Abstract
Pure erythroid leukemia (PEL) is a rare form of acute myeloid leukemia characterized by the neoplastic proliferation of erythroblasts. PEL is associated with inferior survival outcomes, particularly among patients harboring complex karyotype abnormalities. In this case, we present a 21-year-old Sudanese man who presented to our ER with a two-week history of fever, shortness of breath, fatigue, and exercise intolerance. He had no significant personal medical history or family history of malignancy. A bone marrow biopsy revealed hypercellularity and infiltration by cells with an immature appearance. A flow cytometry (FC) analysis of the bone marrow aspirate revealed that approximately 21% of the total nucleated cells were negative for CD45 and positive for CD71, glycophorin A, and CD36 but negative for myeloperoxidase (MPO), CD33, CD13, CD61, CD41, and other lymphoid and myeloid markers. Consistent with the microscopic analysis, <1% of the total cells were identified as CD34/CD13/CD117-positive myeloblasts. Notably, all stains (CD45, MPO, CD34, CD163, CD61, glycophorin A) were negative except E-cadherin, which positively stained >80% of the cells. Our findings suggested a differential diagnosis that included erythroid leukemia and myelodysplastic syndrome (MDS). The morphological, FC, immunohistochemistry, and cytogenetic findings strongly supported a diagnosis of PEL.
Keywords: aml-m6, pure erythroid leukemia, e-cadherin, glycophorin a
Introduction
Pure erythroid leukemia (PEL) is a rare form of acute myeloid leukemia characterized by the neoplastic proliferation of erythroblasts [1]. Two types of erythroleukemia have been described by Di Guglielmo: Di Guglielmo syndrome, which involves myeloblasts and monoblasts, was described in 1917 while Di Guglielmo disease, which includes abnormal erythroblasts, was reported in 1928 [2-3]. Regarding diagnostic methods, flow cytometry (FC) is not particularly valuable for the the diagnosis of erythroleukemias because useful, specific markers for proerythroblasts have not yet been identified [4]. In recent years, however, epithelial calcium-dependent adhesion protein (E-cadherin) was found to be expressed on hematopoietic erythroid precursors in a developmentally regulated pattern, with a distinct expression on early erythroblasts followed by a gradual decrease during cellular maturation [5-6].
Generally, PEL is associated with inferior survival outcomes, particularly among patients harboring complex karyotypic abnormalities. Therefore, allogeneic bone marrow transplant should be considered as the first line of treatment for suitable candidates once remission has been achieved, given the very high risk of relapse and mortality [7]. In this study, we review our experience with the challenging diagnosis of PEL in a patient with a low frequency of proerythroblasts, as well as the difficulty in meeting the current World Health Organization (WHO) diagnostic criteria for this erythroleukemia [8] and the importance of including E-cadherin as a potential diagnostic marker for PEL.
Case presentation
A 21-year-old Sudanese man who resided in Yemen presented to our ER with a two-week history of fever, shortness of breath, fatigue, and exercise intolerance. He denied experiencing night sweats, weight loss, ecchymosis, or bleeding. He had no significant personal medical history or family history of malignancy, was not taking any medications, did not smoke, and did not abuse alcohol or drugs. He had previously been evaluated at a local hospital in Yemen, where he was found to have severe anemia and thrombocytopenia requiring repeated blood transfusions. Although his workup at that institution included bone marrow aspiration, no definite diagnosis was made. Upon physical examination, the patient was pale and febrile without any apparent distress. He did not exhibit petechiae, skin rash, or bleeding of gums. No lymphadenopathy or hepatosplenomegaly. The other physical examination findings were unremarkable.
The initial hematological analysis revealed pancytopenia, with a white blood cell count of 2.32 x 109/L (3.90-11.00 x 109/L), absolute neutrophil count of 0.65 x 109/L (1.35-7.50 x 109/L), hemoglobin level of 9.70 g/dL (13.5-18 g/dL), red blood cell (RBC) count of 2.66 x 1012/L, mean corpuscular volume of 77.40 fl (75.0-95.0 fl), mean corpuscular hemoglobin level of 27.8 pg (24.0-30.0 pg), and platelet count of 3 x 109/L (155.0-435.0 x 109/L). The biochemical analysis revealed mildly elevated levels of lactate dehydrogenase and total bilirubin and renal function and liver function test results within normal limits. A peripheral smear showed slight anisopoikilocytosis with a few schistocytes and teardrop cells but no nucleated red blood cells or circulating blasts.
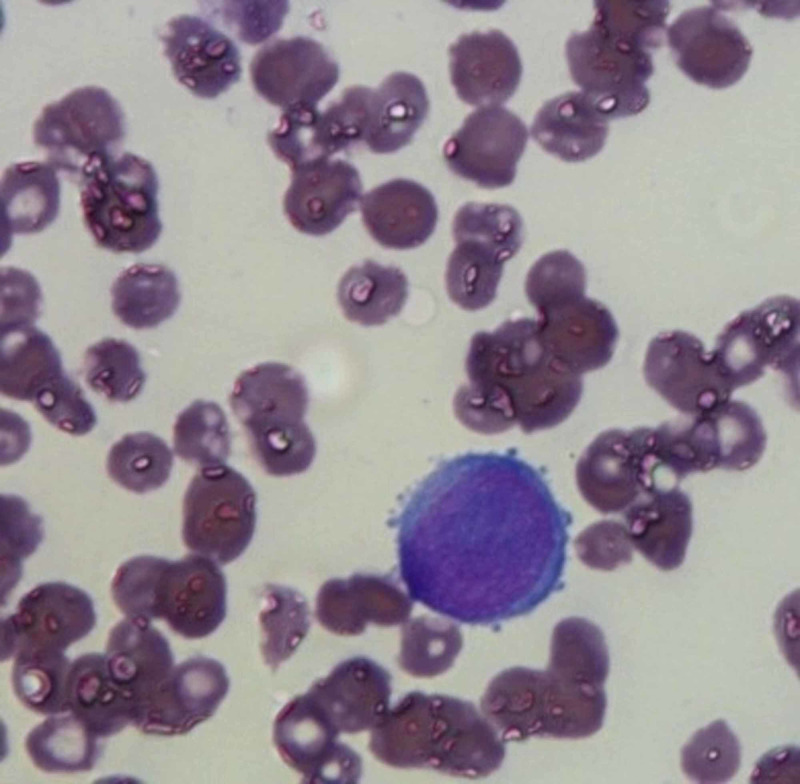
Examination of the initial bone marrow aspirate revealed diluted bone marrow comprising approximately 25% proerythroblasts and erythroid precursors (Figure 1).
Figure 1. Proerythroblast with clear blebs.
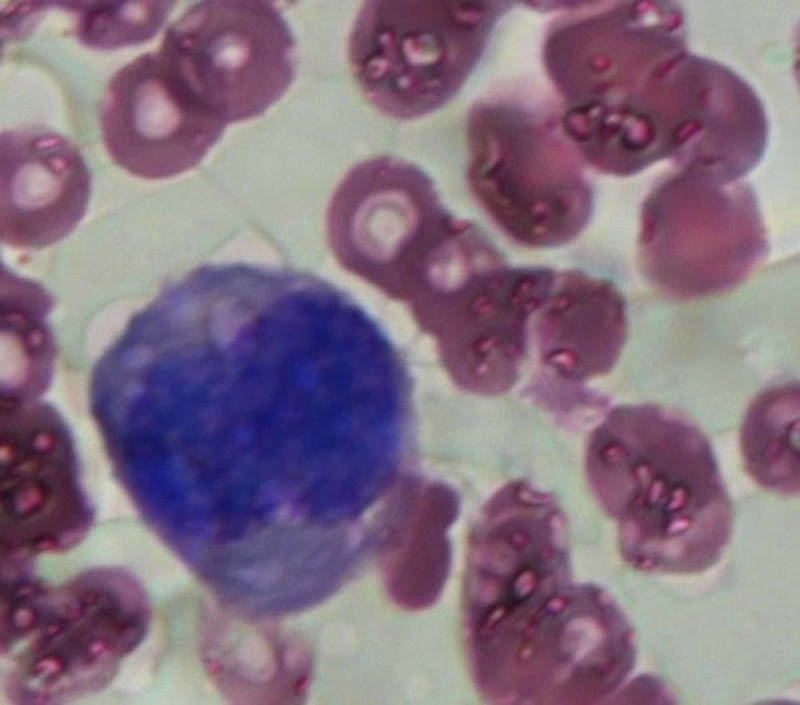
Erythroid precursors exhibiting a dysplastic morphology characterized by megaloblastic features and giant proerythroblasts with multinucleation were identified, in addition to lymphocytes (33%), myeloid cells (12%), monocytes (3%), plasma cells (1%), eosinophils, (1%), and myeloblasts (<1%) (Figure 2).
Figure 2. Large dysplastic erythroblast.
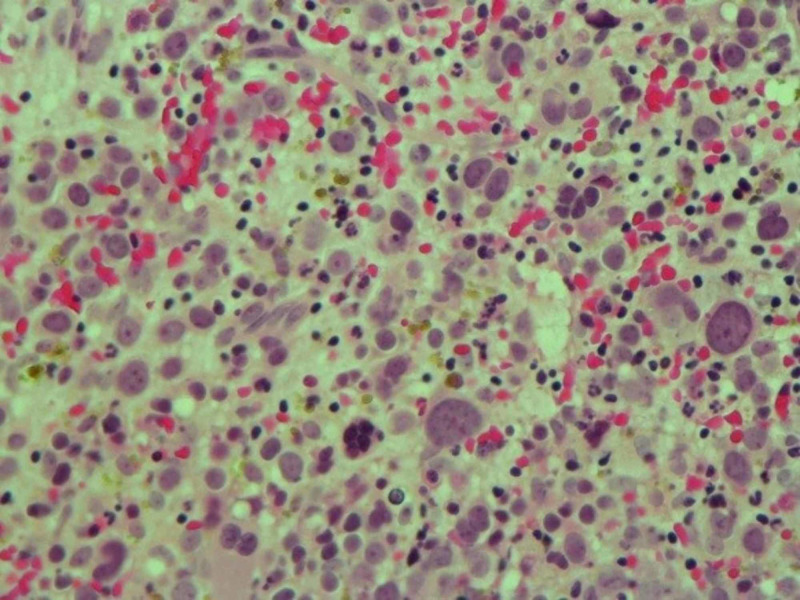
A FC analysis of the bone marrow aspirate revealed that approximately 21% of the total nucleated cells were negative for CD45 and positive for CD71 (30% of gated cells), glycophorin A (7% of gated cells), and CD36 (28% of gated cells) but negative for myeloperoxidase (MPO), CD33, CD13, CD61, CD41, and other lymphoid and myeloid markers. Consistent with the microscopic analysis, <1% of the total cells were identified as CD34/CD13/CD117-positive myeloblasts. A bone marrow biopsy revealed hypercellularity and infiltration by cells with an immature appearance (Figure 3).
Figure 3. H&E stain showing interstitial proerythroblasts.
H&E stain: hematoxylin and eosin stain
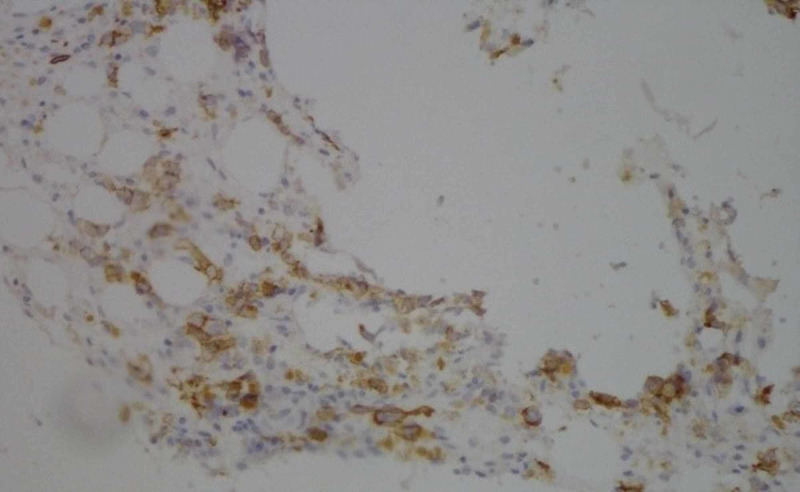
Accordingly, an immunohistochemistry study was performed to characterize these immature cells. Notably, all stains (CD45, MPO, CD34, CD163, CD61, glycophorin A) were negative except E-cadherin, which positively stained >80% of the cells (Figure 4).
Figure 4. Immunohistochemistry for E-cadherin.
Regarding cytogenetic abnormalities, fluorescent in situ hybridization (FISH) analysis revealed the following complex clonal abnormalities in 63% of the scored nuclei: three extra copies of ABL1 (chromosome 9), PML (chromosome 15) L, RUNX1T1 (chromosome 8), MLL (chromosome 11), and five copies of RUNX1 (chromosome 21) in an average of 49% of scored cells. No evidence of deletion was observed on the long arms of chromosomes 5, 7, or 20, although these features are often seen in myelodysplastic syndrome. Molecular studies revealed a lack of mutations in CEBPA, FLT3 ITD, FLT3 TKD\T\, and NPM1, which are commonly seen in other types of acute myeloid leukemia.
The patient was treated with an induction chemotherapy protocol comprising idarubicin (12 mg/m on days 1-3), cytarabine (100 mg/m continuous IV infusion on days 1-7), and etoposide (100 mg/m on days 1-5). The induction course was complicated by febrile neutropenia with negative cultures and grade II oral mucosal effects from which the patient recovered well. Subsequently, voriconazole treatment was escalated from a prophylactic to a therapeutic dose.
On day 28, post-induction, bone marrow hypercellularity (i.e., cellularity = 30%) was observed. Approximately 15% of the proerythroblasts exhibited positive E-cadherin immunostaining, and aggregates of immature erythroid cells were observed. The patient was considered to have primary refractory disease and was administered a second-line chemotherapy regimen comprising 5-azacytidine (75 mg/m2 daily for five days), followed by fludarabine (80 mg/m2 daily on days 1-5), and cytarabine (2 g/m2 daily on days 1-5) with filgrastim for neutropenia.
Three weeks later, a bone marrow analysis again revealed hypercellularity (90%-100% cellularity) with regeneration of tri-lineage hematopoietic cells and 7% proerythroblasts. FC revealed an erythroid element frequency of 8%, with positivity for CD36 and CD71 and negativity for CD34, CD117, and glycophorin A. A FISH analysis revealed persistence of the complex clonal abnormality in an average of 42.9% of the scored nuclei as follows: one additional copy of BCR (9q43), PML (15q22), RUNX1T1 (8q22), and MLL (11q23) and three additional copies of RUNX1 (21q22).
After an additional four weeks, the patient exhibited pancytopenia. A bone marrow analysis revealed approximately 80% cellularity, which comprised 53% E-cadherin-positive proerythroblasts. Given the high risk of relapse, the patient underwent an allogeneic cell transplant from a fully human leukocyte antigen-matched brother during the first remission but has unfortunately expired.
Discussion
The WHO in 2016 [8] defined PEL as “a neoplastic proliferation of immature cells committed exclusively to the erythroid lineage (≥80% of bone marrow cells) with no evidence of a significant myeloblastic component.” However, in the study referred by 2008 WHO for the “description of PEL consisting of eight cases of minimally differentiated erythroleukemia, and the criteria for inclusion contain immature erythroblast ≥50% with <10% 'differentiating' forms” [9]. On the contrary, “Mazzella et al. [10]. have consistently used 30% pronormoblasts of erythroid precursors as the cutoff to define PEL.” In 2002, Domingo-Claros et al. studied 62 cases of acute erythroid neoplastic proliferation [11]. “Fifty-three cases were classified as AEL and two cases of PEL, leaving seven cases unclassifiable.” Two of the seven unclassifiable cases had undifferentiated erythroblasts of 42% and 40%, respectively, while our results investigated that the proerythroblasts and erythroid precursors (immature erythroblasts) were approximately 25%. In PEL patients, the peripheral blood smears show nonspecific changes in most cases, circulating erythroblasts may be observed in about 25% of patients, anemia and thrombocytopenia are commonly observed, which is also supported by our case [12].
In bone marrow aspirate, erythroid precursors with left shift and increased proerythroblasts are the abundant cells (>80%), which are the most immature identified erythroid precursor. They are medium to massive round nucleated blasts with, fine nuclear chromatin, occasional prominent nucleoli, the cytoplasm is dark blue and is agranular with different cytoplasmic vacuolization [13].
Flow cytometry is the primary technique for phenotyping acute erythroid leukemia (AEL), PEL, and other related conditions. The data obtained by FC can be comprehensive enough to completely subtype instances of leukemias/MDS into their myriad classes [14]. The possibility of characterizing individual cells according to their surface antigenicity by FC offers the chance to precisely subclassify the cells and sort them into separate populations [15]. “Glycophorin A positivity has been reported to be consistent with erythroid precursors, CD71 & partial CD36 positivity, also seen in erythroid precursors [16]. In the bone marrow, its expression appears mainly in immature erythroblasts, beta-sialoglycoprotein, carbonic anhydrase1, which can also be used to identify minimally differentiated M6 [17].” Our results revealed that a FC analysis of the bone marrow aspirate showed that approximately 21% of the total nucleated cells were negative for CD45 and positive for CD71 (30% of gated cells), glycophorin A (7% of gated cells), and CD36 (28% of gated cells) but negative for MPO, CD33, CD13, CD61, CD41, and other lymphoid and myeloid markers. Consistent with the microscopic analysis, <1% of the total cells were identified as CD34/CD13/CD117-positive myeloblasts.
“E-cadherin (epithelial cadherin) is a polypeptide protein that belongs to function is to facilitate cell-cell adhesion and recognition. E-cadherin has morphogenesis of cells during migration and acts as a tumor suppressor gene” [18]. In some solid tumors, such as breast cancer, loss of E-cadherin function is often associated with more invasive disease [19]. In our case, approximately 15% of the proerythroblasts exhibited positive E-cadherin immunostaining, and aggregates of immature erythroid cells were observed. In our study (FISH) analysis revealed the following complex clonal abnormalities in 63% of the scored nuclei: three extra copies of ABL1 (chromosome 9), PML (chromosome 15) L, RUNX1T1 (chromosome 8), MLL (chromosome 11), and five copies of RUNX1 (chromosome 21) in an average of 49% of scored cells.
Conclusions
Our findings suggested a differential diagnosis that included erythroid leukemia and MDS. The morphological, FC, immunohistochemistry, and cytogenetic findings strongly supported a diagnosis of PEL.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained by all participants in this study
References
- 1.Pure erythroid leukemia: a reassessment of the entity using the 2008 World Health Organization classification. Liu W, Hasserjian RP, Hu Y, et al. Mod Pathol. 2011;24:375–383. doi: 10.1038/modpathol.2010.194. [DOI] [PubMed] [Google Scholar]
- 2.Di Guglielmo G. Richerche di ematologia I. Vol. 13. Folia Medica (Pavia); 1917. Un caso di eritroleucemia Megacariociti in circolo e loro funzione piastrinopoietico; p. 386. [Google Scholar]
- 3.Le eritremie. DiGuglielmo G. Haematologica. 1928;9:301–312. [Google Scholar]
- 4.Involvement of E-cadherin in the development of erythroid cells; subject heading. Armeanu S, Muller CA, Klein G. Hematology. 2000;5:307–316. doi: 10.1080/10245332.2000.11746524. [DOI] [PubMed] [Google Scholar]
- 5.Molecular players in hematologic tumor cell trafficking. Redondo-Muñoz J, García-Pardo A, Teixidó J. Front Immunol. 2019;10:156. doi: 10.3389/fimmu.2019.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.E-Cadherin is a specific marker for erythroid differentiation and has utility, in combination with CD117 and CD34, for enumerating myeloblasts in hematopoietic neoplasms. Ohgami RS, Chisholm KM, Ma L, et al. Am J Clin Pathol. 2014;141:656–664. doi: 10.1309/AJCP8M4QQTAZPGRP. [DOI] [PubMed] [Google Scholar]
- 7.The pure erythroleukemia: a case report and literature review. Latif N, Salazar E, Khan R, et al. https://www.hematologyandoncology.net/archives/april-2010-2/the-pure-erythroleukemia-a-case-report-and-literature-review-2/ Clin Adv Hematol Oncol. 2010;8:283–290. [PubMed] [Google Scholar]
- 8.The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Arber DA, Orazi A, Hasserjian R, et al. Blood. 2016;127:2391–2405. [Google Scholar]
- 9.Minimally differentiated erythroleukaemia (AML M6 Variant'): a rare subset of AML distinct from AML M6. Garand R, Duchayne E, Blanchard D, et al. Br J Haematol. 1995;90:868–875. doi: 10.1111/j.1365-2141.1995.tb05208.x. [DOI] [PubMed] [Google Scholar]
- 10.The acute erythroleukemias. Mazzella FM, Alvares C, Kowal-Vern A, et al. Clin Lab Med. 2000;20:119–137. [PubMed] [Google Scholar]
- 11.Acute erythroid neoplastic proliferations. A biological study based on 62 patients. Domingo-Claros A, Larriba I, Rozman M, et al. http://www.haematologica.org/content/87/2/148.long. Haematologica. 2002;87:148–153. [PubMed] [Google Scholar]
- 12.Erythroid proliferations in myeloid neoplasms. Wang SA, Hasserjian RP. Hum Pathol. 2012;43:153–164. doi: 10.1016/j.humpath.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Acute erythroid leukemia: a reassessment using criteria refined in the 2008 WHO classification. Hasserjian RP, Zuo Z, Garcia C, et al. Blood. 2010;115:1985–1992. doi: 10.1182/blood-2009-09-243964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Childhood acute erythroleukemia diagnosis by flow cytometry. Sharma A, Buxi G, Walia R, et al. Indian J Pathol Microbiol. 2011;54:173–175. doi: 10.4103/0377-4929.77395. [DOI] [PubMed] [Google Scholar]
- 15.Flow cytometry and effusions in lymphoproliferative processes and other hematologic neoplasias. Bode-Lesniewska B. Acta Cytol. 2016;60:354–364. doi: 10.1159/000448325. [DOI] [PubMed] [Google Scholar]
- 16.Complex karyotype with novel translocation in pure erythroid leukemia patient. Aljabry M. Hematol Rep. 2015;7:5674. doi: 10.4081/hr.2015.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loss of membrane expression of E-cadherin in leukemic erythroblasts. Acs G, LiVolsi VA. Arch Pathol Lab Med. 2001;125:198–201. doi: 10.5858/2001-125-0198-LOMEOE. [DOI] [PubMed] [Google Scholar]
- 18.Expression and significance of cadherins and its subtypes in development and progression of oral cancers: a review. Rajwar YC, Jain N, Bhatia G, et al. J Clin Diagn Res. 2015;9:5–7. doi: 10.7860/JCDR/2015/11964.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.E-cadherin-integrin crosstalk in cancer invasion and metastasis. Canel M, Serrels A, Frame MC, et al. J Cell Sci. 2013;126:393–401. doi: 10.1242/jcs.100115. [DOI] [PubMed] [Google Scholar]