Abstract
Introduction:
In locked plate fixation of proximal humerus fractures, the calcar is an important anchor point for screws providing much-needed medial column support. Most locking plate implants utilize a fixed-trajectory locking screw to achieve this goal. Consequently, adjustments of plate location to account for patient-specific anatomy may result in a screw position outside of the calcar. To date, little is known about the consequences of “missing” the calcar during plate positioning. This study sought to characterize the biomechanics associated with proximal and distal placement of locking plates in a two-part fracture model.
Materials and methods:
This experiment was performed twice, first with elderly cadaveric specimens and again with osteoporotic sawbones. Two-part fractures were simulated and specimens were divided to represent proximal, neutral, and distal plate placements. Non-destructive torsional and axial compression tests were performed prior to an axial fatigue test and a ramp to failure. Torsional stiffness, axial stiffness, humeral head displacement and stiffness during fatigue testing, and ultimate load were compared between groups.
Results:
Cadavers: Proximal implant placement led to trends of decreased mechanical properties, but there were no significant differences found between groups. Sawbones: Distal placement increased torsional stiffness in both directions (p = 0.003, p = 0.034) and axial stiffness (p = 0.018) when compared to proximal placement. Distal placement also increased torsional stiffness in external rotation (p = 0.020), increased axial stiffness (p = 0.024), decreased humeral head displacement during fatigue testing, and increased stiffness during fatigue testing when compared to neutral placement.
Discussion:
The distal and neutral groups had similar mechanical properties in many cadaveric comparisons while the proximal group trended towards decreased construct stiffness.
Results:
from the Sawbones model were more definitive and provided further evidence that proximal calcar screw placements are undesirable and distal implant placement may provide improved construct stability.
Conclusion:
Successful proximal humerus fracture reconstruction is inherent upon anatomic fracture reduction coupled with medial column support. Results from this experiment suggest that missing the calcar proximally is deleterious to fixation strength, while it is safe, and perhaps even desirable, to aim slightly distal to the intended target.
Keywords: Proximal humerus, Fracture, Locking plates, Biomechanics, Fixation strategy
Introduction
Proximal humerus fractures, accounting for over 5% of the fractures in adults [1,2], are the third most common fractures in the elderly [3–5], and are expected to increase 3-fold in the next 30 years [6]. Open reduction and internal fixation (ORIF) is an attractive option for the repair of proximal humerus fractures because it restores native anatomy and allows for early return of function. The recent advent of locking plates in proximal humerus fracture ORIF has improved outcomes [7,8]. Despite the benefits of locking plate fixation, humeral head collapse, fixation failure, and hardware-related complications have led to poor outcome rates between 27% and 59% in some studies [9–12].
Optimization of proximal humerus locking plate design is an avidly researched topic. Previous studies have sought to improve fixation by introducing extra screws or blades into the humeral head [13,14] or by injecting calcium phosphate cement into the cancellous bone [15,16]. The added value of using fibular strut augmentation [13,17,18] and polyaxial screws [19–23] has also been explored. While these studies are valuable, they often utilize additional materials during implantation, which ultimately increases time in the operating room and imposes an additional financial burden.
Several studies have focused on the use of the calcar as an anchor point for screws that are intended to provide medial column support, a technique that has been shown to provide resistance to humeral head collapse [13,24–26]. In many implant designs, humeral head screws have a fixed trajectory relative to the plate. Because the plate and locked screws have a predefined geometry, proximal or distal adjustments of plates may ultimately result in screw purchase outside of the calcar. To date, little is known about the biomechanical consequences of “missing” the calcar during implantation.
The purpose of this study was to characterize the biomechanics of a locked plate construct when the implant is aligned neutrally, distally, and proximally. The goal was to provide surgeons with guidelines for implant placement if optimal calcar screw position is not readily achieved. We hypothesized that missing the calcar by 8 mm in either direction would lead to undesirable changes in fixation strength of the repaired construct. Similarly, we also hypothesized that missing the calcar would lead to increased migration of the humeral head during cyclic testing and decreased failure strength.
Materials and methods
This study was first performed with cadaveric specimens and repeated with Sawbones models. Twelve matched pairs of fresh-frozen cadaveric arm specimens from 8 females and 4 males (average age 78.6 years, range 66 to 96 years) were assigned to the following groups: cadaveric neutral (CN, n = 8); cadaveric proximal (CP, n = 8); and cadaveric distal (CD, n = 8) (Fig. 1). Nine left osteoporotic humerus Sawbones models (#1028-130, Pacific Research Laboratories, Vashon Island, WA) were also used. Specimens were assigned the following groups: Sawbones neutral (SN; n = 3), Sawbones distal (SD; n = 3), and Sawbones proximal (SP; n = 3).
Fig. 1.
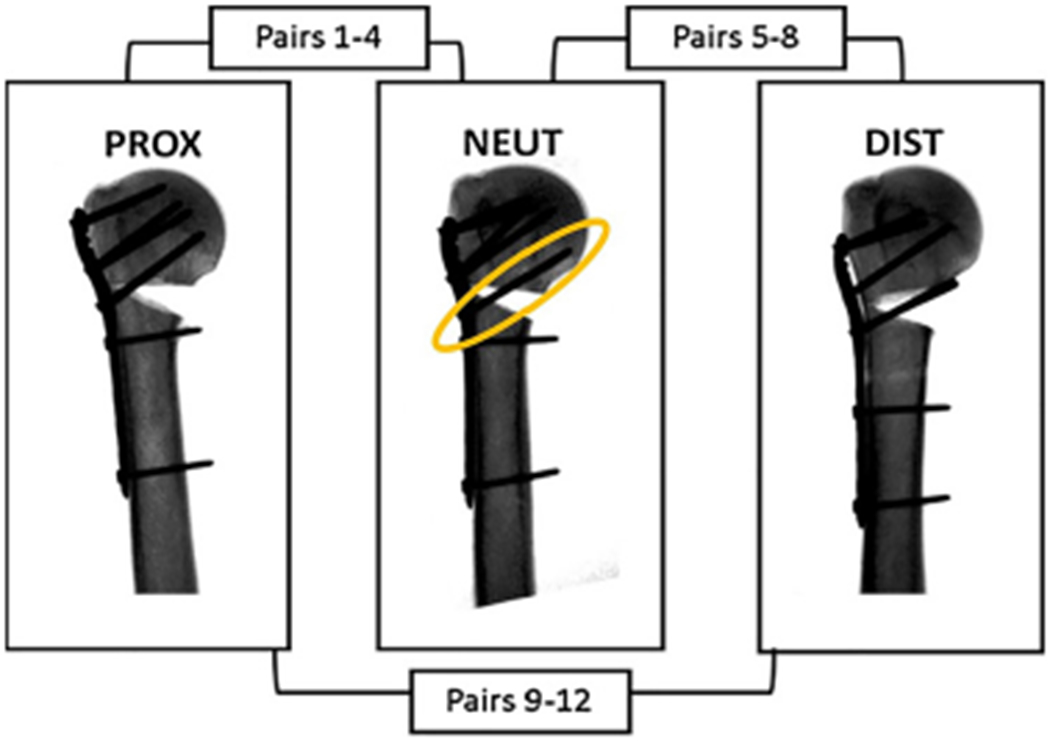
A schematic of the distribution of matched pairs between the proximal, neutral, and distal groups for cadaveric testing. Fluoroscopic images represent how changes in plate placement affect screw purchase into the calcar (circled in yellow). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The number of cadaveric samples used was based on results from a previous study that quantified the biomechanics of proximal humeri with and without calcar screws [24]. We hypothesized that missing the calcar would decrease the previously reported axial stiffness (278.5 N/mm) by at least 25%, while the standard deviation would remain similar to the previous values (40 N/mm). Therefore, the following input parameters were used in an a priori ANOVA sample size analysis: expected difference in mean between groups = 69.6, standard deviation = 40, number of groups = 3, desired power = 0.8, and α = 0.05.
Specimen preparation
Cadaveric specimens were stored at −20 °C and thawed overnight prior to implantation. The humerus was disarticulated from the shoulder joint and transected at the midshaft. In order to simulate an unstable two-part fracture, a defined 30° transverse wedge osteotomy was created with an oscillating saw for all specimens (Fig. 1).
All implantation procedures were performed with a single locking plate design (LCP Proximal Humerus, DePuy Synthes, West Chester, PA). Neutrally aligned plates were positioned according to manufacturer guidelines and care was taken to ensure that pilot holes were drilled directly into the calcar, approximately 3 mm superior to the outer cortex. Drills were left in the specimens and fluoroscopy was used to ensure proper implant placement prior to insertion of screws. The same procedure was used to create proximally and distally placed implants with 8 mm offsets.
Final implantation was achieved with a predefined set of 3.5 mm screws (two 36 mm cortex, two 36 mm locking, two 44 mm locking, and two 48 mm locking). For the cadaveric specimens, screws sizes were selected in a manner to optimize length without violating the articular surface. For the Sawbones models, the screw pattern was kept constant across all specimens. Distal humeri were potted into polycarbonate cylinders filled with rigid epoxy resin (Bondo, 3 M, Maplewood, MN). Cadaveric specimens were refrigerated for no more than 48 h prior to biomechanical testing.
Biomechanical testing
The methods used in this experiment were based on previously published protocols that also sought to characterize the biomechanics of proximal humerus implants [13,27]. All testing was performed in a universal testing frame (TA Instruments ElectroForce 3550, Eden Prairie, MN) equipped with a 15 kN/49 Nm load/torque cell.
First, a non-destructive torsional stiffness test was performed (Fig. 2A). The humeral head was gripped by blunt screws and custom-built aluminum jigs were connected to universal joints so torsion about the long axis of the bone was isolated. Internal and external torques were applied to the humeral head under displacement control at a constant speed of 0.1°/s. Torque limits were set ±3.5 Nm for the cadaveric specimens and ±1.5 Nm for the Sawbones models. The cadaveric torque limits were chosen based on previous estimations of in-vivo measurements during activities of daily living [28] which also falls within the range of torques applied during similar experiments [13,29,30]. The limits were lower for the Sawbones experiment because 3.5 Nm created unrealistically high amounts of angular displacement between the humeral head and shaft. Each specimen was cycled 4 times, and the mean torsional stiffness from the last three cycles was determined by calculating the average slope of the linear portions of torque-angular displacement curves during loading.
Fig. 2.
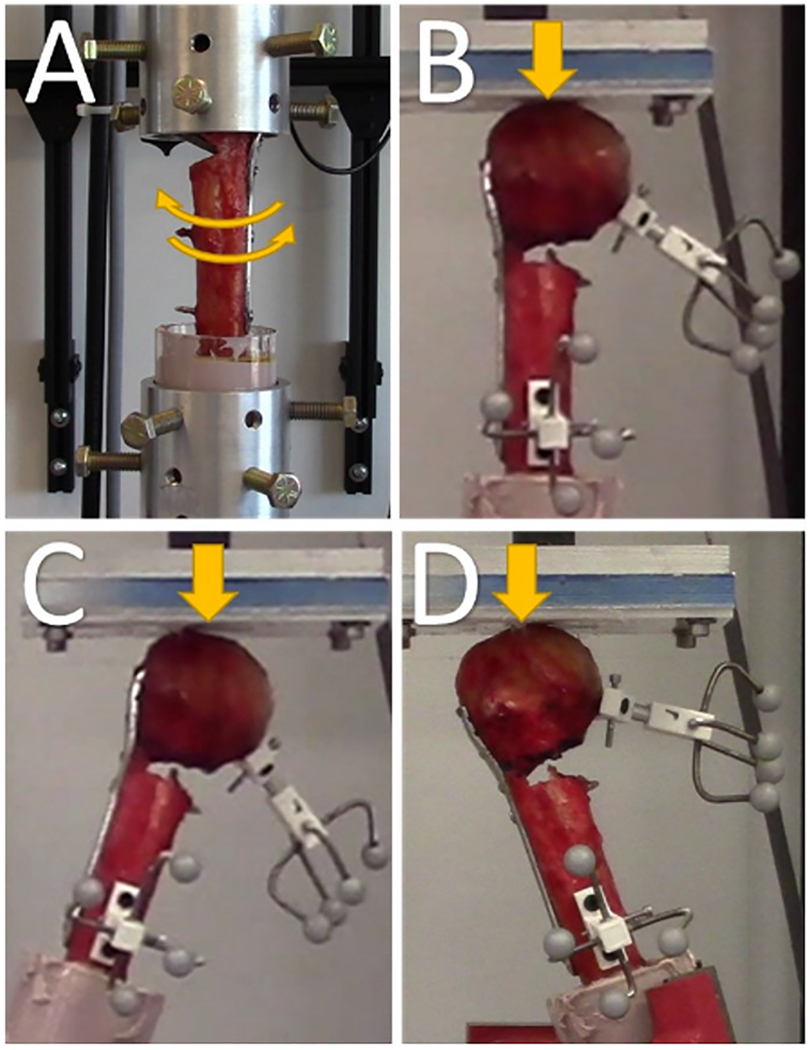
Photographs of the four different testing modalities: (A) torsional testing, (B) Axial (0°) testing, (C) Abduction (+20°) testing, and (D) Adduction (−20°) testing. Cyclic fatigue testing was run in the axial position for 5000 cycles before a ramp to failure was performed.
Next, a battery of nondestructive quasi-static compression tests were performed. The specimens were mounted to a rotating vice and tested at 0°, 20° abduction, and 20° adduction positions (Fig. 2B–D). An aluminum-backed Delryn plate acted as an articulating surface for the humeral head and was coated in petroleum jelly to minimize shear forces. Triangle waveforms were used to impose compressive loads between 15 and 200 N under displacement control at a rate of 0.1 mm/s. Each specimen was cycled 4 times, and the mean stiffness from last three cycles was determined by calculating the average slope of the linear portions of the force-displacement curves. All specimens were all tested in the same order: 0°, 20° abduction, and 20° adduction.
Finally, cyclic loading and ramp to failure tests were performed. Specimens were aligned at 0° (Fig. 2B) and subjected to compressive sinusoidal loads ranging between 50 and 250 N for 5000 cycles at a rate of 1 Hz. The humeral head displacement and stiffness of the construct were recorded at 1,10, 50,100, 500,1000, 2000, 3000, 4000, and 5000 cycles. Immediately following the completion of the fatigue protocol, all specimens were loaded to failure by applying a compressive force under displacement control at a rate of 0.1 mm/s. Ramp to failure stiffness and ultimate load were recorded.
Relative displacements of the humeral head and shaft were recorded with three-dimensional motion tracking techniques during compressive testing. An Optitrack motion capture system (NaturalPoint, Inc., Corvallis, OR) was used and calibrated such that 0.2 mm accuracy of marker tracking was achieved. Individual marker clusters were securely attached to the head and shaft of the humerus (Fig. 2). To quantify the relative displacement between the humeral head and shaft, two local coordinate systems were established such that movement of the humeral head or shaft would result in concomitant movement of their respective local coordinate system. The local coordinate systems were initially oriented such that they overlapped perfectly at the medial edge of the shaft at the wedge osteotomy. Relative displacement between fragments were quantified by calculating the Euclidean distance between the coordinate system origins.
Statistical analyses were conducted using SigmaStat version 4.0 (Systat Software, Inc., Germany). One-way ANOVAs were initially run to determine the presence of significant differences between groups. If significant differences existed, either Mann-Whitney Rank Sum tests or Holm-Sidak tests were performed to make pairwise comparisons.
Results
Cadaveric
CP specimens exhibited non-significant trends of decreased mechanical properties in internal torsional stiffness (Fig. 3) and 0° axial stiffness (Fig. 4). Additionally, the CP group had a significantly higher amount of displacement than CD at the 100th cycle of the fatigue test (Fig. 5). This behavior was not observed at any other time during cyclic testing. Otherwise, there were no significant differences found between groups for any other measure. Means, standard deviations, and p-values for all cadaveric comparisons can be found in the Supplementary materials.
Fig. 3.
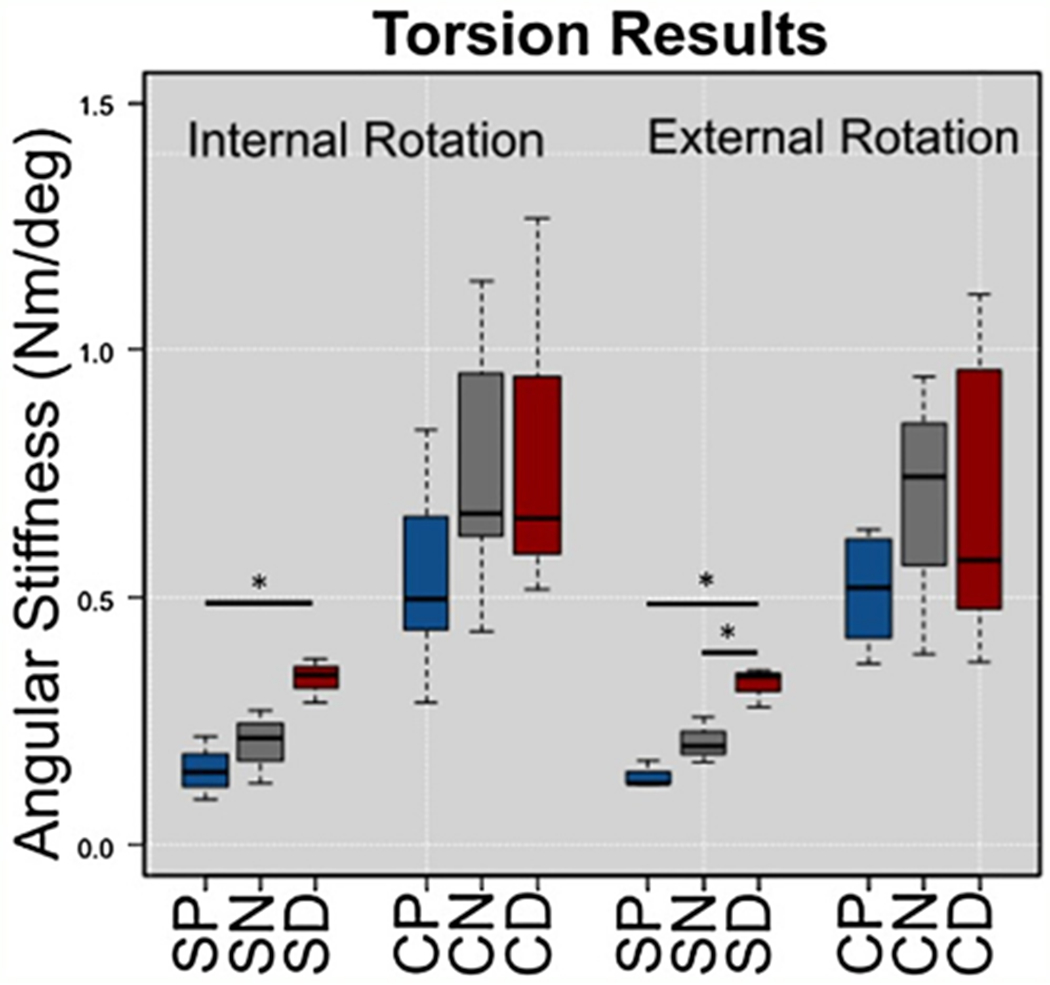
Box and whisker plots from torsional testing for Sawbones (S) and Cadaveric (C) specimens with proximal (P), neutral (N), and distal (D) plate positions. SD was significantly stiffer than SP in both directions, and was also stiffer than SN in external rotation. There were trends, but no significant differences between cadaveric groups.
Fig. 4.
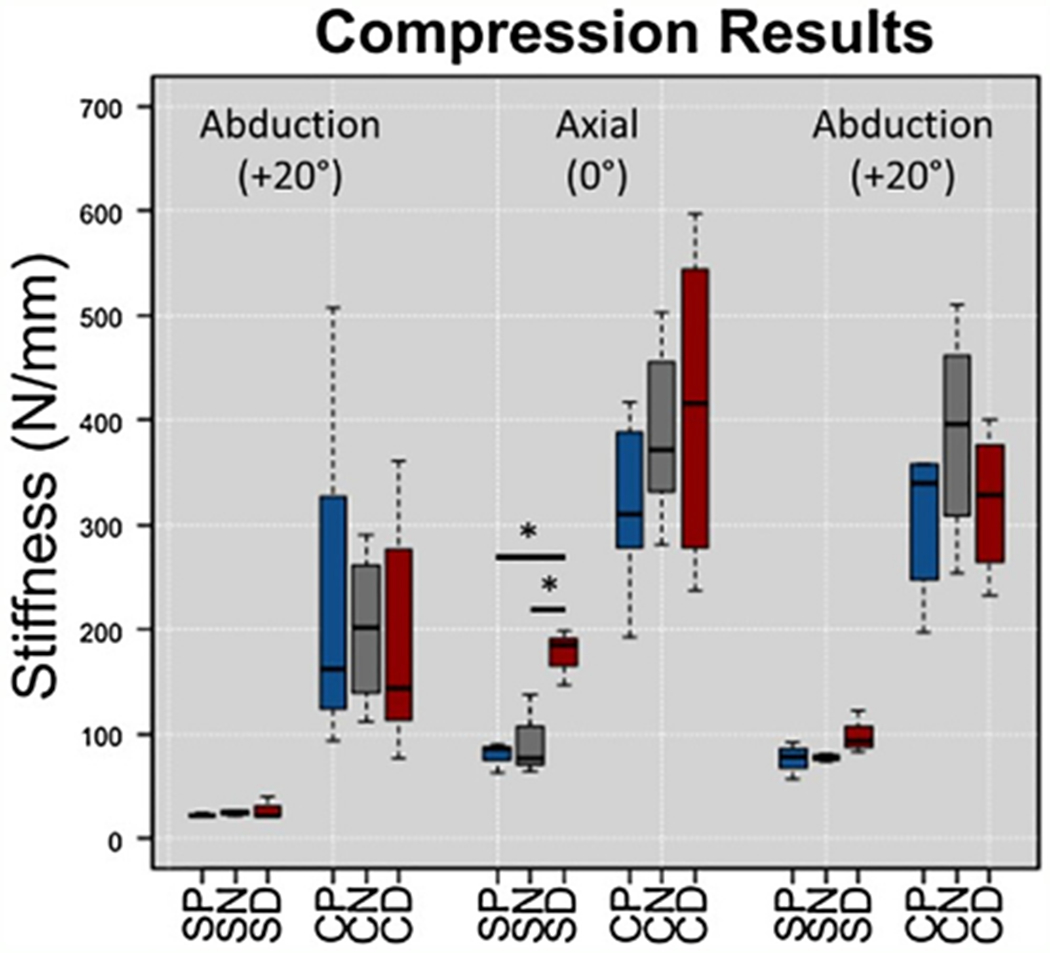
Box and whisker plots from compression testing for Sawbones (S) and Cadaveric (C) specimens with proximal (P), neutral (N), and distal (D) plate positions. SD was significantly stiffer than SP and SN during axial testing. There were no significant differences between cadaveric groups.
Fig. 5.
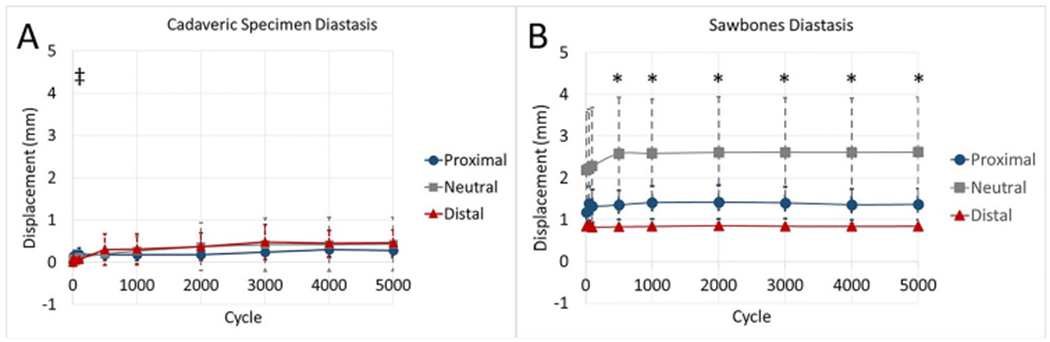
Comparisons of relative displacement between the humeral head and shaft during cyclic testing for (A) cadaveric and (B) Sawbones specimens. During cadaveric testing, the distal group had significantly less diastasis than the proximal group at 100 cycles (‡). During Sawbones testing, the distal group had significantly less diastasis than the neutral group at multiple time points (*). The scale of graphs was kept constant to depict differences between the models, and error bars represent ± 1 standard deviation.
Sawbones
When compared to the SP group, the SD cohort exhibited increased torsional stiffness in both internal and external rotation (p = 0.003 and p = 0.034, respectively) (Fig. 3). Axial stiffness of SD during 0° testing was also higher than SP (p = 0.018) (Fig. 4), and SD was consistently stiffer than SP throughout fatigue testing (Fig. 6).
Fig. 6.
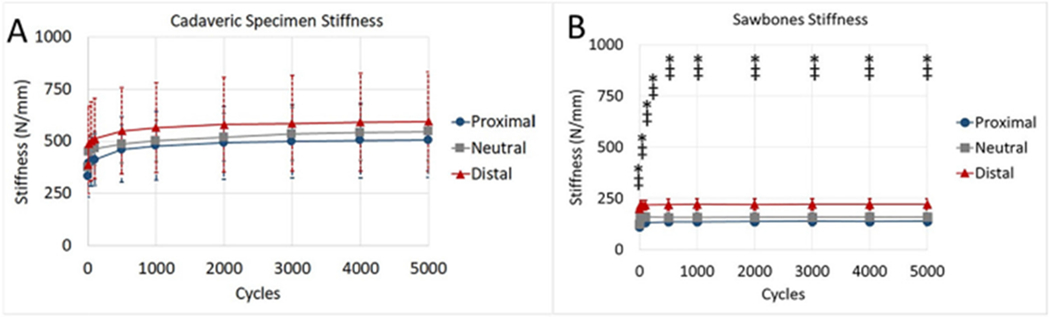
Comparisons of construct stiffness during cyclic testing for (A) cadaveric and (B) Sawbones specimens. There were no significant differences during cadaveric testing. During Sawbones testing, the distal group had significantly more stiffness than the neutral group (*) and the proximal group (‡) at all time points. The scale of graphs was kept constant to depict differences between the models, and error bars represent ± 1 standard deviation.
When compared to the SN group, SD had increased torsional stiffness in external rotation (p = 0.020) (Fig. 3) and increased axial stiffness (p = 0.024) (Fig. 4). Furthermore, the SD group had decreased humeral head displacement and increased stiffness during fatigue testing (Figs. 5 and 6). There were no significant differences in pairwise comparisons of ramp to failure stiffness or ultimate load between groups. Means, standard deviations, and p-values for all Sawbones comparisons can be found in the Supplementary materials.
Discussion
The objective of this study was to characterize the biomechanical effects of “missing” the calcar when implanting a proximal humerus locking plate. The findings, especially from the Sawbones study, indicate that missing the calcar proximally results in significant reductions in axial and torsional stiffness. These results are, in part, consistent with our initial hypothesis. Interestingly, it was also determined that screws positioned just distal the calcar may be beneficial to initial construct stiffness.
The current findings add to the narrative that adequate medial column support is imperative to the success of locked plate fixation in proximal humerus fractures. Results suggest that locking screws inserted proximal to the calcar will result in reduced fixation strength. This conclusion is strengthened by a recent clinical study, where missing the calcar proximally by 12 mm led to statistically higher failure rates [31]. It has also been shown that medial fragment comminution results in significant loss of fixation stiffness [26].
The findings from the initial cadaveric study were obfuscated by differences in human anatomy, which resulted in high amounts of variability between measures. Although the Sawbones models were considerably more compliant than human bones, they eliminated differences between specimens which resulted in tightly bundled data sets. It is notable that the cortical wall of the Sawbones calcar is less than the outer diameter of the screws. Neutral alignment caused the screws to split this wall, eliminating its utility as a medial column support. Alternatively, screws placed inferior to the calcar left the cortical bone intact and instead served as a buttress. This technique may be especially useful if the cortical wall thickness of the calcar is less than the diameter of the screw.
The assays performed in this experiment were the same as those performed by Katthagen et al. on a similar cohort of elderly specimens [13]. In certain aspects, results from the current study agree with the previously published results. For example, 20° abduction stiffness was significantly lower than 20° adduction stiffness in both studies. Also, the majority of humeral head displacement during fatigue testing occurred over the first 500 cycles in both studies. There were several differences in results between the two studies. For example, this study had higher stiffness values and decreased amounts of humeral head displacement. These behaviors may be attributed to the use of different implant alloys (stainless steel v. titanium) or differences in bone mineral density between specimens. It should also be noted that the current study utilized 6 screws in the humeral head and 2 in the shaft, whereas Katthagen et al. used 8 in the humeral head and 3 in the shaft. Thus, it is possible that there may be an upper threshold to the number of screws that can be utilized before implant fixation strength is reduced.
This study has several limitations, most of which are inherent to the cadaveric nature of the study. First, the model represents fracture fixation at “time zero” after operative intervention. It does not take into account the effects of in vivo healing, nor does make an attempt to simulate the small loads experienced when a patient is wearing a sling. When considering elderly populations, however, “time zero” may be of the utmost importance because healing in osteoporotic bone can be slow or incomplete [32] and the presence of implants may increase the risk of healing complications [33]. Second, a simple 2-part fracture of the proximal humerus was used, rather than a more clinically relevant 3- or 4-part fracture. This approach was used because it provides excellent reproducibility and enables direct comparisons of results from an existing study [13]. Finally, the specimens used in this study were not scanned to determine bone mineral densities or T-scores. Although such scans would provide additional information, it was not necessary because matched pairs of specimens were equally distributed between three experimental groups (Fig. 1). Overall, this model was intended to represent an elderly population, not a solely osteoporotic population, and this goal was achieved by controlling for the age of the donor population (average age 78.6 years, range 66–96 years).
Three clinical approaches can be used to optimize calcar screw positioning. First, if the screws are too proximal through the fixed angle implant, the screws can be re-positioned by moving the plate distal on the bone. Second, vectors of the screws can be altered by cross-threading screw heads into the plate. While this workaround may solve the immediate problem of optimizing calcar screw position, this technique greatly reduces the screw’s ability to resist shear loads [22,23,34] and is not recommended. Third, surgeons can utilize implants designs with polyaxial screws and locking caps that do not demonstrate the same loss of strength when directed outside of their neutral axis [22,23]. Unfortunately, a thorough examination of this topic is outside of the scope of the current study.
Conclusions
Successful proximal humerus fracture reconstruction can be performed with a variety of approaches. As has been previously demonstrated, the most important steps in achieving optimal proximal humeral fracture fixation with locking plates include both anatomic fracture reduction and placement of a screw positioned within the calcar [12,26]. This technique helps provide medial column support for the humeral head, even in the absence of complete bone union. Locking plate implant designs are currently evolving so that the trajectory of screws intended for the calcar are not constrained to a predefined axis. Until these new designs become more commonplace, the results of the current study suggest that missing high is deleterious to fixation strength, while it is safe, and perhaps even desirable, to aim slightly distal to the intended target.
Supplementary Material
Acknowledgment
for the study was provided DePuy Synthes, West Chester, PA with grant SR 1005.
Role of funding source
This study was sponsored by DePuy Synthes Grant SR 1005. The study sponsor did not have any involvement in the study design, data collection, data analysis, or interpretation of data. The sponsors were not involved in the writing the manuscript or the decision to submit the manuscript for publication.
Footnotes
Ethics
This study did not involve the use of human subjects or animal models and was therefore exempt from institutional approval for such work.
Conflicts of interest
There are no conflicts of interest that are relevant to this manuscript.
Appendix A.: Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.injury.2018.02.007.
References
- [1].Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury 2006;37:691–7, doi: 10.1016/j.injury.2006.04.130. [DOI] [PubMed] [Google Scholar]
- [2].Helmy N, Hintermann B. New trends in the treatment of proximal humerus fractures. Clin Orthop 2006;442:100–8. [DOI] [PubMed] [Google Scholar]
- [3].Lee SH, Dargent-Molina P, Bréart G, EPIDOS Group Epidemiologie de l’Osteoporose Study. Risk factors for fractures of the proximal humerus: results from the EPIDOS prospective study.J Bone Miner Res Off J Am Soc Bone Miner Res 2002;17:817–25, doi: 10.1359/jbmr.2002.17.5.817. [DOI] [PubMed] [Google Scholar]
- [4].Seeley DG, Browner WS, Nevitt MC, Genant HK, Scott JC, Cummings SR. Which fractures are associated with low appendicular bone mass in elderlywomen? The Study of Osteoporotic Fractures Research Group. Ann Intern Med 1991;115:837–42. [DOI] [PubMed] [Google Scholar]
- [5].Lauritzen JB, Schwarz P, Lund B, McNair P, Transbøl I. Changing incidence and residual lifetime risk of common osteoporosis-related fractures. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA 1993;3:127–32. [DOI] [PubMed] [Google Scholar]
- [6].Aaron D, Shatsky J, Paredes JC, Jiang C, Parsons BO, Flatow EL. Proximal humeral fractures: internal fixation. J Bone Joint Surg Am 2012;94:2280–8. [PubMed] [Google Scholar]
- [7].Gavaskar AS, Chowdary N, Abraham S. Complex proximal humerus fractures treated with locked plating utilizing an extended deltoid split approach with a shoulder strap incision. J Orthop Trauma 2013;27:73–6, doi: 10.1097/BOT.0b013e31825cf545. [DOI] [PubMed] [Google Scholar]
- [8].Ockert B, Siebenbürger G, Kettler M, Braunstein V, Mutschler W. Long-term functional outcomes (median 10 years) after locked plating for displaced fractures of the proximal humerus. J Shoulder Elbow Surg 2014;23:1223–31, doi: 10.1016/j.jse.2013.11.009. [DOI] [PubMed] [Google Scholar]
- [9].Clavert P, Adam P, Bevort A, Bonnomet F, Kempf J-F. Pitfalls and complications with locking plate for proximal humerus fracture. J Shoulder Elbow Surg 2010;19:489–94, doi: 10.1016/j.jse.2009.09.005. [DOI] [PubMed] [Google Scholar]
- [10].Agudelo J, Schürmann M, Stahel P, Helwig P, Morgan SJ, Zechel W, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma 2007;21:676–81, doi: 10.1097/BOT.0b013e31815bb09d. [DOI] [PubMed] [Google Scholar]
- [11].Owsley KC, Gorczyca JT. Displacement/screw cutout after open reduction and locked plate fixation ofhumeral fractures.J Bone Jt Surg 2008;90:233–40, doi: 10.2106/JBJS.F.01351. [DOI] [PubMed] [Google Scholar]
- [12].Schnetzke M, Bockmeyer J, Porschke F, Studier-Fischer S, Grützner P-A, Guehring T. Quality of reduction influences outcome after locked-Plate fixation of proximal humeral type-C fractures. J Bone Jt Surg Am 2016;98:1777–85, doi: 10.2106/JBJS.16.00112. [DOI] [PubMed] [Google Scholar]
- [13].Katthagen JC, Schwarze M, Meyer-Kobbe J, Voigt C, Hurschler C, Lill H. Biomechanical effects of calcar screws and bone block augmentation on medial support in locked plating of proximal humeral fractures. Clin Biomech 2014;29:735–41, doi: 10.1016/j.clinbiomech.2014.06.008. [DOI] [PubMed] [Google Scholar]
- [14].Beirer M, Cronlein M, Venjakob AJ, Saier T, Schmitt-Sody M, Huber-Wagner S, et al. Additional calcar support using a blade device reduces secondary varus displacement following reconstruction of the proximal humerus: a prospective study. Eur J Med Res 2015;20:82, doi: 10.1186/S40001-015-0178-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kennedy J, Molony D, Burke NG, FitzPatrick D, Mullett H. Effect of calcium triphosphate cement on proximal humeral fracture osteosynthesis: a cadaveric biomechanical study. J Orthop Surg Hong Kong 2013;21:173–7. [DOI] [PubMed] [Google Scholar]
- [16].Robinson CM, Page RS. Severely impacted valgus proximal humeral fractures: results of operative treatment. J Bone Joint Surg Am 2003;85-A:1647–55. [DOI] [PubMed] [Google Scholar]
- [17].Neviaser AS, Hettrich CM, Beamer BS, Dines JS, Lorich DG. Endosteal strut augment reduces complications associated with proximal humeral locking plates. Clin Orthop 2011;469:3300–6, doi: 10.1007/s11999-011-1949-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gardner MJ, Boraiah S, Helfet DL, Lorich DG. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant. J Orthop Trauma 2008;22:195–200, doi: 10.1097/BOT.0b013e31815b3922. [DOI] [PubMed] [Google Scholar]
- [19].Ockert B, Pedersen V, Geyer L, Wirth S, Mutschler W, Grote S. Position of polyaxial versus monoaxial screws in locked plating for proximal humeral fractures: analysis of a prospective randomized study. Eur J Orthop Surg Traumatol Orthop Traumatol 2014;24:747–52, doi: 10.1007/s00590-013-1360-5. [DOI] [PubMed] [Google Scholar]
- [20].Zettl R, Müller T, Topp T, Lewan U, Krüger A, Kühne C, et al. Monoaxial versus polyaxial locking systems: a biomechanical analysis of different locking systems for the fixation of proximal humeral fractures. Int Orthop 2011;35:1245–50, doi: 10.1007/s00264-011-1220-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ockert B, Braunstein V, Kirchhoff C, Körner M, Kirchhoff S, Kehr K, et al. Monoaxial versus polyaxial screw insertion in angular stable plate fixation of proximal humeral fractures: radiographic analysis of a prospective randomized study. J Trauma 2010;69:1545–51, doi: 10.1097/TA.0b013e3181c9b8a7. [DOI] [PubMed] [Google Scholar]
- [22].Hebert-Davies J, Laflamme G-Y, Rouleau D, Canet F, Sandman E, Li A, et al. A biomechanical study comparing polyaxial locking screw mechanisms. Injury 2013;44:1358–62, doi: 10.1016/j.injury.2013.06.013. [DOI] [PubMed] [Google Scholar]
- [23].Namdari S, Mehta S, Tierney A, Hast MW. Locking cap designs improve fatigue properties of polyaxial screws in upper extremity applications. J Orthop Trauma 2017;31:275–80, doi: 10.1097/BOT.0000000000000780. [DOI] [PubMed] [Google Scholar]
- [24].Bai L, Fu Z, An S, Zhang P, Zhang D, Jiang B. Effect of calcar screw use in surgical neck fractures of the proximal humerus with unstable medial support: a biomechanical study. J Orthop Trauma 2014;28:452–7, doi: 10.1097/BOT.0000000000000057. [DOI] [PubMed] [Google Scholar]
- [25].Yang P, Zhang Y, Liu J, Xiao J, Ma LM, Zhu CR. Biomechanical effect of medial cortical support and medial screw support on locking plate fixation in proximal humeral fractures with a medial gap: a finite element analysis. Acta Orthop Traumatol Turc 2015;49:203–9. [DOI] [PubMed] [Google Scholar]
- [26].Ponce BA, Thompson KJ, Raghava P, Eberhardt AW, Tate JP, Volgas DA, et al. The role of medial comminution and calcar restoration in varus collapse of proximal humeral fractures treated with locking plates. J Bone Joint Surg Am 2013. 95:, doi: 10.2106/JBJS.K.00202 e113 (1–7). [DOI] [PubMed] [Google Scholar]
- [27].Lescheid J, Zdero R, Shah S, Kuzyk PRT, Schemitsch EH. The biomechanics of locked plating for repairing proximal humerus fractures with or without medial cortical support. J Trauma 2010;69:1235–42, doi: 10.1097/TA.0b013e3181beed96. [DOI] [PubMed] [Google Scholar]
- [28].Bergmann G, Graichen F, Bender A, Rohlmann A, Halder A, Beier A, et al. In vivo gleno-humeral joint loads during forward flexion and abduction. J Biomech 2011;44:1543–52, doi: 10.1016/j.jbiomech.2011.02.142. [DOI] [PubMed] [Google Scholar]
- [29].Schumer RA, Muckley KL, Markert RJ, Prayson MJ, Heflin J, Konstantakos EK, et al. Biomechanical comparison of a proximal humeral locking plate using two methods of head fixation. J Shoulder Elbow Surg 2010;19:495–501, doi: 10.1016/j.jse.2009.11.003. [DOI] [PubMed] [Google Scholar]
- [30].Röderer G, Gebhard F, Krischak G, Wilke H-J, Claes L. Biomechanical in vitro assessment of fixed angle plating using a new concept of locking for the treatment of osteoporotic proximal humerus fractures. Int Orthop 2011;35:535–41, doi: 10.1007/s00264-010-1021-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Padegimas EM, Zmistowski B, Lawrence C, Palmquist A, Nicholson TA, Namdari S. Defining optimal calcar screw positioning in proximal humerus fixation. J Shoulder Elb Surg 2017. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- [32].Giannoudis P, Tzioupis C, Almalki T, Buckley R. Fracture healing in osteoporotic fractures: is it really different? Injury 2007;38:S90–9, doi: 10.1016/j.injury.2007.02.014. [DOI] [PubMed] [Google Scholar]
- [33].Tarantino U, Cerocchi I, Scialdoni A, Saturnino L, Feola M, Celi M, et al. Bone healing and osteoporosis. Aging Clin Exp Res 2011;23:62–4. [PubMed] [Google Scholar]
- [34].Tidwell JE, Roush EP, Ondeck CL, Kunselman AR, Reid JS, Lewis GS. The biomechanical cost of variable angle locking screws. Injury 2016;47:1624–30, doi: 10.1016/j.injury.2016.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


