Abstract
Human immunodeficiency virus (HIV) infection continues to pose a major infectious disease threat worldwide. It is characterized by the depletion of CD4+ T cells, persistent immune activation, and increased susceptibility to secondary infections. Advances in the development of antiretroviral drugs and combination antiretroviral therapy have resulted in a remarkable reduction in HIV-associated morbidity and mortality. Antiretroviral therapy (ART) leads to effective suppression of HIV replication with partial recovery of host immune system and has successfully transformed HIV infection from a fatal disease to a chronic condition. Additionally, antiretroviral drugs have shown promise for prevention in HIV pre-exposure prophylaxis and treatment as prevention. However, ART is unable to cure HIV. Other limitations include drug–drug interactions, drug resistance, cytotoxic side effects, cost, and adherence. Alternative treatment options are being investigated to overcome these challenges including discovery of new molecules with increased anti-viral activity and development of easily administrable drug formulations. In light of the difficulties associated with current HIV treatment measures, and in the continuing absence of a cure, the prevention of new infections has also arisen as a prominent goal among efforts to curtail the worldwide HIV pandemic. In this review, the authors summarize currently available anti-HIV drugs and their combinations for treatment, new molecules under clinical development and prevention methods, and discuss drug delivery formats as well as associated challenges and alternative approaches for the future.
Keywords: antiretroviral drugs, drug delivery, HIV prevention, HIV treatment, pre exposure prophylaxis
1. Introduction
According to the World Health Organization, an estimated 37 million people were living with HIV-1 (hereafter referred to as “HIV”) infection worldwide in 2016 and around half of HIV-positive people are not aware of their status. There are about 2 million new HIV infections occurring annually. Only about 19 million HIV-infected people are currently receiving antiretroviral therapy (ART)[1] and the decline in the incidence of new HIV infections is not optimum. This can be attributed to inadequate access to ART in economically disadvantaged developing countries, lack of adherence or regular clinical monitoring during therapy, and incomplete outreach to communities. The prevalence of HIV is higher in the regions with pronounced gen der imbalance and increases risk of HIV infection among women and their children.[2] Other individuals at high risk of HIV infection worldwide include sex workers, intravenous drug users, transgender people, prisoners, and men who have sex with men (MSM).[3] In the United States, there were 1.1 million people living with HIV infection and 39 000 new HIV infections reported in 2016. A disproportionate increase in HIV infection is found among minority underserved populations including young African Americans and among people aged 13–29.[4]
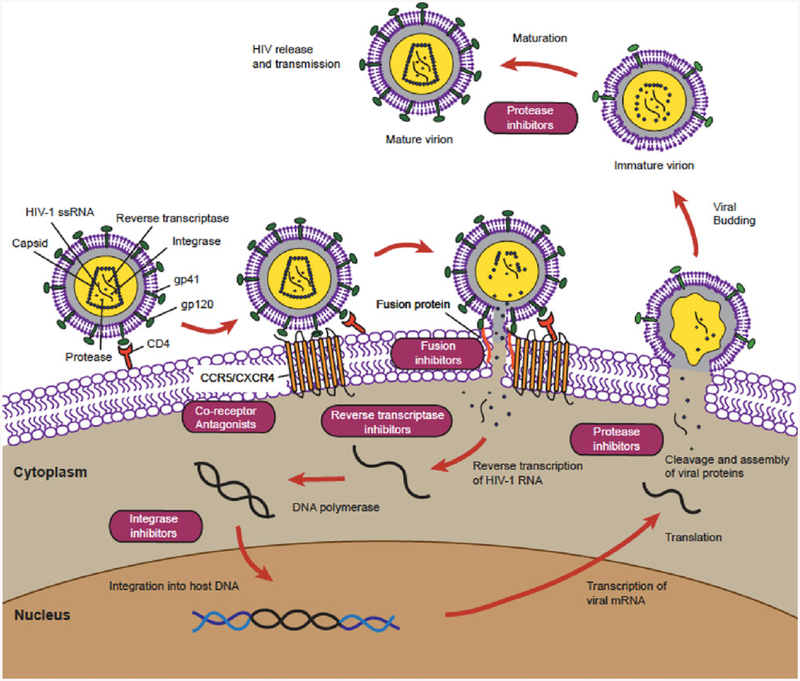
Since the first isolation of HIV over 30 years ago, there has been considerable knowledge gained about the molecular structure and characteristics of the virus, its replication cycle, and pathogenic mechanisms contributing to the development of acquired immune deficiency syndrome (AIDS).[5] The major driver of viral pathogenesis is the ability of HIV to infect and kill CD4+ T cells expressing co-receptor CC-chemokine receptor-5 (CCR5) or CXC receptor-4 (CXCR4) causing CD4+ T cell depletion, which results in the impairment of the host immune system and its functions.[6] Following entry of the virus into the target host cell, the single-stranded HIV RNA genome is reverse transcribed into complementary DNA by reverse transcriptase (RT), transported to the nucleus, and integrated into the host genomic DNA (Figure 1). The integrated provirus can be further transcribed into viral RNA copies and translated to viral proteins. Viral particles are assembled and released from the infected cell and are positioned to infect new target cells including CD4+ T cells.[7] Highly active antiretroviral therapy (HAART) which consists of combination antiretroviral therapy (cART) can effectively suppress HIV replication by targeting multiple specific steps in the viral replication cycle (fusion, reverse transcription, integration, and/or maturation).[8] Advances in HIV research have dramatically improved our understanding of HIV pathogenesis and led to successful efforts in enhancing HIV treatment and prevention resulting in a reduced number of AIDS-related deaths. In the last 2 years, the number of people on HAART has increased by 30%, totaling 19.0 million.[9] However, several limitations in HAART prevent complete eradication of HIV. While HAART suppresses the virus, latent viral reservoirs in lymphoid tissues remain hidden from the immune system and are not eradicated.[10] Inter-ruption of HAART results in a rapid rebound of HIV from latent viral reservoirs.[11] The requirement for lifelong administration of HAART presents a constant challenge for financial affordability and patient compliance and management of secondary effects. New approaches are needed for developing less toxic antiretroviral drugs (ARVs) and therapeutic regimens consisting of decreased dosage and frequency.
Figure 1.
ARVs target specific steps in the HIV replication cycle.
In this review, we present current and cutting-edge HIV prevention and therapeutic regimens, as well as the advantages and limitations of the various approaches and delivery formats. We provide brief historical background on successes and failures in HIV treatment and prevention, as well as a summary of future directions in treatment and prevention efforts aimed at diminishing the HIV pandemic.
2. HIV Treatment
2.1. Challenges in Current Treatment
Current HIV treatment relies heavily on the use of a combination of ARVs, which effectively reduces AIDS-associated morbidity and prolongs lifespan of HIV-infected patients. There are more than 25 ARVs available that encompass multiple different mechanisms of viral inhibition (Table 1) and target different steps in the HIV replication cycle (Figure 1).[12] These anti-HIV drugs can be divided in six groups based on their mechanism of action[13](Table 1).
Table 1.
Currently available ARVs.[14]
| Mechanism of action | Drug | Oral adult dose | Brand name |
|---|---|---|---|
| Nucleoside reverse transcriptase inhibitors (NRTIs) | Abacavir (ABC) | 300 mg twice daily 600 mg once daily | ZIAGEN |
| Didanosine (ddI) | 200 mg twice daily 400 mg once daily | VIDEX EC | |
| Emtricitabine (FTC) | 200 mg once daily | EMTRIVA | |
| Lamivudine (3TC) | 150 mg twice daily 300 mg once daily | EPIVIR | |
| Stavudine (d4T) | 30 mg twice daily | ZERIT | |
| Tenofovir disoproxil fumarate (TDF) | 300 mg once daily | VIREAD | |
| Tenofovir alafenamide (TAF) | 10 or 25 mg once a day, as part of combination therapy | VEMLIDY, component of Descovy, Genvoya, and Biktarvy | |
| Zalcitabine (ddC) | 0.75 mg per 8 h | HIVID | |
| Zidovudine (AZT) | 200 mg three times daily 300 mg twice daily | RETROVIR | |
| Non-nucleoside reverse transcriptase inhibitors (NNRTIs) | Delavirdine (DLV) | 400 mg three times daily | RESCRIPTOR |
| Efavirenz (EFV) | 600 mg once daily | SUSTIVA | |
| Etravirine (TMC125) | 200 mg twice daily | INTELENCE | |
| Nevirapine (NVP) | 200 mg once daily | VIRAMUNE | |
| Rilpivirine (RPV) | 25 mg once daily | EDURANT | |
| Dapivirine (DPV) | N/A—has low oral bioavailability, only used in topical PrEP | N/A (is also known as “TMC-120”) | |
| Protease inhibitors (PIs) | Amprenavir (APV) | 1200 mg twice daily | AGENERASE |
| Atazanavir (ATV) | 150–300 mg daily | REYATAZ | |
| Darunavir (DRV) | 600 mg twice daily | PREZISTA | |
| Fosamprenavir (FOS-APV) | 1400 mg twice daily | LEXIVA | |
| Indinavir (IDV) | 800 mg per 8 h | CIRIXIVAN | |
| Lopinavir (LPV) | 400 mg twice daily 800 mg once daily | KALETRA (In combination with Ritonavir) | |
| Nelfinavir (NFV) | 1250 mg twice daily | VIRACEPT | |
| Ritonavir (RTV) | 600 mg twice daily | NORVIR | |
| Saquinavir (SQV) | 1200 mg three times daily | INVIRASE | |
| Tipranavir (TPV) | 500 mg twice daily | APTIVUS | |
| Fusion inhibitors (FIs; also known as entry inhibitors) | Enfuvirtide (T-20) | 90 mg subcutaneous injection every 12 h | FUZEON |
| CCR5 antagonists (also known as entry inhibitors) | Maraviroc (MVC) | 150–300 mg twice a day | SELZENTRY |
| Integrase inhibitors (IIs) | Raltegravir (RAL) | 400 mg twice a day | ISENTRESS |
| Dolutegravir (DTG) | 50 mg once a day | TIVICAY | |
| Elvitegravir (EVG) | 150 mg once a day, as part of combination therapy | VITEKTA, component of Stribild | |
| Bictegravir (BIC) | 50 mg once a day, as part of combination therapy | (formerly “GS-9883”), component of Biktarvy |
Success of HIV treatment and management has been guided by the availability of cART that uses a combination of two nucleoside/nucleotide reverse transcriptase inhibitors (RTIs) and a drug with different mechanism of action.[15] In 2006, the FDA approved the first “one-pill-daily” tablet Atripla that combined efavirenz (600 mg), emtricitabine (200 mg), and tenofovir disoproxil fumarate (TDF, 300 mg).[16] Atripla has been used as a first line HIV treatment for years until the U.S. Department of Health and Human Services (DHHS) made some changes in 2015 and recommended an alternative treatment with reduced side effects. Current, first line therapeutic regimen includes Stribild (single tablet FDA approved in 2012 as a combination of elvitegravir [EVG, 150 mg]/cobicistat [150 mg]/emtricitabine [200 mg]/TDF [300 mg]),[17] Triumeq (FDA approved in 2014) that combines abacavir (600 mg)/dolutegravir (50 mg)/lamivudine (300 mg) in a single once-daily tablet,[18] and Genvoya (FDA approved in 2015) with EVG (150 mg), cobicistat (150 mg), emtricitabine (200 mg), and tenofovir alafenamide (TAF, 10 mg).[19] How-ever, ART is unable to fully restore immune health in all HIV infected patients. Researchers from Denmark and France have reported that overall life expectancy has significantly increased for HIV+ patients receiving HAART, but it was still lower compared to the HIV-seronegative population.[20] Despite suppressive cART, HIV-infected patients may have incomplete recovery of CD4+ T cell numbers and incomplete resolution of chronic immune activation and experience an increased prevalence of non AIDS diseases such as cancer, osteoporosis, and cardiovascular diseases.[21]
Drug toxicity and the development of drug resistance are major limitations of cART. ARVs usually target either viral or cellular proteins. While drugs directly acting on viral proteins are more specific and have lower toxicity, drug resistance can emerge. Drugs targeting host cellular proteins have a broader spectrum of action and might be less likely to promote development of drug resistance, but tend to have higher toxicity compared to other drugs.[22] The International AIDS Society-USA (IAS-USA) publishes an update on drug resistance mutations in HIV for HIV clinicians.[23] Identification of new viral mutations every year demonstrates limitations of cART. Common side effects include metabolic and liver disorders, muscular dystrophy, peripheral neuropathy,[24] and neurocognitive impairment.[25] Other side effects include constipation and fever that may contribute to the reduced patient compliance and viral rebound. cART can also lead to undesirable drug–drug interactions that might reduce its efficacy. A combination of nevirapine and saquinavir (SQV) presents an example of adverse drug–drug interactions, where nevirapine induced liver cytochrome p450 increasing metabolization and elimination of SQV.[26]
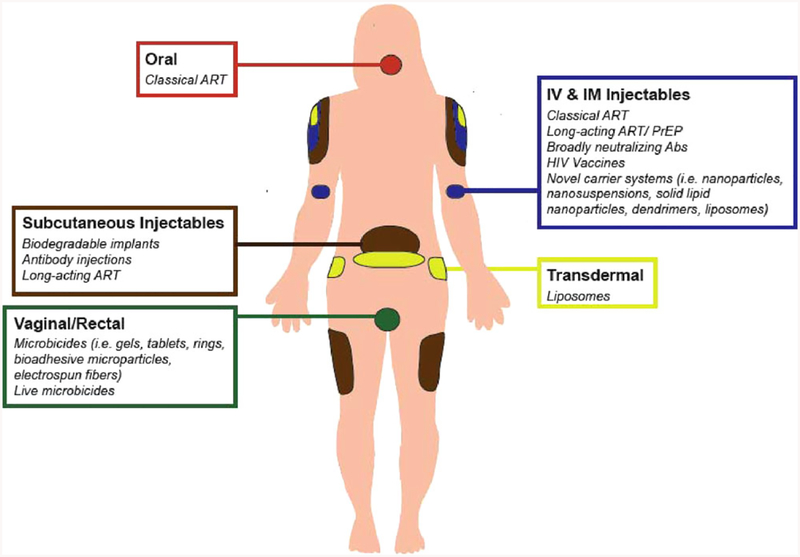
One of the major challenges in ART is the dosing route, as most ARVs have poor solubility and bioavailability. Orally applied solid dosage forms are the most common way to administer ARVs (Figure 2) although they suffer from significant hepatic first-pass effects, and variable absorption and degradation due to enzymes and extreme pH conditions in the gastrointestinal tract, leading to low bioavailability. Frequent dosing (at least once daily) is required as a result of the short half-life of some ARVs, which may cause reduced patient compliance.[27] Furthermore, ARVs may not reliably reach high levels in tissues through the lymphatic system or in the brain across the blood–brain barrier (BBB).[28] Thus, conventional ART fails to target the HIV anatomical (i.e., lymphatic system, central nervous system [CNS], reproductive tract, liver, and lungs) and cellular (i.e., CD4+ T lymphocytes and monocytes, etc.) reservoirs and increases the risk of relapse.[29] Better drug delivery systems and development of new drug molecules with high anti-viral potency and longer half-life would enhance the success of HIV treatment.
Figure 2.
Anti-HIV drug administration routes and delivery systems in the human body.
2.2. New Drugs in Clinical Development
New drug development is focused on minimizing drugassociated toxicity and reducing drug resistance profiles either within the existing ARV classes or with novel molecules with different mechanisms of action, including entry/fusion inhibitors and maturation inhibitors.[15] There are several newdrugs that belong to the previously described ARV classes. Doravirine is a NNRTI that is currently in Phase III clinical evaluation as a single-drug tablet and as part of a doravirine/lamivudine/tenofovir combination tablet.[30] Despite doravirine resistance observed for viruses with the K103N and Y181C mutations in RT, the drug has lower toxicity than efavirenz.[31] Cabotegravir (CAB) is an integrase inhibitor that is in Phase IIb for HIV treatment and Phase IIb/III for HIV prevention. It was reported as orally bioactive with a long halflife.[32] Fostemsavir (BMS-663068) belongs to a relatively newer class known as small-molecule attachment inhibitors (AIs) that bind to gp120, stabilizing a conformation of the viral protein that is unable to recognize host cell CD4 receptors, thus preventing viral entry into the cell; the drug is in Phase III clinical assessment as a later-line therapeutic for heavily treatment experienced (HTE) patients with multidrug-resistant HIV.[33]
For some of these new drugs, long-acting (LA) formulations to be administered as injections are being investigated to improve problems with low drug adherence. The combination of LA CAB and LA rilpivirine (RPV), a non-nucleoside RTI, as intramuscular (IM) injections has progressed to Phase III clinical trials for treatment of HIV-1-infected adults. The most recent study is ATLAS 2M, following up ATLAS, which seeks to compare the efficacy and safety of the combination of CAB LA and RPV LA injections at 4 and 8 week intervals over a 48 week timespan. The estimated date to complete this study is 2022.[34]
Broadly neutralizing antibodies (bNAbs), such as 3BNC117 and VRC01, bind to the HIV envelope (Env) and are among the new candidates for HIV treatment, since they can bind and eliminate infectious viral particles and infected cells. This is also discussed in the context of HIV prevention in Sections 3.1 and 3.2.3. These antibodies (Abs) have been advanced to Phase 1 trials and showed significant HIV suppression, however, there are concerns regarding the development of resistance.[35] Though still in pre-clinical development and testing phase, other potent bNAbs and especially combinations thereof (which will help to combat the selection of strains that are resistant to single bNAbs), are envisioned as future options in HIV treatment (also see Section 3.2.2–Microbicide Candidates for the application of bNAbs in HIV prevention).[36] BMS-936559, an anti-PD-L1 antibody, has been studied in Phase I trials and reported to enhance HIV-1–specific immunity in healthy HIV-1-infected subjects.[37] Pro-140 (developed by CytoDyn) is also an antibody that belongs to the entry and fusion inhibitor class and is currently in Phase IIb/III safety and efficacy trials (since August 2016), administered as a weekly subcutaneous injection of 350 mg. Pro-140 acts by binding to the CCR5 co-receptor on the surface of immune cells and blocking HIV’s ability to infect target cells. Designated a “fast track” product candidate by the FDA, pending success of an ongoing clinical trial, Pro-140 may become the second FDA-approved monoclonal antibody (mAb) therapeutic for HIV,[38] joining the recently approved anti-CD4 mAb ibalizumab (brand name Trogarzo).[39] Bispecific antibodies have also been designed to have two arms that attach to both the target and the effector cells. Bispecific T-cell engagers (BiTEs) and dual-affinity re-targeting (DART) were developed for cancer treatment then redesigned to tackle HIV-1 treatment. HIV-specific BiTEs and DARTs have shown promising results in vitro, however more research is required for evaluation of safety and efficacy of the molecules.[40]
2.3. Delivery Strategies for Anti-HIV Molecules
Current HIV treatments have several shortcomings such as drug resistance, drug–drug interactions, and biological barriers that prevent drug access to potential target tissues. Other drug-related challenges include low solubility, low bioavailability, premature elimination, short shelf life, and off-target side effects. Furthermore, plasma drug concentrations in therapeutic dose ranges are difficult to meet due to the short half-life of these drugs.[41] Phar-maceutical enhancers (boosters) like ritonavir (RTV) and cobicistat (Tybost, FDA approved in 2012) have been used to increase the bioavailability of PIs by inhibiting cytochrome P450 (CYP) 3A enzymes.[42] However, cobicistat interacts with many drugs and can cause life threatening side effects. In addition, there are still questions regarding whether the boosting mechanism itself causes serious long-term side effects.[43]
These issues are being resolved either by discovering new active molecules or targets for HIV treatment, or by improving the delivery of currently available molecules. Several novel drug delivery systems have been tested for delivering anti-HIV agents in order to address the series of concerns:[44]
Increase bioavailability
Decrease metabolization/elimination
Reduce undesired side effects
Improvestabilityandshelflife of drug molecules
Reduce dosing frequency and improve patient compliance
Maintain therapeutic drug level and prevent fluctuation
Improve drug penetration to CNS
Target cells selectively
In this section, we discuss potential targets for anti-HIV drugs, drug targeting strategies, and drug delivery systems that have been studied to enhance HIV treatment.
2.3.1. Targeting Molecules for Drug Delivery
Drug targeting is a relatively a new approach to help optimize the therapeutic index of a drug (efficacy window) by localizing the drug to the site of action. Targeting can help with reducing dosing and related toxicity while increasing the efficacy of the drug. HIV infects immune cells such as macrophages, dendritic cells (DCs), and CD4+ T cells[27b] and these cells should be targeted by HIV therapies.[45]
Targeting anti-HIV drugs to the brain is also a major goal since HIV can migrate and localize in the CNS. The BBB limits ARV delivery to brain due to epithelial tight junctions and the efflux transporters on its surface. Neuronal tissue has bradykinin type II (B2) receptors that are important targets for drug delivery to the CNS. One of the most popular approaches to deliver drugs to the brain involves the use of nanoparticles that can cross the BBB via endocytosis/phagocytosis, coupled with their ability to evade clearance through the efflux systems. Also, the transferrin receptor is an important target to deliver drugs to the CNS since it is highly expressed in the BBB endothelium.[46] Furthermore, the efficiency of nanoparticles in delivering drugs to the BBB can be enhanced with an electromagnetic field, however long-term effects of change in permeability may be detrimental.[47]
Another approach is to target virus (guest) moieties such as envelope glycoproteins gp41 and gp120 regions that are responsible for binding to the CD4+ T cells. Targeting these proteins increases the specificity of the therapeutic approach, but may drive the high mutation rate of HIV creating additional challenges.[48] Following the viral infection, envelope gp120 is exposed on the surface of HIV-infected cells, creating a favorable target to deliver drugs specifically to infected cells. Functionalized liposomes and nanoparticles have been studied for targeting HIV proteins and they were reported to bind gp120.[49] Aptamers were suggested as alternatives to the Abs in order to bind specifically to the target molecules, since they are easier to formulate with their smaller structures.[50] Similar to gp120, gp41 is also a potential target moiety on HIV-infected cells; hence, there have been several studies using anti-gp41antibodies to target HIV-infected cells.[51] Radioactive labeled gp41 Abs were used to target HIV-infected cells in vivo and results showed that the radioactive molecule killed the targeted cells and significantly reduced the HIV load.[52]
The alternative approach to guest targeting is to seek out the host cells, which include HIV-infected cells with unique cellular markers. The most relevant host markers so far are CD4, chemokine receptors (CXCR4 and CCR5), leukocyte function–associated antigen (LFA-1), human leukocyte antigen—antigen D related (HLA-DR), DC-specific ICAM-3-grabbing nonintegrin (DC-SIGN), tuftsin, and carbohydrate binding antigens.[27b] The CD4 and chemokine receptors are expressed on macrophages, T lymphocytes, monocytes, and DCs, and play a role in HIV entry into cells and are utilized for targeting infected cells.[27b] LFA-1 is present in macrophages, neutrophils, and T and B lymphocytes and its expression is increased during HIV infection. Anti-LFA-1 had a double benefit, as it inhibited viral replication while specifically targeting HIV-infected cells.[27b] The HLA-DR is a major histocompatibility complex (MHC) class II protein that is expressed in DCs, macrophages, and B cells. It is an attractive target since it serves as an identifying marker on HIV reservoir cells and attracts CD4+ T helper cells.[53] Liposomes and other nanocarriers have been used to target HLA-DR delivering ARVs to HIV-infected cells and reduce viral spreading.[54] DC-SIGN is a C-type lectin that binds HIV and is one of the major targets for prevention as well as treatment.[55] Cyclodextrin-based glycoconjugates showed high affinity for DC-SIGN and prevented binding to gp120.[56] There are several sugar moieties on the surface of HIV-infected cells that can be targeted by “carbohydrate binding agents.” Mannan and mannose receptors are among the receptors on monocytes and macrophages and may be potential targets for prophylactic protection or to increase the uptake efficiency of anti-HIV drugs. Mannosyl and galactosyl receptors on macrophages have been used for drug targeting via surface modified nanoparticles. Mannosylated gelatin nanoparticles were used for didanosine delivery and showed that ex vivo macrophage uptake was significantly higher when compared to free drug and uncoated nanoparticles.[57] Tuftsin is an immunoglobulin G derivative that binds and activates macrophages and DCs and is a potential target. However, tuftsin targeting is still unexplored— other than reports of tuftsin-dendrimer conjugates for efavirenz delivery.[58] Transferrin, low-density lipoprotein, and aptamers are some of the other potential targets that are subjects of studies for HIV treatment. Increased phagocytic activity of macrophages during HIV infection was also used for passive targeting of drugs such as indinavir, zidovudine, and didanosine to infected cells.[59] Furthermore, RNA interference (RNAi) technology is being developed to silence newly identified targets.[60]
Pharmaceutical scientists have been working on a variety of novel drug delivery systems for delivering anti-HIV molecules to the target moieties that were discussed above. We focus on examples of these carriers in Section 2.3.2.
2.3.2. Novel Drug Delivery Systems for Anti-HIV Molecules
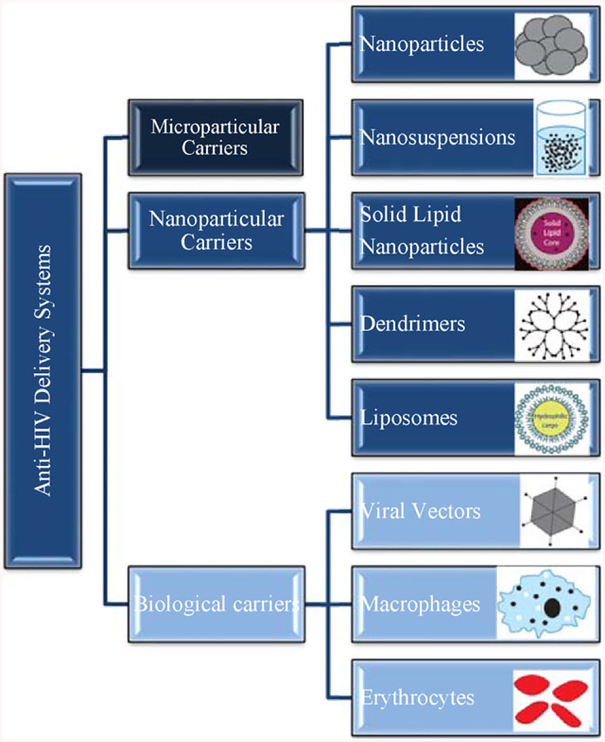
Novel drug carrier systems like microparticles, nanoparticles, liposomes, dendrimers, implants, and drug reservoirs have been studied to overcome the challenges of conventional HIV treatment and prevention. Different delivery routes including longacting injectable formulations were also considered due to the shortcomings of oral delivery of anti-HIV drugs.[61] Sustained transdermal drug delivery can improve bioavailability by overcoming the issues like intestinal absorption, the hepatic first-pass effect, and drug metabolism in the gastrointestinal (GI) track. Percutaneous absorption of ARVs has also been studied and reported to be a promising administration route.[44a,62] A schematic of anti-HIV delivery approaches is presented in Figure 3.
Figure 3.
Schematic of novel anti-HIV delivery systems.
Microparticle carriers have been used for taste masking or controlled drug delivery of ARVs, to reduce dosing frequency and maintain drug plasma levels.[63] Bioadhesive microparticles were designed to prolong retention time of the drugs in the gastrointestinal track and increase drug absorption.[64] Bioadhesive and pH sensitive microparticles have also been studied for vaginal delivery of microbicides for HIV prevention.[65] However there are also some limitations related to the microparticles, such as aggregation and difficulties in handling and storage.[66] Microparticle carriers that have been tested for ARVs are summarized in Table 2.
Table 2.
Microparticle carriers for ARV delivery.
| Drug | Polymer | Results | Reference |
|---|---|---|---|
| Abacavir | Eudragit, ethyl cellulose, HPMC K4M | Controlled release | [67] |
| Efavirenz | HPMC, Carbopol | Enhanced dissolution rate | [68] |
| Enhanced bioavailability | |||
| Indinavir | Eudragit E100 | Controlled release | [69] |
| Taste masking | |||
| Lamivudine | Chitosan | Controlled release | [70] |
| Acrycoat, L30D, S100 | [71] | ||
| Cellulose acetate phthalate, ethyl cellulose | [72] | ||
| Maraviroc | Sodium alginate | Controlled release | [73] |
| Nelfinavir | Cellulose acetate | Controlled release | [74] |
| Stavudine | Ethyl cellulose | Controlled release | [75] |
| Eudragit RS100 | Prolonged gastric retention time | [76] | |
| Sodium alginate | Prolonged gastric retention time | [77] | |
| Enhanced bioavailability | |||
| Zidovudine | Ethyl cellulose | Controlled release | [59b] |
| HPMC | [78] | ||
| Chitosan | [79] | ||
| Eudragit | Prolonged gastric retention time | [80] | |
| Controlled release |
HPMC, hydroxypropyl methylcellulose.
Nanoparticle carriers are solid colloidal particles that range between 1 to 1 000 nm in size and are able to encapsulate drugs. Solid lipid nanoparticles (SLNs) are also nano-sized particulate carriers that consist of biodegradable/biocompatible lipids (such as cetyl palmitate and myristic acid salts) that are solid at physiological temperatures.[81] Both types of nanoparticles have large surface areas that help increase the biological half-lives of drug molecules and reduce dosing frequency. In addition, nanoparticles can encapsulate significant amounts of drugs and improve drug release kinetics,[82] can be internalized by lymphocytes due to their small size and serve as drug depots,[83] and have surfaces that can be modified to target specific cells to increase efficacy and reduce side effects.[84] Nanosystems such as nanoparticles, SLNs, nanosuspensions, dendrimers, and liposomes can help drugs cross the BBB, enter the lymphatic system, or internalize in cells and improve anti-HIV drug delivery efficiency. Advantages of nanoparticles include reproducibility in processing, multiple drug loading (combination therapy), and drug stability.[85] Certain types of nanoparticles can also reverse drug resistance, such as overcoming P-glycoprotein effux in drug-resistant tumors.[86] Most nanoparticle studies have been aimed at targeting cells of the mononuclear phagocytic system or brain, however, several studies have focused on increasing bioavailability of the drugs. We summarize studies that used nanoparticles to improve HIV treatment and the reported outcomes in Table 3.
Table 3.
The use of nanoparticle carriers for anti-HIV drugs.
| Drug | Polymer | Results | Reference |
|---|---|---|---|
| None | Silica | Bindingto HIV-1 gp120 Targeting to the infected cell | [49b] |
| Amprenavir | Quantum rods | Targeting the transferrin receptor Enhanced uptake—in vitro BBB model | [87] |
| Atazanavir | SLNs | Increased BBB permeability | [88] |
| Atazanavir and Ritonavir | Nanosuspension Poloxamer 188 | Neuroprotection in humanized HIV-infected animal model | [89] |
| Delavirdine, Stavudine, and Saquinavir | PBCA, MMA-SPM, and SLNs | Increased in vitro BBB permeability | [47] |
| RMP-7 modified MMA-SPM | Increased in vitro BBB permeability | [90] | |
| Didanosine | Mannosylated gelatin | Increased in vivo macrophage uptake Mannosyl receptor targeting | [57,91] |
| Dolutegravir | DTG prodrugs + Poloxamer nanosuspension | Two weeks of parenteral efficacy | [92] |
| Indinavir | Lipid nanoparticles | Targeting to CD4 binding peptide Increased intracellular concentration High anti-HIV activity in macaques | [93] |
| Lamivudine | PLA/chitosan | Sustained release Protection in gastrointestinal track (in vitro) |
[94] |
| Lamivudine and Zidovudine | PBCA and MMA-SPM | Increased in vitro BBB permeability | [95] |
| Lopinavir, Ritonavir, and Tenofovir | Lipid nanoparticles | 50-fold higher intracellular drug concentration in lymph nodes (in primates) | [96] |
| Nevirapine | PLGA | In vitro transferrin targeting Increased uptake | [97] |
| Nanosuspension Serum albumin, PEG 1000, or dextran 60 surface modifications | Accumulation in brain—in vivo rat model | [98] | |
| Ritonavir | PLA | TAT gene targeting Higher bioavailability Reduced clearance from CNS | [99] |
| Saquinavir | Cyclodextrins | Decreased toxicity on Caco-2 cells | [100] |
| Poly(ethylene oxide) modified poly-e-caprolactone | Higher drug internalization inTHP-1 human monocyte/macrophage cell line Higher intracellular drug concentration | [101] | |
| Quantum rods | Targeting to transferrin receptor Enhanced uptake—in vitro BBB model Decreased HIV-1 replication—in vitro |
[102] | |
| Saquinavir and Zalcitabine | Poly(hexylcyanoacrylate) | Higher efficacy for Saquinavir (induced HIV-1 antigen production) |
[103] |
| Zidovudine | SLN and SLN-PEG | Increased drug concentration in blood with PEGylated SLNs Decreased release rate and prolonged circulation with PEG coating | [104] |
| PACA, PMMA, HSA | Uptake into macrophages isolated from HIV+ patient | [105] | |
| Hexylcyanoacrylate | Higher drug level in liver, blood, and brain following oral administration 18 times higher drug concentration in RES organs following IV administration |
[106,107] | |
| PLA and PLA:PEG | Increased in vitro uptake by polymorphonuclear leukocytes | [108] | |
| Albumin | Targeting to transferrin receptor Increased drug amount in brain cells | [109] |
BBB, blood–brain barrier; HAS, human serum albumin; IV, intravenous; MMA-SPM, methylmethacrylate-sulfopropylmethacrylate; PACA, polyalkylcyanoacrylate; PAMA, poly methylmethacrylate; PBCA, polybutylcyanoacrylate; PEG, poly(ethylene glycol); PLA, poly(lactic acid); PLGA, poly(lactic-co-glycolic acid); RES, reticuloendothelial system; TAT, “Trans-activator of transcription,” an HIV-encoded protein.
Dendrimeric systems have also been explored for anti-HIV drug delivery. Dendrimers are synthetic monodisperse macromolecules with unique tree-like architectures that present several functional end groups for modifications and drug loading.[110] These structures are also called “nanocontainers” since they entrap molecules like a box. Due to their favorable properties, they have been used for controlled release of ARVs as well as targeting by surface modifications. Dendrimers themselves have also been reported as anti-HIV therapeutic agents.[111] Studies using dendrimers for HIV treatment are summarized in Table 4.
Table 4.
The use of dendrimers for anti-HIV drugs.
| Drug | Dendrimer type | Results | Reference |
|---|---|---|---|
| Efavirenz | Fifth-generation PPI | Controlled release with conjugated dendrimers Increased in vitro cellular uptake with mannose-conjugated PPI |
[112] |
| t-Boc-lysine | |||
| Conjugated PPI | |||
| Mannose-conjugated PPI | |||
| Tuftsin-conjugated PPI | In vitro tuftsin targeting | [58] | |
| Lamivudine | PEGylated PAMAM Generations 4 and 5 | Controlled release ofdrug | [113] |
| Lamivudine and Efavirenz | Mannosylated PPI | Mannose targeting Increased uptake in macrophages |
[114] |
| Silver (nano-silver) | Anionic linear globular dendrimer | Significant ARV activity in comparison with Nevirapine | [115] |
| Zidovudine | Sialic acid-conjugated mannosylated PPI | Increased cellular uptake by macrophages In vivo tissue distribution shows efficiency of dual targeting |
[116] |
PAMAM, polyamidoamine; PEG, poly(ethylene glycol); PPI, poly (propyleneimine).
Liposomes are phospholipid-based vesicular carriers (80–100 nm) with a unique structure that can encapsulate both hydrophilic and hydrophobic drugs. Liposomes are easy to surface modify to improve properties or targeting to a specific cell or tissue. Also, PEG-conjugated liposomes are able to avoid recognition by the reticuloendothelial system.[82] Despite disadvantages like poor stability and low encapsulation efficiency, liposomes have been investigated extensively for sustained delivery and targeting of ARVs; various drugs, prodrugs, and conjugates have been encapsulated and tested in vitro and in vivo. Examples of these studies are summarized in Table 5.
Table 5.
The use of liposomes for anti-HIV drugs.
| Drug | Target | Results | Reference |
|---|---|---|---|
| Chimeric HIV entry inhibitor peptide | Two gp41 regions simultaneously | Stabilization of the peptide | [117] |
| Didanosine | - | Longer plasma elimination time Targeting lymph nodes and macrophage rich tissues |
[118] |
| Didanosine glycerolipidic conjugates | - | Enhanced drug bioavailabilityAvoids hepatic first pass | [119] |
| Didanosine triphosphate | Fc receptor | Increased retention time | [120] |
| Indinavir | Anti-HLA-DR | Lower toxicity and immunogenicity in pre-clinical studies | [121] |
| - | Enhanced transdermal delivery (increased in vitro permeation through human cadaver skin) | [122] | |
| P11 | Anti-gp120 | Localization in HIV-1-infected cells—in vitro | [51b] |
| RNAi | LFA-1 | Reduced viral load—in vivo | [123] |
| Stavudine | Mannose | Higher drug concentrations in liver, spleen, and lungs | [124] |
| Galactose | Higher drug concentrations and lower toxicity in liver | [125] | |
| Zalcitabine | - | Extended clearance time in brain tissue in rats (23 h) | [126] |
| Passive targeting (anionic nature) | Rapid uptake by mice macrophages | [127] | |
| Zidovudine | Galactose | In vivo higher drug accumulation and increased half-life in liver No hematological toxicity | [128] |
| Mannose | Higher drug uptake in spleen and lymph nodes | [129] | |
| - | No bone marrow toxicity in mice Enhanced localization in liver, spleen, and lungs |
[130] | |
| Zidovudine 5’-triphosphate | Magnetic targeting across BBB | Increased transmigration across an in vitro BBB model | [131] |
| Zidovudine myristate | - | Higher concentrations in brain and longer elimination time in rats | [132] |
BBB, blood–brain barrier; Fc, fragment crystallizable region; HLA-DR, human leukocyte antigen—antigen D related; LFA-1, leukocyte function–associated antigen.
Biological carriers are used for drug delivery and represent a novel approach and consist of viral vectors, erythrocytes, and macrophages.[44b] HIV-infected macrophages reside in tissues that may not be fully penetrant for ARVs. Based on this idea, a macrophage-based carrier system for indinavir was described.[133] The numbers of HIV-infected cells in peripheral blood, liver, lymph nodes, and spleen were reduced in HIV-1-infected humanized mice. A similar carrier system with the incorporation of Lipoid E80 was prepared and used for sustained indinavir release from macrophages.[134] Chimeric RNA nanoparticles with gp120 binding aptamer showed specific binding to the HIV gp120 expressing cells and internalization to block viral infectivity.[135] In addition, recombinant adeno-associated viral (rAAV) vectors are being actively investigated and developed as vehicles of gene therapy, wherein the rAAV is essentially a protein-based nanoparticle capable of delivering DNA either systemically or to specifically targeted cells (such as skeletal muscle), with consequent expression of a therapeutic gene product.[136] rAAVs are in the developmental and pre-clinical testing phase for gene transfer of anti-HIV bNAbs, with the goal of achieving stable long-term transgene expression and secretion of bNAbs, both for HIV treatment and prevention purposes.[137] If successful, rAAV-mediated antibody gene transfer could eliminate the need for repeated passive infusions of bNAbs in HIV treatment and prevention, by creating a constant, long-term source of therapeutic levels of potent bNAbs in the human body.
Other novel approaches:
In a recent study, albumin-polymerdrug conjugates have been designed to prolong drug residence time and increase lymphatic accumulation of the ARV drugs. Albumin was conjugated to N-(2-hydroxypropyl) methacrylamide (PHPMA) and the conjugate was combined with azidothymidine and lamivudine through copolymerization. The results of in vivo studies showed that albumin-PHPMA-ARV conjugates are able to deliver potent drugs and drug combinations with an increased biodistribution profile.[138] Another study evaluated transdermal films composed of ethyl cellulose and HPMC for delivery of a potent NNRTI IQP-0410 to prevent extensive first-pass metabolism the drug was subject to.[139] The films were found promising car-riers based on in vitro studies, however no in vivo studies were conducted to show the efficacy of the system.
Despite decades of research, discovery of new targets and studies on numerous novel formulations, none of these approaches has yet reached the clinic. However some of these approaches have already demonstrated success in in vivo animal experiments and are currently being further investigated in animal models prior to entering clinical trials.[140] As the search goes on for an alternative treatment for better life quality, there has been a substantial new investment in HIV cure research from governments, foundations, and industry in the past decade. Clearly there are still challenges ahead and much work is needed but if the scientific success of targeting HIV latency can be brought to clinic effectively, an HIV cure may replace lifelong ART.[141]
2.4. Targeting HIV Latency
As noted, cART effectively suppresses the virus but does not eradicate it. In particular, HIV provirus exists in so-called reservoirs, where it reactivates from latency if treatment is suspended, allowing viral rebound.[142] Latency upon HIV infection has been linked to several causes, including insufficient or mutated HIV Tat.[143]
The main latent viral reservoir is generally believed to reside in resting memory CD4+ T cells.[144] However, T follicular helper cells (Tfh) have also been proposed as a major celular reservoir,[145] and non-T cell reservoirs have also been suggested.[146] The cells comprising the reservoir can be found in many areas of the body, particularly the peripheral blood, lymph nodes, and the gut.[147] An additional subject of debate is whether low-level replication continues during cART,[148] leading to the possibility that both latency and low-level replication contribute to HIV persistence.
Elimination of the HIV reservoir is among the highest priorities in HIV research, since removal of this obstacle could lead to a cure. Several strategies have been proposed to eliminate the reservoir. The most studied has been the so-called “shock and kill” strategy,[144c,149] in which a drug is added to latently HIV infected cells that allows activation of transcription (the “shock”), leading to active replication of the HIV genome within the cell. In vivo, this would in turn lead to cellular responses that would allow recognition by cytotoxic immune cells, possibly with the help of additional immune activators, mediating elimination of the infected cell (the “kill”). A second strategy to eliminate the reservoir is to “lock” cells into their latently infected state so that HIV is not transcribed and never re-emerges, even if cART is suspended or stopped completely. Some recent success has been shown for this latter “locking” technique.[150] Finally, the DART strategy has shown success by covalently pairing an antigp120 antibody fragment with an immune activating antibody fragment. This molecule binds the gp120 on the surface of an infected cell and brings it into proximity to an activated immune cell for elimination. However, most DART approaches must be combined with latency-reversing agents (LRAs) because latently infected cells have little or no gp120 on their surface.[151]
Several strategies have evolved for “shock and kill,” with probably the most work being reported on histone deacetylase inhibitors (HDACi). These small molecules are effective reactivating agents, and indeed several different HDACi do activate HIV transcription in cells, including vorinostat, panobinostat, and romidepsin. However, these did not reduce the viral reservoir,[152]and it is possible that the dosages required for low toxicity limit their effectiveness to activate the cells. Other strategies are also being tested as LRAs, including agonists of protein kinase C (PKC);[153] agonists of Toll-like receptor;[154] and immune checkpoint blockade antibodies.[155] These have shown promise, and in particular it has been shown that PKC agonists are able to reactivate cells with several different models of latency.[155] Clinical trials are ongoing with several of these.[156] Finally, HIV Trans Activator of Transcription (Tat) has been shown to reverse latency in HIV reservoirs, either added as a protein or expressed within a cell,[157] although these studies did not target Tat to particular cells; Tat is able to enter cells nonspecifically but is toxic at high concentrations.[158]
3. HIV Prevention
For HIV prevention and treatment to be effective at the population level, individuals need to adhere to the drug usage and dosing regimens in a consistent manner. This poses a great challenge, as demonstrated by the outcomes of many clinical trials that have reported low adherence as shown by low plasma or local drug concentrations. Inconsistent use lowers the apparent efficacy of the drug, which can worsen patient outcomes. There is increasing interest in how users perceive products, termed “user sensory perceptions and experiences,” and how this correlates with adherence to product use.[159] These are important consider-ations for improving acceptability and increasing the efficacy of products for HIV prevention. New systems are needed that are effective, affordable, and convenient to improve user consistency in HIV prevention.
3.1. HIV Vaccines
An effective HIV vaccine would alleviate the burden placed on users to properly and consistently use a product over time to maintain protection. However, despite significant efforts in the past decades, the development of a successful HIV-1 vaccine remains elusive. Correlates of immune protection for HIV vaccines have not been fully defined and variability in HIV genomes present challenges for HIV.[160] Most vaccines allow infection in the portal of entry, but the immune system subsequently neutralizes the infection before it spreads systemically. HIV integrates into the host’s genome, and can exist latently for long periods of time without being detected by the immune system. Once HIV has entered the systemic circulation, viral reservoirs are established quickly in lymphoid tissues and prohibit complete viral eradication despite long-term ART. Therefore, much of the research effort has focused on preventing HIV entry.[160b,161]
The majority of new HIV infections occur via sexual transmission[162] through mucosal tissue in the genital and rectal areas. Thus, mucosal immunity is an important consideration in rational vaccine design, as it is the first line of defense for the body. Mucosal vaccines have been explored, but thus far have not been shown to work better than systematic vaccines. It is thought that a combination of a systematic and mucosal vaccine may result in increased protection compared to either one alone.[163] Mucosa-associated lymphoid tissue (MALT) are the sites where mucosal vaccines can be administered, as this tissue is functionally connected throughout the body.[163] For instance, nasal-associated lymphoid tissue and gut-associated lymphoid tissue are potential sites for induction. However, not all sites provide the same immunity, due to unique local environments of the mucosa.[164] Both human and non-human primate (NHP) studies have identified immune correlates of mucosal protection. These correlates involve both the cellular and humoral arms of the immune system, so the mechanism is not fully clear.[163] NHP and other models have been used to study mucosal vaccines and have shown some success. One study in sheep showed successful elicitation of high levels of antigen-specific mucosal IgA and large amounts of local antigen-reactive B cells after intramuscular injection and prolonged intravaginal administration (via ring) of 167 μg of both CN54gp140 protein and R848 adjuvant.[165] In a mouse study, they demonstrated that immunization with recombinant influenza–HIV vectors via combination intranasal and intravaginal administration resulted in localization of HIV-specific tissue-resident memory CD8 T cells in the vaginal mucosa.[166] Another study involving rhesus macaques, published in 2018, showed that combination adenovirus and protein vaccines were effective in increasing protection in intrarectal challenges.[167] After three intrarectal challenges, all the ten controls were infected, while only three and four out of ten were infected in the two respective vaccinated macaque groups. These studies show some success resulting from mucosal vaccination in non-human models. Mucosal vaccines are in the early stages of human clinical trials, which will give important information about how their safety and elicited immune response differs in humans compared to other models.
Systemic vaccines are further along in development and testing. To date, the most successful vaccine trial has been the Phase III HIV-1 vaccine trial RV144 in Thailand where a 31.2% efficacy in preventing HIV-1 infection compared to placebo was reported.[168] The vaccine was composed of a recombinant canarypox vector vaccine (ALVAC) and a recombinant gp120 subunit vaccine (AIDSVAX B/E). ALVAC was given at weeks 0 and 4. ALVAC/AIDSVAX B/E was given as two booster injections at weeks 12 and 24. Building on the partial success of this study, there is now a Phase IIb/III vaccine trial HVTN 702 in South Africa in which the vaccine regimen in RV144 has been modified. The HVTN 702 vaccine will consist of ALVAC and a two component gp120 subunit vaccine, modified to be specific for HIV-1 subtype C.[169] Additionally, the vaccine includes the adjuvant MF59, which is different from that used in RV144, to induce a greater immune response. The results from this trial are expected in 2020 or 2021.
As mentioned earlier, combining systematic vaccines with other prevention modalities could increase overall protection. We have already discussed the rationale for pairing mucosal vaccines with systematic vaccines. Along a similar line of thinking, combination with ARVs (oral pre-exposure prophylaxis [PrEP] or microbicides) could be useful. It is important to provide protection at the portal of entry to reduce acquisition of the virus. A macaque study demonstrated vaccine and microbicide combinations increase protection against transmission.[170] Two separate experiments were performed, in which either the fusion inhibitor T 1249 or CCR5 inhibitor maraviroc (MVC), applied vaginally at a partially protective concentration, was paired with a T cell–based adenovirus (Ad) vectored vaccine (Ad26/Ad5HVR48 expressing Gag-Pol-Env-Nef) delivered intramuscularly. The T-1249 experiment was the first to be done, and it was paired with a vaccine aimed primarily at reducing post-infection viral loads, thus not expected to prevent infection. This resulted in only two of the six animals in the combination vaccine and microbicide group remaining uninfected following high-dose vaginal challenge with SIVmac251. In the infected vaccinated animals, viral loads remained about tenfold lower than in the control group, with the best outcome observed with the vaccine–microbicide combination. When the vaginal MVC gel was tested in combination with a more potent Ad vaccine (Ad35/Ad26 expressing Gag-Pol-Env) delivered intramuscularly, increased efficacy was observed against high-dose vaginal challenge with SHIV-SF162P3 with intervention efficacies of 43% and 67% for the MVC only and combination MVC plus vaccine groups, respectively. In the infected animals, post-infection viral loads were also significantly reduced with better virologic control obtained with MVC plus vaccine than with MVC only. In both experiments, the combination of vaccine and microbicide provided the best outcome.[170] In another NHP study, the combination of a partially effective dose of 1% tenofovir vaginal gel with an intranasally and intramuscularly administered Env-based vaccine was tested against 12 consecutive low-dose SHIV-SF162P3 vaginal challenges.[171] The vaccine alone provided no protection, and even resulted in a greater infection rate than the control group. The tenofovir group had a 45% and 68% reduction in risk of infection compared to the control and vaccine groups, respectively. Whereas in the combination vaccine–microbicide group 79% and 88% reduction in the per-exposure risk of infection was observed compared to the control and vaccine groups, respectively. Additionally, the protected animals from the combination group were challenged another 12 times afterward, in the absence of the microbicide. The total risk reduction over the 24 exposures for this group was 91%. These studies showed that combining a microbicide with a vaccine provides increased levels of protection compared to either alone.[170,171]
3.2. Pre-Exposure Prophylaxis
In the absence of an effective HIV vaccine, PrEP has emerged as a viable option to help curtail the spread of the virus in the global HIV/AIDS pandemic.[172] PrEP is an HIV prevention strategy wherein HIV-negative individuals ingest (oral PrEP), apply topically (microbicide), or inject (injectable PrEP) anti-HIV compounds prior to exposure to the virus. These compounds become locally or systemically distributed throughout the human body and can reduce the risk of infection, preventing viral dissemination.
3.2.1. Oral Systemic PrEP
ARV-based oral PrEP has been shown to be effective.[172a–c,173]The first FDA-approved drug for oral PrEP is Truvada, which contains two NRTIs, tenofovir and emtricitabine. Clinical trials have primarily been conducted with tenofovir alone or with tenofovir and emtricitabine combined.[172a–c] The Phase III iPrEx trial in six countries with 3 324 MSM participants concluded that the combination of TDF and emtricitabine (FTC) resulted in a 44% reduction of HIV infection rate compared to the placebo.[172a] Higher efficacy was observed in users that were more adherent. In Botswana, the Phase III clinical trial TDF2 studied the effectiveness of TDF-FTC (F/TDF) taken once daily in 1219 heterosexual men and women. It showed an efficacy of 62.2%.[172c] The Partners PrEP Phase III clinical trial in Kenya and Uganda examined the efficacy of TDF alone and F/TDF in 4747 heterosexual serodiscordant (in which only one partner has HIV) couples. TDF alone showed a 67% reduction in HIV transmission compared to the placebo, whereas, F/TDF had a 75% reduction.[172b] The FDA-approved Truvada for use in 2012.[174] The number of people taking Truvada in the United States has greatly increased between 2012 and 2016, by about six times the original number.[175]
Recently, considering the negative effects of Truvada on bone mineral density and the renal system, there has been optimism about emtricitabine combined with TAF, called F/TAF, as an alternative. TAF has been shown to be more stable and potent, thus it requires a lower dose and has lessened side effects compared to TDF.[176] There are two ongoing clinical trials of F/TAF. One of the trials is CONRAD 137, which is Phase I and has 72 women enrolled. They are studying the pharmacological properties and bioavailability of F/TAF[177] using two different dosages, 200/10 mg and 200/25 mg, with Truvada (F/TDF) 200/300 mg as a control. The other ongoing study is the Phase III DISCOVER trial. It has 5400 enrollees from the MSM and transgender women (TGW) who have sex with men populations.[178] DIS-COVER is testing the difference in seroconversion rates between F/TDF and F/TAF.
Besides F/TDF and F/TAF, other drugs with various mechanisms of action have been investigated for oral PrEP use. Raltegravir (RAL), which is an integrase inhibitor, was shown to be effective in preventing infection in humanized mice challenged vaginally with HIV. An ongoing clinical trial ( NCT03205566) is testing the capability of RAL to protect against HIV challenge ex vivo.[179] MVC, a CCR5 antagonist, was also tested in the same humanized mouse model and found to be effective in preventing infection.[180] However, results differed when tested in both NHPs and humans. In one study, macaques were challenged weekly with SHIV rectally.[181] Twenty-four hours before challenge and 2 h post-challenge, the macaques were given a 44 mg kg−1 MVC dose orally (higher than human dose, but equivalent due to faster drug elimination in small mammals). Even though rectal concentrations of MVC were much higher than the concentration needed to block SHIV in vitro, five out of six macaques became infected during the five weekly challenges. While three out of four controls became infected under the same challenge conditions. Additionally, despite high, sustained concentrations of MVC in a human trial consisting of 54 healthy people given a 300 mg MVC dose, no protection was observed against ex vivo challenge of vaginal or rectal biopsies.[182]
3.2.2. Topical PrEP
In addition to oral dosing to prevent or reduce transmission at the mucosal portal of viral entry, anti-HIV drugs have also been formulated for topical vaginal and/or rectal application.
Microbicide Candidates.
A wide range of compounds have been proposed and tested as potential microbicides to prevent HIV infection at sites of mucosal transmission. Although some of the earliest microbicides failed to show adequate protection in humans,[183] there are many promising candidates currently in the pre-clinical study stage and in clinical trials.[184] Micro bicides fall into several different categories including: vaginal milieu (pH) protectors, surfactants, polyanionic polymers, small molecules (entry inhibitors), RTIs, integrase inhibitors (IIs), protease inhibitors (PIs), proteins, and peptides. Vaginal milieu protectors, surfactants, and polyanions are collectively termed “nonspecific agents” given their mechanisms of action in preventing viral transmission: milieu (pH) protectors help to maintain normal vaginal acidity (pH [2264] 5), creating an unfavorable environment for HIV infection,[185] and serving to help destroy bacterial species that cause bacterial vaginosis (dysbiosis); vaginal dysbiosis is associated with increased HIV transmission susceptibility in women.[186] Polyanions (negatively charged) interact with the positively charged V3 loop of HIV-1 gp120, disrupting its interaction with cell receptors,[187] while surfactants solubilize the membranes of bacteria and enveloped viruses (such as HIV), rendering them inactive.[184d] However, in the earlier stages of vaginal microbicide research, numerous clinical trials (including through the Phase III stage) were conducted with these nonspecific agents, demonstrating either a lack of efficacy in preventing viral transmission[184b] or, in the case of the spermicidal surfactant Nonoxynol-9 (N-9), actual increased risk of HIV infection in users due to inflammatory responses in the cervicovaginal mucosa, including ulcer formation.[188]
Following the lack of success with nonspecific agents, the focus shifted to the development of formulations containing ARV-based compounds as microbicides. RTIs such as tenofovir (and its prodrug form, TDF) and dapivirine (DPV) have emerged at the forefront of successful microbicidal device formulation and development.[184b] In addition, the small molecule HIV entry inhibitor, MVC, is also seeing increased inclusion in microbicide devices. Although MVC on its own has shown limited efficacy in pre-clinical studies[189] it is a promising component in combinatorial strategies.[190] Other small molecule entry in-hibitors in development include BMS-378806 and its derivatives, which act by binding gp120 and preventing a conformational change that is necessary for its interaction with CD4.[191] Other small-molecule ARVs in development as microbicides include the NNRTI UC781,[192] several protease inhibitors (PIs, including SQV, darunavir [DRV], lopinavir [LPV], and RTV)[193] and several integrase inhibitors (IIs, including RAL, EVG, and L-870 812).[194] Presentation of clinical studies involving these compounds is given in the proceeding sections for the particular formulation formats in which they have been tested.
In addition to small-molecule compounds, biomolecular (protein and peptide) microbicides have also made progress through the microbicide development pipeline.[184d] These particular microbicide candidates typically function as entry inhibitors by binding to Env or to HIV co-receptors. These biomolecular microbicides typically exhibit low systemic absorption, low toxicity, and reduced side effects.[195] Carbohydrate binding proteins (CBPs, also known as “lectins”) such as scytovirin, cyanovirin, and griffithsin, recognize glycans on the surface of HIV-1 gp120 and are endowed with remarkable anti-HIV activity against a broad range of subtypes and strains.[196] Among these lectins, the homodimeric protein Griffithsin (“Grft”), derived from the red algae Griffithsia sp., has emerged as a potent (at subnanomolar concentrations) and broad-spectrum anti-viral microbicidal candidate[197] that can be produced inexpensively in large quantities.[198]
Other prime candidate protein microbicides include analogs of the chemokine RANTES that potently block CCR5, the primary co-receptor used by HIV to enter and infect human cells.[199] Like Griffithsin, 5P12-RANTES (5P12R) can also be produced inexpensively in large quantities,[200] and has a favorable stability profile over a wide pH range and at elevated temperatures for certain periods of time, as well as demonstrated stability in the presence of both human semen and cervicovaginal lavage.[201] Peptides that mimic HIV gp41 and act as potent fusion inhibitors of the virus, have found use in HIV treatment (such as T-20/Enfuvirtide, see Table 1 above) and several are also being investigated for use as microbicides, including RC-101, T20, T1249, C34, and L’644.[202] It is also noteworthy that chimerization of both 5P12R and Grft, with C-type peptide fusion inhibitors has produced microbicidal candidates with even greater potency and breadth of viral strains inhibited than that exhibited by any single “parent” component,[203] demonstrating the amenability of biomolecular candidates to even greater optimization as microbicides. In addition, some monoclonal antibodies (mAbs) isolated from HIV-positive individuals have demonstrated potent and broadly neutralizing activities against HIV, that have led to their inclusion and development as microbicide candidates, including antibodies such as: b12, 2F5, 2G12, 4E10 (these four are “firstgeneration” bNAbs)[204] and VRC01, 3BNC117, and 10–1074 (all “second-generation” antibodies), for instance.[205] In addition to VRC01, 3BNC117, and 10–1074, other generations of more potent (compared to “first-generation”) bNAbs have been discovered in recent years (including the PG and PGT series[204,206]) and combinations of these have been proven to be remarkably potent and broadly acting.[36b,207] These can be envisioned as possible options in HIV prevention or treatment. Many mAbs can now be expressed via recombinant DNA modes and produced in large quantities.[208] The status of pertinent pre-clinical studies or clinical trials for these biomolecular microbicides is given below under the relevant delivery formats in which they have been evaluated.
Topical Vaginal and Rectal Microbicidal Delivery Systems.
Equally important as the microbicidal agents themselves is consideration of the vehicles by which they will be delivered. Important properties of a delivery system include: microbicide stability within the format, dispersal of the compound from the system, duration of drug release and dosage, impact of the formulation on the mucosal environment and vice versa for mucosal safety, as well as user acceptability of the format. Below, we describe some of the main delivery systems studied to date for topical PrEP and also highlight some of the benefits and limitations of each format (also summarized in Table 6).
Table 6.
Delivery formats for anti-HIV microbicides.[209]
| Delivery format | Advantages | Limitations |
|---|---|---|
| Gels (semi-solid), rectal and vaginal |
|
|
| Vaginal tablets and films |
|
|
| Vaginal rings (VRs) |
|
|
| Nanoparticles (NPs) |
|
|
| Electrospun fibers (EFs) |
|
|
| Live microbicides (commensals) |
|
|
GMOs, genetically modified organisms.
Vaginal and Rectal Gels.
The first and earliest microbicides to be evaluated were formulated as gels intended for daily use. Indeed, semi-solid gels have been the most common and prolific formulation format for delivery of HIV microbicides.[210] These gels are semi-solid polymeric matrix formulations generally composed of cellulose derivatives (such as hydroxyethylcellulose [HEC] or cross-linked polyacrylic acid [Carbopol] polymers), with the active compound usually being dispersed throughout the interstitial liquid phase.[211] Topical vaginal (or rectal) gels have the advantages of being easily administered by the user (though an applicator may be required), low cost of production, rapid dispersal of the active compound to coat the mucosal compartment (conferring protection either immediately or within a few hours of application), and attainment of higher localized drug concentrations than those achieved through systemic approaches. On the other hand, limitations include the vulnerability of active compounds in semi-solid formulations to extreme conditions of temperature and humidity, and the necessity for pre and/or peri-coital dosing; the self-cleansing and flushing nature of the orifices in question results in rapid removal of the active compound from the site of application, thus necessitating more frequent application of the microbicide. Another significant disadvantage of conventional gels is the leakage and potential discomfort for the user.[159b,209a,b]
Following several failed clinical trials involving gel formulations of “nonspecific agent” microbicides, the first positive results in microbicide clinical trials came from the NRTI tenofovir formulated as a 1% gel in HEC. The CAPRISA-004 (Center for the AIDS Program of Research in South Africa) Phase IIb efficacy trial was conducted from May, 2007 to March, 2010 among women (ages 18–40) in South Africa with a dosing regimen called “BAT24”—one application of the gel within 12 h Before intercourse, followed by a second dose within 12 h After, with no more than Two doses in 24 h.[212] The tenofovir gel used in this study reduced HIV infection by 39% overall, and by 54% in women with higher adherence (>80%) to the dosing regimen. Contemporary to CAPRISA-004, another Phase IIb safety and efficacy trial was conducted in sub-Saharan Africa, sponsored by the Microbicide Trials Network (MTN-003)—VOICE (Vaginal and Oral Interventions to Control the Epidemic)—which comprised arms evaluating the efficacy of oral PrEP (Truvada) and of 1% tenofovir gel (with a once daily coitally independent dosing). The results of this trial concluded that the interventions were ineffective against HIV infection due to poor adherence among participants; in fact, the gel arm of the study was prematurely terminated in 2011 due to futility.[213] A Phase III safety and efficacy trial, FACTS-001 (Follow-on African Consortium for Tenofovir Studies), commenced in 2011 in South Africa to evaluate the 1% tenofovir gel with the same “BAT24” dosing regimen as had been employed in CAPRISA-004; the FACTS-001 study concluded that participants were not using the microbicide gel, but that there was correlation between higher user adherence to the dosing regimen and stronger protection against the virus.[214] Thus, user adherence to dosing regimens (and increasing this adherence through better microbicide delivery device design) is of critical importance in the success and efficacy of these microbicides.
In addition, a 1% tenofovir gel has also been evaluated in clinical studies for rectal application. Topical rectal microbicides were initially based upon formulations for the vaginal tract. However, the rate of progress in this particular field is expected to increase due to recognition of fundamental physicochemical differences between the vagina and rectum, with consequent re-formulations specifically for the rectal tract.[215] A topical 1% tenofovir gel for-mulated for rectal application has successfully passed through Phase I safety and pharmacokinetics evaluations in the United States (the MTN-007 and CHARM-01 trials),[216] as well as an extended safety Phase II trial (MTN-017) among international participants.[217] The NNRTI UC781 formulated as a gel has been successfully evaluated in a Phase I safety and acceptability trial as a rectal microbicide (RMP-01/MTN-006),[218] and UC781 micro-bicide gel also retained anti-HIV activity in cervicovaginal lavage fluids collected from women in a Phase I clinical safety trial.[192] Poor solubility and stability issues of the ARV DPV stymied early efforts in its formulation and development as a topical gel, however, micronization of the active compound has allowed its successful formulation in this particular delivery format including its progression into Phase II clinical trials.[209e] Importantly, both UC781 and DPV were abandoned as orally administered drugs for HIV treatment due to their poor systemic absorption, and thus as microbicides, would be orthogonal to drugs currently used in HAART.
Due to the short retention time of gel formulations at the site of application, efforts have been made to increase formula retention and drug residence times through addition of mucoadhesive polymers such as chitosan, carrageenan, or sodium alginate[219]. More recent developments also include expansion of the gel delivery format to include biomolecular microbicides, such as the lectin Griffithsin, the CCR5-targeted HIV entry inhibitor 5P12 RANTES, and anti-HIV bNAbs including VRC01, to name a few. Following successful pre-clinical studies in animal models,[220]a Phase I clinical trial (sponsored by the Population Council) is currently recruiting participants for safety and pharmacokinetics evaluation of a vaginal Griffithsin gel in women (ClinicalTrials.gov Identifier: NCT02875119, with expected completion date in late 2018), and evaluations of a rectal Griffithsin gel are ongoing as part of PREVENT (“Pre-exposure prevention of viral entry”), an integrated pre-clinical/clinical program. The proteinbased HIV entry inhibitor 5P12-RANTES (5P12R) has recently undergone further successful pre-clinical evaluation as a vaginal gel in sheep[221] and has also shown stability in human rectal lavage.[222] According to the Mintaka Foundation, human clinical trials involving the 5P12-RANTES microbicide are expected to begin soon in Geneva, Switzerland. In addition to these examples, pre-clinical studies of the gel-formulated bNAb VRC01 demonstrated protection against HIV vaginal challenge in a humanized mouse model.[205] There has also been a Phase 1 clinical safety and pharmacokinetics trial of a gel (MABGEL) containing a cocktail of three different bNAbs (2F5, 4E10, and 2G12), demonstrating the safety of a daily dosing regimen for a duration of 12 days, as well as potentially sufficient concentrations of bNAbs to block HIV infection.[223]
Vaginal Tablets and Films.
While gels were initially the pharmaceutical dosage form of choice for topical microbicides, due to certain drawbacks of this particular format, other dosage forms also emerged as viable candidates, including vaginal tablets and films. Vaginal tablets offer advantages of precise dosing, easy storage, handling, and application, as well as low cost and stability under different environmental conditions.[210] Conventional fast-dissolving tablets have been formulated containing a number of the same ARV-type microbicides as have been formulated in gels.[224] As in the case of gels, efforts have also been made to prolong the retention time of vaginal tablets and their contained active compounds at the site of application, through inclusion of mucoadhesive polymers in the formulation;[219c] inclusion of a combination of different polymers (hydroxypropylmethyl cellulose and chitosan) has helped to create a tenofovir-releasing tablet that remains adhered to the vaginal mucosa for 96 h, with sustained release of the drug for 72 h.[225] Another more recent development in vaginal tablets and an example of a multipurpose prevention technology (MPT), is the design of a multi-layered tablet capable of simultaneously releasing (at independent rates) the NNRTI DPV, the contraceptive hormone levonorgestrel, and the anti-herpes simplex 2 (HSV-2) drug, acyclovir.[226]
Vaginal films are small, thin sheets of water-soluble polymers that dissolve when placed in contact with the vaginal mucosa, quickly releasing the active compound. Like the tablet format, vaginal films also provide consistent dosage, ease of storage, handling, and application, low unit cost, and improved stability of actives under more extreme temperature and humidity conditions;[227] one drawback of the film format is low overall mass, limiting the amount of an active compound that can be loaded into a single dose. Vaginal quick-dissolve films are usually composed of cellulose derivatives or polyvinyl alcohol.[210] As with other dosage forms, ARVs have also been incorporated into films, including the NNRTI DPV;[228] this film was the subject of a Phase I safety and pharmacokinetics trial (FAME-02) demonstrating safety of the device, but produced much lower effective concentrations of the drug in situ relative to what can be achieved by dapivirine in the gel format.[209e] Polymeric vaginal films containing different dual combinations of ARVs (tenofovir, MVC, and DPV) are also under development; these cellulose/polyvinyl alcohol–based films have demonstrated stability of the contained active compounds for 12 months at ambient temperature.[229]
In the production of conventional vaginal films, once an active compound has been incorporated into the film polymer base, the drying process may occur at room temperature or under vacuum, allowing inclusion of more labile types of drugs in this format, including biomolecules. Developments include formulation of peptide RC-101 (a retrocyclin analog that targets gp41) in a polyvinyl–alcohol vaginal film,[202b] and also formulation of the bNAb VRC01 combined with the anti-HSV mAb HSV8-N into a vaginal film; this latter device will be evaluated in a Phase I safety and pharmacokinetics clinical trial that is currently recruiting participants, with anticipated trial completion later in 2018.[230] In addition to conventional film formulations, development has begun more recently of vaginal and rectal suppository films composed of silk fibroin (SF) from the Bombyx mori silkworm; encapsulation of protein-based microbicides in the SF film format has demonstrated outstanding stabilization of the active compounds (protein microbicides Griffithsin [Grft], 5P12 RANTES [5P12R], and their chimerized variants Grft-linker-C37 and 5P12R-linker-C37), as well as the capacity for sustained release (over the course of 1 month) of Griffithsin.[231] Note that silk fibroin is a highly versatile substance for therapeutic delivery that can also be processed and fashioned into other delivery formats than films, including nanoparticles and electrospun fibers (Table 6), injectable hydrogels and microspheres, implantable tubes and porous scaffolds, and microneedles for transdermal delivery of therapeutics.[232]
Vaginal Rings.
In contrast to vaginal gels, tablets, and films, vaginal rings are insertable devices intended to be worn continuously and are capable of providing sustained and controlled release of an active (microbicidal) compound over the course of several weeks or months, and are thus coitally independent. Early development of vaginal ring technology (beginning in the 1970s) focused primarily on delivery of steroidal compounds for contraception and hormone replacement therapy.[233] Conven-tional vaginal rings (VRs) are comprised of hydrophobic elastomeric polymers such as silicone (polydimethylsiloxane, PDMS) or poly(ethylene-vinyl acetate) copolymer (PEVA), with the simplest VR designs either containing the drug homogeneously dispersed throughout the matrix system, or else located in a drugloaded central core that is covered by a non-medicated polymer membrane.[234]
Sustained release of a microbicide from a silicone elastomer VR was first demonstrated for the first-generation nonionic surfactant-type compound Nonoxynol-9 (N9),[235] however, further development of this device was abandoned following results of clinical evaluations of the gel-formulated active compound that revealed the unsuitability of N9 for mucosal use. Subsequent development of VRs focused on incorporation of ARVs, such as the NNRTI DPV[236] and the NRTI tenofovir.[237] Importantly, conven-tional VRs typically require high (130–190 °C) processing temperatures to create polymeric elastomer devices; fortunately, many of the ARVs examined thus far (including MVC, tenofovir, and NNRTIs UC781 and DPV) have been sufficiently stable to survive temperatures of ≈170 °C for several minutes while the drug is being incorporated into the polymer melt.[234a]
Thus far, DPV is the only candidate microbicide that has been tested in humans—successfully through the Phase III clinical trial stage–in the vaginal ring delivery format. Two Phase III clinical trials were conducted among women (ages 18–45) in subSaharan Africa, involving once-monthly placement of a ring containing 25 mg of DPV, and completed in 2015: “The Ring Study” (IPM-027, sponsored by the International Partnership for Microbicides) and “ASPIRE” (“A Study to Prevent Infection with a Ring for Extended use,” also known as MTN-020 and conducted by the Microbicide Trials Network). The Ring Study (IPM-027) was successful, reporting a 31% reduction in HIV acquisition among participants.[238] The larger ASPIRE study reported an overall 21% reduction in viral transmission, as well as a significant difference in adherence and accompanying protection between age groups, with an adherence rate of over 70% and a 56% protection rate among women older than 21, and low adherence and no protection conferred in the 18–21-year-old group of women.[239] Building upon the results of the ASPIRE trial, in 2016 the NIH announced plans to move forward with an open label extension study of the DPV ring, “MTN-025–HOPE” (HIV Open label Prevention Extension), wherein ASPIRE participants have been offered the opportunity to continue ring use until the trial’s completion date later in 2018; the study will collect data on safety and user adherence and acceptability of the DPV ring. A similar open-label extension trial for participants of “The Ring Study,” named “DREAM” (IPM-032), has also begun in subSaharan Africa. Preliminary results from the Phase 3b “HOPE” trial (presented at the 2018 Conference on Retroviruses and Opportunistic Infections [CROI]) indicate that nearly 90% of participants are using the monthly ring at least some of the time, suggesting that adherence within the HOPE trial is roughly 16% higher than it was in the earlier ASPIRE trial, and the incidence rate of HIV-1 infection among consistent users has been half that expected for ring non-users.[240] Final results from the HOPE trial are expected in 2019. Similar preliminary results have likewise been reported for the “DREAM” trial.[241]
In addition to single microbicide component VRs, combination ARV microbicidal rings are in current development and testing, including a segmented polyurethane ring for combined delivery of the NNRTI DPV and NRTI tenofovir,[242] a silicone elastomer ring containing DPV and small-molecule CCR5-targeted HIV entry inhibitor, MVC,[243] and a matrix-type silicone elastomer ring containing DPV and the protease inhibitor (PI) DRV.[244] The DPV/MVC (25 mg DPV 100 mg MVC) combination ring was the subject of a Phase I safety and pharmacokinetics evaluation in the United States (MTN-013/IPM-026 trial); though the devices tested proved safe and effective inhibitory concentrations of the DPV component were released, it was determined that insufficient amounts of the MVC component were released, requiring further development and improvement of this device prior to any future reevaluation.[245] It is noteworthy that MTN-013/IPM-026 was the first clinical trial of a vaginal microbicide containing two ARVs, and each possessing a different mechanism of HIV interference. Following the combinatorial drug approach employed in HAART,[246] modern and next-generation microbicide candidates and formulations are likely to increasingly contain combinations of compounds that inhibit HIV via different mechanisms and/or at different stages of the viral lifecycle, with activity against a broader range of HIV isolates and less likelihood of favoring the selection and development of drugresistant strains.[190,247]
Additional VR delivery systems are in development and testing for release of biologic microbicides. Due to factors including permeability constraints and the lack of thermal stability of the active at typical processing temperatures required during device manufacture, conventional VRs are not useful for the delivery of biomacromolecules such as proteins, peptides, and nucleic acids.[234b] Such factors have led to the development of novel ring designs, including complex multi-component systems in which the polymer ring form serves as a platform or a holder, for alternative solid dosage forms that are inserted as rods/pods into the ring base; the rods/pods are often directly compressed tablets of lyophilized biomolecule packed with various excipients, and may be coated with semi-permeable polymer, such as polylactic acid (PLA).[234a] It should be noted that this type of processing of the active compound does not involve high temperatures, and the combination of excipients and freeze–drying can serve to stabilize the active component against degradation.[248] The drug pods/inserts are incorporated into the elastomeric polymer rings and the drug is released through a small delivery window in the ring, with release rates of the active being determined by the window diameter, the total number of pods inserted into the ring, etc. Additionally, while the ring form acts as a retainer for the inserts, other microbicides (such as ARVs) could also be loaded into and released from this portion of the device. At present, VRs-releasing protein-based microbicides are still in the pre-clinical and development phases, however, promising results include demonstration of sustained release of two different anti-HIV microbicidal peptides (T-1249 and “JNJ”) over 28 days from a silicone elastomer ring with the actives packed in rod inserts,[249] and also sustained release of the bNAb 2F5 over many days from the insert vaginal ring (“InVR”) design.[250] More recently, sustained controlled release over 21 days of the bNAb VRC01 was demonstrated from intravaginal rings of the pod design (e.g., “pod-IVRs”) in a macaque model, with release rates of up to several milligrams bNAb per day.[251]
Nanoparticles and Electrospun Fibers.
Nanoparticle (NP) delivery systems for microbicides involve attachment of anti-viral molecules to particles made of inorganic material (i.e., a noble metal, such as silver or gold) or comprised of a wide array of cross-linked, biodegradable, and biocompatible polymers, such as polycaprolactone (PCL) and poly(lactic-co-glycolic acid) (PLGA).[252] Advantages of this type of delivery system include protection and stabilization of the active compound(s) against degradation, enhanced solubilization of the attached drugs, and due to their small size, the ability to penetrate through cervicovaginal mucous to reach HIV susceptible sites, as well as internalization in cells to allow intracellular drug delivery.[253] The focus on polymeric nanoparticles as microbicide delivery vehicles is rather recent, but one of the earlier examples was the formulation of PSC-RANTES (an anti-HIV protein and derivative of chemokine RANTES) in co-polymer PLGA nanoparticles, which exhibited sustained release of bioactive PSC-RANTES in vitro over 30 days. It was also shown that the tissue uptake of PSC-RANTES in nanoparticles in ex vivo ectocervical explants was fivefold greater than that of unformulated PSC RANTES, which became distributed and remained only at the superficial epithelial layer, in contrast to the deeper permeation afforded by the NP formulation.[254] Several ARVs have also been formulated into NPs and incorporated into gel and film dosage formats.[255] Several other pertinent examples of nanodelivery systems for HIV microbicides have been detailed in recent reviews.[253,256]
Like nanoparticles, electrospun fibers (EFs) are also a relatively new proposed platform for delivery of HIV microbicides. EFs are polymer fibers produced by electrospinning, in which electrostatic forces are applied to polymer solutions (such as polyglycolic acid, PLA, PCL, or PLGA) to produce dry spun fibers of the polymer.[257] Fibers can be loaded with drug during the spinning process, and the release of drug can be controlled through degradation and erosion of the polymer with subsequent diffu sion of the active compound; the composition and preparation of fibers can be tuned to effect desired release kinetics, including more sustained release (ranging from hours to weeks).[258] Several small-molecule ARV drugs have been formulated in electrospun fibers and evaluated in in vitro assays.[259] Recently, the biomolecular microbicide Griffithsin has also been developed in this format as Griffithsin-coated PLGA electrospun fibers; the Grft-EFs demonstrated efficacy against HIV and were shown to be non-toxic to vaginal epithelial cells in vitro.[260]
Live Microbicides.
An intriguing area of development in anti HIV microbicide delivery systems is the live microbicide approach, wherein commensal bacterial species (such as Lactobacil lus or Bifidobacterium genera, which are common among human vaginal microbiota[261]) are genetically engineered to produce and secrete biomolecular microbicides, such as anti-HIV proteins or peptides. When administered orally or topically, these agents of mucosal drug delivery work by colonization of the intended host compartment with expression and secretion of therapeutics in situ, thereby potentially providing protection for days, weeks, months, or even longer.[262]
A prime example of this approach has been the successful engineering of a vaginal Lactobacillus jensenii strain expressing the anti-HIV lectin Cyanovirin (CV-N).[263] Vaginal inoculation of macaques with this engineered strain resulted in colonization and secretion of CV-N for at least 6 weeks after administration and produced a 63% reduction in infection upon repeated vaginal challenge with SHIV; the presence of the engineered Lacto bacilli and continued secretion of CV-N evidenced no induction of inflammation in the animals.[264] A subsequent study evaluating the safety and toxicity of this live microbicide in macaques recapitulated its safety and also suggested that the Lactobacilli them selves may positively impact the vaginal mucosal environment.[265] This engineered Lactobacillus strain has also been evaluated in an in vitro human vaginal and cervical cell colonization model; similar to the macaque model, bioengineered L. jensenii was able to deliver bioactive microbicidal CV-N protein without inducing cellular toxicity or alterations in levels of biomarkers of inflammation.[266] Thus far, an engineered strain (a.k.a. Muco-Cept, Osel Inc.) of L. jensenii expressing modified CV-N (mCV-N) has been formulated as rapidly disintegrating vaginal tablets that support sustained vaginal colonization comparable to that observed when macaques were inoculated with freshly prepared culture; vaginal tablet administration resulted in colonization in 66% of macaques at 14 days post-dosing and 83% after 21 days, and the prototype tablets showed stability and potency of the active compound for about 1 month at 37 °C and for about 1 year at 25 °C.[267] It is also interesting to note that, when formulated as a yogurt product and fed to macaques, engineered L. jensenii CV-N expression could be detected in rectal lavage specimens up to 7 days after cessation of feeding, and the viral replication levels were reduced by 20-fold in rectal biopsies challenged ex vivo with SHIV. However, complete inhibition of viral infection and replication was not achieved and no animals were colonized by the recombinant bacteria,[268] possibly owing to the fact that L. jensenii is not a prolific native species in the GI tract,[269] whereas it is so in the vagina.[270]
Other developments in the field of live microbicides include engineering of L. jensenii to express and secrete the anti-HIV chemokine protein RANTES as well as C1C5-RANTES, a variant that acts as a CCR5 co-receptor antagonist and lacks proinflammatory activity; both Lactobacilli-expressed proteins were shown in vitro to inhibit HIV infection of CD4+ T cells and macrophages.[271] L. jensenii has also been successfully engineered to secrete or display two-domain CD4 (2D-CD4) proteins that have demonstrated inhibition of virus infectivity of HeLa cells.[272] Escherichia coli have also been engineered to express a fusion peptide of the single-chain variable fragment (scFv) of the VRC01 bNAb (scFv–VRC01) together with the autotransporter β-barrel domain of IgAP gene from Neisseria gonorrhoeae, thus enabling display of the Ab on the surface of the bacterium; the engineered bacteria were demonstrated to capture HIV particles via surface binding and inhibit HIV infection in cell culture.[273] Most recently, L. jensenii was engineered to express and secrete broadly neutralizing single chain and single-domain antibodies (dAbs); these bacterially secreted biotherapeutics demonstrated effective inhibition of infection in single-round TZM-bl assays against a broad range of viral strains.[274] Although microbicidesecreting probiotics are still controversial, and though this type of anti-HIV delivery strategy will require long-term clinical data clearly demonstrating safety and efficacy, the concept of live microbicides is gaining publicity and acceptance.[184e] If successful, these next-generation probiotics have the potential to serve as a sustained, self-replicating delivery system of biomolecular microbicides to combat infection by HIV-1 and potentially other enveloped viruses.
In addition to bacterial strains that have been genetically modified to express and deliver protein microbicides, it is noteworthy that some native (non-engineered) bacterial strains themselves also exhibit inherent microbicidal activity against HIV and other sexually transmitted viruses.[186b,275] In a recent study, six different Lactobacillus strains (including two different strains each of Lactobacillus crispatus, Lactobacillus gasseri, and Lactobacillus vagi nalis) were shown to inhibit HIV-1 replication in human tissues ex vivo; mechanistically, this inhibition was attributed to a combination of milieu acidification and l-lactic acid production, as well as the ability of Lactobacilli to actually adsorb HIV-1 onto their surfaces, thus decreasing the total number of free virions in the system.[209j] Thus, administration of probiotic Lactobacilli—either topically or orally (as orally administered probiotics have been shown to reach the vagina in human studies)[276] could po-tentially constitute an effective adjunct to other current microbicides. Indeed, addressing and amending the vaginal microbiome may even prove to be a necessary antecedent to the efficacious function of certain existent microbicides—as in the case of recently published work done within the context of the CAPRISA 004 trial, demonstrating that vaginal bacteria modulated the efficacy of the microbicide tenofovir gel, with detectable mucosal levels of tenofovir being much lower in women whose vaginal microbiota were not Lactobacillus dominated.[277]
3.2.3. Long-Acting Injectable PrEP
In contrast to other PrEP modalities such as daily oral pills or topical gels, requiring frequent administration by the user, longacting (LA) injectable PrEP offers the advantage of reduced dosing frequency (i.e., once every 2 months) with the attendant potential to increase user adherence and thus, overall efficacy in preventing HIV transmission. All LA injectable anti-HIV agents currently in development require parenteral administration—or, injections delivered via subcutaneous (SC), intramuscular (IM), or intravenous (IV) routes, and are intended to provide sustained drug levels in fluids (serum, plasma) and relevant (vaginal, rectal, penile) tissues.[278] Limitations of this approach include differential acceptability of (potentially painful) needle injections by diverse populations, as well as issues surrounding administration of injections (and the requirement for clinical visits and infrastructure), particularly for those given via the IV or IM routes.[278]
Several long-acting ARV candidates are currently under development as injectables for HIV prevention, all with common attributes of high potency (requiring lower drug doses for efficacy) and favorable pharmacokinetics including relatively long plasma half-lives in the injectable form[279] In addition to its development for HIV treatment,[32] the investigational integrase inhibitor drug CAB is in late-stage clinical development as an every-other-monthly injection for HIV prevention. Formulated as a long-acting injectable nanosuspension (“CAB LA”),[280] CAB is entering into two Phase III clinical trials (HPTN-083 and HPTN-084) to evaluate the safety and efficacy of 600 mg gluteal IM injections administered every 8 weeks, with the first two injections given 4 weeks apart; completion of the studies and final results are expected in 2022. Aside from administration via traditional parenteral routes, there are also LA PrEP delivery methods in pre-clinical development that include biodegradable implants (which would not require terminal device removal) as well as those that are refillable.[281] For instance, the NRTI TAF was formulated in a thin-film polymer device (TFPD) as a biodegradable subcutaneous implant, with a thin-film PCL membrane to control drug release from a reservoir; in vitro studies of the prototypes demonstrated successful linear drug release at 1.2 mg per day (a potentially therapeutically relevant dosage) for up to 90 days.[281a]
In addition to long-acting injectable small-molecule ARVs, the injection of potent, bNAbs is an effective protective measure against HIV infection. This is one option for prevention, albeit expensive. Compared to the cost of the oral PrEP drug combination Truvada (and particularly with recent FDA approval of a generic version), the cost of biologics such as bNAbs greatly exceeds this amount, and though expression in plant-based systems[208] holds the promise of reducing production costs of these biologics, consideration of the sheer volume of individuals currently infected with HIV and needing treatment (19 million worldwide) and the tens of millions more at risk of HIV acquisition and needing PrEP, suggests that these biologics are unlikely to become a globally widespread, mainstream component of anti-HIV efforts. In the absence of an effective vaccine, injecting Abs gives a person almost immediate protection, but for limited periods of time. Still, the protection afforded by these injections requires less frequent dosing (as compared to a daily oral pill). In the absence of an effective vaccine, injecting Abs gives a person almost immediate protection, but for limited periods of time. At present, in addition to the requirement of a cold chain for transport and storage, these Abs (such as VRC01) are primarily being admin istered intravenously, which tends to necessitate certain clinical infrastructure and visits by the user to clinical personnel for dosing (as opposed to the convenience of self-administered IM or SC injections).[279]
Different Abs have been tested in vitro and in vivo for efficacy and safety. An early study showed the anti-V3 domain Ab, Cβ1, protected in vivo when administered intravenously in chimpanzee challenge studies.[282] In another study, they compared the bNAbs VRC01, 10E8, and PG9, which target different regions of gp120, and an anti-CD4 Ab, 2D5, in a mucosal challenge of rhesus macaques with SHIV. VRC01, 10E8, and PG9 bind to the CD4 binding site (CD4bs) in gp120, MPER, and V1V2 glycan regions, respectively. All of the anti-gp120 antibodies were completely protected in the challenge, whereas the anti-CD4 Ab did not provide effective protection. This study suggested that targeting the HIV Env was preferable over targeting the cell surface receptor CD4.[283]
Injection of anti-HIV bNAbs prior to challenge has proven effective in preventing HIV infection. It is of interest to see how long this protection lasts after the injection. One study tested four bNAbs, VRC01, VRC01-LS (containing the mutations M428L and N343S in the Fc domain of VRC01), 3BNC117, and 10–1074, against the clade B SHIV AD8 strain. All of these Abs target the CD4bs on gp120, except 10–1074, which targets the N332 glycan on gp120. Macaques were given a single injection of one of the Abs at a concentration of 20 mg kg−1 1 week prior to intrarectal challenge and were subsequently challenged each week thereafter until the macaque became infected. Control animals became infected in a median of three challenges. In contrast, VRC01, VRC01-LS, 3BNC117, and 10–1074 injected animals became infected after a median of 8, 14.5, 13, and 12.5 challenges, respectively.[284] This study is one of the pieces of evidence show-ing that injection of bNAbs is effective in preventing HIV infection for a significant length of time. In addition, the strategy of introducing mutations in the Fc domains of therapeutic Ab candidates has produced versions of the Abs (such as the VRC01 LS variant) with extended in vivo serum half-lives, potentially expanding the duration of immunoprophylactic coverage provided by these agents.[285] The combination of extended in vivo half-lives of bNAbs, coupled with greater potency (as achieved by the administration of bNAb cocktails rather than single agents) could enable the development of Ab-based HIV prevention products with less frequent dosing requirements (e.g., once every 6 months) that could be administered by subcutaneous injection.[279] The SI route of administration could potentially facilitate self-injections by users via a conventional syringe/needle (as used for routine injections in diabetes treatment), and also invites the possibility of painless delivery via patches of microneedles, which are micron-width needles fabricated from silicon or polymers into arrays that are capable of piercing the skin without stimulating proprioceptive nerves (e.g., averting pain associated with typical large needle insertions) and can deliver a wide array of therapeutics;[286] this new micro-technology is already in early clinical development for dermal vaccine delivery.[287]
VRC01 seems especially promising as an injectable Ab. Structurally, VRC01 somewhat mimics CD4 in binding to the conserved region of the CD4 binding site of gp120, as found with a crystal structure. However, VRC01 binds both CD4-bound and non-CD4-bound conformations (in the absence of CD4) of gp120 with high affinity, unlike CD4 and most other Abs against this region (with the notable exception of b12).[288] VRC01 is entering into two international Phase 2b clinical trials, HVTN 703/HPTN 081 and HVTN 704/HPTN 085, known as the AMP (Ab-mediated prevention) Studies, in which it will be administered intravenously every 8 weeks (over the course of 72 weeks total) to test its safety and efficacy in reducing HIV transmission.[289] Though single bNAb agents are currently being tested in human clinical trials, it is envisioned that combinations of bNAbs (with different epitope specificities against gp120) will prove to have the greatest breadth and potency in preventing HIV transmission.[207,290]
4. Future Directions in HIV Prevention and Treatment
HIV prevention efforts include the use of many drugs and delivery systems. Because users have varying preferences, access to a multitude of product options may improve user consistency and compliance. Multiple products can be also used simultaneously for more comprehensive protection against HIV. Individual product improvement will also help to increase the efficacy of the products and user acceptability.
Regarding HIV vaccine development, the unique combination of characteristics of HIV continues to be a barrier to successful vaccine design. There was some success with the RV144 clinical trial, which demonstrated 31.2% efficacy,[168] and upon which follow-up trials are being pursued, including Phase IIb/III vaccine trial HVTN 702.[169] The knowledge gained from these subsequent trials will broaden our understanding of what contributes to the efficacy of an HIV vaccine. Potent bNAbs against Env will continue to help guide development efforts, however, many combinations of antigens over time still need to be screened in order to optimize the speed and potency of the immune response. Mucosal vaccines are also being investigated further to potentially provide greater protection at the portals of entry. Additionally, combining vaccines with microbicides has been shown to increase protection more than by solely the sum of their efficacies in NHP studies.[170,171] This is an area that will continue to be explored to achieve greater prevention. In the interim, direct injections of long-acting, potent, bNAbs (whose formation, a successful vaccine would illicit) are a promising development in HIV prevention that is rapidly progressing through clinical phase trials.
Also, in the field of HIV prevention, the combination of multiple anti-HIV compounds is of particular interest in the microbicide development pipeline. This approach allows to broaden protection by combining different compounds that act via different mechanisms and at different stages of the viral replication cycle. This approach can potentially lower individual doses of each compound, thus reducing side effects, help provide protection against a broader range of HIV isolates including ARV-resistant mutants and decrease the likelihood of the development of drug resistance against any single compound. This combinatorial strategy applies not only to small-molecule (ARV-type) compounds, but also to protein-based microbicides; for instance, the inhibitor Griffithsin can be combined with other microbicidal proteins, as well as with small-molecule inhibitors.[291]
In addition, one particularly salient point that emerged from the many afore-mentioned clinical trials of microbicides is the importance of user acceptability and adherence to required dosing regimens.[292] Individuals must be offered a variety of dosage forms and delivery formats to meet the varied needs and preferences of a diverse global population. To this end, novel dosage forms have been and will continue to be explored and developed. In addition, there is need for, and ongoing development of dosage forms with less rigorous and frequent dosing regimens for individuals. Clinical trials are increasingly expanding their scope to include examination of issues such as user adherence and accept ability (in addition to safety and efficacy evaluations of devices), and there are data to demonstrate correlation between increasing user adherence and increasing dose duration (i.e., decreased frequency of dosing or user intervention required).[293] Promising developments in this arena have included pre-clinical development and testing (in sheep) of a successful 90-day tenofovir reservoir-type intravaginal ring,[237] and in particular, an upcoming Phase I clinical trial (MTN-036/IPM-047, slated for completion later in 2018) of a DPV vaginal ring that releases effective inhibitory amounts of the drug for 90 days (e.g., ring replacement by the user would potentially only need to occur once every 3 months).
There is also an emerging trend toward the development of multipurpose prevention technologies (MPTs), which include combinations of drugs intended to simultaneously provide dual protection against unintended pregnancies and transmission of HIV and other STIs.[294] It is hypothesized that a device offering prevention of both HIV infection and unintended pregnancy may lead to a higher degree of adherence among female users as compared to microbicide-only products; also, products having the capability of preventing transmission of multiple STIs may also improve the motivation of individuals to use them, as compared to products only preventing HIV transmission. Most efforts to de velop MPTs have focused on combinations of two or more drugs rather than a single multifunctional compound. Newer developments in the MPT field include the successful development of an intravaginal ring demonstrating simultaneous and continuous release over more than 60 days, of both DPV and the contraceptive progestin levonorgestrel;[295] this promising new device entered a Phase I clinical trial (MTN-030/IPM-041) in April 2017, with results from the study expected later in 2018.
In the long-term success of HIV prevention and treatment efficacy, safety and tolerability are key and will likely be the focus of future studies. Targeting different stages of HIV infection will be investigated, since there are several targets like HLA DR, DC-SIGN, and LFA-1. Another challenge is targeting latent HIV in various reservoirs, thus LRAs are being studied to activate the viral reservoirs in order to eradicate the HIV infections completely.
Other sustained delivery options such as transdermal delivery systems and intradermal implants are being investigated to reduce the dosing frequency and improve life quality of the patients. However, these systems will require more potent drugs due to the limited drug loading capacity of these novel delivery systems. There are new ARV molecules with different mechanisms in clinical trials that might be used with these new systems. Both novel prevention and treatment options that are in early stages of testing and long-term commitment and systematic research are necessary to achieve the ultimate goal of HIV eradication.
Acknowledgements
B.Y. and J.L.M contributed equally to this work. The authors thank the NIH for support of some of their work on the subject of this review, including R01AI112011.
Biographies

Dr. Burcin Yavuz is a postdoctoral researcher at Tufts University in the Department of Biomedical Engineer ing. She is a pharmacist with a Ph.D. in pharmaceutical sciences and her research interests are drug delivery, formulation, and stabilization, with fundamental understanding of prop erties and interactions of natural and synthetic polymers such as silk, PLGA, polycaprolactone, cyclodextrins, and dendrimers. Previ ously, she had worked as a research and teaching assistant at Hacettepe University for 9 years during her M.S. and Ph.D. studies.

Patricia LiWang is a professor of molec ular cell biology at the University of California–Merced. As an early mem ber of the faculty of this new campus, Dr. LiWang was the first chair of the MCB unit. Prof. LiWang’s research is directed toward the biochemistry of HIV inhibition and anti-inflammation strategies. Recent work includes the development of protein HIV entry in hibitors in sustained release formats using silk fibroin.

Prof. David L. Kaplan holds an En dowed Chair, the Stern Family Profes sor of Engineering, at Tufts University. He is professor and chair of the Depart ment of Biomedical Engineering and also holds faculty appointments in the School of Medicine, School of Dental Medicine, Department of Chemistry, and the Department of Chemical and Biological Engineering. He directs the NIH P41 Tissue Engineering Resource Center (TERC) that involves Tufts University and Columbia University.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Burcin Yavuz, Department of Biomedical Engineering Tufts University 4 Colby Street, Medford, MA 02155, USA.
Jessica L. Morgan, Department of Molecular Cell Biology University of California–Merced5200 North Lake Road, Merced, CA 95343, USA
Laura Showalter, Department of Molecular Cell Biology University of California–Merced5200 North Lake Road, Merced, CA 95343, USA.
Katti R. Horng, Department of Medical Microbiology and Immunology University of California–Davis 5605 GBSF, 1 Shields Avenue, Davis, CA 95616, USA
Satya Dandekar, Department of Medical Microbiology and Immunology University of California–Davis 5605 GBSF, 1 Shields Avenue, Davis, CA 95616, USA.
Carolina Herrera, Department of Medicine St. Mary’s Campus Imperial College Room 460 Norfolk Place, London W2 1PG, UK.
Patricia LiWang, Department of Molecular Cell Biology University of California–Merced5200 North Lake Road, Merced, CA 95343, USA.
David L. Kaplan, Department of Biomedical Engineering Tufts University 4 Colby Street, Medford, MA 02155, USA
References
- [1].UNAIDS, UNAIDS Data 2017, http://www.unaids.org/sites/default/files/media_asset/20170720_Data_book_2017_en.pdf (accessed: April 17, 2018).
- [2].UNAIDS, AIDS Update 2016, http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf (accessed: April 17, 2018).
- [3].UNAIDS, Key Populations Atlas, http://www.aidsinfoonline.org/kpatlas/document/kp_data_sources.pdf (accessed: April 17, 2018).
- [4].Prejean J, Song R, Hernandez A, Ziebell R, Green T, Walker F, Lin LS, An Q, Mermin J, Lansky A, Hall HI, HIVIStudy Group, PLoS One 2011, 6, e17502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Barré-Sinoussi F, Ross AL, Delfraissy J-F, Nat. Rev. Microbiol 2013, 11, 877. [DOI] [PubMed] [Google Scholar]
- [6].Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR, Proc. Natl. Acad. Sci. U. S. A 1997, 94, 1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wilen CB, Tilton JC, Doms RW, Cold Spring Harb. Perspect. Med 2012, 2, a006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Arts EJ, Hazuda DJ, Cold Spring Harb. Perspect. Med 2012, 2, a007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].UNAIDS, Global AIDS Response Progress Reporting (GARPR) 2016, https://aidsreportingtool.unaids.org/static/docs/GARPR_Guidelines_2016_EN.pdf (accessed: April 17, 2018).
- [10].Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn TC, Chaisson RE, Rosenberg E, Walker B, Gange S, Gallant J, Siliciano RF, Nat. Med 1999, 5, 512. [DOI] [PubMed] [Google Scholar]
- [11].Davey RT Jr., Bhat N, Yoder C, Chun TW, Metcalf JA, Dewar R,Natarajan V, Lempicki RA, Adelsberger JW, Miller KD, Kovacs JA, Polis MA, Walker RE, Falloon J, Masur H, Gee D, Baseler M, Dimitrov DS, Fauci AS, Lane HC, Proc. Natl. Acad. Sci. U. S. A 1999, 96, 15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sierra S, Kupfer B, Kaiser R, J. Clin. Virol 2005, 34, 233. [DOI] [PubMed] [Google Scholar]
- [13].Mehellou Y, De Clercq E, J. Med. Chem 2010, 53, 521. [DOI] [PubMed] [Google Scholar]
- [14].U.S. Department of Health and Human Services, AIDS Info, https://aidsinfo.nih.gov/drugs (accessed: April 17, 2018).
- [15].Cihlar T, Fordyce M, Curr. Opin. Virol 2016, 18, 50. [DOI] [PubMed] [Google Scholar]
- [16].Julg B, Bogner JR, Ther. Clin. Risk Manag 2008, 4, 573. [PMC free article] [PubMed] [Google Scholar]
- [17].Perry CM, Drugs 2014, 74, 75. [DOI] [PubMed] [Google Scholar]
- [18].Greig SL, Deeks ED, Drugs 2015, 75, 503. [DOI] [PubMed] [Google Scholar]
- [19].Angione SA, Cherian SM, Ozdener AE, J. Pharmacy Practice 2018, 31, 216. [DOI] [PubMed] [Google Scholar]
- [20].a) Lohse N, Hansen ABE, Pedersen G, Kronborg G, Gerstoft J, Sorensen HT, Vaeth M, Obel N, Ann. Intern. Med 2007, 146, 87; [DOI] [PubMed] [Google Scholar]; b) Hogg R, Lima V, Sterne JAC, Grabar S, Battegay M, Bonarek M, Monforte AD, Esteve A, Gill MJ, Harris R, Justice, Hayden A, Lampe, Mocroft, Mugavero, Staszewski, Wasmuth JC, van Sighem A, Kitahata M, Guest J, Egger M, May M,Coll ATC, Lancet. 2008, 372, 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Deeks SG, Phillips AN, BMJ 2009, 338, a3172. [DOI] [PubMed] [Google Scholar]
- [22].De Clercq E, Nat. Rev. Drug Discov 2002, 1, 13. [DOI] [PubMed] [Google Scholar]
- [23].Wensing AM, Calvez V, Gunthard HF, Johnson VA, Paredes R,Pillay D, Shafer RW, Richman DD, Top. Antivir. Med 2017, 24, 132. [PMC free article] [PubMed] [Google Scholar]
- [24].Fonsah JY, Njamnshi AK, Kouanfack C, Qiu F, Njamnshi DM, Tagny CT, Nchindap E, Kenmogne L, Mbanya D, Heaton R, Kanmogne GD, PLoS One 2017, 12, e0170893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].a) Marraa CM, Zhao, Clifford DB, Letendre S, Evans S, Henry K, Ellis RJ, Rodriguez B, Coombs RW, Schifitto G, McArthur JC, Robertson K, ACTG Study, AIDS 2009, 23, 1359; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Robertson KR, Su Z, Margolis DM, Krambrink A, Havlir DV, Evans S, Skiest DJ, A. S. Team, Neurology 2010, 74, 1260.20237308 [Google Scholar]
- [26].Gerber JG, Clin. Infect. Dis 2000, 30, S123. [DOI] [PubMed] [Google Scholar]
- [27].a) Eagling VA, Wiltshire H, Whitcombe IWA, Back DJ, Xenobi otica 2002, 32, 1; [DOI] [PubMed] [Google Scholar]; b) Ramana LN, Anand AR, Sethuraman S, Krishnan U,M, J. Controlled Release 2014, 192, 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Calcagno A, Di Perri G, Bonora S, Clin. Pharmacokinet 2014, 53, 891. [DOI] [PubMed] [Google Scholar]
- [29].a) Schweighardt B, Atwood WJ, AIDS Res. Hum. Retrov 2001, 17, 1133; [DOI] [PubMed] [Google Scholar]; b) Gunaseelan S, Gunaseelan K, Deshmukh M, Zhang XP,Sinko PJ, Adv. Drug. Deliver. Rev 2010, 62, 518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].NCT02275780https://www.clinicaltrials.gov/ct2/show/NCT02275780 (accessed: October 26, 2017).
- [31].a) Gatell JM, Morales-Ramirez JO, Hagins DP, Thompson M, Keikawus A, Hoffmann C, Rugina S, Osiyemi O, Escoriu S, Dretler R, Harvey C, Xu X, Teppler H, J. Int. AIDS Soc 2014,17, 28; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lai MT, Feng MZ, Falgueyret JP, Tawa P, Witmer M, DiSte fano D, Li Y, Burch J, Sachs N, Lu MQ, Cauchon E, Campeau LC,Grobler J, Yan YW, Ducharme Y, Cote B, Asante-Appiah E, Hazuda DJ, Miller MD, Antimicrob. Agents Ch 2014, 58, 1652; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Schurmann D, Sobotha C, Gilmartin, Robberechts M, De Lep eleire I, Yee KL, Guo Y, Liu R, Wagner F, Wagner JA, Butterton JR, Anderson MS, AIDS 2016, 30, 57. [DOI] [PubMed] [Google Scholar]
- [32].Spreen W, Min S, Ford SL, Chen S, Lou Y, Bomar M, St Clair M,Piscitelli S, Fujiwara T, HIV Clin. Trials 2013, 14, 192. [DOI] [PubMed] [Google Scholar]
- [33].Meanwell NA, Krystal MR, Nowicka-Sans B, Langley DR, Conlon DA, Eastgate MD, Grasela DM, Timmins P, Wang T, Kadow JF, J. Med. Chem 2018, 61, 62. [DOI] [PubMed] [Google Scholar]
- [34].NCT03299049, https://www.clinicaltrials.gov/ct2/show/record/NCT03299049?termcabotegravir&condhiv&phase=2&rank=2 (accessed: July 2018).
- [35].a) Caskey M, Klein F, Lorenzi JCC, Seaman MS, West AP, Buckley N, Kremer G, Nogueira L, Braunschweig M, Scheid JF, Horwitz JA, Shimeliovich, Ben-Avraham S, Witmer-Pack M, Platten M, Lehmann C, Burke LA, Hawthorne T, Gorelick RJ, Walker BD, Keler T, Gulick RM, Fatkenheuer G, Schlesinger SJ, Nussenzweig MC, Nature 2015, 522, 487; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lynch RM, Boritz E,Coates EE, DeZure A, Madden P, Costner P, Enama ME, Plummer S, Holman L, Hendel CS, Gordon I, Sci. Transl. Med 2015, 7, 319ra206. [DOI] [PubMed] [Google Scholar]
- [36].a) Freund NT, Wang HQ, Scharf L, Nogueira L, Horwitz JA,Bar-On Y, Golijanin J, Sievers SA, Sok D, Cai H, Lorenzi JCC,Halper-Stromberg A, Toth I, Piechocka-Trocha A, Gristick HB,van Gils MJ, Sanders RW, Wang LX, Seaman MS, Burton DR, Gazumyan A, Walker BD, West AP, Bjorkman PJ, Nussenzweig MC, Sci. Transl. Med 2017, 9; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Klein F, Halper Stromberg A, Horwitz JA, Gruell H, Scheid JF, Bournazos S, Mouquet H, Spatz LA, Diskin R, Abadir A, Zang T, Dorner M, Billerbeck E, Labitt RN, Gaebler C, Marcovecchio PM, In cesu RB, Eisenreich TR, Bieniasz PD, Seaman MS, Bjork man PJ, Ravetch JV, Ploss A, Nussenzweig MC, Nature 2012, 492, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gay CL, Bosch RJ, Ritz J, Hataye JM, Aga E, Tressler RL,Mason SW, Hwang CK, Grasela DM, Ray N, Cyktor JC, Coffin JM, Acosta EP, Koup RA, Mellors JW, Eron JJ, Study ACT, J. Infect. Dis 2017, 215, 1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fulton Z, Nat. Rev. Drug Discov 2016, 16, 11. [DOI] [PubMed] [Google Scholar]
- [39].FDA, https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm599657.htm (accessed: April 17, 2018).
- [40].Margolis DM, Koup RA, Ferrari G, Immunol. Rev 2017, 275, 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Li XL, Chan WK, Adv. Drug Deliver. Rev 1999, 39, 81. [DOI] [PubMed] [Google Scholar]
- [42].Deeks ED, Drugs 2014, 74, 195. [DOI] [PubMed] [Google Scholar]
- [43].a) von Hentig N, HIV AIDS Res. Palliat 2016, 8, 1; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Larson KB,Wang K, Delille C, Otofokun I, Acosta EP, Clin. Pharmacokinet 2014, 53, 865. [DOI] [PubMed] [Google Scholar]
- [44].a) Devi KV, Pai RS, Ind. J. Pharm. Sci 2006, 68, 1; [Google Scholar]; b) Gupta U,Jain NK, Adv. Drug Deliver. Rev 2010, 62, 478. [DOI] [PubMed] [Google Scholar]
- [45].Weiss RA, Science 1993, 260, 1273. [DOI] [PubMed] [Google Scholar]
- [46].Gabathuler R, Neurobiol. Dis 2010, 37, 48. [DOI] [PubMed] [Google Scholar]
- [47].Kuo YC, Su FL, Int. J. Pharm 2007, 340, 143. [DOI] [PubMed] [Google Scholar]
- [48].Weissenhorn W, Dessen A, Harrison SC, Skehel JJ, Wiley DC, Nature 1997, 387, 426. [DOI] [PubMed] [Google Scholar]
- [49].a) Schreier H, Moran P, Caras IW, J. Biol. Chem 1994, 269, 9090; [PubMed] [Google Scholar]; b) Cheng K, El-Boubbou K, Landry CC, ACS Appl. Mater. Inter 2012, 4, 235. [DOI] [PubMed] [Google Scholar]
- [50].Dey AK, Griffiths C, Lea SM, James W, RNA 2005, 11, 873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].a) Craig RB, Summa CM, Corti M, Pincus SH, PLoS One 2012, 7; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lu L, Pan CG, Li Y, Lu H, He W, Jiang SB, Retrovirology 2012, 9, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Dadachova E, Patel MC, Toussi S, Apostolidis C, Morgen stern A, Brechbiel MW, Gorny MK, Zolla-Pazner S, Casadevall A, Goldstein H, PLoS Med. 2006, 3, 2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Biancotto A, Iglehart SJ, Vanpouille C, Condack CE, Lisco A, Ruecker E, Hirsch I, Margolis LB, Grivel JC, Blood 2008, 111, 699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].a) Lawn SD, Butera ST, J. Virol 2000, 74, 10256; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Dufresne I,Desormeaux A, Bestman-Smith J, Gourde P, Tremblay MJ, Bergeron MG, BBA Biomembranes 1999, 1421, 284; [DOI] [PubMed] [Google Scholar]; c) Muthu MS, Singh S, Nanomedicine 2009, 4, 105. [DOI] [PubMed] [Google Scholar]
- [55].Jin W, Li C, Du T, Hu K, Huang X, Hu QX, Virology 2014, 458,83. [DOI] [PubMed] [Google Scholar]
- [56].Zhang Q, Su L, Collins J, Chen GS, Wallis R, Mitchell DA, Haddleton DM, Becer CR, J. Am. Chem. Soc 2014, 136, 4325. [DOI] [PubMed] [Google Scholar]
- [57].Jain SK, Gupta Y, Jain A, Saxena AR, Khare P, Jain A, Nanomed. Nanotechnol 2008, 4, 41. [DOI] [PubMed] [Google Scholar]
- [58].Dutta T, Garg M, Jain NK, Eur. J. Pharm. Sci 2008, 34, 181. [DOI] [PubMed] [Google Scholar]
- [59].a) Dou H, Morehead J, Destache CJ, Kingsley JD, Shlyakht enko L, Zhou Y, Chaubal M, Werling J, Kipp J, Rabinow BE, Gendelman HE, Virology 2007, 358, 148; [DOI] [PubMed] [Google Scholar]; b) Jain S, Bishwal I, Dinda A,Moitra SK, Kohli S, Int. J. Pharm. Pharm. Sci 2011, 3, 95. [Google Scholar]
- [60].Vlachakis D, Tsiliki G, Pavlopoulou A, Roubelakis MG, Tsaniras SC, Kossida S, Evol. Bioinform 2013, 9, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Edagwa B, McMillan J, Sillman B, Gendelman HE, Expert Opin. Drug. Del 2017, 14, 1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Thomas NS, Panchagnula R, Eur. J. Pharm. Sci 2003, 18, 71. [DOI] [PubMed] [Google Scholar]
- [63].Gong J, Jaiswal R, Dalla P, Luk F, Bebawy M, Semin. Cell Dev. Biol 2015, 40, 35. [DOI] [PubMed] [Google Scholar]
- [64].Vasir JK, Tambwekar K, Garg S, Int. J. Pharm 2003, 255, 13. [DOI] [PubMed] [Google Scholar]
- [65].Zhang T, Zhang C, Agrahari V, Murowchick JB, Oyler NA, Youan BBC, Antivir. Res 2013, 97, 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Velmurugan S, Ali MA, Kumar P, Int. J. Pharm. Pharm. Sci 2014, 6, 31. [Google Scholar]
- [67].Chandarsekaran N, Balamurugan M, Res. J. Pharm. Technol 2013, 6, 731. [Google Scholar]
- [68].Chowdary KPR, Annamma Devi GS, Res. J. Pharm. Biol. Chem. Sci 2012, 3, 119. [Google Scholar]
- [69].Chiappetta DA, Carcaboso AM, Bregni C, Rubio M, Bra muglia G, Sosnik A, AAPS PharmSciTech. 2009, 10, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Dhanaraju M, Kumar RM, Nithya P, Kishan J, Thirumurugan G, Arch. Appl. Sci. Res 2009, 1, 279. [Google Scholar]
- [71].Shankar NB, Kumar NU, Asian J Pharm. Clin. Res 2009, 2, 55. [Google Scholar]
- [72].Prakash K, Raju PN, Shanta KK, Lakshmi MN, Trop. J. Pharm. Res 2007, 6, 841. [Google Scholar]
- [73].Velmurugan S, Ali MA, Int. J. Pharm. Pharm. Sci 2013, 5, 294. [Google Scholar]
- [74].Pheeba MP, Ganesh SA, Int. J. Pharm. Pharm. Sci 2010, 2, 169. [Google Scholar]
- [75].a) Tanvi R, Anupama D, Int. J. Drug Dev. Res 2011, 2, 211; [Google Scholar]; b) Dey S, Pramanik S, Malgope A, ISRN Pharm. 2011, 2011, 627623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Sahoo SK, Mallick AA, Barik BB, Senapati PC, Trop J Pharm. Res 2005, 4, 369. [Google Scholar]
- [77].Vikram A, Firoz S, Kishore D, Chandramouli Y, Yasmeen BR, Mahitha B, Kumar KLP, Asian J Pharm. Res 2012, 2, 70. [Google Scholar]
- [78].Phalguna Y, Venkateshwarlu BS, Gudas GK, Debnath S, Int. J. Pharm. Pharm. Sci 2010, 2, 41. [Google Scholar]
- [79].Nayak UY, Gopal S, Mutalik S, Ranjith AK, Reddy MS, Gupta P,Udupa N, J. Microencapsul 2009, 26, 214. [DOI] [PubMed] [Google Scholar]
- [80].a) Nath B, Nath LK, Kumar P, Acta Pol. Pharm 2011, 68, 409; [PubMed] [Google Scholar]; b) Yoshida VMH, de Oliveira JM, Goncalves MM, Vila MMDC, Chaud MV, AAPS PharmSciTech. 2011, 12, 658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Mehnert W, Mader K, Adv. Drug Deliver. Rev 2001, 47, 165. [DOI] [PubMed] [Google Scholar]
- [82].Heger Z, Cernei N, Adam V, Kizek R, J. Metallomics Nanotechnol 2015, 1, 20. [Google Scholar]
- [83].Fahmy TM, Fong PM, Park J, Constable T, Saltzman WM, AAPS J. 2007, 9, E171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Immordino ML, Dosio F, Cattel L, Int. J. Nanomed 2006, 1, 297. [PMC free article] [PubMed] [Google Scholar]
- [85].Shahiwala A, Amiji MM, Future HIV Therapy 2007, 1, 49. [Google Scholar]
- [86].Soma CE, Dubernet C, Bentolila D, Benita S, Couvreur P, Bio materials 2000, 21, 1. [DOI] [PubMed] [Google Scholar]
- [87].Mahajan SD, Law WC, Aalinkeel R, Reynolds J, Nair BB, Yong KT, Roy I, Prasad PN, Schwartz SA, Method Enzymol. 2012, 509, 41. [DOI] [PubMed] [Google Scholar]
- [88].Chattopadhyay N, Zastre J, Wong HL, Wu XY, Bendayan R, Pharmaceut. Res 2008, 25, 2262. [DOI] [PubMed] [Google Scholar]
- [89].a) Dash PK, Gendelman HE, Roy U, Balkundi S, Alnouti Y, Mosley RL, Gelbard HA, McMillan J, Gorantla S, Poluektova LY, AIDS 2012, 26, 2135; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Roy U, McMillan J, Alnouti Y, Gautum N,Smith N, Balkundi S, Dash P, Gorantla S, Martinez-Skinner A,Meza, Kanmogne G, Swindells S, Cohen SM, Mosley RL, Poluektova L, Gendelman HE, J. Infect. Dis 2012, 206, 1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Kuo YC, Lee CL, Colloid Surf. B 2012, 90, 75. [DOI] [PubMed] [Google Scholar]
- [91].Kaur A, Jain S, Tiwary AK, JAIDS 2008, 58, 61. [DOI] [PubMed] [Google Scholar]
- [92].Sillman B, Bade AN, Dash PK, Bhargavan B, Kocher T, Mathews S, Su H, Kanmogne GD, Poluektova LY, Gorantla S, McMillan J, Gautam N, Alnouti Y, Edagwa B, Gendelman HE, J. Neuroim mune Pharm 2018, 13, S79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Kinman L, Bui T, Larsen K, Tsai CC, Anderson D, Morton WR,Hu SL, Ho RJY, JAIDS 2006, 42, 155. [DOI] [PubMed] [Google Scholar]
- [94].Dev A, Binulal NS, Anitha A, Nair SV, Furuike T, Tamura H, Jayakumar R, Carbohyd. Polym 2010, 80, 833. [Google Scholar]
- [95].Kuo YC, Chen HH, Int. J. Pharm 2006, 327, 160. [DOI] [PubMed] [Google Scholar]
- [96].Freeling JP, Koehn J, Shu CL, Sun JG, Ho RJY, AIDS 2014, 28, 2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Kuo YC, Lin PI, Wang CC, Nanomedicine 2011, 6, 1011. [DOI] [PubMed] [Google Scholar]
- [98].a) Shegokar R, Jansch M, Singh KK, Muller RH, Nanomed. Nan otechnol 2011, 7, 333; [DOI] [PubMed] [Google Scholar]; b) Shegokar R, Singh KK, Int. J. Pharm 2011, 421, 341. [DOI] [PubMed] [Google Scholar]
- [99].Rao KS, Reddy MK, Horning JL, Labhasetwar V, Biomaterials 2008, 29, 4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Boudad H, Legrand P, Appel M, Coconnier MH, Ponchel G, STP Pharma. Sci 2001, 11, 369. [Google Scholar]
- [101].Shah LK, Amiji MM, Pharmaceut. Res 2006, 23, 2638. [DOI] [PubMed] [Google Scholar]
- [102].Mahajan SD, Roy I, Xu GX, Yong KT, Ding H, Aalinkeel R,Reynolds JL, Sykes DE, Nair BB, Lin EY, Prasad PN, Schwartz SA, Curr. HIV Res 2010, 8, 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Bender AR, vonBriesen H, Kreuter J, Duncan IB, Rubsamen-Waigmann H, Antimicrob. Agents Chemother 1996, 40, 1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Heiati H, Tawashi R, Phillips NC, Int. J. Pharm 1998, 174, 71. [Google Scholar]
- [105].Schafer V, Vonbriesen H, Andreesen R, Steffan AM, Royer C, Troster S, Kreuter J, Rubsamenwaigmann H, Pharmaceut. Res 1992, 9, 541. [DOI] [PubMed] [Google Scholar]
- [106].Lobenberg R, Araujo L, Kreuter J, Eur. J. Pharm. Biopharm 1997, 44, 127. [Google Scholar]
- [107].Lobenberg R, Araujo L, von Briesen H, Rodgers E, Kreuter J, J. Controlled Release 1998, 50, 21. [DOI] [PubMed] [Google Scholar]
- [108].Mainardes RM, Gremiao MPD, Brunetti IL, Da Fonseca LM, Khalil NM, J. Pharm. Sci 2009, 98, 257. [DOI] [PubMed] [Google Scholar]
- [109].Mishra V, Mahor S, Rawat A, Gupta PN, Dubey P, Khatri K, Vyas SP, J. Drug Target 2006, 14, 45. [DOI] [PubMed] [Google Scholar]
- [110].Yavuz B, Pehlivan SB, Unlu N, Sci. World J 2013, DOI: Artn 732340. 10.1155/2013/732340 [DOI] [Google Scholar]
- [111].Witvrouw M, Fikkert V, Pluymers W, Matthews B, Mardel K, Schols D, Raff J, Debyser Z, De Clercq E, Holan G, Pannecouque C, Mol. Pharmacol 2000, 58, 1100. [PubMed] [Google Scholar]
- [112].Dutta T, Agashe HB, Garg M, Balasubramanium P, Kabra M, Jain NK, J. Drug Target 2007, 15, 721. [DOI] [PubMed] [Google Scholar]
- [113].Kumar PD, Kumar PJ, Selvam TP, Sambasiva Rao KRS, Res. Pharm 2013, 3, 8. [Google Scholar]
- [114].Dutta T, Jain NK, BBA Gen. Subjects 2007, 1770, 681. [DOI] [PubMed] [Google Scholar]
- [115].Ardestani MS, Fordoei AS, Abdoli A, Cohan RA, Bahramali G, Sadat SM, Siadat SD, Moloudian H, Koopaei NN, Bolhasani A, Rahimi P, J. Mater. Sci. Mater. Med 2015, 26, 179. [DOI] [PubMed] [Google Scholar]
- [116].Gajbhiye V, Ganesh N, Barve J, Jain NK, Eur. J. Pharm. Sci 2013,48, 668. [DOI] [PubMed] [Google Scholar]
- [117].Gomara MJ, Perez-Pomeda I, Gatell JM, Sanchez-Merino V, Yuste E, Haro I, Nanomed. Nanotechnol 2017, 13, 601. [DOI] [PubMed] [Google Scholar]
- [118].a) Harvie P, Desormeaux A, Gagne N, Tremblay M, Poulin L, Beauchamp D, Bergeron MG, AIDS 1995, 9, 701; [DOI] [PubMed] [Google Scholar]; b) Harvie P, Desormeaux A, Bergeron MC, Tremblay M, Beauchamp D, Poulin L, Bergeron MG, Antimicrob. Agents Chemother 1996, 40, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Lalanne M, Paci A, Andrieux K, Dereuddre-Bosquet N, Clayette P, Deroussent A, Re M, Vassal G, Couvreur P, Desmaele D, Bioorg. Med. Chem. Lett 2007, 17, 2237. [DOI] [PubMed] [Google Scholar]
- [120].Betageri GV, Burrell LS, Int. J. Pharm 1993, 98, 149. [Google Scholar]
- [121].Gagne JF, Desormeaux A, Perron S, Tremblay MJ, Bergeron MG, BBA-Biomembranes 2002, 1558, 198. [DOI] [PubMed] [Google Scholar]
- [122].Dubey V, Mishra D, Nahar M, Jain V, Jain NK, Nanomed. Nanotechnol 2010, 6, 590. [DOI] [PubMed] [Google Scholar]
- [123].Kim SS, Peer D, Kumar P, Subramanya S, Wu HQ, Asthana D, Habiro K, Yang YG, Manjunath N, Shimaoka M, Shankar P, Mol. Ther 2010, 18, 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Garg M, Asthana A, Agashe HB, Agrawal GP, Jain NK, J.Pharm. Pharmacol 2006, 58, 605. [DOI] [PubMed] [Google Scholar]
- [125].Garg M, Dutta T, Jain NK, Eur. J. Pharm. Biopharm 2007, 67, 76. [DOI] [PubMed] [Google Scholar]
- [126].Kim S, Scheerer S, Geyer MA, Howell SB, J. Infect. Dis 1990,162, 750. [DOI] [PubMed] [Google Scholar]
- [127].Makabi-Panzu B, Gourde P, Desormeaux A, Bergeron MG, Cell Mol. Biol 1998, 44, 277. [PubMed] [Google Scholar]
- [128].Garg M, Jain NK, J. Drug Target 2006, 14, 1. [DOI] [PubMed] [Google Scholar]
- [129].Kaur CD, Nahar M, Jain NK, J. Drug Target 2008, 16, 798. [DOI] [PubMed] [Google Scholar]
- [130].a) Phillips NC, Skamene E, Tsoukas C, J. Acq. Immun. Def. Synd 1991, 4, 959; [PubMed] [Google Scholar]; b) Phillips NC, Tsoukas C, Blood 1992, 79, 1137. [PubMed] [Google Scholar]
- [131].Saiyed ZM, Gandhi NH, Nair MPN, Int. J. Nanomed 2010, 5,157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Jin SX, Bi DZ, Wang J, Wang YZ, Hu HG, Deng YH, Pharmazie 2005, 60, 840. [PubMed] [Google Scholar]
- [133].Dou H, Destache CJ, Morehead JR, Mosley RL, Boska MD, Kingsley J, Gorantla S, Poluektova L, Nelson JA, Chaubal M, Werling J, Kipp J, Rabinow BE, Gendelman HE, Blood 2006, 108, 2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Dou HY, Morehead J, Destache CJ, Kingsley JD, Shlyakhtenko L, Zhou Y, Chaubal M, Werling J, Kipp J, Rabinow BE, Gendelman HE, Virology 2007, 358, 148. [DOI] [PubMed] [Google Scholar]
- [135].Zhou JH, Shu Y, Guo PX, Smith DD, Rossi JJ, Methods 2011. 54, 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Naso MF, Tomkowicz B, Perry WL, Strohl WR, Biodrugs 2017, 31, 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Fuchs SP, Desrosiers RC, Mol. Ther. Meth. Clin. Dev 2016, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Andersen AHF, Riber CF, Zuwala K, Tolstrup M, Dagnæs-Hansen F, Denton PW, Zelikin AN, ACS Macro. Lett 2018, 7, 587. [DOI] [PubMed] [Google Scholar]
- [139].Ham AS, Lustig W, Yang L, Boczar A, Buckheit KW, Buckheit RW, PLoS One 2013, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Chupradit K, Moonmuang S, Nangola S, Kitidee K, Yasamut U, Mougel M, Tayapiwatana C, Viruses 2017, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Margolis DM, Garcia JV, Hazuda DJ, Haynes BF, Science 2016,353, aaf6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Deeks SG, Nat. Rev. Immunol 2012, 12, 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Weinberger LS, Burnett JC, Toettcher JE, Arkin AP, Schaffer DV, Cell 2005, 122, 169. [DOI] [PubMed] [Google Scholar]
- [144].a) Deeks SG, Lewin SR, Ross AL, Ananworanich J, Benkirane M, Cannon P, Chomont N, Douek D, Lifson JD, Lo YR, Kuritzkes D, Margolis D, Mellors J, Persaud D, Tucker JD, Barre-Sinoussi F, Alter G, Auerbach J, Autran B, Barouch DH, Behrens G, Cavazzana M, Chen ZW, Cohen EA, Corbelli GM, Eholie S, Eyal N, Fidler S, Garcia L, Grossman C, Henderson G, Henrich TJ, Jefferys R, Kiem HP, McCune J, Moodley K, Newman PA, Nijhuis M, Nsubuga MS, Ott M, Palmer S, Richman D, Saez-Cirion A, Sharp M, Siliciano J, Silvestri G, Singh J, Spire B, Taylor J, Tolstrup M, Valente S, van Lunzen J, Walensky R, Wilson I, Zack J, Grp IASCW, Nat. Med 2016, 22, 839; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Perreau M, Banga R, Pantaleo G, Trends Mol. Med 2017, 23, 945; [DOI] [PubMed] [Google Scholar]; c) Darcis G, Van Driessche B, Van Lint C, Trends Immunol. 2017, 38, 217. [DOI] [PubMed] [Google Scholar]
- [145].Banga R, Procopio FA, Noto A, Pollakis G, Cavassini M, Ohmiti K, Corpataux JM, de Leval L, Pantaleo G, Perreau M, Nat. Med 2016, 22, 754. [DOI] [PubMed] [Google Scholar]
- [146].Kandathil AJ, Sugawara S, Balagopal A, Retrovirology 2016, 13, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].a) Barton K, Winckelmann A, Palmer S, Trends Microbiol. 2016, 24, 345; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Estes JD, Kityo C, Ssali F, Swainson L, Makamdop KN, Del Prete GQ, Deeks SG, Luciw PA, Chipman JG, Beilman GJ, Hoskuldsson T, Khoruts A, Anderson J, Deleage C, Jasurda J, Schmidt TE, Hafertepe M, Callisto SP, Pearson H, Reimann T, Schuster J, Schoephoerster J, Southern P, Perkey K, Shang L, Wietgrefe SW, Fletcher CV, Lifson JD, Douek DC, McCune JM, Haase AT, Schacker TW, Nat. Med 2017, 23, 1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].a) Lorenzo-Redondo R, Fryer HR, Bedford T, Kim EY, Archer J, Pond SLK, Chung YS, Penugonda S, Chipman JG, Fletcher CV, Schacker TW, Malim MH, Rambaut A, Haase AT, McLean AR,Wolinsky SM, Nature 2017, 551, E10; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Rosenbloom DIS, Hill AL, Laskey SB, Siliciano RF, Nature 2017, 551, E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Schwartz C, Bduchat S, Marban C, Gautier V, Van Lint C, Rohr O,Le Douce V, Biochem. Pharmacol 2017, 146, 10. [DOI] [PubMed] [Google Scholar]
- [150].a) Mousseau G, Kessing CF, Fromentin R, Trautmann L, Chomont N, Valente ST, Mbio 2015, 6, e00465–15; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kim H, Choi MS, Inn KS, Kim BJ, Sci. Rep 2016, 6, 28896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].a) Pegu A, Asokan M, Wu L, Wang KY, Hataye J, Casazza JP, Guo XT, Shi W, Georgiev I, Zhou TQ, Chen XJ, O’Dell S, Todd JP, Kwong PD, Rao SS, Yang ZY, Koup RA, Mascola JR, Nabel GJ, Nat. Commun 2015, 6, 8447; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Sung JAM, Pickeral J,Liu LQ, Stanfield-Oakley SA, Lam CYK, Garrido C, Pollara J,LaBranche C, Bonsignori M, Moody MA, Yang YH, Parks R, Archin N, Allard B, Kirchherr J, Kuruc JD, Gay CL, Cohen MS, Ochsenbauer C, Soderberg K, Liao HX, Montefiori D, Moore P, Johnson, Koenig, Haynes, Nordstrom JL, Margolis DM,Ferrari G, J. Clin. Invest 2015, 125, 4077; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Sloan DD, Lam CYK Irrinki A, Liu LQ, Tsai A, Pace CS, Kaur J, Murry JP, Balakrishnan M, Moore PA, Johnson S, Nordstrom JL, Cihlar T, Koenig S, PLoS Pathog. 2015, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].a) Archin NM, Bateson R, Tripathy MK, Crooks AM, Yang KH, Dahl NP, Kearney MF, Anderson EM, Coffin JM, Strain MC, Richman DD, Robertson KR, Kashuba AD, Bosch RJ,Hazuda DJ, Kuruc JD, Eron JJ, Margolis DM, J. Infect. Dis 2014, 210, 728; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Rasmussen TA, Tolstrup M, Brinkmann CR,Olesen R, Erikstrup, Solomon A, Winckelmann A, Palmer,Dinarello C, Buzon M, Lichterfeld M, Lewin SR, Ostergaard L,Sogaard OS, Lancet Hiv. 2014, 1, E13; [DOI] [PubMed] [Google Scholar]; c) Sogaard OS, Graversen ME, Leth S, Olesen R, Brinkmann CR, Nissen SK, Kjaer AS, Schleimann MH, Denton PW, Hey-Cunningham WJ,Koelsch KK, Pantaleo G, Krogsgaard K, Sommerfelt M, Fromentin R, Chomont N, Rasmussen TA, Ostergaard L, Tolstrup M, PLoS Pathog. 2015, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Mehla R, Bivalkar-Mehla S, Zhang RN, Handy I, Albrecht H,Giri S, Nagarkatti P, Nagarkatti M, Chauhan A, PLoS One 2010, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Novis CL, Archin NM, Buzon MJ, Verdin E, Round JL, Lichterfeld M, Margolis DM, Planelles V, Bosque A, Retrovirology 2013, 10, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Wightman F, Solomon A, Kumar SS, Urriola N, Gallagher K, Hiener B, Palmer S, Mcneil C, Garsia R, Lewin SR, AIDS 2015, 29, 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Spina CA, Anderson J, Archin NM, Bosque A, Chan J, Famiglietti M, Greene WC, Kashuba A, Lewin SR, Margolis DM,Mau M, Ruelas D, Saleh S, Shirakawa K, Siliciano RF, Singhania A, Soto PC, Terry VH, Verdin E, Woelk C, Wooden S, Xing SF, Planelles V, PLoS Pathogens 2013, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].a) Donahue DA, Kuhl BD, Sloan RD, Wainberg MA, J. Virol 2012, 86, 3253; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Geng GN, Liu BF, Chen CC, Wu K, Liu J, Zhang YJ, Pan T, Li J, Yin Y, Zhang JS, Huang F, Yu F, Chen JL,Ma XC, Zhou J, Kuang ES, Liu C, Cai WP, Zhang H, Mol. Ther 2016, 24, 1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].a) Chen D, Wang M, Zhou S, Zhou Q, EMBO J. 2002, 21, 6801; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Rozzi SJ, Avdoshina V, Fields JA, Trejo M, Ton HT, Ahern GP,Mocchetti I, Neurotox. Res 2017, 32, 723; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Erazo-Oliveras A, Najjar K, La Dayani L, Wang TY, Johnson GA, Pellois JP, Nat. Methods 2014, 11, 861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].a) Guthrie KM, Vargas S, Shaw JG, Rosen RK, van denBerg JJ, Kiser PF, Buckheit K, Bregman D, Thompson L, Jensen K,Johnson T, PLoS One 2015, 10, e0145642; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Guthrie KM, Dun siger S, Vargas SE, Fava JL, Shaw JG, Rosen RK, Kiser PF, Kojic EM, Friend DR, Katz DF, AIDS Res. Hum. Retrov 2016, 32, 1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].a) Johnston MI, Fauci AS New Engl. J. Med 2007, 356, 2073; [DOI] [PubMed] [Google Scholar]; b) Burton DR, Ahmed R, Barouch DH, Butera ST, Crotty S,Godzik A, Kaufmann DE, McElrath MJ, Nussenzweig MC, Pulendran B, Scanlan CN, Schief WR, Silvestri G, Streeck H, Walker BD, Walker LM, Ward AB, Wilson IA, Wyatt R, Cell Host Microbe 2012, 12, 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Haynes BF, Mascola JR, Immunol. Rev 2017, 275, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].a) Gouws E, White PJ, Stover J, Brown T, Sex Transm. Infect 2006, 82(Suppl 3), iii51; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Centers for Disease Control and Prevention, HIV Surveillance Report, 2015, http://www.aidsinfoonline.org/kpatlas/document/kp_data_sources.pdf (accessed: April 18, 2018). [Google Scholar]
- [163].Tuero I, Robert-Guroff M, Viruses 2014, 6, 3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Wijesundara DK, Ranasinghe C, Grubor-Bauk B, Gowans EJ,Front. Microbiol 2017, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Mckay PF, Mann JFS, Pattani A, Kett V, Aldon Y, King D, Malcolm RK, Shattock RJ, J. Controlled Release 2017, 249, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Tan HX, Wheatley AK, Esterbauer R, Jegaskanda S, Glass JJ,Masopust D, De Rose R, Kent SJ, Mucosal. Immunol 2017, 10.1038/mi.2017.89 [DOI] [PubMed] [Google Scholar]
- [167].Malherbe DC, Mendy J, Vang L, Barnette PT, Reed J, Lakhashe SK, Owuor J, Gach JS, Legasse AW, Axthelm MK, LaBranche CC, Montefiori D, Forthal DN, Park B, Wilson JM, McLinden JH, Xiang JH, Stapleton JT, Sacha JB, Haynes BF,Liao HX, Ruprecht RM, Smith J, Gurwith M, Haigwood NL, Alexander J, J. Virol 2018, 92, JVI-01092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E,Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH, New Engl. J. Med 2009, 361, 2209. [DOI] [PubMed] [Google Scholar]
- [169].NIH-NIAID, http://www.niaid.nih.gov/news-events/first-new-hivvaccine-efficacy-study-seven-years-has-begun (accessed: April 17, 2018).
- [170].Barouch DH, Klasse PJ, Dufour J, Veazey RS, Moore JP, Proc. Natl. Acad. Sci. U. S. A 2012, 109, 8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Le Grand R, Dereuddre-Bosquet N, Dispinseri S, Gosse L, Desjardins D, Shen XY, Tolazzi M, Ochsenbauer C, Saidi H, Tomaras G, Prague M, Barnett SW, Thiebaut R, Cope A, Scar latti G, Shattock RJ, J. Virol 2016, 90, 5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].a) Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY,Vargas L, Goicochea P, Casapía M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernández T, Veloso VG,Buchbinder SP, Chariyalertsak S, Schechter M, Bekker L-G, Mayer KH, Kallás EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang F, McConnell JJ, Zheng J-H, Lee J, Rooney JF, Jaffe HS, Martinez AI,Burns DN, Glidden DV, New Engl. J. Med 2010, 363, 2587;21091279 [Google Scholar]; b) Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, Tappero JW, Bukusi EA, Cohen CR, Katabira E, Ronald A, Tumwesigye E, Were E, Fife KH, Kiarie J, Farquhar C, John-Stewart G, Kakia A, Odoyo J, Mucunguzi A, Nakku-Joloba E,Twesigye R, Ngure K, Apaka C, Tamooh H, Gabona F, Mujugira A, Panteleeff D, Thomas KK, Kidoguchi L, Krows M, Revall J,Morrison S, Haugen H, Emmanuel-Ogier M, Ondrejcek L, Coombs RW, Frenkel L, Hendrix C, Bumpus NN, Bangsberg D, Haberer JE, Stevens WS, Lingappa JR, Celum C, New Engl. J. Med 2012, 367, 399;22784037 [Google Scholar]; c) Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, Henderson FL, Pathak SR, Soud FA, Chillag KL, Mutanhaurwa R, Chirwa LI, Kasonde M, Abebe D, Buliva E, Gvetadze RJ, Johnson S, Sukalac T, Thomas VT,Hart C, Johnson JA, Malotte CK, Hendrix CW, Brooks JT, New. Engl. J. Med 2012, 367, 423;22784038 [Google Scholar]; d) Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JHS, Godbole SV,Mehendale S, Chariyalertsak S, Santos BR, Mayer KH, Hoffman IF, Eshleman SH, Piwowar-Manning E, Wang L, Makhema J,Mills LA, de Bruyn G, Sanne I, Eron J, Gallant J, Havlir D, Swindells S, Ribaudo H, Elharrar V, Burns D, Taha TE, Nielsen Saines K, Celentano D, Essex M, Fleming TR, New. Engl. J. Med 2011, 365, 493.21767103 [Google Scholar]
- [173].Davies O, Ustianowski A, Fox J, Infect. Dis. Ther 2016, 5, 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].FDA, https://aidsinfo.nih.gov/news/1254/fda-approves-first-drugfor-reducing-the-risk-of-sexually-acquired-hiv-infection (accessed: July 2018).
- [175].Giler RM, Magnuson D, Trevor H, Bush S, Rawlings K, McCallister S, in 9th International AIDS Society Conference on HIV Science, France 2017. [Google Scholar]
- [176].Ray AS, Fordyce MW, Hitchcock MJ, Antiviral Res. 2016, 125,63. [DOI] [PubMed] [Google Scholar]
- [177].CONRAD 137, https://www.avac.org/trial/conrad-137 (accessed: April 17, 2018).
- [178].DISCOVER, https://www.avac.org/trial/discover (accessed: April 17, 2018).
- [179].NCT03205566, https://clinicaltrials.gov/ct2/show/NCT03205566?term=Raltegravir+prep&cond=HIV&rank=1 (accessed: July 2018).
- [180].Neff CP, Ndolo T, Tandon A, Habu Y, Akkina R, PLoS One 2010,5, e15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Massud I, Aung W, Martin A, Bachman S, Mitchell J, Aubert R,Solomon Tsegaye T, Kersh E, Pau CP, Heneine W, Garcia Lerma JG, J. Virol 2013, 87, 8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Fox J, Tiraboschi JM, Herrera C, Else L, Egan D, Dickinson L, Jackson A, Olejniczak N, Back D, Khoo S, Shattock R, Boffito M, J. Acquir. Immune Defic. Syndr 2016, 73, 252. [DOI] [PubMed] [Google Scholar]
- [183].Obiero J, Mwethera PG, Wiysonge CS, Cochrane Database Syst. Rev 2012, 6, CD007961. [DOI] [PubMed] [Google Scholar]
- [184].a) Shattock RJ, Rosenberg Z, Cold Spring Harb. Perspect. Med 2012, 2, a007385; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Friend DR, Kiser PF, Antiviral Res 2013, 99, 391; [DOI] [PubMed] [Google Scholar]; c) Herrera C, Shattock RJ, Curr. Top Microbiol. Immunol 2014, 383, 1; [DOI] [PubMed] [Google Scholar]; d) Alexandre KB, Mufhandu HT, London GM, Chakauya E, Khati M, Virology 2016, 497, 69; [DOI] [PubMed] [Google Scholar]; e) Notario-Perez F,Ruiz-Caro R, Veiga-Ochoa MD, Drug Des. Devel. Ther 2017, 11, 1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Olmsted SS, Khanna KV, Ng EM, Whitten ST, Johnson ON 3rd, Markham RB, Cone RA, Moench TR, BMC Infect. Dis 2005, 5, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].a) Nunn KL, Wang YY, Harit D, Humphrys MS, Ma B, Cone R, Ravel J, Lai SK, MBio. 2015, 6, e01084; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Borgdorff H, Tsivtsivadze E, Verhelst R, Marzorati M, Jurriaans S, Ndayisaba GF, Schuren FH, van de Wijgert JHHM, ISME J. 2014, 8, 1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Mitsuya H, Looney DJ, Kuno S, Ueno R, Wong-Staal F, Broder S,Science 1988, 240, 646. [DOI] [PubMed] [Google Scholar]
- [188].a) Stafford MK, Ward H, Flanagan A, Rosenstein IJ, Taylor-Robinson D, Smith JR, Weber J, Kitchen VS, J. Acquir. Immune Defic. Syndr. Hum. Retrovirol 1998, 17, 327; [DOI] [PubMed] [Google Scholar]; b) Fichorova RN, Tucker LD, Anderson DJ, J. Infect. Dis 2001, 184, 418; [DOI] [PubMed] [Google Scholar]; c) Van Damme L, Ramjee, Alary M, Vuylsteke B, Chandeying V, Rees H,Sirivongrangson P, Mukenge-Tshibaka L, Ettiegne-Traore V, Uaheowitchai, Karim SS, Masse B, Perriens J, Laga M, C O L-1492 Study Group, Lancet 2002, 360, 971. [DOI] [PubMed] [Google Scholar]
- [189].Fletcher P, Herrera C, Armanasco N, Nuttall J, Shattock RJ, AIDS Res. Hum. Retroviruses 2016, 32, 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Herrera C, Armanasco N, Garcia-Perez J, Ziprin P, Olejniczak N,Alcami J, Nuttall J, Shattock RJ, AIDS 2016, 30, 1015. [DOI] [PubMed] [Google Scholar]
- [191].Liu T, Huang B, Zhan P, De Clercq E, Liu X, Eur. J. Med. Chem 2014, 86, 481. [DOI] [PubMed] [Google Scholar]
- [192].Haaland RE, Evans-Strickfaden T, Holder A, Pau CP, Mc-Nicholl JM, Chaikummao S, Chonwattana W, Hart CE, Antimicrob. Agents Chemother 2012, 56, 3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Herrera C, Shattock RJ, Curr. Hiv. Res 2012, 10, 42. [DOI] [PubMed] [Google Scholar]
- [194].Herrera C, Shattock RJ, Curr. Top. Microbiol 2014, 383, 1. [DOI] [PubMed] [Google Scholar]
- [195].Steinbach JM, Cell. Mol. Life Sci 2015, 72, 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].a) Alexandre KB, Gray ES, Lambson BE, Moore PL, Choge IA,Mlisana K, Karim SS, McMahon J, O’Keefe B, Chikwamba R, Morris L, Virology 2010, 402, 187; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Balzarini J, Antiviral. Res 2006, 71, 237; [DOI] [PubMed] [Google Scholar]; c) Xue J, Gao YG, Hoorelbeke B, Kagiampakis I, Zhao B,Demeler B, Bazarini J, LiWang PJ, Mol. Pharmaceut 2012, 9, 2613; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Xue J, Hoorelbeke B, Kagiampakis I, Demeler B, Balzarini J, LiWang PJ, Antimicrob. Agents Chemother 2013, 57, 3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Lusvarghi S, Bewley CA, Viruses 2016, 8, 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].a) O’Keefe BR, Vojdani F, Buffa V, Shattock RJ, Montefiori DC,Bakke J, Mirsalis J, d’Andrea AL, Hume SD, Bratcher B, Saucedo CJ, McMahon JB, Pogue GP, Palmer KE, Proc. Natl. Acad. Sci. U. S. A 2009, 106, 6099; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Fuqua JL, Wanga V, Palmer KE, BMC Biotechnol. 2015, 15, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Gaertner H, Cerini F, Escola JM, Kuenzi G, Melotti A, Offord R,Rossitto-Borlat I, Nedellec R, Salkowitz J, Gorochov G, Mosier D,Hartley O, Proc. Natl. Acad. Sci. U. S. A 2008, 105, 17706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Cerini F, Gaertner H, Madden K, Tolstorukov I, Brown S, Laukens B, Callewaert N, Harner JC, Oommen AM, Harms JT, Sump AR, Sealock RC, Peterson DJ, Johnson SK, Abramson SB, Meagher M, Offord R, Hartley O, Protein. Expr. Purif 2016, 119, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Cerini F, Landay A, Gichinga C, Lederman MM, Flyckt R, Starks D, Offord RE, Le Gal F, Hartley O, J. Acquir. Immune. Defic. Syndr 2008, 49, 472. [DOI] [PubMed] [Google Scholar]
- [202].a) Pang W, Tam SC, Zheng YT, Antivir. Chem. Chemother 2009, 20, 1; [DOI] [PubMed] [Google Scholar]; b) Sassi AB, Cost MR, Cole AL, Cole AM, Patton DL, Gupta P, Rohan LC, Antimicrob. Agents Chemother. 2011, 55, 2282; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Harman S, Herrera C, Armanasco N, Nuttall J, Shattock RJ, Antimicrob. Agents Chemother. 2012, 56, 2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].a) Kagiampakis I, Gharibi A, Mankowski MK, Snyder BA, Ptak RG, Alatas K, LiWang PJ, Antimicrob. Agents Chemother. 2011, 55, 264; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhao B, Mankowski MK, Snyder BA, Ptak RG, Liwang PJ, J. Biol. Chem 2011, 286, 28370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Burton DR, Hangartner L, Annu. Rev. Immunol 2016, 34, 635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Veselinovic M, Neff CP, Mulder LR, Akkina R, Virology 2012,432, 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Sok D, Doores KJ, Briney B, Le KM, Saye-Francisco KL, Ramos A, Kulp DW, Julien JP, Menis S, Wickramasinghe L, Seaman MS, Schief WR, Wilson IA, Poignard P, Burton DR, Sci. Transl. Med 2014, 6, 23ra63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Julg B, Liu PT, Wagh K, Fischer WM, Abbink P, Mercado NB,Whitney JB, Nkolola JP, McMahan K, Tartaglia LJ, Bor ducchi EN, Khatiwada S, Kamath M, LeSuer JA, Seaman MS, Schmidt SD, Mascola JR, Burton DR, Korber BT, Barouch DH, Sci. Transl. Med 2017, 9, eaao4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].a) Rosenberg Y, Sack M, Montefiori D, Forthal D, Mao LJ, Hernandez-Abanto S, Urban L, Landucci G, Fischer R, Jiang XM, PLoS One 2013, 8, e58724; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Teh AYH, Maresch D, Klein K, Ma JKC, Plant Biotechnol. J 2014, 12, 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].a) Rosen RK, Morrow KM, Carballo-Dieguez A, Mantell JE,Hoffman S, Gai F, Maslankowski L, El-Sadr WM, Mayer KH,J. Womens Health 2008, 17, 383; [DOI] [PubMed] [Google Scholar]; b) Weld ED, Hiruy H, Guthrie KM, Fava JL, Vargas SE, Buckheit K, Buckheit R, Spiegel H,Breakey J, Fuchs EJ, Hendrix CW, AIDS Res. Hum. Retrov 2017, 33, 440; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Machado RM, Palmeira-De-Oliveira A, Martinez-De Oliveira J, Palmeira-de-Oliveira R, J. Pharm. Sci 2013, 102, 2069; [DOI] [PubMed] [Google Scholar]; d) Malcolm RK, Boyd PJ, McCoy CF, Murphy DJ, Adv. Drug De liver. Rev 2016, 103, 33; [DOI] [PubMed] [Google Scholar]; e) das Neves J, Nunes R, Rodrigues F, Sarmento B, Adv. Drug Deliver. Rev 2016, 103, 57; [DOI] [PubMed] [Google Scholar]; f) Blakney AK, Ball C, Krogstad EA, Woodrow KA, Antivir. Res 2013, 100, S9; [DOI] [PubMed] [Google Scholar]; g) Grooms TN, Vuong HR, Tyo KM, Malik DA, Sims LB, Whittington CP, Palmer KE, Matoba N, Steinbach-Rankins JM, Antimicrob. Agents Chemother 2016, 60, 6518; [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Alvarez B, Fernandez LA, Microb. Biotechnol 2017, 10, 1057; [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Brichacek B, Lagenaur LA, Lee PP, Venzon D, Hamer DH, PLoS One 2013, 8, e78817; [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Palomino RAN, Zicari S, Vanpouille C, Vitali B, Margolis L, Front. Microbiol 2017, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Agashe H, Hu M, Rohan L, Curr. Hiv. Res 2012, 10, 88. [DOI] [PubMed] [Google Scholar]
- [211].das Neves J, Bahia MF, Int. J. Pharm 2006, 318, 1. [DOI] [PubMed] [Google Scholar]
- [212].Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC,Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M,Morris L, Taylor D, Group CT, Science 2010, 329, 1168.20643915 [Google Scholar]
- [213].Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N,Nair G, Palanee T, Nakabiito C, van der Straten A, Noguchi L, Hendrix CW, Dai JY, Ganesh S, Mkhize B, Taljaard M, Parikh UM,Piper J, Masse B, Grossman C, Rooney J, Schwartz JL, Watts H,Marzinke MA, Hillier SL, McGowan IM, Chirenje ZM, Team VS, Engl N. J. Med 2015, 372, 509. [Google Scholar]
- [214].Rees H, Delaney-Moretlwe SA, Lombard C, Baron D, Panchia R,Myer L, Schwartz JL, Doncel GF, Gray G, in CROI. Foundation and International Antiviral Society ” San Francisco, CA: 2015, Abstract Number 26LB. [Google Scholar]
- [215].Nunes R, Sarmento B, das Neves J, J. Control Release 2014, 194,278. [DOI] [PubMed] [Google Scholar]
- [216].a) McGowan I, Hoesley C, Cranston RD, Andrew P, Janocko L,Dai JY, Carballo-Dieguez A, Ayudhya RK, Piper J, Hladik F, Mayer K, PLoS One 2013, 8, e60147; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) McGowan I, Cranston RD,Duffill K, Siegel A, Engstrom JC, Nikiforov A, Jacobson C, Rehman KK, Elliott J, Khanukhova, Abebe K, Mauck C, Spiegel HM, Dezzutti CS, Rohan, Marzinke MA, Hiruy H, Hendrix CW, Richardson-Harman N, Anton PA, PLoS One 2015, 10, e0125363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Cranston RD, Lama JR, Richardson BA, Carballo-Dieguez A, Kunjara Na Ayudhya RP, Liu K, Patterson KB, Leu CS, Galaska B,Jacobson CE, Parikh UM, Marzinke MA, Hendrix CW, Johnson S, Piper JM, Grossman C, Ho KS, Lucas J, Pickett J, Bekker LG, Chariyalertsak S, Chitwarakorn A, Gonzales P, Holtz TH,Liu AY, Mayer KH, Zorrilla C, Schwartz JL, Rooney J, McGowan I, Team MTNP, Clin. Infect. Dis 2017, 64, 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Richardson-Harman N, Mauck C, McGowan I, Anton P, AIDS Res. Hum. Retrov 2012, 28, 1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].a) Valenta C, Adv. Drug Deliv. Rev 2005, 57, 1692; [DOI] [PubMed] [Google Scholar]; b) Perioli L, Ambrogi V, Venezia L, Pagano C, Ricci M, Rossi C, Colloids Surf. B Biointerfaces 2008, 66, 141; [DOI] [PubMed] [Google Scholar]; c) Baloglu E, Senyigit ZA, Kara vana SY, Bernkop-Schnurch A, J. Pharm. Pharm. Sci 2009, 12, 312. [DOI] [PubMed] [Google Scholar]
- [220].a) Kouokam JC, Lasnik AB, Palmer KE, Viruses 2016, 8, 311; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Barton C, Kouokam JC, Hurst H, Palmer KE, Viruses 2016, 8, 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].McBride JW, Dias N, Cameron D, Offord RE, Hartley O, Boyd P,Kett VL, Malcolm RK, Antimicrob. Agents Chemother 2017, 61, e00965–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Cerini F, Offord R, McGowan I, Hartley O, AIDS Res. Hum. Retro viruses 2017, 33, 768. [DOI] [PubMed] [Google Scholar]
- [223].Morris GC, Wiggins RC, Woodhall SC, Bland JM, Taylor CR,Jespers V, Vcelar BA, Lacey CJ, PLoS One 2014, 9, e116153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Clark MR, Peet MM, Davis S, Doncel GF, Friend DR, Pharmaceutics 2014, 6, 616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Notario-Perez F, Cazorla-Luna R, Martin-Illana A, Ruiz-Caro R, Tamayo A, Rubio J, Veiga MD, Carbohydr. Polym 2018, 179, 305. [DOI] [PubMed] [Google Scholar]
- [226].McConville C, Major I, Devlin B, Brimer A, Eur. J. Pharm. Biopharm 2016, 104, 171. [DOI] [PubMed] [Google Scholar]
- [227].Machado RM, Palmeira-de-Oliveira A, Martinez-De-Oliveira J, Palmeira-de-Oliveira R, J. Pharm. Sci 2013, 102, 2069. [DOI] [PubMed] [Google Scholar]
- [228].Akil A, Parniak MA, Dezzuitti CS, Moncla BJ, Cost MR, Li M, Rohan LC, Drug Deliv. Transl. Res 2011, 1, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Akil A, Agashe H, Dezzutti CS, Moncla BJ, Hillier SL, Devlin B,Shi Y, Uranker K, Rohan LC, Pharm. Res 2015, 32, 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].NCT02579083, https://clinicaltrials.gov/ct2/show/NCT02579083(accessed; July 2018).
- [231].Zhang L, Herrera C, Coburn J, Olejniczak N, Ziprin P, Ka plan DL, LiWang PJ, ACS Biomater. Sci. Eng 2017, 3, 1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].a) Yucel T, Lovett ML, Keplan DL, J. Controlled Release 2014, 190,381; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Seib FP, Pritchard EM, Kaplan DL, Adv. Funct. Mater 2013, 23, 58; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Chiu B, Coburn J, Pilichowska M, Holcroft C, Seib FP, Charest A, Kaplan DL, Brit. J. Cancer 2014, 111, 708; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Yucel T, Lovett ML, Giangregorio R, Coonahan E, Kaplan DL, Biomaterials 2014, 35, 8613; [DOI] [PubMed] [Google Scholar]; e) Mwangi TK, Bowles RD, Tainter DM,Bell RD, Kaplan DL, Setton LA, Int. J. Pharm 2015, 485, 7; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Seib FP, Coburn J, Konrad I, Klebanov N, Jones GT, Black wood B, Charest A, Kaplan DL, Chiu B, Acta Biomaterialia 2015, 20, 32; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Coburn J, Harris J, Zakharov AD, Poirier J, Ikegaki N,Kajdacsy-Balla A, Pilichowska M, Lyubimov AV, Shimada H, Kaplan DL, Chiu B, Int. J. Cancer 2017, 140, 726; [DOI] [PubMed] [Google Scholar]; h) Stinson J, Raja WK, Lee S, Kim HB, Diwan I, Tutunjian, Panilaitis B, Omenetto FG, Tzipori S, Kaplan DL, ACS Biomater. Sci. Eng 2017, 3, 360. [DOI] [PubMed] [Google Scholar]
- [233].Mishell DR, Lumkin ME, Fertil. Steril 1970, 21, 99. [DOI] [PubMed] [Google Scholar]
- [234].a) Malcolm RK, Edwards KL, Kiser P, Romano J, Smith TJ,Antiviral. Res 2010, 88(Suppl 1), S30; [DOI] [PubMed] [Google Scholar]; b) Malcolm RK, Boyd PJ,McCoy CF, Murphy DJ, Adv. Drug Deliv. Rev 2016, 103, 33. [DOI] [PubMed] [Google Scholar]
- [235].Malcolm K, Woolfson D, Russell J, Andrews C, J. Control Release 2003, 91, 355. [DOI] [PubMed] [Google Scholar]
- [236].a) Malcolm RK, Woolfson AD, Toner CF, Morrow RJ, McCullagh SD, J. Antimicrob. Chemother 2005, 56, 954; [DOI] [PubMed] [Google Scholar]; b) Devlin B, Nuttall J, Wilder S, Woodsong C, Rosenberg Z, Antiviral. Res 2013, 100(Suppl), S3. [DOI] [PubMed] [Google Scholar]
- [237].Johnson TJ, Clark MR, Albright TH, Nebeker JS, Tuitupou AL, Clark JT, Fabian J, McCabe RT, Chandra N, Doncel GF, Friend DR, Kiser PF, Antimicrob. Agents Chemother 2012, 56, 6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Nel A, van Niekerk N, Kapiga S, Bekker LG, Gama C, Gill K, Kamali A, Kotze P, Louw C, Mabude Z, Miti N, Kusemererwa S, Tempelman H, Carstens H, Devlin B, Isaacs M, Malherbe M, Mans W, Nuttall J, Russell M, Ntshele S, Smit M, Solai L, Spence P, Steytler J, Windle K, Borremans M, Resseler S, Van Roey J, Parys W, Vangeneugden T, Van Baelen B, Rosenberg Z, Ring Study T, Engl N. J. Med 2016, 375, 2133. [Google Scholar]
- [239].Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, Mgodi NM, Matovu Kiweewa F, Nair G, Mhlanga F, Siva S, Bekker LG, Jeenarain N, Gaffoor Z, Martin son F, Makanani B, Pather A, Naidoo L, Husnik M, Richard son BA, Parikh UM, Mellors JW, Marzinke MA, Hendrix CW, van der Straten A, Ramjee G, Chirenje ZM, Nakabiito C, Taha TE, Jones J, Mayo A, Scheckter R, Berthiaume J, Livant E, Jacob son C, Ndase P, White R, Patterson K, Germuga D, Galaska B, Bunge K, Singh D, Szydlo DW, Montgomery ET, Mensch BS, Torjesen K, Grossman CI, Chakhtoura N, Nel A, Rosenberg Z,McGowan I, Hillier S, Team M-AS, Engl N. J. Med 2016, 375, 2121. [Google Scholar]
- [240].Baeten J, presented at Conf. Retroviruses and Opportunistic Infections,Boston, MA, March 2018. [Google Scholar]
- [241].Nel A, Van Niekerk N, Van Baelen B, Rosenberg Z, HIV Incidence and Adherence in Dream: An Open-Label Trial of Dapivirine Vaginal Ring. Conf. Retroviruses and Opportunistic Infections, Boston, MA, March 4–7, 2018. [Google Scholar]
- [242].Johnson TJ, Gupta KM, Fabian J, Albright TH, Kiser PF, Eur. J. Pharm. Sci 2010, 39, 203. [DOI] [PubMed] [Google Scholar]
- [243].Fetherston SM, Boyd P, McCoy CF, McBride MC, Edwards KL,Ampofo S, Malcolm RK, Eur. J. Pharm. Sci 2013, 48, 406. [DOI] [PubMed] [Google Scholar]
- [244].Murphy DJ, Desjardins D, Dereuddre-Bosquet N, Brochard P,Perrot L, Pruvost A, Le Grand R, Lagatie O, Vanhooren L, Feyaerts M, van Roey J, Malcolm RK, J. Antimicrob. Chemother 2014, 69, 2477. [DOI] [PubMed] [Google Scholar]
- [245].Chen BA, Panther L, Marzinke MA, Hendrix CW, Hoesley CJ,van der Straten A, Husnik MJ, Soto-Torres L, Nel A, Johnson S,Richardson-Harman N, Rabe LK, Dezzutti CS, J. Acquir. Immune. Defic. Syndr 2015, 70, 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].De Clercq E, Int. J. Antimicrob. Agents 2009, 33, 307. [DOI] [PubMed] [Google Scholar]
- [247].a) Herrera C, Cranage M, McGowan I, Anton P, Shattock RJ,AIDS 2011, 25, 1971; [DOI] [PubMed] [Google Scholar]; b) Dezzutti CS, Yandura S, Wang L, Moncla B, Teeple EA, Devlin B, Nuttall J, Brown ER, Rohan LC, Pharm. Res 2015, 32, 3768; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Yang Y, Zhu, Hassink M, Jenkins LMM, Wan Y, Appella DH, Xu J, Appella E, Zhang X, Emerg. Microbes. Infect 2017, 6, e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Pattani A, Lowry D, Curran RM, McGrath S, Kett VL, An drews GP, Malcolm RK, Int. J. Pharm 2012, 430, 89. [DOI] [PubMed] [Google Scholar]
- [249].Murphy DJ, Amssoms K, Pille G, Clarke A, O’Hara M, vanRoey J, Malcolm RK, Drug Deliv. Transl. Res 2016, 6, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Morrow RJ, Woolfson AD, Donnelly L, Curran R, Andrews G,Katinger D, Malcolm RK, Eur. J. Pharm. Biopharm 2011, 77, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Zhao C, Gunawardana M, Villinger F, Baum MM, Remedios-Chan M, Moench TR, Zeitlin L, Whaley KJ, Bohorov O, Smith TJ,Anderson DJ, Moss JA, Antimicrob. Agents Chemother 2017,61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Ensign LM, Cone R, Hanes J, J. Control Release 2014, 190, 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].das Neves J, Nunes R, Rodrigues F, Sarmento B, Adv. Drug. Deliv. Rev 2016, 103, 57. [DOI] [PubMed] [Google Scholar]
- [254].Ham AS, Cost MR, Sassi AB, Dezzutti CS, Rohan LC, Pharm. Res 2009, 26, 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].a) Date AA, Shibata A, McMullen E, La Bruzzo K, Bruck P, Belshan M, Zhou Y, Destache CJ, J. Biomed. Nanotechnol 2015, 11, 416; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) das Neves J, Sarmento B, Acta Biomater. 2015, 18, 77; [DOI] [PubMed] [Google Scholar]; c) Machado A, Cunha-Reis C, Araujo F, Nunes R, Seabra V, Ferreira D, das Neves J, Sarmento B, Acta Biomater. 2016, 44, 332; [DOI] [PubMed] [Google Scholar]; d) Cunha-Reis C, Machado A, Barreiros L, Araujo F, Nunes R, Seabra V, Ferreira D, Segundo MA, Sarmento B, das Neves J, J. Control Release 2016, 243, 43. [DOI] [PubMed] [Google Scholar]
- [256].a) D’Cruz OJ, Uckun FM, Expert Opin. Drug Deliv 2014, 11, 723; [DOI] [PubMed] [Google Scholar]; b) Antimisiaris SG, Mourtas S, Adv. Drug Deliv. Rev 2015, 92, 123. [DOI] [PubMed] [Google Scholar]
- [257].Blakney AK, Ball C, Krogstad EA, Woodrow KA, Antiviral Res.2013, 100(Suppl), S9. [DOI] [PubMed] [Google Scholar]
- [258].a) Chou SF, Carson D, Woodrow KA, J. Control Release 2015,220, 584; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Sebe I, Szabo P, Kallai-Szabo B, Zelko R, Int. J. Pharm 2015, 494, 516. [DOI] [PubMed] [Google Scholar]
- [259].a) Krogstad EA, Woodrow KA, Int. J. Pharm 2014, 475, 282; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ball C, Chou SF, Jiang Y, Woodrow KA, Mater. Sci. Eng C Mater. Biol. Appl 2016, 63, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Grooms TN, Vuong HR, Tyo KM, Malik DA, Sims LB, Whittington CP, Palmer KE, Matoba N, Steinbach-Rankins JM, Antimicrob. Agents Chemother 2016, 60, 6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Mendling W, Adv. Exp. Med. Biol 2016, 902, 83. [DOI] [PubMed] [Google Scholar]
- [262].Alvarez B, Fernandez LA, Microb. Biotechnol 2017, 10, 1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Liu X, Lagenaur LA, Simpson DA, Essenmacher KP, Frazier-Parker CL, Liu Y, Tsai D, Rao SS, Hamer DH, Parks TP, Lee PP, Xu Q, Antimicrob. Agents Chemother 2006, 50, 3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Lagenaur LA, Sanders-Beer BE, Brichacek B, Pal R, Liu X, Liu Y,Yu R, Venzon D, Lee PP, Hamer DH, Mucosal Immunol. 2011, 4, 648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Brichacek B, Lagenaur LA, Lee PP, Venzon D, Hamer DH,PLoS One 2013, 8, e78817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Yamamoto HS, Xu Q, Fichorova RN, BMC Microbiol. 2013, 13,4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Lagenaur LA, Swedek I, Lee PP, Parks TP, PLoS One 2015, 10, e0122730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Li M, Patton DL, Cosgrove-Sweeney Y, Ratner D, Rohan LC, Cole AM, Tarwater PM, Gupta P, Ramratnam B, J. Acquir. Immune. Defic. Syndr 2011, 58, 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].a) Harmsen HJ, de Goffau MC, Adv. Exp. Med. Biol 2016, 902,95; [DOI] [PubMed] [Google Scholar]; b) Walter J, Appl. Environ. Microbiol 2008, 74, 4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].a) Mirmonsef P, Spear GT, Am. J. Reprod. Immunol 2014, 71, 531; [DOI] [PubMed] [Google Scholar]; b) Borgdorff H, Tsivtsivadze E, Verhelst R, Marzorati M, Jurriaans S, Ndayisaba GF, Schuren FH, van de Wijgert JH, ISME J. 2014, 8, 1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Vangelista L, Secchi M, Liu X, Bachi A, Jia L, Xu Q, Lusso P,Antimicrob. Agents Chemother 2010, 54, 2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].a) Chang TL, Chang CH, Simpson DA, Xu Q, Martin PK, Lagenaur LA, Schoolnik GK, Ho DD, Hillier SL, Holodniy M, Lewicki JA, Lee PP, Proc. Natl. Acad. Sci. U. S. A 2003, 100, 11672; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liu X, Lagenaur LA, Lee PP, Xu Q, Appl. Environ. Microbiol 2008, 74, 4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Wang LX, Mellon M, Bowder D, Quinn M, Shea D, Wood C, Xiang SH, Virology 2015, 475, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Marcobal A, Liu X, Zhang W, Dimitrov AS, Jia L, Lee PP, Fouts TR, Parks TP, Lagenaur LA, AIDS Res. Hum. Retroviruses 2016, 32, 964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Nunn KL, Wang YY, Harit D, Humphrys MS, Ma B, Cone R,Ravel J, Lai SK, Mbio. 2015, 6, e01084–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Petricevic L, Unger FM, Viernstein H, Kiss H, Eur. J. Obstet. Gynecol. Reprod. Biol 2008, 141, 54. [DOI] [PubMed] [Google Scholar]
- [277].Klatt NR, Cheu R, Birse K, Zevin AS, Perner M, Noel-Romas L,Grobler A, Westmacott G, Xie IY, Butler J, Mansoor L, McK innon LR, Passmore JAS, Karim QA, Karim SSA, Burgener AD, Science 2017, 356, 938. [DOI] [PubMed] [Google Scholar]
- [278].Landovitz RJ, Kofron R, McCauley M, Curr. Opin. HIV AIDS 2016,11, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Barnhart M, Glob. Health Sci. Prac 2017, 5, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Trezza C, Ford SL, Spreen W, Pan R, Piscitelli S, Curr. Opin. Hiv. AIDS 2015, 10, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].a) Schlesinger E, Johengen D, Luecke E, Rothrock G, McGowan I,van der Straten A, Desai T, Pharmaceut. Res 2016, 33, 1649; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Chua CX, Jain P, Hu M, Anderson PL, Kimata JT, Sastry J, Arduino R, Grattoni A, in 24th Conference on Retroviruses and Oppor tunistic Infections (CROI 2017), Seattle, WA 2017. [Google Scholar]
- [282].Emini EA, Schleif WA, Nunberg JH, Conley AJ, Eda Y, Tokiyoshi S, Putney SD, Matsushrta S, Cobb KE, Jett CM, Eichberg JW, Murthy KK, Nature 1992, 355, 728. [DOI] [PubMed] [Google Scholar]
- [283].Pegu A, Yang Z.-y., Boyington JC, Wu L, Ko S-Y, Schmidt SD, McKee K, Kong W-P, Shi W, Chen X, Todd J-P, Letvin NL,Huang J, Nason MC, Hoxie JA, Kwong PD, Connors M,Rao SS, Mascola JR, Nabel GJ, Sci. Transl. Med 2014, 6, 243ra88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Gautam R, Nishimura Y, Pegu A, Nason MC, Klein F, Gazumyan A, Golijanin J, Buckler-White A, Sadjadpour R, Wang K,Mankoff Z, Schmidt SD, Lifson JD, Mascola JR, Nussen zweig MC, Martin MA, Nature 2016, 533, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Ko SY, Pegu A, Rudicell RS, Yang ZY, Joyce MG, Chen XJ,Wang KY, Bao S, Kraemer TD, Rath T, Zeng M, Schmidt SD,Todd JP, Penzak SR, Saunders KO, Nason MC, Haase AT,Rao SS, Blumberg RS, Mascola JR, Nabel GJ, Nature 2014, 514, 642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Ita K, Pharmaceutics 2015, 7, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Leone M, Monkare J, Bouwstra J, Kersten G, Pharmaceut. Res 2017, 34, 2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Zhou T, Georgiev I, Wu X, Yang Z-Y, Dai K, Finzi A, Do Kwon Y,Scheid JF, Shi W, Xu L, Yang Y, Zhu J, Nussenzweig MC, Sodroski J, Shapiro L, Nabel GJ, Mascola JR, Kwong PD, Science 2010, 329, 811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [289].NIH, https://www.niaid.nih.gov/news-events/nih-launches-largeclinical-trials-antibody-based-hiv-prevention (accessed: July 2018).
- [290].Wagh K, Bhattacharya T, Williamson C, Robles A, Bayne M, Gar rity J, Rist M, Rademeyer C, Yoon H, Lapedes A, Gao H, Greene K,Louder MK, Kong R, Karim SA, Burton DR, Barouch DH,Nussenzweig MC, Mascola JR, Morris L, Montefiori DC, Korber B, Seaman MS, PLoS Pathog. 2016, 12, e1005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].a) Ferir G, Palmer KE, Schols D, Virology 2011, 417, 253; [DOI] [PubMed] [Google Scholar]; b) Ferir G, Huskens D, Palmer KE, Boudreaux DM, Swanson MD,Markovitz DM, Balzarini J, Schols D, AIDS Res. Hum. Retroviruses 2012, 28, 1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].van der Straten A, Van Damme L, Haberer JE, Bangsberg DR, AIDS 2012, 26, F13. [DOI] [PubMed] [Google Scholar]
- [293].Kruk ME, Schwalbe N, Clin. Ther 2006, 28, 1989. [DOI] [PubMed] [Google Scholar]
- [294].a) Harrison PF, Hemmerling A, Romano J, Whaley KJ, YoungHolt B, AIDS Res. Treat 2013, 2013, 790154; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Friend DR, Expert Opin. Drug Deliv. 2016, 13, 533; [DOI] [PubMed] [Google Scholar]; c) Fernandez-Romero JA, Deal C,Herold BC, Schiller J, Patton D, Zydowsky T, Romano J, Petro CD, Narasimhan M, Trends Microbiol. 2015, 23, 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].Boyd P, Fetherston SM, McCoy CF, Major I, Murphy DJ, Kumar S, Holt J, Brimer A, Blanda W, Devlin B, Malcolm RK, Int. J. Pharm 2016, 511, 619. [DOI] [PubMed] [Google Scholar]