Abstract
The female reproductive system consists of the ovaries, the female gonads, and the reproductive track organs of the fallopian tubes, uterus, cervix, and vagina. It functions to provide hormonal support and anatomical structure for the production of new offspring. A number of endogenous and exogenous factors can impact female reproductive health and fertility, including genetic vulnerability, medications, environmental exposures, age, nutrition, and diseases, etc. To date, due to the ethical concerns of using human subjects in biomedical research, the majority of studies use in vivo animal models and 2D cell/tissue culture models to study female reproduction. However, the complexity and species difference of the female reproductive system in humans makes it difficult to compare to those of animals. Moreover, the monolayered cells cultured on flat plastics or glass lose their 3D architecture as well as the physical and/or biochemical contacts with other cells in vivo. Further, all reproductive organs do not work alone but interconnect with each other and also with non-reproductive organs to support female reproductive, endocrine, and systemic health. These facts suggest that there is an urgent and unmet need to develop representative, effective, and efficient in vitro models for studying human female reproduction. The prodigious advancements of bioengineering (e.g. biomaterials, 3D printing, and organ-on-a-chip) allow us to study female reproduction in an entirely new way. Here, we review recent advances that use bioengineering methods to study female reproduction, including the bioengineering models of the ovary, fallopian tube, uterus, embryo implantation, placenta, and reproductive disease.
Keywords: female reproduction, bioengineering, biomaterials, 3D printing, microfluidics
1. Introduction
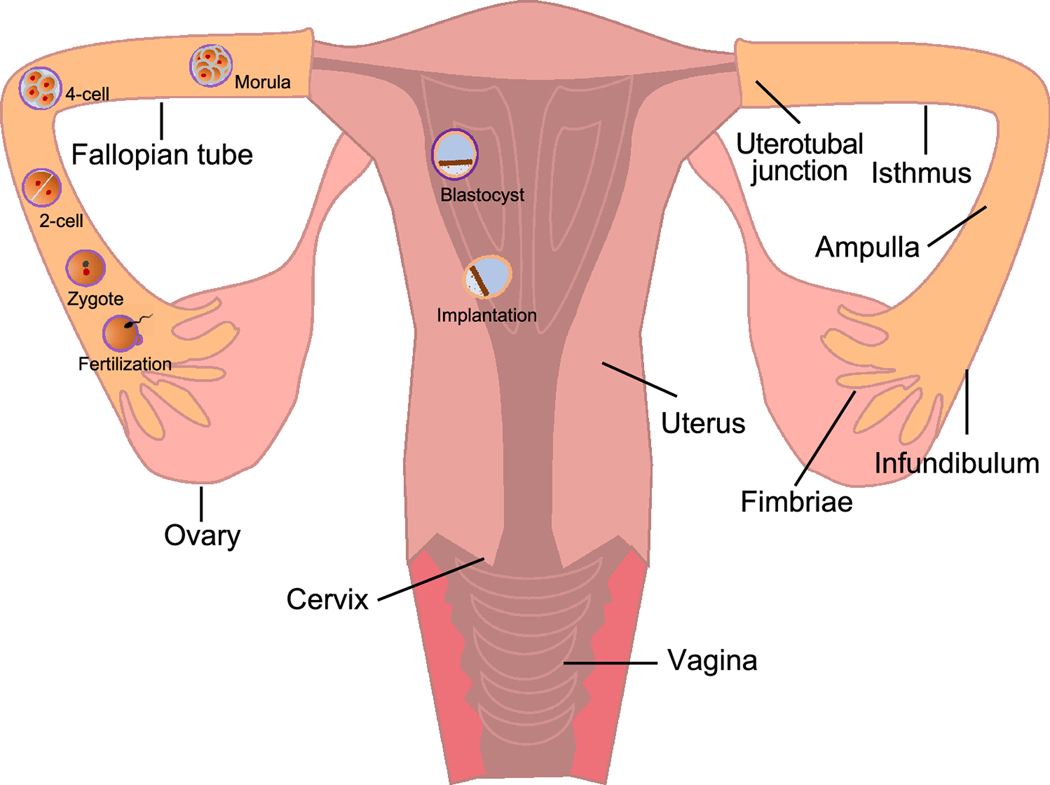
The female reproductive system is composed of the female gonads, the ovaries, and the female reproductive track, which includes the fallopian tubes (termed oviducts in non-primate species), uterus, uterine cervix, and vagina (Figure. 1). It functions to provide hormonal support and anatomical structure for the production of new offspring. In addition to fertility, it is also important for women’s systemic health because hormones secreted from the ovaries contribute to the general health of their endocrine, cardiovascular, skeletal, and immune systems, etc [1].
Figure 1.
The anatomy of human female reproductive system and early pregnant events. The female reproductive system consists of the ovaries, fallopian tubes, uterus, cervix, and vagina. Following fertilization in the fallopian tube, the zygote undergoes 5–6 miotic cell division to form morula, a 16-cell embryo. Simultaneously, the developing embryo continues to be projected toward the uterus in the fallopian tube and reaches the uterus approximately 3 days after fertilization. Once inside the uterus, the morula continues to divide and produce an approximately 100-cell embryo, which called blastocyst. The blastocyst consists of two major structures: the mass of cells inside of blastocyst is called inner cell mass (ICM) which will become the embryo; the cells that form the outer shell of the blastocyst are trophoblasts which will develop into chorionic sac and fetal portion of placenta. The trophoblasts of blastocyst then secrete enzymes to degrade zona pellucida, which is referred to as hatching. At about day 7 after fertilization, the hatched blastocyst adheres to the uterine endometrium for embryo implantation, which is complete at about day 10 after fertilization.
Abnormal female reproduction is caused by a number of factors including genetic vulnerability, medical treatments, environmental exposures, age, nutrition, and diseases. In the United States, about 16.2% of married women within reproductive age (15–49 years old) have impaired fecundity, 8.8% of them are diagnosed as infertile, and 12.7% of them have received fertility treatments such as in vitro fertilization (IVF) [2]. Moreover, female reproductive diseases such as the endometriosis and polycystic ovary syndrome (PCOS) are becoming more and more prevalent [3]. Furthermore, gynecologic cancers do not only affect women’s fertility but also threaten their lives. For example, ovarian cancer is the fifth leading cause of cancer death among women and cervical cancer is the fourth most frequent female cancer disease [4]. The female reproductive system is also one of the major off-targets of clinical drugs. Both chemotherapy and irradiation have been demonstrated to exhibit highly detrimental effects on the ovaries and increase childhood and young adult female cancer patients’ risks of ovarian failure, early menopause, and infertility [5–7]. Our recent studies also suggested that doxorubicin (DOX), a commonly used chemotherapeutic chemical, permanently altered the uterine response to estrogen, an ovarian steroid hormone, indicating that anticancer agents can also directly target the uterus to impair female reproductive health and fertility [8]. In addition to pharmaceutical compounds, increasing evidence suggests that the environmental chemicals, particularly those identified as endocrine disrupting chemicals (EDCs) such as the bisphenols, phthalates, and flame retardants, etc, can also cause female reproductive toxicities.
Thus far, due to the ethical concerns of using human subjects to study female reproduction, particularly when women are pregnant, the majority of the explorations for female reproductive physiology, pathology, and toxicology have been through in vivo animal models and two-dimensional (2D) cell or tissue culture models. However, the complexity and species difference of the female reproductive system in humans makes it difficult to compare to those of animals. For example, the average ovarian cycle is 4–5 days for rodents but 28 days for humans, and the average of gestation period is 20–23 days for rodents but 40 weeks for humans. Moreover, in vivo animal models are time consuming and costly, and it is unethical to sacrifice a large number of animals for human benefit. Regarding 2D culture models, the cells cultured on flat plastics or glass lose their 3D architecture as well as the physical or biochemical contacts with other cells in vivo. For instance, ovarian cell lines, including both somatic cells and oocytes, have been cultured in vitro [9–11]. However, these individual cell lines lack the 3D cell/tissue architecture and also the bidirectional communications between somatic cells and their enclosed oocytes [12–16], which are required for supporting normal ovarian development and functions [17–21]. These facts indicate that there is an urgent and unmet need to develop representative and effective models for studying human female reproduction.
Bioengineering aims to use the principles and technologies of engineering to solve problems in biology and medicine. Over the past decades, the prodigious advancements of bioengineering (e.g. biomaterials, 3D printing, and microfluidics) allow us to study the female reproductive system and reproductive diseases in an entirely new way. Here, we review recent advances of bioengineering models of female reproductive tissues and functions, which provide us with encouraging research models to study the female reproductive science and medicine. We first discuss the research related to bioengineering and the ovaries, the primary female reproductive organs, which is followed by the downstream female reproductive tract including the fallopian tube, uterus, and placenta. Further, we reviewed current research that used bioengineering methods to study female reproductive diseases including endometriosis and gynecologic cancers.
2. Bioengineering models of ovaries
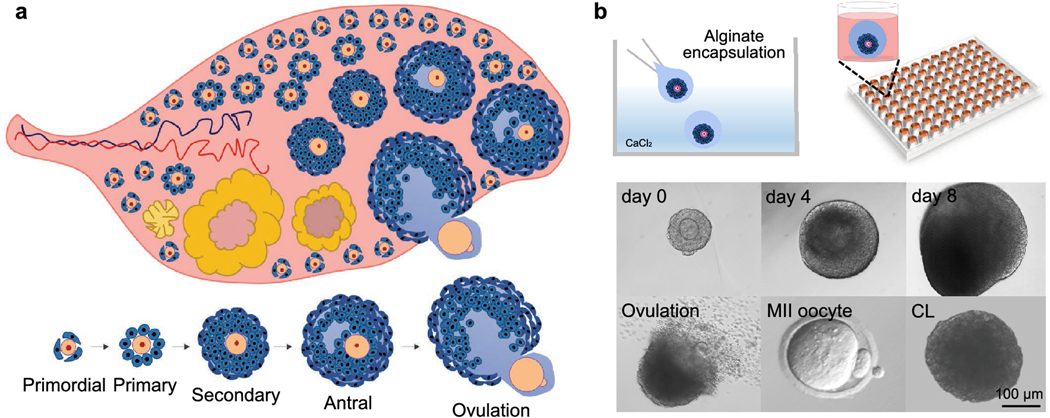
The ovary is the female gonad and contains various developmental stages of follicles as the functional units (Figure. 2A). Each follicle consists of a central germ cell, the oocyte, and the surrounding somatic cells. It is believed that the ovarian follicle pool is established prior to birth and is non-renewable [22,23]. Thus, the diseases, medications, environmental exposures or other factors that compromise the quantity and quality of follicles and/or oocytes will increase women’s risks of premature ovarian insufficiency (POI), hormonal imbalance, and infertility [24]. Growing ovarian tissues or follicles in vitro has a long history because it provides valuable research models and significant applications in female reproductive science and medicine, including understanding the basic ovarian biology, preserving and restoring women’s fertility and endocrine functions after cancer therapy, and ovarian toxicity screening. However, when follicles are cultured on flat plastics or glass, the connections between the oocyte and somatic cells will be crushed and follicles will lose their 3D architecture and die. Here, we review the existing bioengineering methods that have been applied to grow ovarian tissues or follicles in 3D, including the static ovarian tissue/ follicle culture models using hydrogel encapsulation, decellularized ECM, and 3D bio printed scaffold, as well as the dynamic culture models using microfluidic system.
Figure 2.
The ovary anatomy, folliculogenesis, oogenesis, and encapsulate in vitro follicle growth (eIVFG). (A) The earliest stage of ovarian follicles are termed primordial follicles which remain quiescent to represent ovarian reserve. After birth, the primordial follicles are activated and grow to the primary, secondary, and antral stages for ovulation until menopause, which is termed folliculogenesis. In parallel to folliculogenesis, the oocytes increase in size and store mRNAs and proteins to gain meiotic and developmental competence, which is termed oogenesis. The ovulation is characterized by the rupture of antral follicles and release of oocytes into the fallopian tube. During ovulation, the oocytes resume meiosis and progress to metaphase II (MII) stage. After ovulation, the remaining granulosa cells and theca cells differentiate into luteal cells, form corpus luteum (CL), and secrete progesterone. (B) The eIVFG maintains the 3D architecture of ovarian follicles and supports mouse follicle development from multilayered secondary stage to antral stage for maturation, ovulation, oocyte meiotic division, and luteinization.
2.1. Hydrogel encapsulation
Hydrogel encapsulation have been increasingly used and become a promising bioengineering method to support 3D in vitro cell or tissue culture. The liquid precursor solutions of hydrogels can become solid hydrogels after crosslinking with curing agents, which is termed gelation. The gelated hydrogels are water swellable and the formed hydrogels exhibit tissue-like elastic properties as well as allow for the diffusion of nutrients, wastes, and oxygen for the encapsulated cells or tissues. These attributes make hydrogels ideal scaffolds to mimic the native extracellular matrices (ECMs), provide tunable stiffness similar to the cellular environment in vivo, and maintain the 3D architecture of encapsulated cells or tissues [25,26].
Several research groups have used hydrogels (e.g. alginate, collagen, and fibrin) to grow ovarian tissues or follicles in vitro and obtained remarkable advancements. A good example is that in 2003, Dr. Woodruff and her team started to use alginate hydrogel, a polysaccharide derived from brown algae, to encapsulate and culture mouse ovarian follicles, which is termed encapsulated in vitro follicle growth (eIVFG) [27]. Their results showed that the 3D architecture of follicular complex was well-maintained during eIVFG, the granulosa cells proliferated, and the oocytes grew in volume and obtained structural characteristics of mature oocytes such as the developed zona pellucida, presence of gap junctions between granulosa cells and oocytes, and meiosis resumption after human chorionic gonadotropin (hCG) stimulation [27]. Their following studies further demonstrated that eIVFG recapitulates all key events of mouse folliculogenesis and oogenesis in vivo, including the follicle development from preantral to antral stage, differentiation of mural granulosa cells and cumulus cells, development of the theca cell layer, ovarian hormone synthesis and secretion, oocyte maturation and ovulation, and luteinization (Figure. 2B). Moreover, the ovulated oocytes from eIVFG were able to be fertilized after IVF and produced live birth after embryo transfer [28,29]. In addition to supporting mouse ovarian follicles, eIVFG has also been successful in other species including rat, dog, sheep, non-human primate, and human. In the Supplemental Table 1, we summarized previous works that used hydrogel encapsulation method to grow ovarian tissues or follicles in vitro, including species, hydrogels, ovarian tissue types, and achieved reproductive outcomes.
In addition to supporting follicle development and oocyte maturation processes in vitro, eIVFG is also a valuable research model to explore key ovarian biology questions that have been challenging using in vivo models. For example, by encapsulating and culturing multiple primary mouse follicles in a single alginate bead, it was found that follicles communicated with each other to develop and mature [30]. Although the underlying mechanism is not fully understood, the conditioned culture medium from eIVFG provides a valuable resource to identify the interfollicular factors, which may include miRNAs, exosomes, bioactive lipids, or other active peptide or protein factors. Another example to study ovarian biology using eIVFG is that the ovarian physical environment determines follicle fate. It has been demonstrated that changing the rigidity or stiffness of alginate hydrogel impacted follicle and oocyte reproductive outcomes. Specifically, decreasing the stiffness of alginate hydrogels enhanced granulosa cell proliferation, estradiol secretion, and oocyte maturation. In contrast, the rigid matrix environment induced higher production of androgen as well as compromised oocyte developmental competence [31,32]. These results are consistent with the phenotypes of aging or cystic ovaries, which have more fibrotic ovarian stroma tissues, compromised follicle/oocyte quality, and high androgen production. Such results suggest that the ovarian physical environment plays an essential role in determining the quality of follicles and oocytes. These research findings have also been translated from mouse to human. For example, since human preantral follicles require a much longer time to develop to antral stage for maturation, we developed a two-step human follicle culture method that provided a more rigid growth environment during preantral stage and then a permissive environment after follicles grow to antral stage. Using this two-step and dynamic follicle culture regimen, we were able to, for the first time, generated human MII oocytes using eVIFG [33]. In summary, these studies indicate that the hydrogel encapsulation method is a robust bioengineering model to grow and mature ovarian follicles in vitro, which can be used for multiple reproductive biology and medicine applications
2.2. Decellularized ECM scaffold
Decellularization is a process of using the physical, chemical, or enzymatic methods to remove cellular components and only preserve the intact ECM scaffold of the original tissue [34]. ECM does not only provide a natural physical environment for the cells, but also plays essential biochemical roles in regulating a number of cellular functions, such as the cell adhesion, migration, proliferation, and differentiation [35]. Following injecting the decellularized ECM scaffold with targeting cells (e.g. stem cells, primary cells, or cell lines), the recellularized ECM scaffold has been increasingly researched for regenerative medicine and organ transplantation.
Making an artificial ovary using the decellularization method has been studied as a potential fertility preservation option for young female cancer patients. Compared to the conventional ovarian tissue transplantation, the decellularized ECM scaffold allows for the malignant cell-free recellularization and transplantation, eliminating the risk of reintroduction of cancer cells to cancer survivors. Laronda et al. used to seed primary murine ovarian cells into the decellularized bovine ECM scaffolds to reconstruct mouse ovaries. They demonstrated that the recellularized ECM scaffolds were able to produce estradiol in vitro and also induced puberty in ovariectomized mice following renal graft [36]. Moreover, the same research group further used bovine ovary ECM powders to fabricate ovarian tissue pieces, which were termed ovarian tissue papers (OTPs) [37]. The OTPs were used to reconstitute human ovaries by co-culturing with human ovarian cortical strip. Their results showed that the reconstituted human ovaries supported follicle viability and hormone secretion. However, whether the reconstituted human ovaries can support more advanced folliculogenesis and oogenesis requires further investigations. More profoundly, Oktay et al. transplanted human decellularized ECM scaffolds seeded with ovarian cortical tissues to two patients who had POI after cancer treatments [38,39]. Results showed that the ECM scaffolds supported vascularization in the grafted ovarian tissues and follicles secreted estradiol and developed to antral stage in both patients. Moreover, both patients had pregnancy following IVF and embryo transfer and one of them successfully delivered a healthy baby. Recently, another research group also used the decellularized human ovary ECM scaffold and isolated human preantral follicles to reconstruct human ovaries [40]. After the reconstructed human ovaries were subcutaneously grafted to the immunodeficient mice for 3 weeks, about 40% of preantral follicles developed to antral stage, indicating that the decellularized ovarian scaffold provides a promising environment to support follicle growth and development. Taken together, these studies demonstrate that decellularized ECM scaffolds provide a promising method to reconstitute ovaries and also ovarian functions for restoring female fertility and endocrine functions.
2.3. 3D printed scaffold
3D printing is novel emerging tissue engineering method that has remarkable potential in regenerative medicine and tissue transplantation. Compared to the decellularized ECM scaffold that depends on natural tissue structurality, 3D printing uses biomaterials to fabricate tissue scaffolds, which allows for precise control of the scaffold shape, size, geometry, porosity, and other physical and biochemical properties to meet the specific research and medical needs. Researchers have used various biomaterials and 3D printing approaches to fabricate live tissues and organs, such as the heart [41], blood vessel [42], aortic valve [43], skin [44], and bone and cartilage [45], etc.
With respect to the 3D printed female reproductive tissues, one good example is that Laronda et al created bioprosthetic ovaries using the 3D printed microporous gelatin scaffolds [46]. It was found that the pore geometry of the printed scaffolds influenced the seeded follicle reproductive outcomes. The 30° and 60° with underlying struts better supported follicle development and survival than the 90° without underlying struts. After in vivo transplantation using ovariectomized female mice, the reconstructed ovaries became highly vascularized and animals fully restored their ovarian functions and delivered live pups by natural mating. In addition to gelatin hydrogel, other researchers also used the mixture of poly (epsilon caprolactone) (PCL), a biodegradable polyester, and gelatin for scaffold fabrication to reduce the hydrophobicity as well as improve the biocompatibility of the 3D printed scaffold [47,48]. Results revealed that the unique presences of fibers in the middle of scaffold macropores promoted the adhesion, infiltration, and growth of seeded porcine ovarian follicles. Another emerging tissue engineering method based on 3D printing is bioprinting. Different from the conventional 3D printing using biomaterials only, bioprinting refers to the use of the mixture of both biomaterials and encapsulated cells, termed bioink, to print tissue constructs [49]. The bioink can be stabilized through crosslinking during or immediately after bioprinting to generate the desired size, shape and architecture of engineered tissues or organs. In summary, both the 3D printing and bioprinting indicate a significant potential to reconstruct and build artificial, functional, and implantable human ovaries that allow prepubertal girls and young adult women to restore their fertility and endocrine functions.
2.4. Microfluidic system
The bioengineering models described above are considered as static models. Microfluidics is another emerging bioengineering method that can provide cells or tissues with a dynamic culture environment. It is defined as the application of a simplified 3D cell/tissue culture under the fluidic flow in a micron-sized channel to yield a functional tissue unit. This can recapitulate the entire organ-level of functionalities and responses. The functional unit together with the microfluidic system is also called ‘organ-on-a-chip’ [50–52]. Compared to static 2D or conventional 3D cultures, the microfluidic culture provides the benefits of dynamic and desired oxygen and nutrient delivery, prompt waste removal, and mechanical input by controlling the volume or rate of fluidic flow. Another unique feature of the microfluidic system is the interconnection of multiple types of tissues together to build an organ system-on-a-chip or even an entire body-on-a-chip, which will be discussed in another section of this review.
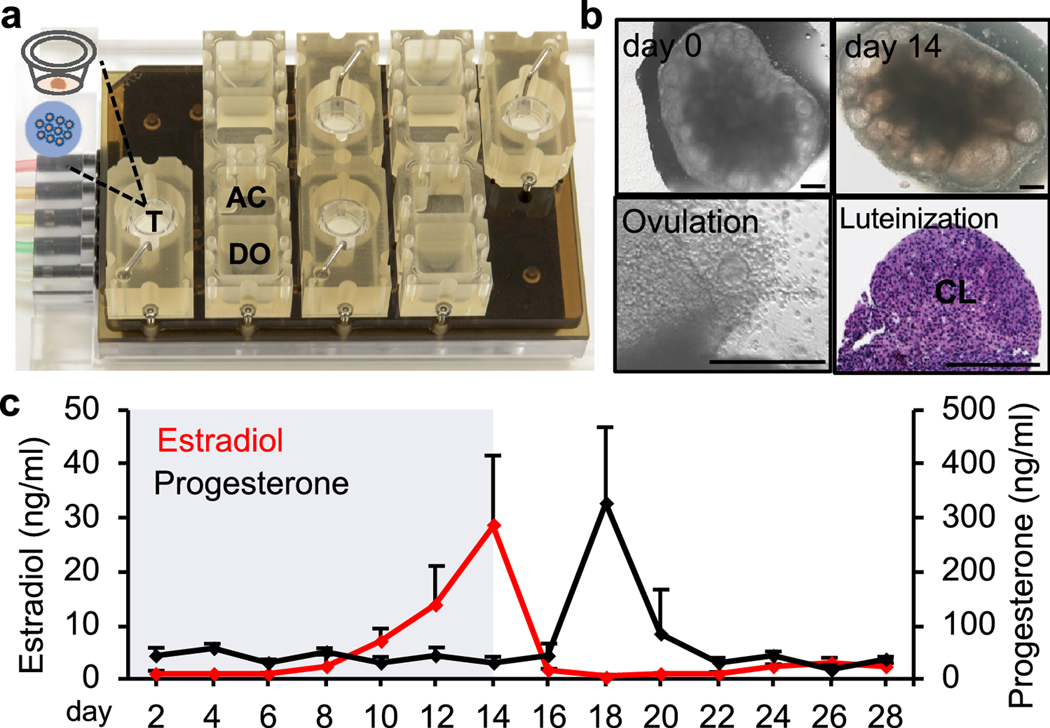
With respect to the ovary, we collaborated with bioengineers and created a microfluidic platform to culture both individual follicles and ovarian explants, which is shown in Figure 3A. Our results showed that the microfluidic culture of mouse primary and early secondary follicles completely recapitulated follicle development and oocyte maturation as eIVFG does. More profoundly, the follicles cultured in the microfluidic environment further promoted follicle survival as well as the production of both steroid and peptide hormones, producing a human 28-day menstrual cycle-like hormone profile [53]. Furthermore, the microfluidic platform also supported a long-term (28 days) culture of mouse ovarian explants that contain all developmental stages of immature follicles, which has been challenging due to the insufficient oxygen and nutrient diffusion in static cultures (Figure. 3B). In addition to mouse ovarian tissues, the microfluidic culture has also been applied to large mammalian species. Nagashima et al. used the microfluidic system to culture both individual follicles and ovarian cortex tissues isolated from domestic cats and dogs [54]. The dynamic fluidic flow significantly promoted the growth of both primordial follicles and preantral growing follicles, indicating a promising exploration toward the development and maturation of ovarian tissues or follicles of large mammalian species. These results gained from rodent and large mammalian species using microfluidic technology provide encouraging clue to create a human-ovary-on-a-chip.
Figure 3.
The ovary-on-a-chip. (A) Each microfluidic platform consists of 4 replicates of a fluidic circuit and each replicate has 3 connected modules: inlet media donor module (DO), tissue culture module (T), and outlet media acceptor module (AC). The microfluidic system can introduce a unidirectional transport fresh medium at a controlled flow rate from the DO to the T, and then remove the conditioned media and secreted hormones and other factors from the T to the AC. (B) The ovary-on-a-chip supported long-term culture of mouse ovarian explants which supported follicle development, oocyte maturation, ovulation, and luteinization. CL: corpus luteum. Scale bar: 300 μm. (C) The ovary-on-a-chip produced a 28-day menstrual cycle-like hormone secretion profile including both follicular phase and luteal phase.
3. Bioengineering models of fallopian tubes
The fallopian tubes are paired tubes with one end of the tube adjacent to the ovary and the other end extended to the uterus (Figure. 1). They provide space and a biological environment to support several essential events during early pregnancy, including fertilization, embryo development from a fertilized oocyte to morula stage, and transport of developing embryos to the uterus [55]. The fallopian tube consists of five segments: fimbriae (opening to the ovary), infundibulum, ampulla, isthmus, and uterotubal junction (Figure. 1). There are three main layers of the fallopian tube: epithelium, stroma, and inner circular and outer longitudinal layers of the smooth muscle. The epithelium layer of the fallopian tubes contains both epithelial cells and cilia. The coordination of cilia beating and smooth muscle contraction controls the uptake of ovulated oocytes and transport of developing embryos to the uterus. Nowadays, although the artificial reproductive technologies (ARTs) allow fertilization and early embryogenesis to fully complete in vitro without the fallopian tube, it is increasingly believed that the hormones, growth factors, and other components in the fallopian tubes have important roles in supporting fertilization and embryo development and transport [56,57]. Below, we reviewed previous studies that used bioengineering methods to recapitulate fallopian tubes or oviducts and their associated functions in vitro, which is particularly essential for improving the IVF success of humans, agricultural animals, and some endangered species. Compared to the ovaries, since there are limited studies focusing on the bioengineering models of fallopian tubes and the other downstream reproductive organs and diseases, we did not separate different model types as we did for the ovaries.
Human fallopian tube fimbriae have been encapsulated in 0.5% alginate hydrogel and cultured in vitro for 7 days [58]. Compared to a control group without alginate encapsulation, the alginate matrix retained the 3D architecture of fimbriae tissue and morphologically normal epithelial and stromal compartments. Further, unlike the loss of ciliated epithelia cultured in 2D, the alginate encapsulation retained both ciliated and secretory features of fallopian tube epithelium. Furthermore, we and others also used transwell inserts and a microfluidic platform to co-culture human fallopian tube epithelium and murine ovarian follicles [59,53]. It was found that the fallopian tube cilia beating and secretion of oviduct-specific glycoprotein (OVGP1) were regulated by the dynamic ovarian secretion of estradiol. Moreover, the co-culture of fallopian tube-ovary also prolonged the secretion of progesterone of the formed CL. These results illustrate the crosstalk between the ovaries and fallopian tubes. Additionally, another microfluidic platform has also been designed to build a bovine oviduct-on-a-chip, which maintained the polarization and differentiation of bovine oviductal epithelial cells for 6 weeks [60,35]. Further, the oviduct-on-a-chip improved bovine IVF outcomes such as preventing poly-sperm fertilization and parthenogenic activation and producing in vivo like-zygote transcriptome and epigenome, compared to static bovine IVF system. These studies indicate that the bioengineered fallopian tube or oviduct has significant potential to provide a more physiological IVF and embryo culture system.
4. Bioengineering models of uterus, embryo implantation, and beyond
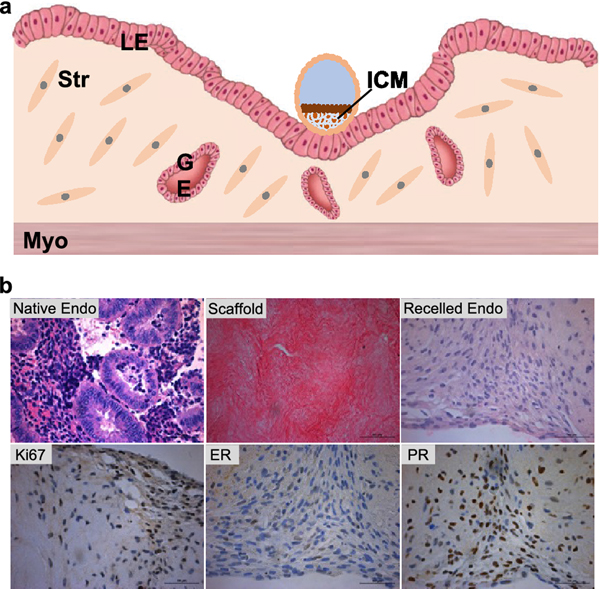
The uterus is where the developing embryo or fetus resides during pregnancy. Histologically, the uterus consists of three main layers: luminal epithelium (LE), stroma, and myometrium (Figure. 4A). The uterine endometrium forms the uterine cavity and includes both LE and stroma layers. In mammals, the thickness and components of uterine endometrium change with the menstrual cycle. After fertilization, the uterus stops its transition to the next cycle and prepares for embryo implantation, a process by which a competent embryo implants into the receptive uterus. Upon embryo implantation, the uterine LE cells at the embryo attachment site undergo apoptosis to allow for further penetration of the implanting embryo, and the stromal cells differentiate into secretory decidual cells to provide a nutritive and immune-privileged matrix to support embryo and placenta development. The transition of uterus from a non-receptive to receptive status is highly regulated by the coordination of ovarian hormones of estradiol and progesterone [61]. It has been estimated that 75% of pregnancy loss is caused by implantation failure and is not clinically recognized [62,63]. Therefore, understanding the mechanisms of uterine receptivity and embryo implantation is critical for reproductive biology and medicine.
Figure 4.
(A) Uterine anatomy and embryo implantation. The blastocyst initiates the implantation process through embryo apposition, adhesion, and penetration to the uterine luminal epithelium (LE). The LE cells around the attachment site will start apoptosis upon blastocyst attachment, and help the blastocyst penetrate the LE layer into the stroma. ICM: inner cell mass. Str: stroma cells. GE: glandular epithelium. Myo: myometrium. Modified based reference [91]. (B) Bioengineering model of human uterine endometrium. Human endometrium tissue before and after the decellularization, after recellularization, and immunohistochemistry staining of Ki67, estrogen receptor (ER), and progesterone receptor (PR) on day 14 (28 days in microfluidic culture) in EVATAR.
Thus far, due to the complexity of both embryo and uterus as well as their elaborate interactions during implantation, the gold standard for studying embryo implantation relies on in vivo animal models, in particular of mice. However, generating early pregnant animals is time and effort consuming and costly and the species difference also underscores the translation of research findings from mouse to human. The traditional 2D culture of human uterine cell lines (e.g. ECC-1 and Ishikawa cells) have been used to study uterine biology in vitro. However, these monolayer cells cultured in 2D miss the 3D tissue-specific architecture as well as the interconnection of different cell types. In recent years, uterine explants or bioengineered uterine tissues have been used for recapitulating human uterine functions in vitro. One example is that Cook et al. co-cultured human uterine epithelial cells on top of the polyethylene glycol (PEG) hydrogels encapsulated with uterine stromal cells [64]. Their results showed that the bioengineered uterine endometrium formed in vivo-like tissue architecture and also displayed hormone-mediated differentiation such as the secretion of prolactin and IGFBP-1 (Insulin Like Growth Factor Binding Protein 1), two decidualization markers. Moreover, we and others have combined the microfluidics and decellularized ECM scaffold methods to engineer human uterine endometrium (Figure. 4B) [53,65]. Specifically, we repopulated decellularized human uterine endometrium scaffolds with primary human uterine epithelial and stroma cells and demonstrated that the seeded endometrial cells remain viable for a 28-day microfluidic culture period. These cells also expressed endometrial cell markers of estrogen receptor (ER) and progesterone receptor (PR), secreted uterine decidualization markers of prolactin and IGFBP-1, and responded to the upstream ovarian hormones. Another research group designed a polydimethylsiloxane (PDMS)-based microfluidic device that allowed for the co-culture of primary human endometrial stromal cells and endothelial cells isolated from umbilical vein or uterine blood vessels [66,67]. Their results revealed that the co-culture system successfully differentiated the stroma cells into decidual cells and the decidualization was also significantly enhanced by the co-cultured endothelial cells, suggesting that the uterine vascularization plays an essential role in human stroma decidualization during peri-implantation period.
In addition to the uterus, a competent embryo is another critical factor to secure successful embryo implantation. Because of the extremely limited resource of human embryos and ethical considerations of human embryo research, using human pluripotent stem cells (hESCs) to develop embryo-like organoids has significant potential to study human embryo implantation [68]. For instance, Zheng et al. designed a microfluidic device which created a 3D environment and supported the growth and differentiation of loaded hESCs into embryonic-sac-like-structures [69]. The formed embryo-like organoids recapitulated many key events of embryos during the peri-implantation period, such as the anteriorization, posteriorization, and initial germ cell layer differentiation, indicating a valuable model to study embryo implantation in vitro. However, whether the induced ‘embryos’ are able to attach to uterus lining for implantation and post-implantation differentiation requires further studies. On the other hand, although embryos can be cultured and cryopreserved up until the prezygote, 8 cell, or blastocyst stage [70], there remains no in vitro models to support embryo or fetus development after this. Some research has investigated post-implantation embryo or fetal development through engineering an artificial uterus that is able to closely reproduce the environment of the womb. A good example is that a device has been constructed for a fetus of a lamb and incorporated an umbilical cord structure with an amniotic fluid like system [71]. This device included a pumpless arterial–venous circuit, a closed fluid environment with continuous fluid exchange, and umbilical vascular access. Their results demonstrated that the system kept the fetus alive for four weeks without organ failure.
5. Bioengineering models of placenta and maternal-fetal interface
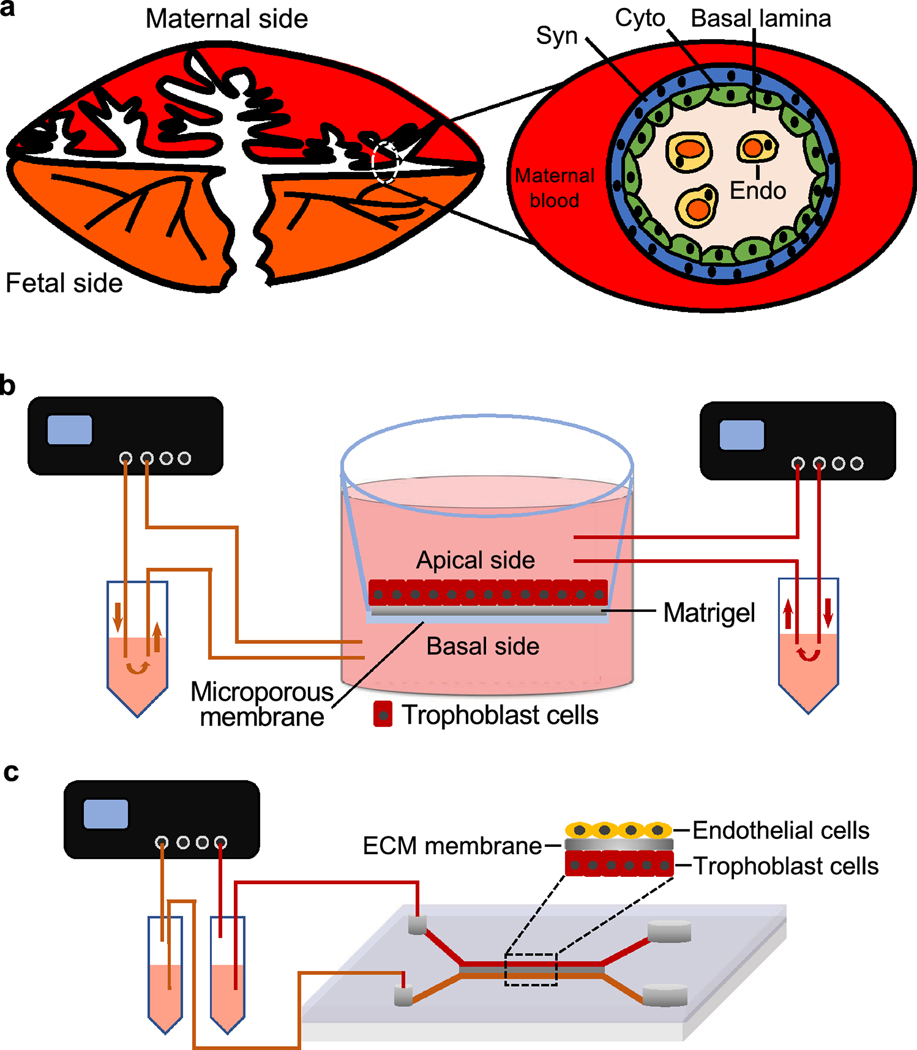
The placenta is another essential female reproductive organ inside the uterus during pregnancy. The primary function of the placenta is to serve as the interface to separate the maternal and fetal circulation and also mediate the exchange of oxygen, nutrients, and fetal waste. The exchange of these substances is through a multilayered membranous structure which is often called blood placenta barrier (BPB, Figure. 5A). The BPB is composed of trophoblasts (syncytiotrophoblasts and cytotrophoblasts), basal lamina, and fetal capillary endothelium. The abnormal placenta development and functions are associated with a number of pregnancy complications, such as the preeclampsia, fetal growth restriction, miscarriage, and still birth [72].
Figure 5.
(A) The anatomy of blood-placenta-barrier (BPB) that consists of trophoblasts (syncytiotrophoblasts, Syn, and cytotrophoblasts, Cyto), basal lamina, and fetal capillary endothelium (Endo). (B) The illustration of perfusion ex-vivo placenta model with the fetal reservoir on the left side and maternal reservoir on the right side. (C) The illustration of placenta-on-a-chip based on microfluidic technology, which consists of the upper villous endothelial cells (maternal side), middle semipermeable membrane, and lower trophoblast cells (fetal side).
Because the ethical considerations prevent researchers from using human placenta during pregnancy and the placenta tissues collected after birth cannot fully represent the placenta functions during gestation, there is a long history of research to develop in vitro human placenta models. For example, previous studies developed in vitro trophoblast cell culture on transwells and ex vivo placental perfusion systems to study the placenta physiology and pathology (Figure. 5B) [73–75]. However, these models cannot completely recapitulate the complex structure of the multilayered BPB as well as the transport and metabolism of both endogenous and exogenous substances across the placenta. The recent advances of microfluidic or organ-on-a-chip technologies allow for the bioengineering of human placenta in a more complex and representative manner. Blundell et al. created a microfluidic device to reproduce the trophoblast-endothelial interface by culturing human trophoblast BeWo cell line and human primary villous endothelial cells appositely using a semipermeable ECM membrane under flow conditions, which was called placenta-on-a-chip (Figure. 5C) [76]. This microfluidic culture system maintained the long-term viability of placental cells, which produced highly confluent monolayers on both sides of the placental membrane. More importantly, the bioengineered placenta barrier allowed for a more physiologically relevant glucose transport from the maternal to the fetal side compared to the other two in vitro models made of a bare membrane and a monolayer of BeWo cells without the co-cultured endothelial cells. Additionally, this microfluidic system was also used to culture another two types of placental cells, human trophoblast JEG-E cells line and human primary umbilical vein endothelial cells, to engineer a placenta-on-a-chip, receiving similar results [77]. The same research group also used the created placenta-on-a-chip to study how placenta regulates the maternal-to-fetal transfer of clinical drugs that are commonly used during pregnancy [78]. They demonstrated that the bioengineered placenta maintained the barrier integrity of BPB and also prevented the passage of heparin, a widely used medication for the treatment of deep vein thrombosis and pulmonary embolism during pregnancy. Additionally, the bioengineered BPB also expressed functional breast cancer resistance protein (BCRP), an important efflux drug transporter in the apical membrane of trophoblast cells in human placenta. In summary, these results suggest the microfluidic platform allows for the bioengineer of human placenta-on-a-chip, which can recapitulate both in vivo placenta barrier structure and transporter functions.
In addition to the ‘barrier’ functions, another critical role of the placenta is to provide developing fetus with oxygen and also accept carbon dioxide from the fetus, letting fetus ‘breathe’. Preterm neonates that suffer from respiratory distress syndrome require support from ventilators, which can lead to long term complications. Another microfluidic system has been created and combined with a lung support device to improve oxygenation status in preterm neonates [79]. The creation of this artificial placenta type microfluidic oxygenator allows for gas permeable membranes that work through arterio-venous pressure difference. This oxygenates the blood through exposure directly to the air without any pumping. The device has the ability to support 30% of the oxygen needs of a pre-term neonate. Reasons for premature birth are complex, but engineered artificial placenta could help us create better outcomes for premature neonates.
6. Bioengineering models of female reproductive diseases
Although engineered tissues, organs and organ system models have been applied for a variety of research, studies regarding bioengineering and female reproductive diseases remain limited. Several studies have been published using microfluidics and scaffold engineering to understand endometriosis [80,81] and reproductive cancers [82,83], though none to study polycystic ovary syndrome (PCOS).
6.1. Bioengineering and endometriosis
Endometriosis occurs when endometrial tissues are outside of the uterine cavity. It is one of the most common gynecological diseases in women of reproductive age worldwide [84]. An in vitro model was used to investigate the cell interactions between endometrial stromal cells (ESCs) and human peritoneal mesothelial cells (HPMCs) that are similar to endometriosis conditions [80]. Microfluidic channels and cover slips were used to observe interactions between ESCs and HPMCs, mimicking the physiological processes of peritoneal endometriosis. Control HPMCs resisted introduction of ESCs from both control and endometriotic individuals. However, HPMCs from endometriotic individuals were unable to resist ESCs from both normal and endometriotic individuals. This study developed an adaptable and simple in vitro method for real-time monitoring of interactions between ESCs and HPMCs. This approach can be used for demonstrating interactions among three or more types of cells and for investigating organ development of other diseases. The data suggest that endometriosis is related not only to the condition of endometrial cells, but also to the locations where there is the disease. Another study used the microfluidic system to investigate the drug pathologies and biomarkers for those with endometriosis vs. those without [81]. Authors aimed to evaluate an in vitro model that was designed to look at inflammation and could be applied to endometriosis tissues. Results showed that the developed droplet-based microfluidic platform allowed for the observation of hundreds of protease enzyme activity reactions for hours, creating physiologically relevant differences in controls and those with endometriosis [81].
6.2. Bioengineering and female reproductive cancers
Traditional cell cultures fail to repeat the natural tumor microenvironment. Recently, functional 3D in vitro models have been investigated and engineered for studying cervical cancers [82]. Normal epithelial and immortalized cervical epithelial carcinoma cell lines were used to mimic 3D artificial normal cervical and cervical cancerous tissues. Human skin cells were used as a scaffold for both models. Results indicated that the created 3D in vitro cervical cancer model showed stratified epithelial layers and expressed the same types and patterns of differentiation marker proteins as seen in corresponding in vivo tissue in either normal cervical or cervical cancerous tissues. Additionally, other 3D microfluidic systems have been investigated to mimic ovarian cancer environments [83,85,58]. For example, a microfluidic platform of the peritoneum was constructed to mimic ovarian cancer spheroids in the peritoneal cavity with mesothelial cells under hydrodynamic conditions. The interactions between cancer cells and mesothelial cells were analyzed. This model can help future researchers understand mechanisms of metastatic progression and assist with therapeutic development [83]. High-grade serous carcinoma (HGSC), which is the most common type of ovarian cancer, originates in epithelial cells in the fallopian tube. Several studies use oviductal epithelia cultured in a dynamic microfluidic chip to create an in vitro model that recapitulated human carcinoma [86,87]. These in vitro models can allow for the study of biomarkers for early detection of cancer and for improved therapeutic treatments. Theory shows that cortical inclusion cysts (CICs) in the ovary play a role in HGSC progression. Other studies use human samples to engineer an in vitro model that mimics the size, shape, and extracellular matrix properties of CICs [88,89]. Taken together, these engineered platforms can provide new knowledge on basic gynecologic cancer biology and pathology and for potential drug screening and development.
7. Co-culture of reproductive and non-reproductive organs in a microfluidic setting
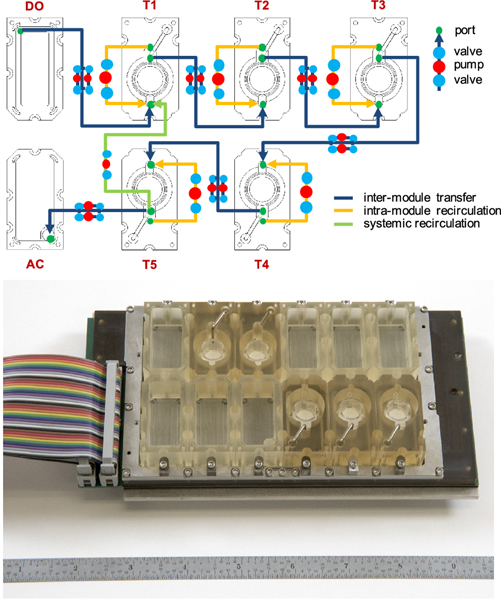
One unique feature of the female reproductive system is that no reproductive organ functions alone. For example, the ovarian follicle development and ovulation are highly regulated by the pituitary hormones of follicle stimulating hormone (FSH) and luteinizing hormone (LH). The ovarian hormones of estradiol and progesterone control the downstream reproductive track organs. As we described above, we have used the transwell insert or the microfluidic system to co-culture ovarian tissues and fallopian tube epithelium and demonstrated the crosstalk between these two female reproductive organs [59,53]. Furthermore, we collaborated with bioengineers and created a novel microfluidic device that can interconnect five different organs in one platform, which is termed ‘EVATAR’ (Figure. 6) [53]. The five organs we cultured using EVATAR were human liver spheroids, mouse ovarian explants, human fallopian tube epithelium, human endometrium, and human cervix tissues. The EVATAR interconnected these reproductive and non-reproductive tissues through embedded microfluidic channels and the universal culture medium. The results generated from this bioengineered female reproductive system-on-a-chip indicated that the dynamic fluidic flow maintained the viability of all culture tissues for 28 days and the downstream reproductive track tissues responded to the upstream ovarian hormones. Additionally, compared to ovarian tissue culture alone, the co-culture system also produced a more physiologically relevant human 28-day menstrual cycle hormone profiles.
Figure 6.
The microfluidic platform of ‘EVATAR’ that can interconnect different tissues/organs to make a female reproductive system-on-a-chip [53]. The system was designed based on pneumatic actuation technology. The interconnection between different modules was accomplished by embedding electromagnetically actuated micro-pumps and microfluidic channels. Each module allowed for recirculation within each module (orange), ensuring that the system was well mixed and enabling homogenous exposure of cultured tissues to factors within the media. Additionally, the fluidic path design also allowed for whole-system recirculation (blue), which enabled a well-mixed system within and across all tissue/organ modules. DO: donor module, T: tissue module, AC: acceptor module.
Another important application of the microfluidic system is to introduce liver metabolism for drug screening or toxicity testing. In our previous works using EVATAR [53], the liver spheroids remained viable and secreted albumin over the entire 28 days culture period with other co-cultured female reproductive tissues; however, further studies are necessary to determine whether the co-cultured liver spheroids were able to metabolize the hormone and other secreted factors from reproductive tissues and whether the reproductive tissues can influence the metabolic activities of co-cultured liver tissues. A number of clinical drugs or environmental chemicals require liver metabolism to exhibit therapeutic or toxic effects. For example, cyclophosphamide, a widely used chemotherapeutic drug, needs to be metabolized to phosphoramide mustard to exhibit both anti-cancer effects and ovarian toxicities. Di(2-ethylhexyl)phthalate (DEHP), a plasticizer and well-identified EDC, only exhibits female reproductive toxicities when it is metabolized to the Mono-(2-ethylhexyl) phthalate (MEHP) [90]. The co-culture of liver and female reproductive tissues in a microfluidic setting will allow us to introduce pharmacokinetics or toxicokinetics of the tested compounds, providing a more complex and representative in vitro models.
8. Conclusion, challenge, and future direction
There are limited ways to study female reproduction because of the complex interactions among cells, organs, hormones, and organ systems. Recent advances that use bioengineering methods to study female reproduction allow for a better understanding of the ovary, fallopian tube, uterus, embryo implantation, placenta, and reproductive disease. Bioengineering methods have been applied to study basic female reproductive biology, reproductive medicine, and toxicity screening. Hydrogel encapsulation, decellularized ECM scaffolds, 3D printing, microfluidic platform and other engineered advances indicate significant potentials to reconstruct and build artificial, functional, and implantable reproductive organs that may allow prepubertal girls and young adult women to restore their fertility and endocrine functions. Additionally, advances in in vitro systems like artificial wombs can help build better outcomes for preterm neonates. Furthermore, these engineered platforms can provide new knowledge on basic gynecologic cancer biology and pathology and for potential drug screening and development. While remarkable advancements have been achieved, most of these bioengineering models copy female reproduction at organotypic or cellular levels. Therefore, more in-depth analyses are required to investigate whether these bioengineering methods can recapitulate female reproduction at the molecular, genetic, and epigenetic levels, etc. For example, it is not well understood that whether the reconstructed ovaries using decellularized or 3D printed ECM scaffolds completely support the acquisition of oocyte transcriptome profiling to ensure its meiotic and developmental competence and whether the engineered oocyte or embryos fully preserve in vivo epigenetic reprogramming signatures. Compared to static culture environment, the microfluidic platform significantly promotes reproductive cell proliferation and differentiation. However, different microfluidic designed have been used and the precise microfluidic settings such as the fluid flow pattern, flow rate, and shear stress and whether these settings depend on cell/tissue types have not been well determined. Further, the safety assessment of applied biomaterials and fabrication methods also need to be considered when bioengineering female reproductive organs and function. In conclusion, although many endeavors are required in future studies, the intersection of bioengineering and female reproductive biology and medicine provides great potential to advance the knowledge of female fertility, genetic vulnerability, medications, environmental exposures and toxicities, aging, nutrition, and diseases.
Supplementary Material
Acknowledgements:
This work is supported by the National Institutes of Health (NIH K01ES030014 and P01ES028942) and National Science Foundation (NSF 183291) to S. Xiao. We thank Jingshan Xu for contributing to the Figures and Supplemental Table 1. As the limited space, we apologize for not being able to include all previously published studies that contributed to the understanding of the bioengineering of female reproduction.
Footnotes
Competing interests: The authors declare no conflict of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Buyuk E, Nejat E, Neal-Perry G (2010) Determinants of female reproductive senescence: differential roles for the ovary and the neuroendocrine axis. Semin Reprod Med 28 (5):370–379. doi: 10.1055/s-0030-1262896 [DOI] [PubMed] [Google Scholar]
- 2.Prevention CfDCa (November 8, 2019) Key Statistics from the National Survey of Family Growth - I Listing. https://www.cdc.gov/nchs/nsfg/key_statistics/i_2015-2017.htm#infertility. 2020
- 3.Prevention CfDCa (2018) Common Reproductive Health Concerns for Women. https://www.cdc.gov/reproductivehealth/womensrh/healthconcerns.html.
- 4.Organization WH (2020) Cervical cancer. https://www.who.int/cancer/prevention/diagnosis-screening/cervical-cancer/en/. 2020
- 5.Tao JJ, Visvanathan K, Wolff AC (2015) Long term side effects of adjuvant chemotherapy in patients with early breast cancer. Breast 24 Suppl 2:S149–153. doi: 10.1016/j.breast.2015.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin NE, D’Amico AV (2014) Progress and controversies: Radiation therapy for prostate cancer. CA Cancer J Clin 64 (6):389–407. doi: 10.3322/caac.21250 [DOI] [PubMed] [Google Scholar]
- 7.Jeruss JS, Woodruff TK (2009) Preservation of fertility in patients with cancer. N Engl J Med 360 (9):902–911. doi: 10.1056/NEJMra0801454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersen CL, Liu M, Wang Z, Ye X, Xiao S (2018) Chemotherapeutic agent doxorubicin alters uterine gene expression in response to estrogen in ovariectomized CD-1 adult mice. Biol Reprod. doi: 10.1093/biolre/ioy259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Havelock JC, Rainey WE, Carr BR (2004) Ovarian granulosa cell lines. Mol Cell Endocrinol 228 (1–2):67–78. doi: 10.1016/j.mce.2004.04.018 [DOI] [PubMed] [Google Scholar]
- 10.Pocar P, Augustin R, Gandolfi F, Fischer B (2003) Toxic effects of in vitro exposure to p-tert-octylphenol on bovine oocyte maturation and developmental competence. Biol Reprod 69 (2):462–468. doi: 10.1095/biolreprod.102.010355 [DOI] [PubMed] [Google Scholar]
- 11.Pocar P, Perazzoli F, Luciano AM, Gandolfi F (2001) In vitro reproductive toxicity of polychlorinated biphenyls: effects on oocyte maturation and developmental competence in cattle. Mol Reprod Dev 58 (4):411–416. doi: [DOI] [PubMed] [Google Scholar]
- 12.Matzuk MM, Burns KH, Viveiros MM, Eppig JJ (2002) Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science 296 (5576):2178–2180. doi: 10.1126/science.1071965 [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Torcia S, Xie F, Lin CJ, Cakmak H, Franciosi F, Horner K, Onodera C, Song JS, Cedars MI, Ramalho-Santos M, Conti M (2013) Somatic cells regulate maternal mRNA translation and developmental competence of mouse oocytes. Nature cell biology 15 (12):1415–1423. doi: 10.1038/ncb2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su YQ, Sugiura K, Eppig JJ (2009) Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Seminars in reproductive medicine 27 (1):32–42. doi: 10.1055/s-0028-1108008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugiura K, Pendola FL, Eppig JJ (2005) Oocyte control of metabolic cooperativity between oocytes and companion granulosa cells: energy metabolism. Developmental biology 279 (1):20–30. doi: 10.1016/j.ydbio.2004.11.027 [DOI] [PubMed] [Google Scholar]
- 16.Gilchrist RB, Ritter LJ, Myllymaa S, Kaivo-Oja N, Dragovic RA, Hickey TE, Ritvos O, Mottershead DG (2006) Molecular basis of oocyte-paracrine signalling that promotes granulosa cell proliferation. Journal of cell science 119 (Pt 18):3811–3821. doi: 10.1242/jcs.03105 [DOI] [PubMed] [Google Scholar]
- 17.Eppig JJ, Pendola FL, Wigglesworth K, Pendola JK (2005) Mouse oocytes regulate metabolic cooperativity between granulosa cells and oocytes: amino acid transport. Biol Reprod 73 (2):351–357. doi: 10.1095/biolreprod.105.041798 [DOI] [PubMed] [Google Scholar]
- 18.Biggers JD, Whittingham DG, Donahue RP (1967) The pattern of energy metabolism in the mouse oocyte and zygote. Proceedings of the National Academy of Sciences of the United States of America 58 (2):560–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su YQ, Sugiura K, Wigglesworth K, O’Brien MJ, Affourtit JP, Pangas SA, Matzuk MM, Eppig JJ (2008) Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development 135 (1):111–121. doi: 10.1242/dev.009068 [DOI] [PubMed] [Google Scholar]
- 20.Buccione R, Schroeder AC, Eppig JJ (1990) Interactions between Somatic-Cells and Germ-Cells Throughout Mammalian Oogenesis. Biol Reprod 43 (4):543–547. doi:Doi 10.1095/Biolreprod43.4.543 [DOI] [PubMed] [Google Scholar]
- 21.Gui UM, Joyce LA (2005) RNA interference evidence that growth differentiation factor-9 mediates clocyte regulation of cumulus expansion in mice. Biol Reprod 72 (1):195–199. doi:Doi 10.1095/Biolreprod.104.033357 [DOI] [PubMed] [Google Scholar]
- 22.Oatley J, Hunt PA (2012) Of mice and (wo)men: purified oogonial stem cells from mouse and human ovaries. Biol Reprod 86 (6):196. doi: 10.1095/biolreprod.112.100297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horan CJ, Williams SA (2017) Oocyte stem cells: fact or fantasy? Reproduction 154 (1):R23–R35. doi: 10.1530/REP-17-0008 [DOI] [PubMed] [Google Scholar]
- 24.Goswami D, Conway GS (2005) Premature ovarian failure. Hum Reprod Update 11 (4):391–410. doi: 10.1093/humupd/dmi012 [DOI] [PubMed] [Google Scholar]
- 25.Tibbitt MW, Anseth KS (2009) Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng 103 (4):655–663. doi: 10.1002/bit.22361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orive G, Hernandez RM, Gascon AR, Calafiore R, Chang TM, De Vos P, Hortelano G, Hunkeler D, Lacik I, Shapiro AM, Pedraz JL (2003) Cell encapsulation: promise and progress. Nat Med 9 (1):104–107. doi: 10.1038/nm0103-104 [DOI] [PubMed] [Google Scholar]
- 27.Pangas SA, Saudye H, Shea LD, Woodruff TK (2003) Novel approach for the three-dimensional culture of granulosa cell-oocyte complexes. Tissue Eng 9 (5):1013–1021. doi: 10.1089/107632703322495655 [DOI] [PubMed] [Google Scholar]
- 28.Xu M, Kreeger PK, Shea LD, Woodruff TK (2006) Tissue-engineered follicles produce live, fertile offspring. Tissue Eng 12 (10):2739–2746. doi: 10.1089/ten.2006.12.2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kreeger PK, Fernandes NN, Woodruff TK, Shea LD (2005) Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biol Reprod 73 (5):942–950. doi: 10.1095/biolreprod.105.042390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hornick JE, Duncan FE, Shea LD, Woodruff TK (2013) Multiple follicle culture supports primary follicle growth through paracrine-acting signals. Reproduction 145 (1):19–32. doi: 10.1530/REP-12-0233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.West ER, Xu M, Woodruff TK, Shea LD (2007) Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials 28 (30):4439–4448. doi: 10.1016/j.biomaterials.2007.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu M, West E, Shea LD, Woodruff TK (2006) Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod 75 (6):916–923. doi: 10.1095/biolreprod.106.054833 [DOI] [PubMed] [Google Scholar]
- 33.Xiao S, Zhang J, Romero MM, Smith KN, Shea LD, Woodruff TK (2015) In vitro follicle growth supports human oocyte meiotic maturation. Sci Rep 5:17323. doi: 10.1038/srep17323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crapo PM, Gilbert TW, Badylak SF (2011) An overview of tissue and whole organ decellularization processes. Biomaterials 32 (12):3233–3243. doi: 10.1016/j.biomaterials.2011.01.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frantz C, Stewart KM, Weaver VM (2010) The extracellular matrix at a glance. J Cell Sci 123 (Pt 24):4195–4200. doi: 10.1242/jcs.023820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laronda MM, Jakus AE, Whelan KA, Wertheim JA, Shah RN, Woodruff TK (2015) Initiation of puberty in mice following decellularized ovary transplant. Biomaterials 50:20–29. doi: 10.1016/j.biomaterials.2015.01.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jakus AE, Laronda MM, Rashedi AS, Robinson CM, Lee C, Jordan SW, Orwig KE, Woodruff TK, Shah RN (2017) “Tissue Papers” from Organ-Specific Decellularized Extracellular Matrices. Adv Funct Mater 27 (3). doi: 10.1002/adfm.201700992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oktay K, Bedoschi G, Pacheco F, Turan V, Emirdar V (2016) First pregnancies, live birth, and in vitro fertilization outcomes after transplantation of frozen-banked ovarian tissue with a human extracellular matrix scaffold using robot-assisted minimally invasive surgery. Am J Obstet Gynecol 214 (1):94 e91–99. doi: 10.1016/j.ajog.2015.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oktay K, Taylan E, Kawahara T, Cillo GM (2019) Robot-assisted orthotopic and heterotopic ovarian tissue transplantation techniques: surgical advances since our first success in 2000. Fertil Steril 111 (3):604–606. doi: 10.1016/j.fertnstert.2018.11.042 [DOI] [PubMed] [Google Scholar]
- 40.Pors SE, Ramlose M, Nikiforov D, Lundsgaard K, Cheng J, Andersen CY, Kristensen SG (2019) Initial steps in reconstruction of the human ovary: survival of preantral stage follicles in a decellularized human ovarian scaffold. Hum Reprod 34 (8):1523–1535. doi: 10.1093/humrep/dez077 [DOI] [PubMed] [Google Scholar]
- 41.Lee A, Hudson AR, Shiwarski DJ, Tashman JW, Hinton TJ, Yerneni S, Bliley JM, Campbell PG, Feinberg AW (2019) 3D bioprinting of collagen to rebuild components of the human heart. Science 365 (6452):482–487. doi: 10.1126/science.aav9051 [DOI] [PubMed] [Google Scholar]
- 42.Papaioannou TG, Manolesou D, Dimakakos E, Tsoucalas G, Vavuranakis M, Tousoulis D (2019) 3D Bioprinting Methods and Techniques: Applications on Artificial Blood Vessel Fabrication. Acta Cardiol Sin 35 (3):284–289. doi: 10.6515/ACS.201905_35(3).20181115A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duan B, Hockaday LA, Kang KH, Butcher JT (2013) 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mater Res A 101 (5):1255–1264. doi: 10.1002/jbm.a.34420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee V, Singh G, Trasatti JP, Bjornsson C, Xu X, Tran TN, Yoo SS, Dai G, Karande P (2014) Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng Part C Methods 20 (6):473–484. doi: 10.1089/ten.TEC.2013.0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Markstedt K, Mantas A, Tournier I, Martinez Avila H, Hagg D, Gatenholm P (2015) 3D Bioprinting Human Chondrocytes with Nanocellulose-Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 16 (5):1489–1496. doi: 10.1021/acs.biomac.5b00188 [DOI] [PubMed] [Google Scholar]
- 46.Laronda MM, Rutz AL, Xiao S, Whelan KA, Duncan FE, Roth EW, Woodruff TK, Shah RN (2017) A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat Commun 8:15261. doi: 10.1038/ncomms15261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raffel N, Dittrich R, Bauerle T, Seyler L, Fattahi A, Hoffmann I, Leal-Egana A, Beckmann MW, Boccaccini AR, Liverani L (2019) Novel approach for the assessment of ovarian follicles infiltration in polymeric electrospun patterned scaffolds. PLoS One 14 (4):e0215985. doi: 10.1371/journal.pone.0215985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liverani L, Raffel N, Fattahi A, Preis A, Hoffmann I, Boccaccini AR, Beckmann MW, Dittrich R (2019) Electrospun patterned porous scaffolds for the support of ovarian follicles growth: a feasibility study. Sci Rep 9 (1):1150. doi: 10.1038/s41598-018-37640-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gungor-Ozkerim PS, Inci I, Zhang YS, Khademhosseini A, Dokmeci MR (2018) Bioinks for 3D bioprinting: an overview. Biomater Sci 6 (5):915–946. doi: 10.1039/c7bm00765e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhatia SN, Ingber DE (2014) Microfluidic organs-on-chips. Nat Biotechnol 32 (8):760–772. doi: 10.1038/nbt.2989 [DOI] [PubMed] [Google Scholar]
- 51.Ronaldson-Bouchard K, Vunjak-Novakovic G (2018) Organs-on-a-Chip: A Fast Track for Engineered Human Tissues in Drug Development. Cell Stem Cell 22 (3):310–324. doi: 10.1016/j.stem.2018.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sosa-Hernandez JE, Villalba-Rodriguez AM, Romero-Castillo KD, Aguilar-Aguila-Isaias MA, Garcia-Reyes IE, Hernandez-Antonio A, Ahmed I, Sharma A, Parra-Saldivar R, Iqbal HMN (2018) Organs-on-a-Chip Module: A Review from the Development and Applications Perspective. Micromachines (Basel) 9 (10). doi: 10.3390/mi9100536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao S, Coppeta JR, Rogers HB, Isenberg BC, Zhu J, Olalekan SA, McKinnon KE, Dokic D, Rashedi AS, Haisenleder DJ, Malpani SS, Arnold-Murray CA, Chen K, Jiang M, Bai L, Nguyen CT, Zhang J, Laronda MM, Hope TJ, Maniar KP, Pavone ME, Avram MJ, Sefton EC, Getsios S, Burdette JE, Kim JJ, Borenstein JT, Woodruff TK (2017) A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat Commun 8:14584. doi: 10.1038/ncomms14584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nagashima JB, El Assal R, Songsasen N, Demirci U (2018) Evaluation of an ovary-on-a-chip in large mammalian models: Species specificity and influence of follicle isolation status. J Tissue Eng Regen Med 12 (4):e1926-e1935. doi: 10.1002/term.2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eddy CA, Pauerstein CJ (1980) Anatomy and physiology of the fallopian tube. Clinical obstetrics and gynecology 23 (4):1177–1193 [DOI] [PubMed] [Google Scholar]
- 56.Aviles M, Gutierrez-Adan A, Coy P (2010) Oviductal secretions: will they be key factors for the future ARTs? Mol Hum Reprod 16 (12):896–906. doi: 10.1093/molehr/gaq056 [DOI] [PubMed] [Google Scholar]
- 57.Li S, Winuthayanon W (2017) Oviduct: roles in fertilization and early embryo development. J Endocrinol 232 (1):R1–R26. doi: 10.1530/JOE-16-0302 [DOI] [PubMed] [Google Scholar]
- 58.Eddie SL, Quartuccio SM, Zhu J, Shepherd JA, Kothari R, Kim JJ, Woodruff TK, Burdette JE (2015) Three-dimensional modeling of the human fallopian tube fimbriae. Gynecol Oncol 136 (2):348–354. doi: 10.1016/j.ygyno.2014.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu J, Xu Y, Rashedi AS, Pavone ME, Kim JJ, Woodruff TK, Burdette JE (2016) Human fallopian tube epithelium co-culture with murine ovarian follicles reveals crosstalk in the reproductive cycle. Mol Hum Reprod 22 (11):756–767. doi: 10.1093/molehr/gaw041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferraz M, Rho HS, Hemerich D, Henning HHW, van Tol HTA, Holker M, Besenfelder U, Mokry M, Vos P, Stout TAE, Le Gac S, Gadella BM (2018) An oviduct-on-a-chip provides an enhanced in vitro environment for zygote genome reprogramming. Nat Commun 9 (1):4934. doi: 10.1038/s41467-018-07119-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Psychoyos A (1973) Endocrine control of egg implantation. In Handbook of Physiology 2:187–215 [Google Scholar]
- 62.Norwitz ER, Schust DJ, Fisher SJ (2001) Implantation and the survival of early pregnancy. The New England journal of medicine 345 (19):1400–1408. doi: 10.1056/NEJMra000763 [DOI] [PubMed] [Google Scholar]
- 63.Wilcox AJ, Weinberg CR, O’Connor JF, Baird DD, Schlatterer JP, Canfield RE, Armstrong EG, Nisula BC (1988) Incidence of early loss of pregnancy. The New England journal of medicine 319 (4):189–194. doi: 10.1056/NEJM198807283190401 [DOI] [PubMed] [Google Scholar]
- 64.Cook CD, Hill AS, Guo M, Stockdale L, Papps JP, Isaacson KB, Lauffenburger DA, Griffith LG (2017) Local remodeling of synthetic extracellular matrix microenvironments by co-cultured endometrial epithelial and stromal cells enables long-term dynamic physiological function. Integr Biol (Camb) 9 (4):271–289. doi: 10.1039/c6ib00245e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olalekan SA, Burdette JE, Getsios S, Woodruff TK, Kim JJ (2017) Development of a novel human recellularized endometrium that responds to a 28-day hormone treatment. Biol Reprod 96 (5):971–981. doi: 10.1093/biolre/iox039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gnecco JS, Ding T, Smith C, Lu J, Bruner-Tran KL, Osteen KG (2019) Hemodynamic forces enhance decidualization via endothelial-derived prostaglandin E2 and prostacyclin in a microfluidic model of the human endometrium. Hum Reprod 34 (4):702–714. doi: 10.1093/humrep/dez003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gnecco JS, Pensabene V, Li DJ, Ding T, Hui EE, Bruner-Tran KL, Osteen KG (2017) Compartmentalized Culture of Perivascular Stroma and Endothelial Cells in a Microfluidic Model of the Human Endometrium. Ann Biomed Eng 45 (7):1758–1769. doi: 10.1007/s10439-017-1797-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pera MF (2017) Human embryo research and the 14-day rule. Development 144 (11):1923–1925. doi: 10.1242/dev.151191 [DOI] [PubMed] [Google Scholar]
- 69.Zheng Y, Xue X, Shao Y, Wang S, Esfahani SN, Li Z, Muncie JM, Lakins JN, Weaver VM, Gumucio DL, Fu J (2019) Controlled modelling of human epiblast and amnion development using stem cells. Nature 573 (7774):421–425. doi: 10.1038/s41586-019-1535-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gracia C, Woodruff TK (2012) Oncofertility medical practice : clinical issues and implementation. Springer, New York [Google Scholar]
- 71.Partridge EA, Davey MG, Hornick MA, McGovern PE, Mejaddam AY, Vrecenak JD, Mesas-Burgos C, Olive A, Caskey RC, Weiland TR, Han J, Schupper AJ, Connelly JT, Dysart KC, Rychik J, Hedrick HL, Peranteau WH, Flake AW (2017) An extra-uterine system to physiologically support the extreme premature lamb. Nat Commun 8:15112. doi: 10.1038/ncomms15112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ilekis JV, Tsilou E, Fisher S, Abrahams VM, Soares MJ, Cross JC, Zamudio S, Illsley NP, Myatt L, Colvis C, Costantine MM, Haas DM, Sadovsky Y, Weiner C, Rytting E, Bidwell G (2016) Placental origins of adverse pregnancy outcomes: potential molecular targets: an Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Am J Obstet Gynecol 215 (1 Suppl):S1–S46. doi: 10.1016/j.ajog.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mathiesen L, Mose T, Morck TJ, Nielsen JK, Nielsen LK, Maroun LL, Dziegiel MH, Larsen LG, Knudsen LE (2010) Quality assessment of a placental perfusion protocol. Reprod Toxicol 30 (1):138–146. doi: 10.1016/j.reprotox.2010.01.006 [DOI] [PubMed] [Google Scholar]
- 74.Bode CJ, Jin H, Rytting E, Silverstein PS, Young AM, Audus KL (2006) In vitro models for studying trophoblast transcellular transport. Methods Mol Med 122:225–239. doi: 10.1385/1-59259-989-3:225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang X, Luthi M, Ontsouka EC, Kallol S, Baumann MU, Surbek DV, Albrecht C (2016) Establishment of a confluent monolayer model with human primary trophoblast cells: novel insights into placental glucose transport. Mol Hum Reprod 22 (6):442–456. doi: 10.1093/molehr/gaw018 [DOI] [PubMed] [Google Scholar]
- 76.Lee JS, Romero R, Han YM, Kim HC, Kim CJ, Hong JS, Huh D (2016) Placenta-on-a-chip: a novel platform to study the biology of the human placenta. J Matern Fetal Neonatal Med 29 (7):1046–1054. doi: 10.3109/14767058.2015.1038518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blundell C, Tess ER, Schanzer AS, Coutifaris C, Su EJ, Parry S, Huh D (2016) A microphysiological model of the human placental barrier. Lab Chip 16 (16):3065–3073. doi: 10.1039/c6lc00259e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blundell C, Yi YS, Ma L, Tess ER, Farrell MJ, Georgescu A, Aleksunes LM, Huh D (2018) Placental Drug Transport-on-a-Chip: A Microengineered In Vitro Model of Transporter-Mediated Drug Efflux in the Human Placental Barrier. Adv Healthc Mater 7 (2). doi: 10.1002/adhm.201700786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dabaghi M, Fusch G, Saraei N, Rochow N, Brash JL, Fusch C, Ravi Selvaganapathy P (2018) An artificial placenta type microfluidic blood oxygenator with double-sided gas transfer microchannels and its integration as a neonatal lung assist device. Biomicrofluidics 12 (4):044101. doi: 10.1063/1.5034791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen Z, Dai Y, Dong Z, Li M, Mu X, Zhang R, Wang Z, Zhang W, Lang J, Leng J, Jiang X (2012) Co-cultured endometrial stromal cells and peritoneal mesothelial cells for an in vitro model of endometriosis. Integr Biol (Camb) 4 (9):1090–1095. doi: 10.1039/c2ib00172a [DOI] [PubMed] [Google Scholar]
- 81.Chen CH, Miller MA, Sarkar A, Beste MT, Isaacson KB, Lauffenburger DA, Griffith LG, Han J (2013) Multiplexed protease activity assay for low-volume clinical samples using droplet-based microfluidics and its application to endometriosis. J Am Chem Soc 135 (5):1645–1648. doi: 10.1021/ja307866z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Karolina Zuk A, Wen X, Dilworth S, Li D, Ghali L (2017) Modeling and validating three dimensional human normal cervix and cervical cancer tissues in vitro. J Biomed Res 31 (3):240–247. doi: 10.7555/JBR.31.20160150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li SS, Ip CK, Tang MY, Sy SK, Yung S, Chan TM, Yang M, Shum HC, Wong AS (2017) Modeling Ovarian Cancer Multicellular Spheroid Behavior in a Dynamic 3D Peritoneal Microdevice. J Vis Exp (120). doi: 10.3791/55337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kuznetsov L, Dworzynski K, Davies M, Overton C, Guideline C (2017) Diagnosis and management of endometriosis: summary of NICE guidance. BMJ 358:j3935. doi: 10.1136/bmj.j3935 [DOI] [PubMed] [Google Scholar]
- 85.Watters KM, Bajwa P, Kenny HA (2018) Organotypic 3D Models of the Ovarian Cancer Tumor Microenvironment. Cancers (Basel) 10 (8). doi: 10.3390/cancers10080265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.de Almeida Monteiro Melo Ferraz M, Nagashima JB, Venzac B, Le Gac S, Songsasen N (2020) Author Correction: A dog oviduct-on-a-chip model of serous tubal intraepithelial carcinoma. Sci Rep 10 (1):4733. doi: 10.1038/s41598-020-61782-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dorayappan KDP, Gardner ML, Hisey CL, Zingarelli RA, Smith BQ, Lightfoot MDS, Gogna R, Flannery MM, Hays J, Hansford DJ, Freitas MA, Yu L, Cohn DE, Selvendiran K (2019) A Microfluidic Chip Enables Isolation of Exosomes and Establishment of Their Protein Profiles and Associated Signaling Pathways in Ovarian Cancer. Cancer Res 79 (13):3503–3513. doi: 10.1158/0008-5472.CAN-18-3538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fleszar AJ, Walker A, Kreeger PK, Notbohm J (2019) Substrate curvature induces fallopian tube epithelial cell invasion via cell-cell tension in a model of ovarian cortical inclusion cysts. Integr Biol (Camb) 11 (8):342–352. doi: 10.1093/intbio/zyz028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fleszar AJ, Walker A, Porubsky V, Flanigan W, James D, Campagnola PJ, Weisman PS, Kreeger PK (2018) The Extracellular Matrix of Ovarian Cortical Inclusion Cysts Modulates Invasion of Fallopian Tube Epithelial Cells. APL Bioeng 2 (3). doi: 10.1063/1.5022595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hannon PR, Flaws JA (2015) The effects of phthalates on the ovary. Front Endocrinol (Lausanne) 6:8. doi: 10.3389/fendo.2015.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cha J, Sun X, Dey SK (2012) Mechanisms of implantation: strategies for successful pregnancy. Nature medicine 18 (12):1754–1767. doi: 10.1038/nm.3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.