Abstract
Technology-assisted cognitive-behavioral therapy (CBT) interventions have been conducted for symptoms including depression, pain, and fatigue in patients with chronic illnesses but not in end-stage renal disease (ESRD). The purpose of this study was to pilot the feasibility and acceptability of a technology-assisted CBT intervention in ESRD patients on hemodialysis (HD), share design and implementation lessons learned, and provide preliminary results on changes in select patient-reported symptoms. This was a single-center pilot feasibility study of adult ESRD patients on HD. Study eligibility required clinically elevated levels of at least one symptom (depression, pain, or fatigue). Patients met weekly with a CBT therapist for eight sessions, each 45–60 min, during HD sessions via a video-conferencing platform. Symptom questionnaires were completed at baseline and 3 months follow-up. Of 10 patients screened, 100% screened positive for at least one symptom, 100% of eligible patients consented, and eight (of 10) completed the intervention (mean age 59 years, 50% male, 50% African American). Patient adherence and satisfaction was high, and seven of the eight patients completed all eight prescribed sessions. Minimal interference with HD was reported. Preliminary results indicate no statistically significant changes in depression, fatigue, or pain at follow-up. However, there was small improvement in SF-36 Physical Component score [t(7) = −2.60, p = .035], and four of the six patients (67%) with clinically elevated pain at baseline reported improvement at follow-up. A technology-assisted CBT intervention for ESRD patients was feasible, well-accepted, and required minimal additional resources in the HD setting. Larger, adequately powered clinical trials are needed to evaluate the effect on ESRD patient-reported outcomes.
Keywords: ESRD, Hemodialysis, Depression, Pain, Fatigue, Telemedicine, CBT
Implications.
Practice: Technology-assisted cognitive-behavioral therapy may be a feasible option to reduce symptoms of depression, pain, and fatigue, and to increase quality of life in patients with end-stage renal disease (ESRD) who are being treated at a hemodialysis (HD) facility.
Policy: Policymakers who want to address the Kidney Disease Improving Global Outcomes (KDIGO) call in 2015 for integration of symptom assessment and management in routine ESRD care should consider novel technology-assisted interventions for addressing psychosocial symptoms.
Research: Future studies are needed in larger samples to examine scalability and effectiveness of the intervention and generalizability across gender and racial/ethnic subgroups.
INTRODUCTION
Despite advances in hemodialysis (HD) treatment, the majority of patients with ESRD report a high-symptom burden that is comparable to patients with advanced cancer [1–3]. Depression, pain, and fatigue are among the most common symptoms in this population, reported by 20%–90% of patients [4–6]. These symptoms are often comorbid and may exacerbate one another [7]. Symptoms of depression, pain, and fatigue are robust predictors of poor health-related quality of life (HRQOL) in HD patients [3, 6, 8]. Furthermore, these symptoms are associated with medication nonadherence and missed and shortened HD sessions [9–11], which in turn may lead to increased hospitalization and mortality [9, 12, 13].
Despite the negative impact of these symptoms on HRQOL and health outcomes, many symptoms go untreated despite available treatment options. For example, in a study of ESRD patients starting dialysis, only 16% of patients with depressive symptoms were engaged in mental health treatment [14]. Similarly, 75% of 200 HD patients with pain were found to have ineffective pain management [15]. Pharmacotherapy alone for depression and pain does not appear to be a viable option, as HD patients are reluctant to accept it for managing symptoms, and it may even impair their HRQOL secondary to burden, side-effects, or medication interactions. For these reasons, ESRD patients may be more likely to accept behavioral interventions, such as cognitive-behavioral therapy (CBT) [11, 16]. For example, a 3 month trial of CBT for depression in 59 HD patients reported a recruitment rate of >95% and a volitional drop-out rate of <1%, despite a high prevalence of personality disorders in the sample [16]. This high adherence to behavioral therapy is in stark contrast to the rates of medication nonadherence in ESRD patients, which are estimated to be >90% in some studies [11].
Telemedicine may provide a novel platform to deliver CBT and can be tailored to address the unique challenges faced by ESRD patients. For example, it can be conducted in the HD clinic, allowing for face-to-face patient engagement without additional visits to mental health providers, reducing burden on patients and caregivers. Adherence to CBT treatment during HD may also be higher as it is utilizing patients’ time while undergoing dialysis and not encroaching on their precious nondialysis time. Additionally, telemedicine-CBT can provide access to, and make efficient use of, trained CBT therapists, and thus may be a scalable strategy. In contrast, CBT interventions conducted “chairside” in the HD facility, such as the ASCEND trial [17], are limited by patient access and availability of CBT providers willing to travel to HD units.
Given the high-symptom burden, its negative health consequences, and lack of optimal treatment options, there is an urgent need to test novel approaches for symptom management for HD patients. Thus, to improve efficiency in the delivery of CBT in HD patients and reduce patient and provider burden, we tested CBT delivery using live video-conferencing in HD units in a single-center single arm feasibility study. CBT was utilized to address symptoms of depression, pain, and fatigue. Our aims were three-fold: (a) To assess feasibility and acceptability of a technology-assisted CBT intervention in ESRD patients on HD; (b) to share design and implementation lessons learned; and (c) to provide preliminary results regarding changes in select patient-reported symptoms (depression, pain, fatigue, and HRQOL).
METHODS
Participants
ESRD patients on HD at a single Dialysis Clinic, Inc. (DCI) clinic were enrolled from April 2016 to October 2016. Consecutive HD patients at a single dialysis unit were approached by the study coordinator during their regular dialysis treatments and those that were interested in participating were screened for eligibility. Inclusion criteria included: (a) adults ≥18 years, (b) undergoing thrice-weekly maintenance HD for >3 months, and (c) clinically elevated levels of at least one symptom: depressive symptoms (>3 on the PHQ-2 [18]); pain (≥4 on a 1–10 scale regarding worst pain over the past 2 weeks); and/or fatigue (≥4 on a 1–10 scale regarding fatigue over the past 2 weeks). Exclusion criteria included: (a) thought disorder, delusions, or active suicidal ideation; (b) active substance abuse; and (c) too ill or cognitively impaired to participate based on clinicians’ judgment. Patients with prior or current treatment with antidepressant or analgesic medication were eligible if they screened positive for clinically significant levels of symptoms. The rationale for this was that a large number of these patients are reportedly undertreated for their symptoms [14, 15] and may experience improvements in symptoms with the addition of a CBT intervention. Eligible participants who met screening criteria were invited to participate in the study. The study was approved by the Institutional Review Board and DCI, and all participants provided informed consent.
Intervention
CBT intervention sessions were conducted by three Masters-level trained behavioral therapists under the supervision of a licensed clinical psychologist (J.L.S.) using an approved, Health Information Portability and Accountability Act (HIPAA) compliant, secure online video-conferencing platform (Vidyo). Each clinician worked with three or four patients and the same clinician followed a patient for the entire duration of the intervention to maintain continuity. The protocol included eight sessions which were conducted during regularly scheduled HD sessions. Each session lasted 45–60 min, with shorter sessions resulting from patient fatigue, as determined by clinician judgment, or the end of dialysis treatment for that day. The entire protocol lasted 8–10 weeks and was based on the number of symptoms being addressed, patient hospitalizations, or constraints in the clinician schedule. During sessions, patients used a study laptop with headphones and microphone and connected in real-time to therapists over a secure Wi-Fi hotspot.
Therapists were trained in CBT prior to the initiation of the intervention and employed cognitive behavioral strategies according to a standard protocolized manual used for a collaborative care intervention designed for cancer patients [19, 20]. The manual was modified to reflect the challenges and cognitions associated with ESRD and HD-dependence, including: (a) psycho-education regarding how ESRD diagnosis and treatment may contribute to symptoms of pain, depression, and fatigue (e.g., energy conservation and the rest-activity cycle); (b) identification and cognitive restructuring of negative thought patterns regarding these symptoms; (c) developing pleasant activities and strategies while considering the limitations of patients with ESRD on HD; (d) identifying and resolving communication difficulties that may exist between the patient and his/her family or medical team that prevent symptom management; (e) relaxation; and (f) facilitating understanding of how core beliefs and assumptions associated with ESRD and/or HD may prevent effective symptom management. In addition, the Oxford Guide to CBT for People with Cancer (2nd ed.) [21] was utilized to provide further guidance for the delivery of CBT in patients with chronic illness. The fidelity of the intervention was monitored by J.L.S.
Instruments
Baseline sociodemographic and clinical data
Baseline data were collected from a standardized health interview and chart review and included sociodemographic characteristics, comorbidities, laboratory tests, and medications.
Symptom questionnaires
Patients were administered a battery of validated symptom questionnaires at baseline and at 3 month follow-up. Fatigue was measured using the 13-item Functional Assessment of Chronic Illness Therapy Fatigue (FACIT-F) [22]. Scores can range between 0 and 52 with higher scores indicating lesser fatigue. A score of <24 was used to classify fatigue, based on the average score of anemic cancer patients [23]. Depressive symptoms were measured using the 20-item Center for Epidemiological Studies-Depression (CES-D) [24]. Scores can range between 0 and 60, with higher scores indicating more depressive symptoms. A score of ≥16 represents depressive symptoms in the clinical range. Pain was measured using the Brief Pain Inventory (BPI) [25], a 15-item scale that measures the pain intensity and interference in the patient’s life. A score of ≥3 for “average level of pain over last 2 weeks” was used to classify pain. Lastly, quality of life was assessed by the Medical Outcomes Short Form 36 Health Survey (SF-36) [26]. The Mental and Physical Component Summary scores were calculated. Scores can range between 0 and 100, with higher scores indicating better HRQOL. These four measures are widely used in HD patients and have demonstrated construct validity, reliability, and responsiveness to treatment [22, 27, 28].
Satisfaction with the intervention
Patient satisfaction was assessed at the end of the intervention via open-ended questions asked by the research coordinator. Nephrologist satisfaction was determined as obtaining approval for approaching their patient(s) about the intervention. Interference with HD was assessed by checking in with patients and dialysis staff and reviewing dialysis flowsheets.
Data analysis
Descriptive statistics (M and SD for continuous variables and proportions for categorical variables) were used to summarize the baseline sociodemographic, comorbidity, and lab characteristics. Rates for recruitment, retention, and adherence were calculated. The rates of depressive symptoms, pain, and fatigue at or above the aforementioned cut-off scores were calculated at baseline. Paired t-tests were used to calculate change in scores from baseline to the 3 month follow-up for each symptom category. Data analysis was completed in R [29].
RESULTS
Feasibility and retention
The recruitment and retention rate for our pilot study was excellent. Of the 10 patients approached, 100% met eligibility criteria and agreed to be screened for depression, pain, and fatigue symptoms. Furthermore, 100% of patients screened positive for clinical levels of at least one symptom, and all consented for the study. Eight patients (80%) remained in the study for the entire intervention and two patients discontinued the study prior to completing any research procedures (one patient moved to a different HD unit and one withdrew consent).
Baseline sample characteristics
The participants had a mean age of 58.7 years, 50% were male and 50% were African American (Table 1). Ninety per cent of the sample had at least a high school education, and 100% of the sample was retired or not working due to chronic disease or disability. Among the 10 patients, six reported use of current antidepressant medications, whereas only one reported use of analgesic medications. The majority of participants had comorbid diagnoses of cardiovascular disease (80%), hypertension (90%), and diabetes (50%).
Table 1.
Sample characteristics at baseline (N = 10)
| N (%) or M (SD) | |
|---|---|
| Gender (male) | 5 (50%) |
| Age (years) | 58.70 (12.16) |
| Race | |
| Caucasian | 5 (50%) |
| African American | 5 (50%) |
| High school degree or greater | 9 (90%) |
| Employment | |
| Retired | 2 (20%) |
| Disabled due to chronic disease or other reasons | 8 (80%) |
| Income | |
| US$10,000 to US$20,000 | 3 (30%) |
| US$20,000 to US$30,000 | 4 (40%) |
| US$40,000 to >US$70,000 | 3 (30%) |
| Smoking history | |
| Never smoked | 4 (40%) |
| Currently smokes | 1 (10%) |
| Use to smoke | 5 (50%) |
| Current antidepressant medication use | 6 (60%) |
| Current analgesic medication use | 1 (10%) |
| Time on dialysis (years) | 8.03 (12.77) |
| Diabetes | 5 (50%) |
| Hypertension | 9 (90%) |
| Cardiovascular disease | 8 (80%) |
| Laboratory measures | |
| Albumin | 3.65 (.36) |
| Hemoglobin | 10.69 (1.23) |
| Creatinine | 7.66 (2.12) |
| Phosphorus | 5.73 (2.71) |
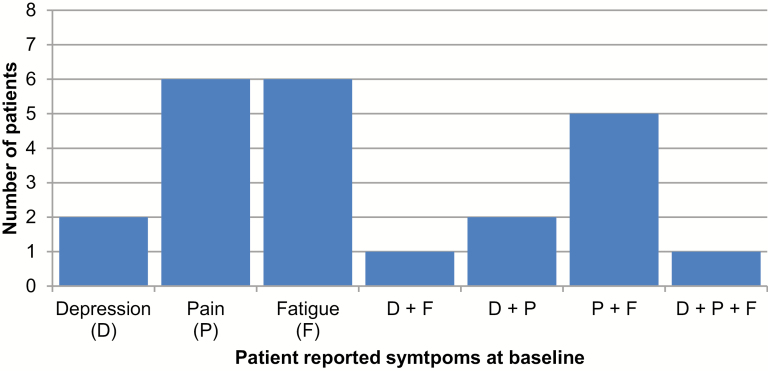
Baseline symptom prevalence and clustering was high (Fig. 1). Of the eight patients who completed the intervention sessions, six (75%) reported pain, six (75%) reported fatigue, whereas two (25%) patients reported depressive symptoms. Symptoms often co-existed—five of the eight patients reported both pain and fatigue, two reported both pain and depressive symptoms, and one patient reported elevations in all three symptoms.
Fig. 1.
Baseline prevalence of patients reporting clinically elevated symptoms (N = 8). The threshold for defining elevated levels of symptoms was as follows: (1) Depressive symptoms (D) = CES-D ≥ 16; (2) Pain (P) = BPI average pain ≥ 3; (3) Fatigue (F) = FACIT-F ≥ 24 (reverse scored: smaller values = more fatigue).
Study acceptability
Patient adherence to the intervention was high, with seven of the eight patients completing all eight prescribed sessions, each session lasting 45–60 min (one patient completed only three of the eight sessions). Furthermore, patients reported high acceptability and satisfaction with the video-conferencing sessions: “I thought the therapy was helpful. It gave me a chance to get some stuff off my back.” and “Being able to talk one-on-one with someone was helpful.” Importantly, patients were comfortable engaging with their therapist using headphones and a study laptop in the HD unit and denied concerns related to privacy. The intervention was also well received by nephrologists, and none were opposed to their patients’ recruitment and participation in the study. There was no reported interference with HD treatment due to the intervention.
Preliminary results on changes in patient-reported outcomes
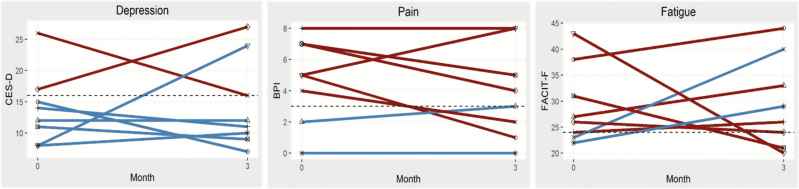
Our preliminary results suggest statistically significant but perhaps not clinically meaningful improvements on the SF-36 Physical Component score, a measure of HRQOL, from baseline to 3 months follow-up [t(7) = −2.60, p = .035]. No significant changes in depression, pain, or fatigue were observed from baseline to 3 months follow-up, although there was a trend towards improvement in pain. Of the six patients who reported clinically elevated pain at baseline, four (67%) reported reduced pain at follow-up. Although this improvement is modest (M = 2.75, SD = .96), it is notable for a short intervention that focused on three symptoms. See Fig. 2 for patient-level symptom changes. However, our study was not powered to test efficacy, and these results should be considered preliminary and interpreted with caution.
Fig. 2.
Individual change in depression, pain, and fatigue symptoms from baseline to 3 month follow-up (N = 8). Red lines = patients with elevated baseline symptom levels. Blue lines = patients without elevated baseline symptom levels. Each line indicates an individual patient. For each panel, the horizontal dashed line indicates threshold for defining elevated levels of symptoms: (1) CES-D ≥ 16, (2) BPI average pain ≥ 3, (3) FACIT-F ≥ 24 (reverse scored: smaller values = more fatigue).
DISCUSSION
Our feasibility study showed that a technology-assisted CBT intervention for ESRD patients was feasible and acceptable, as indicated by: high recruitment, retention, and adherence rates; minimal equipment and dialysis staff involvement; therapist ability to engage patients on video-conferencing; lack of patient-reported privacy concerns in the HD facility; and lack of interference with HD treatment. Additionally, 100% of the patients approached met criteria for inclusion, which demonstrates the need for interventions focused on depression, pain, fatigue, and quality of life in ESRD patients.
Although our preliminary results examining the changes in patient-reported outcomes are promising and hypothesis-generating, they are also limited given the small sample size. We observed a trend toward decrease in pain, which reflects evidence supporting CBT as an effective nonpharmacological treatment for chronic pain [30]. There was also an increase in physical functioning quality of life, possibly due to CBT giving patients’ generalizable skills for problem solving, coping, and mood management across multiple aspects of their lives, including interpersonal relationships. In contrast, there was no impact of the intervention on depression scores, which may be due to low baseline levels of depression and short intervention duration in our study. Similarly, the intervention did not lead to a reduction in fatigue, which may be due to the multifactorial etiology of fatigue in this population, but future studies may use CBT skills to focus on sleep in addition to fatigue. These preliminary findings warrant evaluation in larger randomized clinical trials including an attentional control group (e.g., health education), as well as a longer follow-up period.
The present study used a novel intervention and technology-assisted platform, in light of known challenges to engaging in traditional in-person CBT for ESRD patients. Barriers to seeking face-to-face psychological treatment include overall symptom burden (e.g., fatigue); treatment inertia; and financial, time, and transportation constraints, particularly in a patient population who already attends thrice-weekly HD sessions in addition to other routine medical appointments. Thus, it is encouraging that growing evidence from randomized controlled trials suggests that mental health interventions delivered via interactive video-conferencing are as efficacious as face-to-face interventions [31, 32]. Although computerized CBT programs exist (e.g., Beating the Blues) and are effective for treating depression in primary care patients [33], they are unlikely to be effective for HD patients, who have a high prevalence of poor health literacy and limited use of and accessibility to technology-assisted health resources [34, 35]. Such barriers to self-guided computerized CBT programs call for novel ways to deliver CBT, such as telemedicine, so it can be scalable to the hundreds and thousands of HD patients that need it.
Our technology-assisted approach to CBT also has several potential benefits compared to interventions conducted “chairside” in the HD facility [16, 17]. Although chairside CBT reduces the number of medical visits by combining psychological treatment and dialysis, there are potential limitations of patient adherence and ability of providers to travel to HD units. Indeed, results from this pilot study suggest that telemedicine may be a feasible platform for scalability, and this intervention is being tested in an ongoing trial (Technology Assisted Stepped Collaborative Care Intervention) including 150 patients with ESRD on HD who will be randomized to a technology-assisted stepped collaborative care intervention versus a technology-delivered health education (ClinicalTrials.gov NCT03440853).
Several lessons regarding screening, technology, and feasibility were learned from this pilot study. First, it would be beneficial to screen patients’ readiness for behavior change, based on the transtheoretical model [36], given the impact of stage of change on psychotherapy outcomes [37]. Although this study did not measure stage of change, it is possible that some patients who screened positive for symptoms and who consented to participation were not necessarily ready to make behavior changes, which may have influenced their ability to benefit from the intervention. Future work might consider enrolling only patients in the contemplation stage or higher, to reduce confounding of intervention results by baseline stage of change. Second, one-click access to the Vidyo software ensured ease of use for patients with minimal technology experience while reducing staff burden. Third, logistical lessons included having a secure and reliable Wi-Fi hotspot, phone/in-person support from the research team to respond to technology issues, and coordination of HD center staff to facilitate accessibility of the laptop/tablet to patients and cleaning of the equipment in between uses. Finally, privacy concerns were minimized by providing patients with a microphone and headphones, and in some instances, screens or private rooms at the dialysis clinic. Overall, this intervention required minimal additional resources in the outpatient HD setting.
There were several limitations to this pilot study, most notably the small sample size, which precludes the ability to make definitive conclusions regarding the efficacy of the intervention and to examine differences in symptom levels across subgroups, such as race, gender, socio-economic status, or current use of antidepressant medications (which was reported by 60% of the sample). Although the small sample size may limit conclusions about recruitment and retention in a larger trial, the feasibility findings related to using technology to deliver CBT in dialysis units are likely to be applicable to other dialysis units given similar workflow practices. Of relevance, 90% of the sample reported at least a high school education; thus, this protocol may not be generalizable to individuals with less education. Finally, this pilot study included a relatively short follow-up period (3 months) and therefore maintenance of the benefits of the intervention is not known.
In conclusion, our pilot study demonstrates that conducting CBT via video-conferencing in the dialysis unit is feasible, acceptable, and has high rates of both recruitment and retention, highlighting the need for such interventions. By overcoming patient and provider barriers, telemedicine may provide a novel way of delivering a scalable CBT intervention targeting common mental and physical health symptoms in ESRD patients. Future randomized controlled trials are needed to evaluate the efficacy of technology-assisted CBT in improving patient-centered outcomes and HRQOL across gender and racial/ethnic subgroups.
Compliance with Ethical Standards
Funding: This study was funded by NIDDK grant P30-DK-079307 (Renal-Electrolyte Division, University of Pittsburgh School of Medicine), AHA grant 11FTF7520014 (M. Jhamb), and NIH/NIDDK grant R01DK114085 (M. Jhamb). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflicts of Interest: All authors have no conflicts of interest to disclose.
Primary Data: Portions of these data were presented at the American Psychosomatic Society 2017 Annual Meeting, Sevilla, Spain.
Authors’ Contributions: MJ and JLS conceived the study and participated in the design, coordination and data interpretation. KPJ, SG, and LEO conducted behavioral therapy sessions under supervision and training from JLS. JY performed the analysis. FB helped in data interpretation. KPJ, MJ, and JLS drafted the manuscript. All authors reviewed the manuscript and approved the final version.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the University of Pittsburgh IRB and Dialysis Clinic, Inc. This article does not contain any studies with animals performed by any of the authors.
Informed Consent: Informed consent was obtained from all individual participants in the study.
References
- 1. Abdel-Kader K, Unruh ML, Weisbord SD. Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol. 2009;4(6):1057–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moens K, Higginson IJ, Harding R; EURO IMPACT Are there differences in the prevalence of palliative care-related problems in people living with advanced cancer and eight non-cancer conditions? A systematic review. J Pain Symptom Manage. 2014;48(4):660–677. [DOI] [PubMed] [Google Scholar]
- 3. Davison SN, Jhangri GS. Impact of pain and symptom burden on the health-related quality of life of hemodialysis patients. J Pain Symptom Manage. 2010;39(3):477–485. [DOI] [PubMed] [Google Scholar]
- 4. Davison SN, Koncicki H, Brennan F. Pain in chronic kidney disease: a scoping review. Semin Dial. 2014;27(2):188–204. [DOI] [PubMed] [Google Scholar]
- 5. Jhamb M, Weisbord SD, Steel JL, Unruh M. Fatigue in patients receiving maintenance dialysis: a review of definitions, measures, and contributing factors. Am J Kidney Dis. 2008;52(2):353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weisbord SD. Patient-centered dialysis care: depression, pain, and quality of life. Semin Dial. 2016;29(2):158–164. [DOI] [PubMed] [Google Scholar]
- 7. O’Connor NR, Corcoran AM. End-stage renal disease: symptom management and advance care planning. Am Fam Physician. 2012;85(7):705–710. [PubMed] [Google Scholar]
- 8. Jhamb M, Pike F, Ramer S, et al. Impact of fatigue on outcomes in the hemodialysis (HEMO) study. Am J Nephrol. 2011;33(6): 515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weisbord SD, Mor MK, Sevick MA, et al. Associations of depressive symptoms and pain with dialysis adherence, health resource utilization, and mortality in patients receiving chronic hemodialysis. Clin J Am Soc Nephrol. 2014;9(9):1594–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Artom M, Moss-Morris R, Caskey F, Chilcot J. Fatigue in advanced kidney disease. Kidney Int. 2014;86(3):497–505. [DOI] [PubMed] [Google Scholar]
- 11. Ghimire S, Castelino RL, Lioufas NM, Peterson GM, Zaidi ST. Nonadherence to medication therapy in haemodialysis patients: a systematic review. Plos One. 2015;10(12):e0144119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lopes AA, Bragg J, Young E, et al. ; Dialysis Outcomes and Practice Patterns Study (DOPPS) Depression as a predictor of mortality and hospitalization among hemodialysis patients in the United States and Europe. Kidney Int. 2002;62(1):199–207. [DOI] [PubMed] [Google Scholar]
- 13. Hedayati SS, Grambow SC, Szczech LA, Stechuchak KM, Allen AS, Bosworth HB. Physician-diagnosed depression as a correlate of hospitalizations in patients receiving long-term hemodialysis. Am J Kidney Dis. 2005;46(4):642–649. [DOI] [PubMed] [Google Scholar]
- 14. Watnick S, Kirwin P, Mahnensmith R, Concato J. The prevalence and treatment of depression among patients starting dialysis. Am J Kidney Dis. 2003;41(1):105–110. [DOI] [PubMed] [Google Scholar]
- 15. Davison SN. Pain in hemodialysis patients: prevalence, cause, severity, and management. Am J Kidney Dis. 2003;42(6):1239–1247. [DOI] [PubMed] [Google Scholar]
- 16. Cukor D, Ver Halen N, Asher DR, et al. Psychosocial intervention improves depression, quality of life, and fluid adherence in hemodialysis. J Am Soc Nephrol. 2014;25(1):196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hedayati SS, Daniel DM, Cohen S, et al. Rationale and design of a trial of sertraline vs. Cognitive behavioral therapy for end-stage renal disease patients with depression (ASCEND). Contemp Clin Trials. 2016;47:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kroenke K, Spitzer RL, Williams JB. The patient health questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284–1292. [DOI] [PubMed] [Google Scholar]
- 19. Steel JL, Geller DA, Kim KH, et al. Web-based collaborative care intervention to manage cancer-related symptoms in the palliative care setting. Cancer. 2016;122(8):1270–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Steel J, Geller DA, Tsung A, et al. Randomized controlled trial of a collaborative care intervention to manage cancer-related symptoms: lessons learned. Clin Trials. 2011;8(3):298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moorey S, Greer S. 2012. Oxford Guide to CBT for People with Cancer (2nd ed.). New York: Oxford University Press Inc. [Google Scholar]
- 22. Jhamb M, Liang K, Yabes J, et al. Prevalence and correlates of fatigue in chronic kidney disease and end-stage renal disease: are sleep disorders a key to understanding fatigue? Am J Nephrol. 2013;38(6):489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94(2):528–538. [DOI] [PubMed] [Google Scholar]
- 24. Radloff LS. The CES scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 25. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 26. Ware JE, Snow KK, Kosinski M, Gandek B. 1993. SF-36 Health Survey Manual and Interpretation Guide. Boston: The Health Institute. [Google Scholar]
- 27. Upadhyay C, Cameron K, Murphy L, Battistella M. Measuring pain in patients undergoing hemodialysis: a review of pain assessment tools. Clin Kidney J. 2014;7(4):367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jhamb M, Tamura MK, Gassman J, et al. ; Frequent Hemodialysis Network Trial Group Design and rationale of health-related quality of life and patient-reported outcomes assessment in the Frequent Hemodialysis Network trials. Blood Purif. 2011;31(1-3):151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; Available at www.R-project.org. [Google Scholar]
- 30. Morley S, Eccleston C, Williams A. Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain. 1999;80(1-2):1–13. [DOI] [PubMed] [Google Scholar]
- 31. De Las Cuevas C, Arredondo MT, Cabrera MF, Sulzenbacher H, Meise U. Randomized clinical trial of telepsychiatry through videoconference versus face-to-face conventional psychiatric treatment. Telemed J E Health. 2006;12(3):341–350. [DOI] [PubMed] [Google Scholar]
- 32. O’Reilly R, Bishop J, Maddox K, Hutchinson L, Fisman M, Takhar J. Is telepsychiatry equivalent to face-to-face psychiatry? Results from a randomized controlled equivalence trial. Psychiatr Serv. 2007;58(6):836–843. [DOI] [PubMed] [Google Scholar]
- 33. Online Treatments for Mood and Anxiety Disorders in Primary Care Available at www.clinicaltrials.gov/ct2/show/NCT01482806. Accessibility verified May 2019.
- 34. Green JA, Mor MK, Shields AM, et al. Prevalence and demographic and clinical associations of health literacy in patients on maintenance hemodialysis. Clin J Am Soc Nephrol. 2011;6(6):1354–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jhamb M, Cavanaugh KL, Bian A, et al. Disparities in electronic health record patient portal use in nephrology clinics. Clin J Am Soc Nephrol. 2015;10(11):2013–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51(3):390–395. [DOI] [PubMed] [Google Scholar]
- 37. Norcross JC, Krebs PM, Prochaska JO. Stages of change. J Clin Psychol. 2011;67(2):143–154. [DOI] [PubMed] [Google Scholar]