gardin T., Farhadian S., Kuruvilla D.E., Spudich S. Neuropathogenesis and Neurologic Manifestations of the Coronaviruses in the Age of Coronavirus Disease 2019: A Review. JAMA Neurol. 2020 May 29 doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Madia F., Merico B., Primiano G., Cutuli S.L., De Pascale G., Servidei S. Acute myopathic quadriplegia in COVID-19 patients in the intensive care unit. Neurology. 2020 Jun 29:10. doi: 10.1212/WNL.0000000000010280. [DOI] [PubMed] [Google Scholar]
- 251.Tankisi H., Tankisi A., Harbo T., Markvardsen L.K., Andersen H., Pedersen T.H. Critical illness myopathy as a consequence of Covid-19 infection. Clin Neurophysiol. 2020 Jun 12;131(8):1931–1932. doi: 10.1016/j.clinph.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zhang H., Charmchi Z., Seidman R.J., Anziska Y., Velayudhan V., Perk J. COVID-19 associated myositis with severe proximal and bulbar weakness. Muscle Nerve. 2020 Jun 14 doi: 10.1002/mus.27003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Delly F., Syed M.J., Lisak R.P., Zutshi D. Myasthenic crisis in COVID-19. J Neurol Sci. 2020 May 6;414 doi: 10.1016/j.jns.2020.116888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kajani R., Apramian A., Vega A., Ubhayakar N., Xu P., Liu A. Neuroleptic malignant syndrome in a COVID-19 patient. Brain Behav Immun. 2020;88:28–29. doi: 10.1016/j.bbi.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]