Abstract
The clinical use of the hormone leptin, a key regulator of food intake, to treat the most common instances of obesity has so far failed. In this issue, Zhao et al. (2019) report that, paradoxically, reducing leptin levels in obese mice increases their sensitivity to the concentrations that remain, and leads to reductions in weight gain, thus suggesting why these earlier trials may have failed and possibly a new approach to treating obesity.
The protein hormone leptin, which is produced and released by adipose tissue, provides a readout of the overall energy stores of the body, while also being a key regulator of food intake. Based on the biology of the Lepob/ob mouse, from which the gene for leptin was cloned, and on the rescue of the obesity in these mice by administration of leptin, the prevailing— and generally correct—view is that deficiency of the hormone leads to hyperphagic obesity, and supraphysiological levels lead to loss of body fat. But in this issue of the journal, Zhao et al. (2019) convincingly show that in some mouse models of obesity, lowering leptin causes weight loss and elevating leptin causes weight gain. Is the conventional model wrong, then? Do we need to revise our understanding of the physiology of this important component of the system that regulates body weight in rodents and in humans? The answer is no; as for insulin, so it is for leptin. Namely, metabolic context is key with regard to understanding the hormone's functional biology.
Leptin may be regarded as an evolutionary solution to a mammal’s need to ensure sufficient fat stores to survive in circumstances of insufficient environmental calories, and in this context not to commit to energy-costly physiological endeavors; for example, pregnancy without sufficient calories to support the resulting fetuses and neonates. One way to control energy stores is to regulate feeding behavior. Thus, the circulating concentration of leptin, which is proportional to aggregate adipocyte size and number, informs the brain and its feeding circuitry of the status of stored fat. Behavioral and metabolic homeostatic processes, mediated by those circuits, respond to that information. Evolutionary considerations would suggest that the circuiťs main role would be defense of fat stores, not their reduction. Thus, the system would be physiologically asymmetrical, responding much more powerfully to reductions than increases in fat stores. In fact, due to the risk to survival, the circuit would auto-suppress in the context of “supraphysiological” concentrations of leptin (Figure 1). Using these concepts as context, the results reported by Zhao et al. make sense—at least for mice. What they have done is to test these systems at the inflection points of the functional valences of leptin’s actions and shown that, under some circumstances, these valences can be reversed.
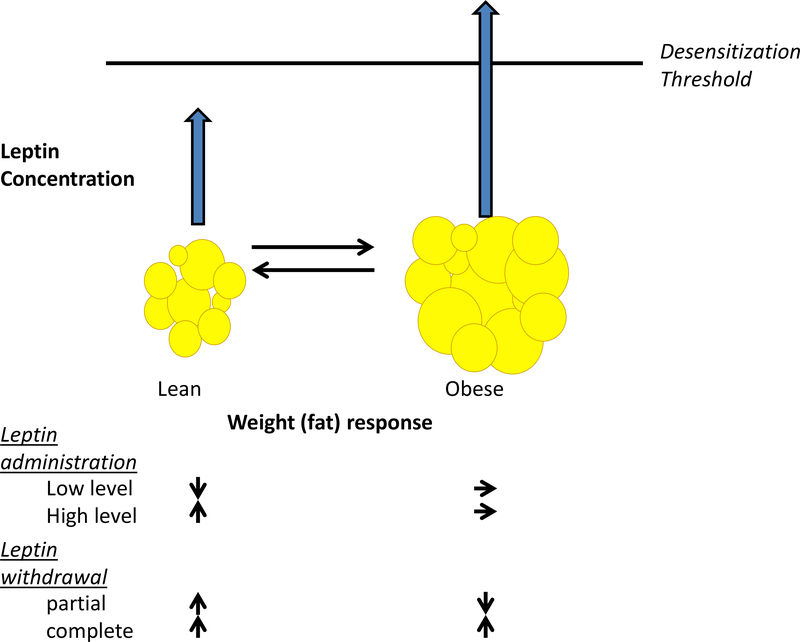
Figure 1. Valence of Physiological Responses to Changes in Ambient Leptin Is Dependent on the Relationship of the New Concentration to Cellular Desensitization Threshold.
There is a level of circulating leptin, above which the mouse or human hypothalamus (and probably other brain regions) is desensitized. This threshold is determined by concurrent metabolic circumstance (related to adiposity) and probably by developmental and genetic factors, as well as cell-type specificities. In the lean state, adding a small amount of leptin does not raise the concentration above the threshold, so weight is lost; adding a large amount of leptin raises concentrations above the desensitization threshold: the axis becomes insensitive and weight is gained. If the individual is sufficiently obese, the leptin threshold may be exceeded so that the organism is desensitized to additional leptin. Withdrawing leptin in the lean state leads to weight gain. In the obese state, partially withdrawing leptin restores sensitivity to ambient leptin and leads to weight loss. In both states, complete withdrawal leads to weight gain. This contextual dependence accounts for the apparently paradoxical effects on energy intake and expenditure of raising or lowering ambient leptin in obese or lean individuals.
Obesity raises circulating leptin concentrations to the point where the neural responses auto-suppress (Balland and Cowley, 2015; Pan and Myers, 2018), using molecular mechanisms that are only partially understood. If, in these circumstances, leptin concentrations are functionally reduced—in this case by clever intercurrent transgenesis or by use of blocking antibodies—the responsiveness of neural circuits is relieved and ambient leptin concentrations restore ingestive and neuroendocrine homeostasis. That is, in specific circumstances, less leptin restores its own conventional physiological functions. In these circumstances, similar effects occur if leptin receptor function is partially impaired (Balland et al., 2019). Conversely, and by reciprocal biological effects, transgenic elevations of leptin can result in acute desensitization and weight gain. So don’t discard your leptin textbooks.
Even so, using transgenic manipulations of endogenous adipocyte leptin production, Zhao et al. report seemingly heterodox responses to transient reductions and elevations of circulating leptin concentration in adult male mice. Two techniques were used to reduce circulating leptin concentrations: transgenic mice with doxycycline-inhibited alleles of leptin and neutralizing anti-leptin antibodies. Using these methods to moderately reduce functional circulating leptin inmature animals,the authors found that obese mice, being fed high-fat chow, gained less body fat when circulating leptin was reduced. When they decreased leptin more completely, using a leptin-floxed mouse and a higher dosage of doxycycline, these mice gained more body fat relative to the control mice, indicating the system’s sensitivity to the relative degree of leptin reduction. The authors also made a mouse that modestly overexpresses leptin using an inducible adipocyte-specific leptin transgene. This mouse, when dosed with doxycycline, increased circulating leptin to levels comparable to those seen in obese animals; the leptin-overexpressing mice gained more weight than the control mice when exposed to high-fat chow. Importantly, all of these leptin manipulations were conducted in adult mice with mature feeding circuitry. Since leptin participates in the maturation of its own hypothalamic circuitry (Bouret et al., 2004), responses in congenital leptin-deficient animals/individuals to manipulations such as those described here would be anticipated to be (and are) different.
What is most interesting here is the ability of these mouse models to separate metabolic effects of obesity from the associated elevations of circulating leptin, and to thereby implicate the functions of the molecule itself in the induction and relief of resistance to its effects in the hypothalamus. Many studies have implicated the metabolic consequences of obesity (elevated blood lipids, neuro-inflammation, etc.) in the induction of relative hypothalamic leptin insensitivity (Pan and Myers, 2018). These are certainly playing a role, but as the studies reported here suggest, leptin itself may be a major mediator of these effects. And if so, there are potential clinical implications.
The clinical literature is consistent with some, but not all, inferences that might be drawn from this report. Human defense mechanisms against hyperleptinemia appear to be hypersensitive (Rosenbaum et al., 2005). Even modest increases in circulating leptin levels may blunt responses to the hormone, accounting for the failures of (relatively) too highdose leptin interventions for the treatment of existing obesity (Heymsfield et al., 1999). However, as predicted, weight-reduced humans in whom circulating leptin concentrations are lowered due to the loss of fat mass show diminished hunger and increased energy expenditure in response to leptin (Rosenbaum et al., 2005). Importantly, however, in humans, weight loss and lowered ambient leptin do not themselves restore auto-sensitivity to endogenous leptin, unlike Zhao’s animals with chronic transgenic hypoleptinemia, which is not induced by weight reduction. In humans there is an apparent interaction of hypoleptinemia with loss of fat mass, possibly implicating other adipokines in this axis (Ravussin et al., 2014).
If modest hyperleptinemia can desensitize this axis, why don’t all of us get fat? The axis must, for example, permit regulated fat gain in childhood and pregnancy. How are these processes controlled? The majority of genes implicated in polygenic human obesity are expressed primarily in the brain (Willer et al., 2009). We consider that they participate in circuits that are directly or indirectly leptin-responsive. Perhaps some of these genes affect the mechanisms that mediate auto-inhibition of leptin’s actions. Such differences would be consistent with the significant difference among individuals in responses to leptin administration in various clinical contexts (Rosenbaum and Leibel, 2018).
Since environmentally driven weight gain and self-imposed weight reduction are events unanticipated by our evolution, we should perhaps pay more attention to the implications of studies like those of Zhao et al. that inform the physiology of weight homeostasis at these inflection points of physiology.
ACKNOWLEDGMENTS
Funding was provided by the Russell Berrie Foundation (Teaneck, NJ, USA) and NIH grants RO1-DK52431 and P30-DK26687.
REFERENCES
- Balland E, and Cowley MA (2015). New insights in leptin resistance mechanisms in mice. Front. Neuroendocrinol. 39, 59–65. [DOI] [PubMed] [Google Scholar]
- Balland E, Chen W, Dodd GT, Conductier G, Coppari R, Tiganis T, and Cowley MA (2019). Leptin signaling in the arcuate nucleus reduces insulin’s capacity to suppress hepatic glucose production in obese mice. Cell Rep. 26, 346–355.e3. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, and Simerly RB (2004). Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 304, 108–110. [DOI] [PubMed] [Google Scholar]
- Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, and McCamish M (1999). Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 282, 1568–1575. [DOI] [PubMed] [Google Scholar]
- Pan WW, and Myers MG Jr. (2018). Leptin and the maintenance of elevated body weight. Nat. Rev. Neurosci. 19, 95–105. [DOI] [PubMed] [Google Scholar]
- Ravussin Y, Leibel RL, and Ferrante AW Jr. (2014). A missing link in body weight homeostasis: the catabolic signal of the overfed state. Cell Metab. 20, 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum M, and Leibel RL (2018). Physiological responses to leptin levels in lipodystrophy: a model for other hypoleptinemias? J. Clin. Invest. 128, 3237–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, and Leibel RL (2005). Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J. Clin. Invest. 115, 3579–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, Berndt SI, Elliott AL, Jackson AU, Lamina C, et al. ; Wellcome Trust Case Control Consortium; Genetic Investigation of ANthropometric Traits Consortium (2009). Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat. Genet. 41, 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Zhu Y, Schultz RD, Li N, He Z, Zhang Z, Caron A, Zhu Q, Sun K, Xiong W, et al. (2019). Partial leptin reduction as an effective weight loss strategy. Cell Metab. 30, this issue, 706–719. [DOI] [PMC free article] [PubMed] [Google Scholar]