Abstract
Background
The United States is currently enduring an opioid crisis. Identifying cost-effective, easy-to-implement behavioral measures that predict treatment outcomes in opioid misusers is a crucial scientific, therapeutic, and epidemiological goal.
Methods
The current study used a mixed cross-sectional and longitudinal design to test whether a behavioral choice task, previously validated in stimulant users, was associated with increased opioid misuse severity at baseline, and whether it predicted change in opioid misuse severity at follow-up. At baseline, data from 100 prescription opioid-treated chronic pain patients were analyzed; at follow-up, data were analyzed in 34 of these participants who were non-misusers at baseline. During the choice task, participants chose under probabilistic contingencies whether to view opioid-related images in comparison with affectively pleasant, unpleasant, and neutral images. Following previous procedures, we also assessed insight into choice behavior, operationalized as whether (yes/no) participants correctly self-reported the image category they chose most often.
Results
At baseline, higher choice for viewing opioid images in direct comparison with pleasant images was associated with opioid misuse and impaired insight into choice behavior; the combination of these produced especially elevated opioid-related choice behavior. In longitudinal analyses of individuals who were initially non-misusers, higher baseline opioid versus pleasant choice behavior predicted more opioid misuse behaviors at follow-up.
Conclusions
These results indicate that greater relative allocation of behavior toward opioid stimuli and away from stimuli depicting natural reinforcement is associated with concurrent opioid misuse and portends vulnerability toward future misuse. The choice task may provide important medical information to guide opioid-prescribing practices.
Keywords: Opioid misuse, Chronic pain, Choice behavior, Insight, Treatment outcome, Reward, Allostasis
INTRODUCTION
The current opioid crisis claims the lives of 115 Americans each day, making it the leading cause of accidental death in the United States (Hedegaard et al., 2017). In 2016, this epidemic produced an estimated 2.1 million diagnoses of opioid use disorder (Rudd et al., 2016). The prescription of opioid analgesic medications for chronic pain management has contributed to the opioid crisis. Indeed, recent estimates suggest that approximately 21% to 29% of chronic pain patients receiving long-term opioid analgesic pharmacotherapy develop substance misuse, and approximately 8% to 12% develop substance use disorder (SUD) (Vowles et al., 2015). Identifying and validating cost-effective, easy-to-implement behavioral models that characterize vulnerability and predict outcomes in opioid misusers is a crucial scientific, therapeutic, and epidemiological goal.
Prominent theories of opioid misuse specifically, and SUD in general, emphasize that in susceptible individuals addictive drugs come to assume heightened motivational significance at the expense of natural reinforcement (Zilverstand et al., 2018, Garland et al., 2013a). One ecologically valid paradigm to measure shifts in the value of these reinforcers is the drug-choice procedure (Banks et al., 2015). During drug-choice procedures, an individual selects between receiving a drug reinforcer and a non-drug reinforcer (e.g., money or chocolate) (Lawn et al., 2015, Donny et al., 2004, Hogarth and Chase, 2011), with choice for the drugs over the alternative marking greater SUD severity (Hogarth and Chase, 2011, Lenoir et al., 2013). To adapt this standard drug-choice procedure for use in abstinent or treatment-seeking individuals (who usually cannot self-administer actual drugs in the laboratory) (Moeller and Stoops, 2015), we created simulated drug-choice tasks in which choices between tangible reinforcers (e.g., drugs versus money) are replaced by choices between abstract reinforcers (i.e., drug images versus affectively pleasant images). In our prior work, chronic cocaine users chose to view more drug-related images, and fewer pleasant images, than healthy controls (Moeller et al., 2009). In turn, heightened choice for drug images over pleasant images correlated with greater recent and prospective drug use outside the laboratory (Moeller et al., 2013a, Moeller et al., 2010). Related choice tasks recently have been used in smokers and heavy users of alcohol (Hardy and Hogarth, 2017, Hogarth and Hardy, 2018, Hogarth et al., 2017), but to our knowledge have not been used in a prescription opioid-using population.
The current study used a combined cross-sectional, longitudinal approach to validate the simulated (picture-viewing) drug-choice paradigm in chronic pain patients who use prescription opioids. In the cross-sectional study component, 100 patients studied at baseline completed a probabilistic learning choice task that compared their selections for pleasant, unpleasant, neutral, and opioid pill images under uncertain task contingencies. Our cross-sectional study goal was to examine whether opioid-choice selections would be elevated in participants presenting with a more severe clinical phenotype. For this purpose, we examined the following variables as correlates of choice behavior: first, opioid misuser status, indexed by the Current Opioid Misuse Measure (COMM) (Butler et al., 2007); and second, our well-established measure of drug-choice “insight” (Moeller et al., 2010, Moeller et al., 2014, Moeller et al., 2012), where an underestimation of drug selections has been associated with a worse SUD phenotype (more money spent on drugs per use) (Moeller et al., 2010). Although we expected that pleasant images would be chosen most frequently overall (Moeller et al., 2009, Moeller et al., 2018), in line with our own work described above and other work showing an attentional bias for rewarding stimuli in SUD (Anderson et al., 2013), we expected that pleasant and opioid choices would be modulated by severity. More specifically, we hypothesized that lower pleasant selections and higher opioid selections would correlate with opioid misuse status (Hypothesis 1A) and impaired insight into choice behavior (Hypothesis 1B); analyses considered the independent and multiplicative contributions of these variables. In the longitudinal arm of the study, a subset of 34 participants with initially no opioid misuse was prospectively followed and assessed for onset of misuse behaviors. We hypothesized that baseline choice for opioid images in preference to pleasant images would predict an increase in opioid misuse over time (Hypothesis 2), validating the choice task as a marker of premorbid vulnerability. Indeed, there is an urgent need for new measures predicting misuse outcomes in vulnerable opioid-prescribed populations, given that existing risk questionnaires have often fared poorly in this regard (Kaye et al., 2017).
METHODS
Participants
At baseline, 100 prescription opioid users participated. Participants were recruited from primary care and pain clinics in Salt Lake City, UT through electronic health record review, opt out letters, flyers, and radio advertisements. Advertisements recruited individuals who suffered from, and were prescribed opioid medications for, chronic pain to participate in a study investigating ways to better address problems with chronic pain and prescription pain medication. Participants were mostly female, white, and primarily experienced lower back pain (but also included patients with head/cervical pain, joint pain, fibromyalgia, and other ailments) (Table 1). Following psychiatric screening, eligible and consenting participants completed several validated task and self-report measures, and provided demographic and clinical information. Participants were paid $25 for completing the study protocol. The Institutional Review Board at the University of Utah reviewed and approved the study. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Table 1.
Demographics and clinical characteristics of the study sample at baseline assessment.
| Measure | Non-misusers (N=43) | Misusers (N=57) | Statistical test |
|---|---|---|---|
| Gender (M/F) | χ2=2.13 | ||
| Female N (%) | 27 (62.8) | 34 (60.7) | |
| Age (M ± SD) | 57.0 ± 12.1 | 51.3 ± 11.8 | t=2.37* |
| Race/Ethnicity | χ2=2.15 | ||
| Caucasian N (%) | 40 (93.0) | 53 (94.6) | |
| African American N (%) | - | 1 (1.8) | |
| Latino N (%) | 1 (2.3) | 1 (1.8) | |
| Asian/Pacific Islander N (%) | 1 (2.3) | - | |
| Other | 1 (2.3) | 1 (1.8) | |
| Primary pain condition | χ2=4.41 | ||
| Lower back N (%) | 22 (51.2) | 30 (53.6) | |
| Joint/arthritis N (%) | 5 (11.6) | 10 (17.9) | |
| Head/neck N (%) | 2 (4.7) | 6 (10.7) | |
| Fibromyalgia N (%) | 5 (11.6) | 5 (8.9) | |
| Other | 9 (20.9) | 5 (8.9) | |
| Clinical characteristics (M ± SD) | |||
| COMM | 9.2 ± 3.1 | 21.1 ± 7.1 | t=10.29* |
| Opioid use disorder symptoms | 2.2 ± 2.3 | 4.1 ± 2.9 | t=3.50* |
| Morphine equivalent units per day | 79.4 ± 103.1 | 89.9 ± 136.8 | t=0.42 |
| BPI, Pain Severity | 5.0 ± 1.4 | 5.2 ± 1.4 | t=0.47 |
| BPI, Pain Interference | 5.8 ± 1.8 | 6.3 ± 1.9 | t=1.44 |
Note. Characterization of opioid non-misusers and misusers was based on the Current Opioid Misuse Measure (COMM), where the threshold for misuser was a score of ≥13.
Of these 100 baseline participants, 34 provided longitudinal follow-up data 8 weeks later on opioid misuse. All 34 participants were selected for initially low levels of opioid misuse at baseline to allow us to establish whether baseline choice predicts onset of opioid misuse behaviors. The clinical outcomes of individuals with high opioid misuse scores, who were also followed over time, will be reported separately as part of an ongoing clinical trial. The 34 participants included at follow-up were demographically similar to the larger sample. They were 57 ± 10.1 years old, and they were mostly female (62%), white (94%), and had lower back pain (56%).
Probabilistic Picture-Choice Task
The probabilistic picture-choice task has been previously used and validated in cocaine users (Moeller et al., 2009, Moeller et al., 2010, Moeller et al., 2013b, Moeller et al., 2013a) and methamphetamine users (Moeller et al., 2018), and here was adapted for the first time in chronic pain patients who use opioids. Images in three categories were selected from the International Affective Image System (IAPS) (Lang et al., 2008): pleasant (e.g., smiling babies), unpleasant (e.g., mutilation), and neutral (e.g., household items) images. Another image category depicted opioid pills and individuals taking opioid pills, collected from freely available online repositories. The pill images were matched to the IAPS images on size and ratio of human/non-human content, as we have done previously in the cocaine and methamphetamine SUD studies.
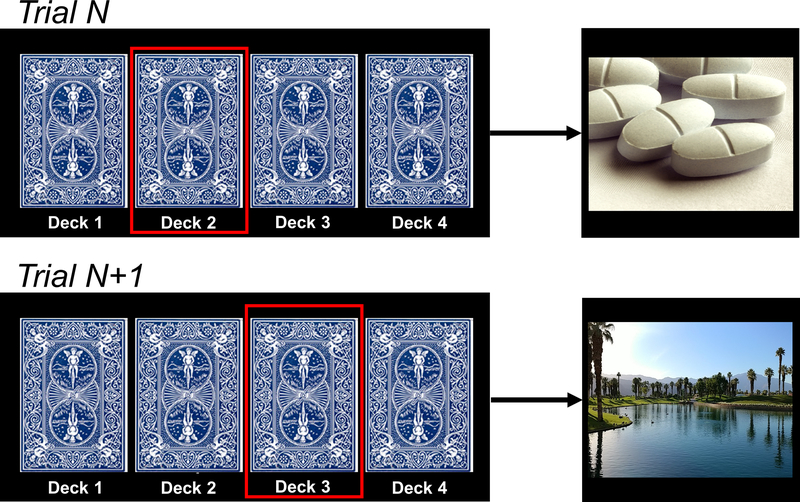
On each trial, participants used a button press to choose to view images on flipped-over cards, arranged in four decks. Immediately after selection from a particular deck, an image was revealed, which then covered the entire screen for passive viewing (2000 ms) (Figure 1). Participants were instructed that there were no correct or incorrect responses, and that they should simply choose their preferred decks. The images were arranged probabilistically: each deck contained 26 (of 30) images from a particular category (e.g., pleasant), allowing images from other categories to be interspersed within each deck (two images from a secondary category, e.g., opioid pills; and one image from each of the two remaining categories). After selection from a particular deck eight times (corresponding to one task run), deck location of the four image categories shifted to further reduce awareness of deck identity, and the participant’s deck preference needed to be re-learned. For analysis, we summed the number of cards selected per image category across four task runs. Because the task included four runs, and because each run terminated when a participant chose a particular deck eight times, the total number of possible trials per run was between 8 (8 choices of one deck and 0 choices of the other three decks) and 29 (8 choices of one deck and 7 choices from each of the other three decks) (total range: 32–116 choices). In this sample, the mean number of selections was 64.7 (SD=18.2), corresponding to 23.4 pleasant choices (SD=6.7), 12.4 unpleasant choices (SD=7.4), 17.9 neutral choices (SD=7.6), and 11.0 opioid pill choices (SD=7.2).
Figure 1.
Task schematic. Participants chose whether to view opioid pill images in comparison with standardized pleasant, unpleasant, and neutral images (from the IAPS collection; unpleasant and neutral not shown). Images were arranged probabilistically under flipped-over cards; the desired outcome (image type) of the choice was likely (87%), but not certain.
We used the same probabilistic choice task to assess insight, using procedures validated in cocaine users (Moeller et al., 2010, Moeller et al., 2012, Moeller et al., 2014). Immediately at the conclusion of the choice task, participants pressed a button corresponding to one of the four picture categories to indicate what they perceived was their most selected picture type. We then compared participants’ self-report of choice behavior with their actual choice behavior (i.e., participants’ most selected picture category versus what they perceived was their most selected picture category). Participants who showed correspondence between these subjective and objective measures (e.g., executing the highest number of presses for pleasant images and responding ‘pleasant’ to the question above) were classified as having intact insight (N=62), whereas participants lacking correspondence (e.g., executing the highest number of presses for pleasant images and responding ‘pill’ to the question above) were classified as having impaired insight (N=38) (Figure 2). In this way, the measure taps into self-awareness of a key psychiatric symptom in this population (drug-seeking behavior), showing relevance to clinical insight. In prior work, lack of insight was not fully explained by performance on classical neuropsychological tests, indicating that insight is a unique domain (Moeller et al., 2010).
Figure 2.
Insight measure. As done in our previous research, insight was operationalized as whether (yes/no) participants correctly self-reported the image category they chose most often during the task. In the abbreviated example shown where opioid selections exceed pleasant selections (unpleasant and neutral not shown), a response that pleasant images were chosen most frequently would be scored as impaired insight; a response that opioid images were chosen most frequently would be scored as intact insight.
Measures of Opioid Severity
COMM
The COMM consists of 17 items, rated on a Likert-type scale (0=never, 4=very often), asking how often in the past 30 days participants engaged in behaviors linked with opioid misuse (e.g., took medication in ways other than how it was prescribed, etc.) (Butler et al., 2007). At baseline, we used the COMM to group participants into opioid misusers (COMM score ≥13) and non-misusers (COMM score ≤12), where receiver operator characteristic curve analyses revealed that a score of 13 or higher on the COMM had maximum sensitivity and specificity to identify prescription opioid use disorder among chronic pain patients in primary care settings (Meltzer et al., 2011).
We then used COMM group membership (misuser, non-misuser) as a moderator in our cross-sectional analyses, and as the method to stratify patients in our longitudinal analyses; for the latter, note that this COMM cutoff is also being used to stratify patients in the parent clinical trial from which these data were derived. Furthermore, the dichotomization of the COMM is in keeping with its primary purpose as a screening measure, which provides a straightforward clinical cutpoint for clinicians allowing them to determine whether their patient may be engaged in aberrant drug-related behavior. By dichotomizing the COMM, we also maintain consistency with the other studies in the literature, including its original psychometric validation papers (Butler et al., 2007, Meltzer et al., 2011, Garland et al., 2014, Garland et al., 2015a).
Addiction Behaviors Checklist
Because the COMM established baseline group membership, we used a different measure, the Addictions Behavior Checklist (ABC) (Wu et al., 2006), to assess misuse severity in the longitudinal analyses. The ABC combines patient self-reports, clinician ratings, and chart review to assess aberrant medication-related behaviors suggestive of misuse (range: 0–20; higher scores indicate more problems).
Pain Measures
Pain Severity
Pain severity was measured with the 4-item pain severity subscale from the Brief Pain Inventory (BPI) a well-validated measure of acute and chronic pain (Cleeland, 1994). Participants reported their worst pain during the past week, least pain during the past week, average pain, and current pain. Response options ranged from 0 (no pain) to 10 (pain as bad as I can imagine).
Pain Interference
Pain-related functional interference was assessed with the pain interference subscale of the BPI. Participants rated on a 0 (does not interfere) to 10 (completely interferes) scale the extent to which pain had interfered with each of seven domains of normal functioning in the past week, including: general activity, mood, walking ability, normal work, relations with other people, sleep and enjoyment of life.
Statistical Analysis
Baseline Choice Behavior
We examined overall choice behavior and its potential moderation by a more severe clinical phenotype. Data were analyzed using a 4 (Image: pleasant, unpleasant, neutral, opioid) × 2 (Severity Status: misuser, non-misuser) × 2 (Insight: impaired, intact) mixed ANOVA, with Image as a within-person factor, and with Severity Status and Insight as between-person factors. Significant interactions were followed by posthoc comparisons to localize their source. To examine the three-way interaction specifically, we created four study groups corresponding to the four cells associated with Severity Status and Insight, and tested how these groups differed on choice behavior. Cell sizes for the respective groups were: 17 impaired insight, non-misuser; 21 impaired insight, misuser; 26 intact insight, non-misuser; and 36 intact insight, misuser. Furthermore, and in line with our a priori hypotheses and our prior reports using this task (Moeller et al., 2009, Moeller et al., 2010, Moeller et al., 2018, Moeller et al., 2012), we also created a targeted contrast whereby pleasant selections were directly subtracted from opioid selections (opioid>pleasant), and this opioid>pleasant contrast was also compared among the four Severity × Insight study groups. Finally, we tested the robustness of the effects by covarying for age, which differed between opioid misusers and non-misusers (Table 1), and by covarying for BPI Severity and BPI Interference scores. P<0.05 was considered significant.
Longitudinal Prediction of Outcome
These analyses were conducted in the 34 individuals who were opioid non-misusers at baseline, based on the COMM cutoff for misuse. We performed a multiple regression analysis in which opioid>pleasant selections and baseline ABC opioid misuse were entered as simultaneous predictors of follow-up ABC opioid misuse severity. Following a significant correlation with opioid>pleasant selections, additional regressions were performed using the constituent opioid and (separately) pleasant scores, to localize the source of the difference score effect. We also performed a secondary regression in which Insight was added to model.
RESULTS
Baseline Task Behavior
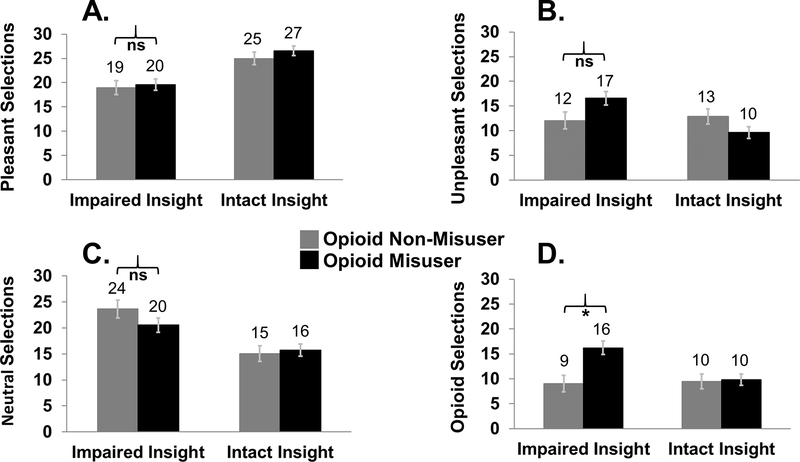
The 4 (Image: pleasant, unpleasant, neutral, opioid) × 2 (Severity Status: misuser, non-misuser) × 2 (Insight: impaired, intact) revealed a main effect of Image (F3,94=58.94, p<0.001): pleasant selections exceeded neutral selections, which exceeded unpleasant selections, which exceeded opioid pill selections (pairwise comparisons: all p<0.016). Significant two-way interactions emerged between Image and Insight (F3,94=19.13, p<0.001), and between Image and Severity Status (F3,94=4.25, p=0.007). These two-way interactions were further qualified by the significant three-way interaction between Image, Severity Status, and Insight (F3,94=3.83, p=0.012). Posthoc comparisons showed that, as anticipated, participants with high opioid misuse and impaired insight made more opioid selections (pairwise comparisons: all p<0.002) and more opioid>pleasant selections (pairwise comparisons: all p<0.018) than the other three Insight-Severity Status subgroups. This pattern was not evident for pleasant, unpleasant, and neutral selections. More specifically, among those with impaired insight, opioid misusers differed from non-misusers in their selections for opioid pill images (and opioid>pleasant images), but not in their selections for any other image category. Figure 3 displays the Group-Insight cell means for each image category. The three-way interaction was robust to correction for age (p=0.012), and robust to correction for ratings of BPI Severity (p=0.008) and BPI Interference (p=0.010); within these models, neither BPI Severity nor BPI Interference interacted with Image (p>0.072).
Figure 3.
Task behavior, moderated by opioid misuse on the Current Opioid Misuse Measure (COMM) and by insight. The three-way interaction between image choice, opioid misuse, and insight was partially driven by especially high opioid selections among individuals with high opioid severity and impaired insight. Panels display choice behavior for (A) pleasant images, (B) unpleasant images, (C) neutral images, and (D) opioid pill images.
Prediction of Clinical Outcome
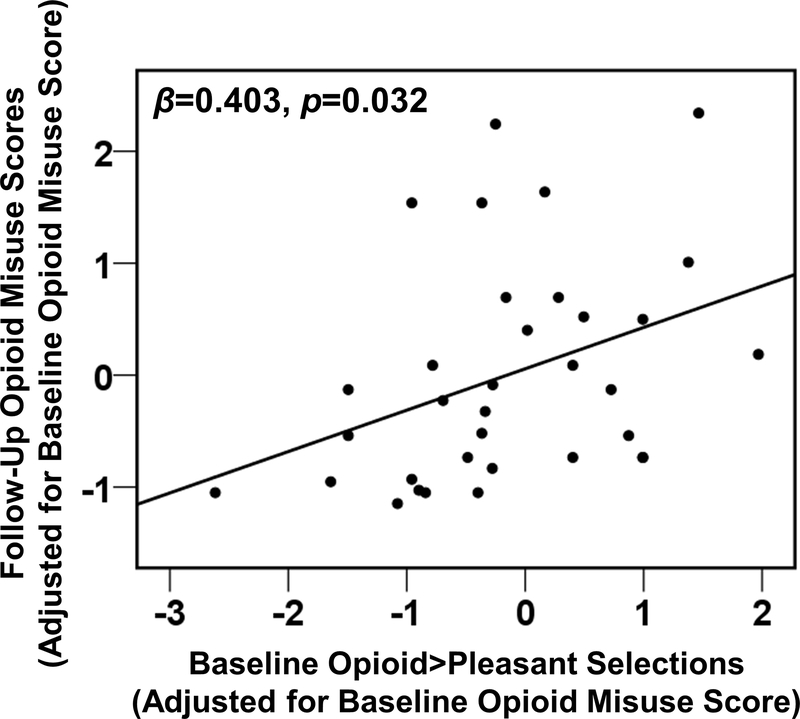
Controlling for pre-treatment opioid misuse severity, higher baseline opioid>pleasant selections predicted higher post-treatment opioid misuse at follow-up as hypothesized (β=0.403, p=0.032, N=34) (Figure 4). Analyses of the constituent scores (pleasant selections and opioid selections, respectively) showed that change in opioid misuse was predicted neither by baseline opioid selections (β=0.091, p=0.61, N=34) nor by baseline pleasant selections, although there was a trend for the latter in the expected direction (β=−0.357, p=0.059, N=34). Thus, greater allocation of behavior toward opioid reinforcement and away from natural reinforcement, but not either of these alone, predicted greater opioid misuse over time. Adding insight to the model did not alter the predictive effect of opioid>pleasant choice (β=0.413, p=0.037, N=34); insight was not a significant predictor on its own (p=0.85). Adding BPI Severity or (separately) BPI Interference to the model similarly did not alter the predictive effect of opioid>pleasant choice (βs>0.406, p<0.034, N=34); neither BPI Severity nor BPI Interference were significant predictors on their own (p>0.37).
Figure 4.
Longitudinal prediction of future opioid misuse, measured by the Addiction Behaviors Checklist (ABC), among individuals with initially low severity. As calculated with binary logistic regression, there was an association between opioid versus pleasant selections on the task at baseline and opioid misuse status at follow-up, controlling for opioid misuse status at baseline. For display purposes only, opioid misuse is provided using continuous scoring.
DISCUSSION
In this study, individuals with chronic pain prescribed long-term opioid analgesic medications completed a simulated drug-choice task to measure their preference for viewing images depicting opioid pills in comparison with their preference for viewing standardized pleasant, unpleasant, and neutral valenced images. In the cross-sectional component of this study, differences in opioid-related choice behavior were measured as a function of whether participants were opioid misusers (or not), and whether participants were (or were not) self-aware of the choices they made during the task (insight). In the longitudinal component of this study, opioid-related choice behavior was used to measure longitudinal change in opioid misuse from baseline.
Consistent with Hypothesis 1A and 1B, opioid misuse severity status and insight both emerged as significant moderating variables in affecting choice behavior. Links between drug misuse/disordered use and higher drug-related choice, especially when compared with pleasant choice, are well-established. For example, in studies of smokers or alcohol users, the choice for the respective drug over another natural reinforcer (chocolate) differentiated occasional users from dependent users (Lawn et al., 2015), and was positively associated with individual differences in SUD severity scores (Hogarth and Chase, 2011, Hogarth and Hardy, 2018). In a study using an abstract/simulated choice task, the more that hazardous alcohol users chose to enlarge alcohol- versus food images, the greater was their severity of dependence (Hardy and Hogarth, 2017). A novel finding of our study was that the contribution of opioid misuse severity to drug-choice behavior may be compounded by problems with insight and self-awareness, where we found that impaired insight interacted with high severity to produce especially high opioid-related choice. This finding is in line with prior work showing that insight and other self-monitoring abnormalities exacerbate disease severity, both in SUD (Moeller and Goldstein, 2014, Goldstein et al., 2009, Castine et al., 2018, Lindgren et al., 2017) and in other psychopathologies such as schizophrenia (David et al., 2012, van der Meer et al., 2010, Keefe et al., 2006, Lysaker et al., 2018). Lower insight has also been linked with poorer executive functioning and greater externalizing behavior (Volz-Sidiropoulou et al., 2016, Dean et al., 2015, Pujol et al., 2014, Blume et al., 2005, Le Berre et al., 2012). As in these other psychopathological conditions, it has been theorized that prescription opioid misusers have poor insight into their behavior, resulting from deficits in interoceptive and self-referential awareness. Prescription opioid misusers often believe they take opioids for pain management, when in fact their behavior is being driven by an appetitive impulse irrespective of the need for pain relief (Garland et al., 2013a). Future studies should employ interoceptive and other self-awareness probes, possibly in combination with neuroimaging, to assess whether the observed lack of insight might be undergirded by deficient capacity for interoception and self-awareness.
Interestingly, despite the modulation of opioid choice by variables suggesting a more severe opioid phenotype, the overall choice for opioid images was lower than for all other image categories. In our prior studies of stimulant users (Moeller et al., 2009, Moeller et al., 2018), drug-related images were comparably chosen to neutral images, but chosen more frequently than unpleasant images. One clear difference between this opioid-using sample and our prior stimulant-using samples is that here a large proportion of the sample (43.0%) took their opioid medications as prescribed and had no SUD or misuse; in prior studies, all participants met diagnostic criteria for stimulant use disorder. As the choice task is specifically designed to pit the choice for opioids against the choice for natural reinforcers as a marker of SUD severity, the comparatively low choice for viewing opioid images among this sample is not unsurprising. A sizable proportion of the stimulant users in our prior studies were also polysubstance users, and thus they may have been more impaired or more impulsive than the pure opioid users in this study (Schmidt et al., 2017). Emerging research has also begun revealing neurobehavioral differences between opioid and stimulant users more generally (Badiani et al., 2011, Ahn and Vassileva, 2016). Finally, as this was a chronic pain population, there may also have been ancillary behavioral effects. For example, seeing opioid pills may have reminded participants of their pain (Evans and Cahill, 2016), causing aversive reactions that reduced choice and overall motivation. Importantly, however, pain ratings did not explain our findings, speaking against this possibility.
In the longitudinal study component, opioid-related choice behavior predicted change in opioid misuse severity over time, consistent with Hypothesis 2. That is, among a sample of prescription opioid users with initially low misuse severity, baseline choice for opioids versus natural reinforcers predicted an escalation of opioid misuse behaviors. Importantly, this effect was above and beyond the influence of baseline severity measured by the ABC and baseline pain ratings measured by the BPI, indicating a vulnerability that was uniquely tapped by the task. These results support the view that our task, which partially relies on implicit processes related to drug-seeking, is providing complementary information that is not captured by more explicit measures (Nosek et al., 2011, Lindgren et al., 2016, Greenwald et al., 2009, Reich et al., 2010). Given that the current task is short and easy to administer, it is reasonable for future studies to validate the task longitudinally among individuals with greater SUD severity than those studied here (i.e., those with severe opioid use disorder). The task potentially could serve to predict future opioid abstinence, track treatment-related shifts in behavioral preference linked with salutary clinical outcomes, and/or identify individuals who might benefit from additional therapeutic resources in treatment settings. In that regard, evidence indicates individuals at elevated risk for opioid misuse are dispositionally anhedonic and prone to negative affectivity, neuroticism, and psychopathology (Wasan et al., 2015, Kornor and Nordvik, 2007, Clark et al., 2017). Such vulnerable patients may therefore come to misuse opioids as a means of self-medicating negative affective states and compensating for hedonic deficits (Garland et al., 2013a). Indeed, rates of opioid self-medication of negative affect are high among individuals with opioid use disorder (Garland et al., 2015b). Opioid use in the context of negative affective states may be negatively reinforcing and thereby come to increase the salience of opioid-related cues, driving an opioid attentional bias that magnifies craving and increases the likelihood of future opioid misuse (Garland et al., 2013b, Garland and Howard, 2014).
Of note, it was the opioid>pleasant difference score, but not its constituent scores (opioid or pleasant selections alone), that predicted prospective clinical outcome. We reported a similar finding in our prior longitudinal work in initially treatment-seeking cocaine users, where cocaine>pleasant choice predicted the number of drug use days over the next six months (Moeller et al., 2013a). More broadly, this finding supports a core tenet of SUD that drugs assume heightened motivational significance while natural reinforcers diminish in importance (Zilverstand et al., 2018, Garland et al., 2013a). The pleasant alternative putatively distinguishes between individuals who are true drug misusers from others who would self-administer drugs for a lack of viable alternatives (e.g., due to boredom) (Ahmed, 2010). For clinical practice, the drug versus pleasant choice is important for medication development, as the ideal therapeutic will decrease drug-related responding while simultaneously increasing allocation of behavior to non-drug reinforcers (i.e., thereby showing preserved behavioral motivation) (Moeller and Stoops, 2015). An efficacious therapy for opioid misuse among chronic pain patients, Mindfulness-Oriented Recovery Enhancement (MORE) (Garland et al., 2014), has been shown to shift relative physiological responsiveness to natural reinforcement versus drug-related reinforcement (Garland et al., 2017), with these shifts predicting decreases in subsequent opioid misuse behavior; it would be interesting to test if opioid-related choice is sensitive to this therapy and decreases in parallel with clinical improvement. Future studies might also examine in larger opioid-using samples, and possibly even across multiple addictive substances, whether there is an identifiable percent difference (or other relative difference metric) in choice (i.e., between drug and pleasant) that could be established as a cut-point for use in the clinic to describe problematic opioid behaviors. Indeed, the task appears sufficiently sensitive to detect variance in clinical outcomes, even as there was a small difference in opioid versus pleasant image selections in the sample.
Limitations of the current study include the following. First, we studied a heterogeneous sample of prescription opioid users. Although a heterogeneous sample was recruited by design to validate the task across a range of opioid use severities, current results likely may not generalize to a clinical sample of individuals with opioid use disorder, whom we anticipate would show higher levels of opioid-related choice than seen here. Current results also may not generalize to users of illicit opioids, such as heroin. Future studies should aim to validate the task in such samples, using appropriate substance-specific images. Second, it is possible that social desirability or other demand characteristics may have impacted our results, lowering the choice for viewing opioid images. However, the study was protected by a federal certificate of confidentiality, and experimenters emphasized that there were no right or wrong answers on the task and that participants’ responses would not affect their pain treatment or study payment. Finally, the increased opioid selections were specific to participants with impaired insight, who misremembered how often they chose drug images. This would argue against a conscientious-participant account of our findings. Third, for the longitudinal study component, given that we studied individuals who were opioid non-misusers at baseline, we are unable to generalize choice prediction to individuals with initially high levels of misuse or SUD. Insofar as we previously reported choice prediction of clinical outcome in individuals with cocaine use disorder (Moeller et al., 2013a), we would expect to find parallel results in individuals with opioid use disorder. Fourth, our method of assessing insight does not capture all aspects of the construct as classically defined in psychiatry (David et al., 2012, Amador et al., 1993). Incorporating additional metrics, such as metacognition (Moeller et al., 2016) or readiness to change behavior (Dean et al., 2015), into future studies could improve behavior prediction, perhaps helping to reveal a predictive signal for insight on drug-related outcomes that was not detected here.
In conclusion, we validated a simulated drug-choice task for use in a prescription opioid-using chronic pain population with a broad range of opioid misuse severity. Although opioid-related choice was low overall, it was comparatively elevated in individuals with a more severely addicted phenotype (supported by moderations with severity and insight, respectively), validating the task in this population for the first time. Opioid-related choice behavior also predicted change in misuse over time among individuals with initially low opioid misuse. As such, our results offer the intriguing possibility that simulated opioid choice could be used to objectively, yet noninvasively help to identify which individuals using prescription opioids might have the highest vulnerability for progressing into opioid use disorder and other deleterious outcomes.
Acknowledgements
This work was supported by R01DA042033 (PI: Garland) and K01DA037452 (PI: Moeller) from the National Institute on Drug Abuse; and a grant from the Fahs Beck Fund for Research and Experimentation (PI: Garland). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest: None
References
- AHMED SH 2010. Validation crisis in animal models of drug addiction: beyond non-disordered drug use toward drug addiction. Neurosci Biobehav Rev, 35, 172–84. [DOI] [PubMed] [Google Scholar]
- AHN WY & VASSILEVA J 2016. Machine-learning identifies substance-specific behavioral markers for opiate and stimulant dependence. Drug Alcohol Depend, 161, 247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMADOR XF, STRAUSS D,H, YALE S, FLAUM M, ENDICOTT J & GORMAN J 1993. Assessment of insight in psychosis. American Journal of Psychiatry, 150, 873–879. [DOI] [PubMed] [Google Scholar]
- ANDERSON BA, FAULKNER ML, RILEE JJ, YANTIS S & MARVEL CL 2013. Attentional bias for nondrug reward is magnified in addiction. Exp Clin Psychopharmacol, 21, 499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BADIANI A, BELIN D, EPSTEIN D, CALU D & SHAHAM Y 2011. Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci, 12, 685–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANKS ML, HUTSELL BA, SCHWIENTECK KL & NEGUS SS 2015. Use of Preclinical Drug vs. Food Choice Procedures to Evaluate Candidate Medications for Cocaine Addiction. Curr Treat Options Psychiatry, 2, 136–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLUME AW, SCHMALING KB & MARLATT GA 2005. Memory, executive cognitive function, and readiness to change drinking behavior. Addict Behav, 30, 301–14. [DOI] [PubMed] [Google Scholar]
- BUTLER SF, BUDMAN SH, FERNANDEZ KC, HOULE B, BENOIT C, KATZ N & JAMISON RN 2007. Development and validation of the Current Opioid Misuse Measure. Pain, 130, 144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTINE BR, ALBEIN-URIOS N, LOZANO-ROJAS O, MARTINEZ-GONZALEZ JM, HOHWY J & VERDEJO-GARCIA A 2018. Self-awareness deficits associated with lower treatment motivation in cocaine addiction. Am J Drug Alcohol Abuse, 1–7. [DOI] [PubMed] [Google Scholar]
- CLARK JM, CAO Y & KRAUSE JS 2017. Risk of Pain Medication Misuse After Spinal Cord Injury: The Role of Substance Use, Personality, and Depression. J Pain, 18, 166–177. [DOI] [PubMed] [Google Scholar]
- CLEELAND CS 1994. Brief Pain Inventory–Short Form (BPI–SF) Houston, TX, University of Texas M. D. Anderson Cancer Center. [Google Scholar]
- DAVID AS, BEDFORD N, WIFFEN B & GILLEEN J 2012. Failures of metacognition and lack of insight in neuropsychiatric disorders. Philos Trans R Soc Lond B Biol Sci, 367, 1379–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEAN AC, KOHNO M, MORALES AM, GHAHREMANI DG & LONDON ED 2015. Denial in methamphetamine users: Associations with cognition and functional connectivity in brain. Drug Alcohol Depend, 151, 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONNY EC, BIGELOW GE & WALSH SL 2004. Assessing the initiation of cocaine self-administration in humans during abstinence: effects of dose, alternative reinforcement, and priming. Psychopharmacology (Berl), 172, 316–23. [DOI] [PubMed] [Google Scholar]
- EVANS CJ & CAHILL CM 2016. Neurobiology of opioid dependence in creating addiction vulnerability. F1000Res, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARLAND EL, FROELIGER B & HOWARD MO 2015a. Allostatic dysregulation of natural reward processing in prescription opioid misuse: autonomic and attentional evidence. Biol Psychol, 105, 124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARLAND EL, FROELIGER B, ZEIDAN F, PARTIN K & HOWARD MO 2013a. The downward spiral of chronic pain, prescription opioid misuse, and addiction: cognitive, affective, and neuropsychopharmacologic pathways. Neurosci Biobehav Rev, 37, 2597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARLAND EL, FROELIGER BE, PASSIK SD & HOWARD MO 2013b. Attentional bias for prescription opioid cues among opioid dependent chronic pain patients. J Behav Med, 36, 611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARLAND EL, HANLEY AW, THOMAS EA, KNOLL P & FERRARO J 2015b. Low dispositional mindfulness predicts self-medication of negative emotion with prescription opioids. J Addict Med, 9, 61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARLAND EL & HOWARD MO 2014. Opioid attentional bias and cue-elicited craving predict future risk of prescription opioid misuse among chronic pain patients. Drug Alcohol Depend, 144, 283–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARLAND EL, HOWARD MO, ZUBIETA JK & FROELIGER B 2017. Restructuring Hedonic Dysregulation in Chronic Pain and Prescription Opioid Misuse: Effects of Mindfulness-Oriented Recovery Enhancement on Responsiveness to Drug Cues and Natural Rewards. Psychother Psychosom, 86, 111–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARLAND EL, MANUSOV EG, FROELIGER B, KELLY A, WILLIAMS JM & HOWARD MO 2014. Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: results from an early-stage randomized controlled trial. J Consult Clin Psychol, 82, 448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDSTEIN RZ, CRAIG AD, BECHARA A, GARAVAN H, CHILDRESS AR, PAULUS MP & VOLKOW ND 2009. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci, 13, 372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWALD AG, POEHLMAN TA, UHLMANN EL & BANAJI MR 2009. Understanding and using the Implicit Association Test: III. Meta-analysis of predictive validity. J Pers Soc Psychol, 97, 17–41. [DOI] [PubMed] [Google Scholar]
- HARDY L & HOGARTH L 2017. A novel concurrent pictorial choice model of mood-induced relapse in hazardous drinkers. Exp Clin Psychopharmacol, 25, 448–455. [DOI] [PubMed] [Google Scholar]
- HEDEGAARD H, WARNER M & MINIÑO AM 2017. Drug overdose deaths in the United States, 1999–2016. In: HEALTH NCF & STATISTICS (eds.). Hyattsville, MD. [Google Scholar]
- HOGARTH L & CHASE HW 2011. Parallel goal-directed and habitual control of human drug-seeking: implications for dependence vulnerability. J Exp Psychol Anim Behav Process, 37, 261–76. [DOI] [PubMed] [Google Scholar]
- HOGARTH L & HARDY L 2018. Depressive statements prime goal-directed alcohol-seeking in individuals who report drinking to cope with negative affect. Psychopharmacology (Berl), 235, 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOGARTH L, MATHEW AR & HITSMAN B 2017. Current major depression is associated with greater sensitivity to the motivational effect of both negative mood induction and abstinence on tobacco-seeking behavior. Drug Alcohol Depend, 176, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAYE AD, JONES MR, KAYE AM, RIPOLL JG, GALAN V, BEAKLEY BD, CALIXTO F, BOLDEN JL, URMAN RD & MANCHIKANTI L 2017. Prescription Opioid Abuse in Chronic Pain: An Updated Review of Opioid Abuse Predictors and Strategies to Curb Opioid Abuse: Part 1. Pain Physician, 20, S93–s109. [PubMed] [Google Scholar]
- KEEFE RS, POE M, WALKER TM, KANG JW & HARVEY PD 2006. The Schizophrenia Cognition Rating Scale: an interview-based assessment and its relationship to cognition, real-world functioning, and functional capacity. Am J Psychiatry, 163, 426–32. [DOI] [PubMed] [Google Scholar]
- KORNOR H & NORDVIK H 2007. Five-factor model personality traits in opioid dependence. BMC Psychiatry, 7, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANG PJ, BRADLEY MM & CUTHBERT BN 2008. International Affective Picture System (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8., Gainsville, FL, University of Florida. [Google Scholar]
- LAWN W, FREEMAN TP, HINDOCHA C, MOKRYSZ C, DAS RK, MORGAN CJ & CURRAN HV 2015. The effects of nicotine dependence and acute abstinence on the processing of drug and non-drug rewards. Psychopharmacology (Berl), 232, 2503–17. [DOI] [PubMed] [Google Scholar]
- LE BERRE AP, VABRET F, CAUVIN C, PINON K, ALLAIN P, PITEL AL, EUSTACHE F & BEAUNIEUX H 2012. Cognitive barriers to readiness to change in alcohol-dependent patients. Alcohol Clin Exp Res, 36, 1542–9. [DOI] [PubMed] [Google Scholar]
- LENOIR M, AUGIER E, VOUILLAC C & AHMED SH 2013. A choice-based screening method for compulsive drug users in rats. Curr Protoc Neurosci, Chapter 9, Unit 9 44. [DOI] [PubMed] [Google Scholar]
- LINDGREN KP, NEIGHBORS C, GASSER ML, RAMIREZ JJ & CVENCEK D 2017. A review of implicit and explicit substance self-concept as a predictor of alcohol and tobacco use and misuse. Am J Drug Alcohol Abuse, 43, 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDGREN KP, NEIGHBORS C, TEACHMAN BA, BALDWIN SA, NORRIS J, KAYSEN D, GASSER ML & WIERS RW 2016. Implicit alcohol associations, especially drinking identity, predict drinking over time. Health Psychol, 35, 908–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LYSAKER PH, PATTISON ML, LEONHARDT BL, PHELPS S & VOHS JL 2018. Insight in schizophrenia spectrum disorders: relationship with behavior, mood and perceived quality of life, underlying causes and emerging treatments. World Psychiatry, 17, 12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELTZER EC, RYBIN D, SAITZ R, SAMET JH, SCHWARTZ SL, BUTLER SF & LIEBSCHUTZ JM 2011. Identifying prescription opioid use disorder in primary care: diagnostic characteristics of the Current Opioid Misuse Measure (COMM). Pain, 152, 397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOELLER SJ, BEEBE-WANG N, WOICIK PA, KONOVA AB, MALONEY T & GOLDSTEIN RZ 2013a. Choice to view cocaine images predicts concurrent and prospective drug use in cocaine addiction. Drug Alcohol Depend, 130, 178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOELLER SJ, FLEMING SM, GAN G, ZILVERSTAND A, MALAKER P, DOLEIRE UQUILLAS F, SCHNEIDER KE, PRESTON-CAMPBELL RN, PARVAZ MA, MALONEY T, ALIA-KLEIN N & GOLDSTEIN RZ 2016. Metacognitive impairment in active cocaine use disorder is associated with individual differences in brain structure. Eur Neuropsychopharmacol, 26, 653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOELLER SJ & GOLDSTEIN RZ 2014. Impaired self-awareness in human addiction: deficient attribution of personal relevance. Trends Cogn Sci, 18, 635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOELLER SJ, HAJCAK G, PARVAZ MA, DUNNING JP, VOLKOW ND & GOLDSTEIN RZ 2012. Psychophysiological prediction of choice: relevance to insight and drug addiction. Brain, 135, 3481–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOELLER SJ, KONOVA AB, PARVAZ MA, TOMASI D, LANE RD, FORT C & GOLDSTEIN RZ 2014. Functional, structural, and emotional correlates of impaired insight in cocaine addiction. JAMA Psychiatry, 71, 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOELLER SJ, MALONEY T, PARVAZ MA, ALIA-KLEIN N, WOICIK PA, TELANG F, WANG GJ, VOLKOW ND & GOLDSTEIN RZ 2010. Impaired insight in cocaine addiction: laboratory evidence and effects on cocaine-seeking behaviour. Brain, 133, 1484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOELLER SJ, MALONEY T, PARVAZ MA, DUNNING JP, ALIA-KLEIN N, WOICIK PA, HAJCAK G, TELANG F, WANG GJ, VOLKOW ND & GOLDSTEIN RZ 2009. Enhanced choice for viewing cocaine pictures in cocaine addiction. Biol Psychiatry, 66, 169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOELLER SJ, OKITA K, ROBERTSON CL, BALLARD ME, KONOVA AB, GOLDSTEIN RZ, MANDELKERN MA & LONDON ED 2018. Low Striatal Dopamine D2-type Receptor Availability is Linked to Simulated Drug Choice in Methamphetamine Users. Neuropsychopharmacology, 43, 751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOELLER SJ, PARVAZ MA, SHUMAY E, BEEBE-WANG N, KONOVA AB, ALIA-KLEIN N, VOLKOW ND & GOLDSTEIN RZ 2013b. Gene x Abstinence Effects on Drug Cue Reactivity in Addiction: Multimodal Evidence. J Neurosci, 33, 10027–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOELLER SJ & STOOPS WW 2015. Cocaine choice procedures in animals, humans, and treatment-seekers: Can we bridge the divide? Pharmacol Biochem Behav, 138, 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOSEK BA, HAWKINS CB & FRAZIER RS 2011. Implicit social cognition: from measures to mechanisms. Trends Cogn Sci, 15, 152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUJOL J, BLANCO-HINOJO L, BATALLA A, LOPEZ-SOLA M, HARRISON BJ, SORIANO-MAS C, CRIPPA JA, FAGUNDO AB, DEUS J, DE LA TORRE R, NOGUE S, FARRE M, TORRENS M & MARTIN-SANTOS R 2014. Functional connectivity alterations in brain networks relevant to self-awareness in chronic cannabis users. J Psychiatr Res, 51, 68–78. [DOI] [PubMed] [Google Scholar]
- REICH RR, BELOW MC & GOLDMAN MS 2010. Explicit and implicit measures of expectancy and related alcohol cognitions: a meta-analytic comparison. Psychol Addict Behav, 24, 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUDD RA, SETH P, DAVID F & SCHOLL L 2016. Increases in Drug and Opioid-Involved Overdose Deaths - United States, 2010–2015. MMWR Morb Mortal Wkly Rep, 65, 1445–1452. [DOI] [PubMed] [Google Scholar]
- SCHMIDT TP, PENNINGTON DL, CARDOOS SL, DURAZZO TC & MEYERHOFF DJ 2017. Neurocognition and inhibitory control in polysubstance use disorders: Comparison with alcohol use disorders and changes with abstinence. J Clin Exp Neuropsychol, 39, 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DER MEER L, COSTAFREDA S, ALEMAN A & DAVID AS 2010. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci Biobehav Rev, 34, 935–46. [DOI] [PubMed] [Google Scholar]
- VOLZ-SIDIROPOULOU E, BOECKER M & GAUGGEL S 2016. The Positive Illusory Bias in Children and Adolescents With ADHD: Further Evidence. J Atten Disord, 20, 178–86. [DOI] [PubMed] [Google Scholar]
- VOWLES KE, MCENTEE ML, JULNES PS, FROHE T, NEY JP & VAN DER GOES DN 2015. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain, 156, 569–76. [DOI] [PubMed] [Google Scholar]
- WASAN AD, MICHNA E, EDWARDS RR, KATZ JN, NEDELJKOVIC SS, DOLMAN AJ, JANFAZA D, ISAAC Z & JAMISON RN 2015. Psychiatric Comorbidity Is Associated Prospectively with Diminished Opioid Analgesia and Increased Opioid Misuse in Patients with Chronic Low Back Pain. Anesthesiology, 123, 861–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU SM, COMPTON P, BOLUS R, SCHIEFFER B, PHAM Q, BARIA A, VAN VORT W, DAVIS F, SHEKELLE P & NALIBOFF BD 2006. The addiction behaviors checklist: validation of a new clinician-based measure of inappropriate opioid use in chronic pain. J Pain Symptom Manage, 32, 342–51. [DOI] [PubMed] [Google Scholar]
- ZILVERSTAND A, HUANG AS, ALIA-KLEIN N & GOLDSTEIN RZ 2018. Neuroimaging Impaired Response Inhibition and Salience Attribution in Human Drug Addiction: A Systematic Review. Neuron, 98, 886–903. [DOI] [PMC free article] [PubMed] [Google Scholar]