Abstract
Human immunodeficiency virus 1 (HIV-1) is a life-threatening pathogen that still lacks a curative therapy or vaccine. Despite the reduction in AIDS-related deaths achieved by current antiretroviral therapies, drawbacks including drug resistance and the failure to eradicate infection highlight the need to identify new pathways to target the infection. Circadian rhythms are endogenous 24-h oscillations which regulate physiological processes including immune responses to infection, and there is an emerging role for the circadian components in regulating viral replication. The molecular clock consists of transcriptional/translational feedback loops that generate rhythms. In mammals, BMAL1 and CLOCK activate rhythmic transcription of genes including the nuclear receptor REV-ERBα, which represses BMAL1 and plays an essential role in sustaining a functional clock. We investigated whether REV-ERB activity regulates HIV-1 replication and found REV-ERB agonists inhibited HIV-1 promoter activity in cell lines, primary human CD4 T cells and macrophages, whilst antagonism or genetic disruption of REV-ERB increased promoter activity. The REV-ERB agonist SR9009 inhibited promoter activity of diverse HIV-subtypes and HIV-1 replication in primary T cells. This study shows a role for REV-ERB synthetic agonists to inhibit HIV-1 LTR promoter activity and viral replication, supporting a role for circadian clock components in regulating HIV-1 replication.
Subject terms: Virology, Cell biology, Drug discovery, Microbiology, Infectious diseases
Introduction
All life forms have evolved under a rhythmically changing light/dark cycle due to the Earth’s rotation. From bacteria to man, all organisms possess an internal clock that oscillates in a 24-h manner to anticipate environmental changes. The central clock and peripheral oscillators share a common molecular architecture and consist of transcriptional/translational feedback loops that regulate rhythmic gene expression1. In mammals, BMAL1 and CLOCK dimerize and the complex can bind E-box motifs in the promoter/enhancer of various clock genes, including Per and Cry, to activate their transcription. In turn, the PER and CRY proteins repress BMAL1/CLOCK function and thereby shut down their own transcription. An additional feedback loop involves the nuclear receptors REV-ERBα and RORα. RORα competes with REV-ERBα for binding to the Bmal1 promoter ROR element (RORE) site and activates Bmal1 transcription. REV-ERBα and RORα coordinate a regulatory loop which is crucial for stabilizing the core clock machinery2 (Fig. 1).
Figure 1.
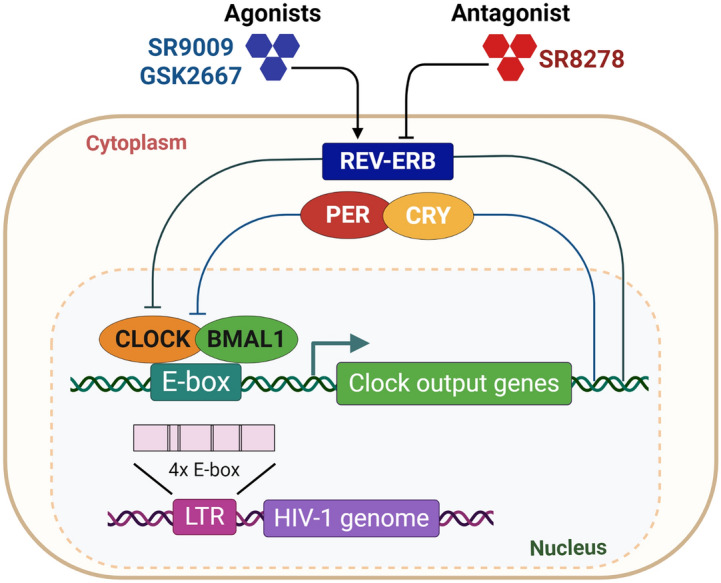
Schematic diagram illustrating the strategy for pharmacological modulation of REV-ERB.
The circadian system regulates host innate and adaptive immune responses to microbial pathogens3–5 and host susceptibility to an infectious agent is not only dependent on the inoculum size, transmission route and length of exposure, but on the time of day when the pathogen is encountered6. Recent clinical studies show that the time of vaccination can influence host immune responses and vaccine efficacy7. Viruses are obligate parasites that rely on host cell synthesis machinery for their replication, survival and dissemination. The potential for circadian pathways to regulate viral infection is an emerging research field6,8–10. We recently reported a role for REV-ERB to regulate flavivirus replication and particle assembly, including hepatitis C virus, dengue virus and Zika virus11.
Human immunodeficiency virus 1 (HIV-1) is the aetiologic agent of AIDS, one of the most devastating viral pandemics. Current therapies suppress HIV-1 replication and prevent the development of AIDS, but do not eradicate infection altogether. HIV-1 establishes latent sites of infection that promote viral persistence and evasion of host immune responses and antiviral therapies12. HIV-1 primarily replicates in CD4 T cells and macrophages which display intrinsic rhythms of clock genes and cytokine expression13–15. Despite reports of disrupted circadian rhythms in HIV-1 infected patients16–18, there is limited evidence supporting a direct role for circadian components in regulating HIV-1 replication. The HIV-1 long terminal repeat (LTR) promoter encodes regulatory elements that bind viral or cellular trans-activating factors that regulate its activity19, demonstrating the innate dependency of the virus on host cell components to replicate. Chang et al. recently reported that BMAL1 positively regulates the HIV-1 LTR activity through E-box motifs therein20 (Fig. 1).
REV-ERBα/β are members of the nuclear hormone receptor family that are involved in the molecular clock circuits. Raghuram et al. identified haem as the physiological ligand of REV-ERB, showing that haem was required for recruiting the co-repressors: Nuclear Receptor CoRepressor (NCoR) and Histone Deacetylase 3 (HDAC3)21. Several synthetic ligands targeting REV-ERB have been developed, including the agonists (GSK411222, SR900923 and GSK266724) and antagonist (SR827825). Since REV-ERB can repress BMAL1 expression, these compounds are useful tools for examining circadian modulation and its effect on HIV-1 replication. In this paper, we show that REV-ERB synthetic ligands inhibit HIV-1 LTR promoter activity and viral replication, supporting a role for circadian clock transcription factors in regulating HIV-1 replication. This study highlights a novel research area with potential for discovery of new pathways that may impact on the replication of not only HIV-1, but also other viruses.
Results and discussion
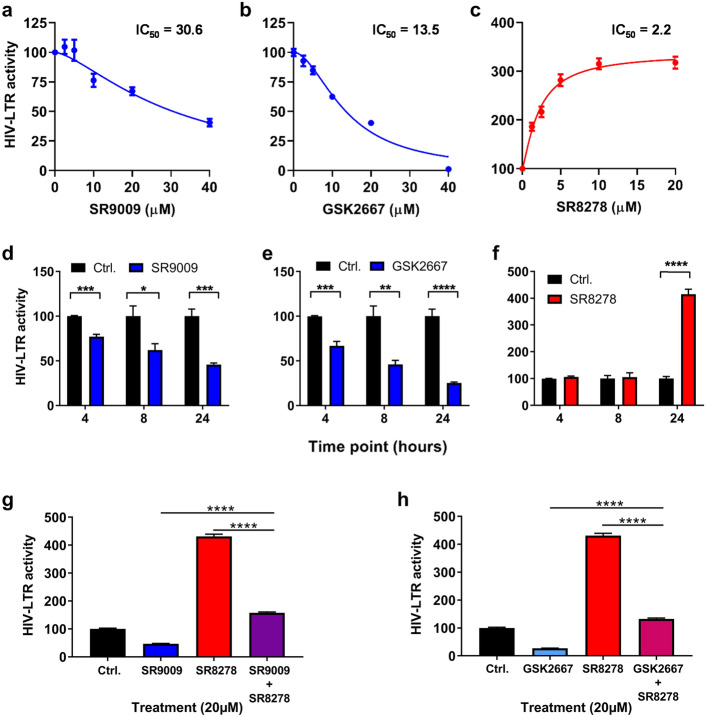
A recent study reported that overexpression of BMAL1/CLOCK increased HIV-1 LTR activity20, suggesting the potential for employing circadian modulators to inhibit BMAL1 and thereby reduce HIV-1 LTR activity. Since BMAL1 and CLOCK are basic helix-loop-helix PER-ARNT-SIM (bHLH-PAS) transcription factors, which are commonly considered undruggable, we tested the well characterized synthetic REV-ERB agonists SR9009 and GSK2667 and antagonist SR8278 for their ability to modulate HIV-1 LTR activity (Fig. 2). Initial experiments were performed using the Hela TZM-bl cell line, which encodes integrated copies of the luciferase gene under the control of the HIV-1 LTR26. We confirmed these drugs had no cytotoxic effects on TZM-bl cells in the dose range and treatment length tested (Supplementary Fig. 1). Both REV-ERB agonists (SR9009 and GSK2667) inhibited basal HIV-1 LTR activity in a dose-dependent manner (Fig. 2a,b), whilst the REV-ERB antagonist SR8278 increased LTR activity (Fig. 2c). When treatment lengths of 4 h, 8 h and 24 h were evaluated, the peak antiviral activity was seen after 24 h of treatment for all three compounds (Fig. 2d–f). To validate the specificity of the drugs, we treated TZM-bl cells with the agonists SR9009 or GSK2667 alone or in combination with antagonist SR8278. We observed a modest effect on basal LTR activity in the combined treatment group compared to single treatments (Fig. 2g,h), demonstrating that the compounds target a common molecular pathway.
Figure 2.
Pharmacological modulation of REV-ERB affects HIV-1 promoter activity. TZM-bl cells were treated with REV-ERB agonists SR9009 (a), GSK2667 (b) or the antagonist SR8278 (c) at a range of doses for 24 h and HIV-1 promoter activity measured by quantifying luciferase activity (mean ± S. E.M., n = 4). TZM-bl cells were treated with SR9009 (d) or GSK2667 (e) or SR8278 (f) at 20 µM for 4 h, 8 h or 24 h and HIV-1 promoter activity measured by quantifying luciferase activity. Data are expressed relative to the control untreated cells at each time point (mean ± S.E.M., n = 4, Two-way ANOVA). (g) TZM-bl cells were treated with media containing SR9009 or SR8278, or simultaneously treated with SR9009 and SR8278 for 24 h and HIV-1 promoter activity measured by quantifying luciferase activity (mean ± S.E.M., n = 8, One-way ANOVA). (h) TZM-bl cells were treated with GSK2667 or SR8278, or simultaneously treated with GSK2667 and SR8278 for 24 h and HIV-1 promoter activity measured by quantifying luciferase activity (mean ± S.E.M., n = 8, One-way ANOVA). All data are expressed relative to the control untreated cells.
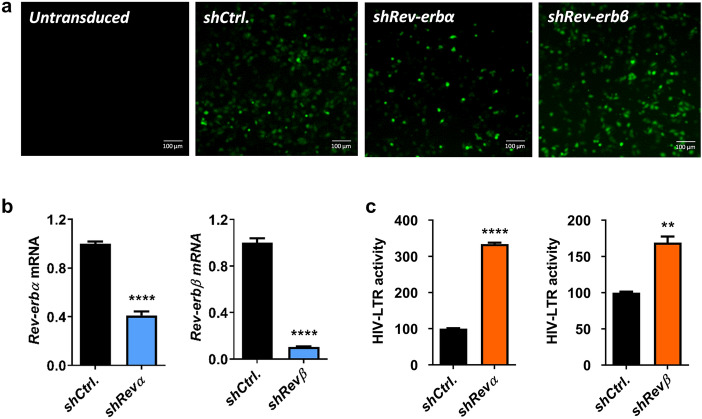
Since the REV-ERB compounds target both REV-ERB isoforms, we investigated the effect of individual REV-ERBs on HIV-1-LTR activity. We silenced Rev-erbα or Rev-erbβ in TZM-bl cells using lentivectors expressing shRNA targeting either isoform. Since the lentivectors encode a GFP reporter, the transduction efficiency was confirmed by imaging GFP expressing cells (Fig. 3a). The knockdown efficiency was confirmed by qPCR of Rev-erbα or Rev-erbβ mRNA 48 h post-transduction (Fig. 3b). Silencing Rev-erbα or Rev-erbβ increased HIV-1 LTR activity (Fig. 3c), suggesting a role for REV-ERBs in repressing HIV-1 promoter activity.
Figure 3.
Silencing Rev-erbs increases HIV-1 promoter activity. (a) TZM-bl cells were transduced with control or shRev-erbα or shRev-erbβ-encoding lentiviral vectors that also expressed a GFP reporter, and fluorescent images of GFP were obtained 48 h later to confirm transduction. (b) Total RNA was extracted from control or Rev-erb silenced TZM-bl cells at 48 h post-transduction and Rev-erbα or Rev-erbβ mRNA levels quantified by qRT-PCR (mean ± S.E.M., n = 3, unpaired t test). (c) Control or Rev-erb silenced TZM-bl cells were lysed 48 h post-transduction and HIV-1 promoter activity measured by quantifying luciferase activity (mean ± S.E.M., n = 3, unpaired t test).
To extend these observations to more physiologically relevant cell types, Jurkat cells (a CD4 T cell line) were infected with VSV-G complemented HIV NL4.3 R-E- reporter virus that undergoes a single cycle of replication that can be assessed by measuring luciferase activity. SR9009 significantly reduced HIV-1 replication and SR8278 showed an opposite effect (Fig. 4a). Reassuringly, we show that these REV-ERB ligands regulate Bmal1 promoter activity in Jurkat cells (Supplementary Fig. 2). Importantly, these observations were replicated in activated primary human CD4 T cells and macrophages derived from human induced pluripotent stem cells (iPSC) (Fig. 4b,c). Of note, these compounds showed no detectable cytotoxicity in any of the cell types used (Supplementary Figs. 3–5).
Figure. 4.
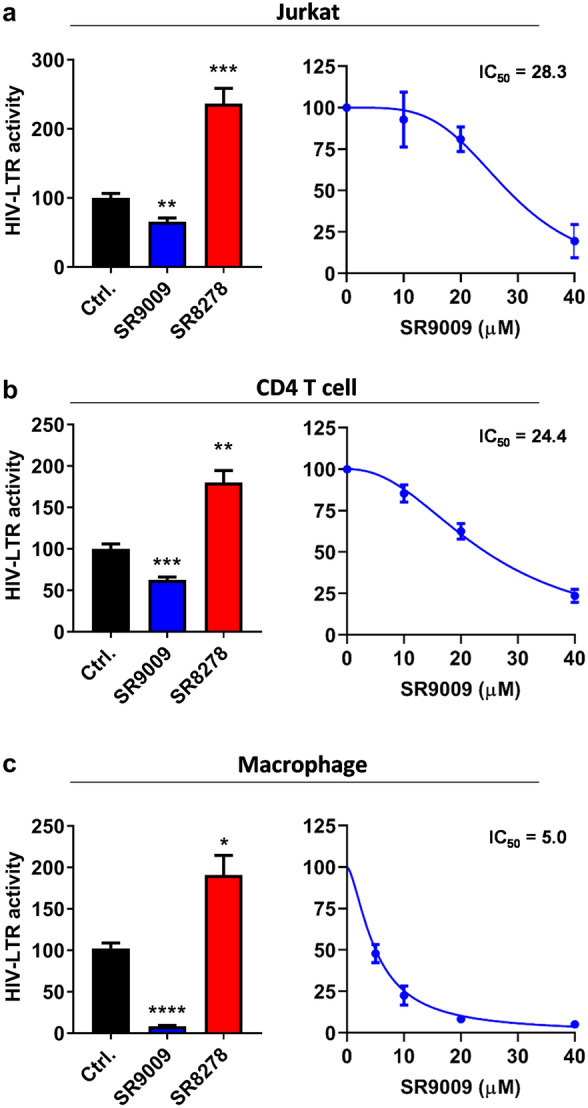
Pharmacological modulation of REV-ERB inhibits HIV-1 replication in T cells and macrophages. (a) Jurkat cells were infected with VSV-G-pseudotyped HIV-1 NL4.3-Luc for 24 h. Infected cells were treated with SR9009 (20 µM) or SR8278 (20 µM) for 24 h and LTR activity measured (mean ± S.E.M., n = 3, One-way ANOVA). The IC50 of SR9009 was determined at 24 h post treatment by quantifying luciferase activity. (b) Primary CD4 T cells were activated for 3 days with anti-CD3/CD28 and infected with VSV-G-pseudotyped HIV-1 NL4.3-Luc for 24 h and treated with SR9009 (20 µM) or SR8278 (10 µM) for 24 h and LTR activity measured (n = 4, mean ± S.E.M, One-way ANOVA). The IC50 of SR9009 was determined at 24 h post treatment by quantifying luciferase activity. (c) Human induced pluripotent stem cells (iPSCs) derived macrophages were infected with VSV-G-pseudotyped HIV-1 NL4.3-Luc for 24 h and treated with SR9009 (10 µM) or SR8278 (5 µM) for 24 h and LTR activity measured (n = 3, mean ± S.E.M, One-way ANOVA). The IC50 of SR9009 was determined at 24 h post treatment by quantifying luciferase activity. All data are expressed relative to the control untreated cells.
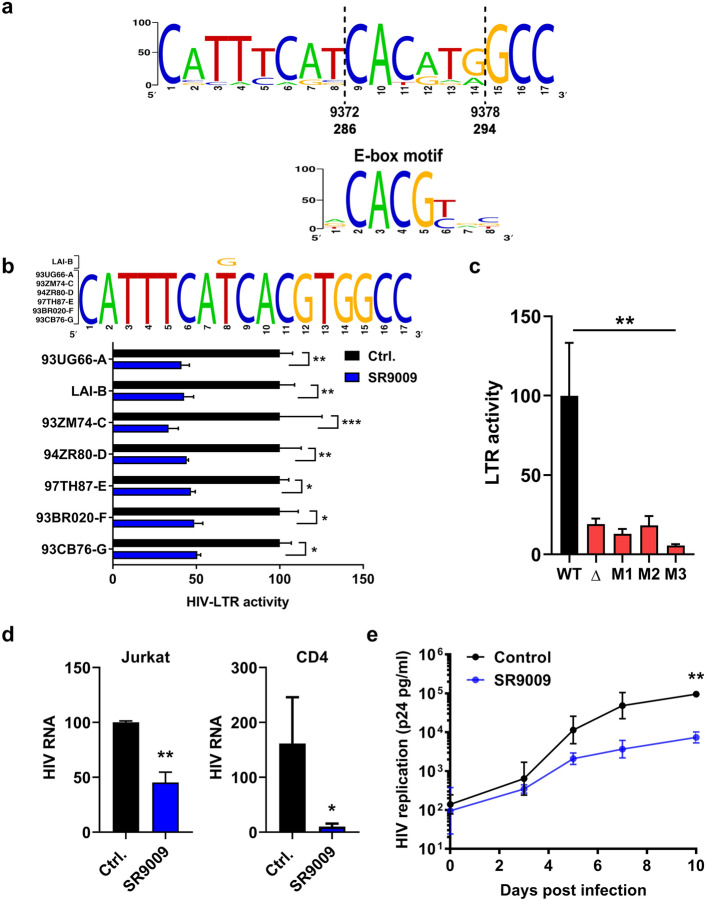
The BMAL1/CLOCK complex binds a canonical CACGTG motif E-box motif and screening HIV-1 sequences deposited in the Los Alamos Database revealed that approximately one third of sequences (467/1263) encode an E-box at position 294–286 in the U3 region of the LTR (Fig. 5a). Furthermore, three non-canonical (CANNTG) E-box motifs were found in the LTR at positions 151–156, 416–421, 433–438 that all showed a high level of conservation (> 70%) amongst published HIV-1 LTR sequences, providing potential alternative target motifs20. To investigate whether REV-ERB mediated-repression of the LTR was conserved across diverse HIV-1 subtypes, we transfected Jurkat cells with a panel of LTR-Luc reporter plasmids encoding promoter regions cloned from diverse HIV-1 clades27. All of the HIV-1 subtype LTR-Luc reporters used in these experiments encode the canonical E-box motif (Fig. 5b). As expected, SR9009 repressed the activity of all LTRs, demonstrating pan-subtype anti-viral activity (Fig. 5b). To evaluate whether this E-box motif is essential for LTR activity, we generated a panel of HIV LTR-Luc reporters where the E-box was deleted (Δ) or mutated to tgCGTG (M1), tcacgG (M2) or gACGTG (M3) and evaluated their effect on promoter activity in Jurkat cells. Mutating or deleting the E-box in the LTR significantly reduced luciferase activity (Fig. 5c), suggesting that this motif or sequence regulates HIV transcription.
Figure 5.
REV-ERB agonist SR9009 has pan-subtype antiviral activity. (a) Consensus plot illustrating the conserved nature of the E-box in the HIV-1 LTR based on the 897 HIV-1 sequences available in the LANL repository (analyzed with LANL QuikAlign/AnalyzeAlign software, coordinates are from the HXB2 referent). The level of conservation is reflected by the height of the bases (y axis 0–100%)39,40, with the consensus E-box motif (JASPAR database)40 shown below. (b) Conserved E-box motifs in the HIV-1 strains chosen to study, where HIV LAI has a variant, G, at position 8. Jurkat cells were transfected with HIV-1 promoter reporter constructs for 24 h. Transfected cells were treated with SR9009 at 20 µM for 24 h and LTR activity measured by quantifying luciferase activity (mean ± S.E.M., n = 4, Two-way ANOVA). (c) E-box mutation reduces HIV-LTR activity. Jurkat cells were transfected with wild type (LAI-B) or E-box mutated HIV-LTR constructs (where Δ represents a deletion and M represents a mutation). LTR activity was measured 48 h later and data are expressed relative to the wild type control. (mean ± S.E.M., n = 8–11, One-way ANOVA analysis). (d) Jurkat or activated CD4 T cells were infected with HIV-1 for 72 h. Infected cells were cultured in medium with or without SR9009 and viral RNA measured 48 h later. Data are expressed relative to the control untreated cells (mean ± S.E.M., n = 5–6, Mann–Whitney analysis). (e) CD8 depleted Human peripheral blood mononuclear cells (PBMCs) were infected with HIV-1 and cultured in medium with or without SR9009. Extracellular p24 levels were measured at intervals across a 10-day time period. Mean values from n = 3 biological repeats are shown, with error bars reflecting the geometric standard deviation. The first biological repeat employed cells from a single HIV-seronegative donor, whilst repeats two and three employed cells pooled from three healthy donors. The statistical significance of differences in mean extracellular p24 values between SR9009 and control cells at each time point was assessed using a paired t test.
The low basal activity of the mutated LTRs precluded experiments to evaluate the repressive effects of REV-ERB agonists. Given the low frequency of HIV infected primary CD4 T cells it was technically challenging to obtain direct evidence of BMAL1 binding the LTR by current ChIP-qPCR methodologies. It is worth noting that other host transcription factors, including CAAAT/EBP US2 could interact with this region28. Further analysis of these HIV-1 subtypes revealed additional conserved circadian regulatory elements in the LTR region, including the REV-ERB binding site RORE motif and the glucocorticoid response element (Supplementary Fig. 6). Cortisol production and secretion is tightly regulated by the circadian system and coordinates the synchronization of clock genes in peripheral tissues29. These data suggest a possibility where HIV-1 replication may be synchronized at certain times of day. A recent study demonstrated a physical interaction of glucocorticoid receptor with REV-ERBα in the liver, providing an alternative mechanism for REV-ERB to regulate HIV-1 replication30.
To confirm whether REV-ERB regulates HIV-1 replication at the transcriptional level, we quantified HIV-1 RNA in control or SR9009 treated infected Jurkat and primary CD4 T cells. SR9009 significantly reduced viral RNA levels in infected cells (Fig. 5d). Finally, we addressed the impact of SR9009 on the replication of an infectious virus in primary CD4 T cells over time. Activated CD8-depleted peripheral blood mononuclear cells (PBMCs) from HIV-seronegative individuals were infected with a patient-derived subtype B virus (CH058) and cultured in the presence or absence of SR9009 (20 µM) for 10 days, monitoring secreted p24 antigen as a measure of viral replication. While there was no impact on cell viability (Supplementary Fig. 7), SR9009 reduced HIV-1 replication with a statistically significant reduction in p24 levels at day 10 post infection (Fig. 5e).
Our analysis of the E-box motifs in the HIV-1 LTR suggests the viral transcription is likely to be affected by the host circadian rhythm and more specifically by BMAL1. Cleret-Buhot et al. reported that CD4 Th17 cells are more permissive to HIV infection than other CD4 T subsets and showed increased Bmal1 mRNA in CD4 Th17 lymphocytes31, consistent with a positive regulatory role for BMAL1 in the HIV-1 replication cycle. One could speculate, since BMAL1 expression oscillates throughout 24 h, HIV-1 may have evolved to adapt to this host rhythm by time of day dependent productive replication, to avoid host immune responses and to maximize viral persistence. Nevertheless our simplistic model system only demonstrated one aspect of the circadian regulation of HIV-1 which may under-represent the circadian impact if studied in a whole organism which warrants further investigation.
Since viruses are dependent on their host’s cellular machinery to replicate, it is likely that they have evolved to utilize host pathways to their advantage. In line with this, our data show that manipulating the host core circadian component REV-ERB can inhibit HIV-1 promoter activity. Importantly, our results suggest that REV-ERB ligands could be developed as a new class of HIV-1 replication inhibitors, which may synergize with current therapies. Of note, a recent report demonstrated REV-ERB dependent and independent effects of SR900932, and therefore it is reassuring that Rev-Erbα/β silencing, along with an additional REV-ERB agonist GSK2667 and the antagonist SR8278 had consistent phenotypes to support a REV-ERB mediated effect. Our study highlights a novel and exciting area of HIV research and provides a rationale for future work to identify novel circadian modulators with antiviral properties that may have potential to augment existing antiviral regimens.
Current therapeutically-used combination anti-retroviral therapies effectively inhibit HIV-1 replication and delay progression of the disease. However viral resistance to antiviral agents can undermine their efficacy33. Instead of targeting the virus, the REV-ERB ligands target an intrinsic host pathway which is entirely different to the direct antiviral activity of the agents currently employed to block HIV-1 replication. REV-ERB agonists may provide an additional drug class as adjuvants or to aid in current combination therapy, especially in cases where there is a persistent low-level viraemia, or in patients where multi-class antiretroviral resistance is an obstacle to effective combination therapy. Moreover, REV-ERB antagonists could have potential utility in ‘shock and kill’ HIV eradication strategies34, as targeting this pathway may lead to fewer side effects than the agents currently being investigated in this approach.
Methods
Cell lines and primary cells
TZM-bl cells were provided by Professor Bill Paxton (University of Liverpool, Liverpool, UK) and cultured in DMEM medium supplemented with 10% heat-inactivated FBS (hiFBS, Sigma, UK), 1% penicillin/streptomycin and 1% L-glutamine. Jurkat cells were provided by Professor Xiaoning Xu (Imperial College, London) and maintained in RPMI medium containing 10% hiFBS. Jurkat cells stably expressing a Bmal1-luciferase promoter were generated using a well characterized lentiviral plasmid as previously described35.
Peripheral blood mononuclear cells (PBMCs) were isolated from leukapheresis cones purchased from NHS Blood and Transplant (Oxford, UK), who obtained ethical approval for their research use from local NHS Research Ethics committees. All donors provided written informed consent. All work was compliant with institutional guidelines. CD4 T cells were isolated using the CD4 + T cell isolation kit from Miltenyi Biotec, Germany. Cells were stimulated with 50 IU/ml IL-2 (Proleukin; Novartis), 0.01 ug/ml soluble human anti-CD3 (R&D; clone UCHT1) and 0.1 ug/ml soluble human anti-CD28 (Life Technologies; clone CD28.2) at a concentration of 1 × 106 cells/ml in RPMI-1640 (Life Technologies, UK) containing 10% hiFBS, 1% penicillin/streptomycin (Sigma, UK), 1% sodium pyruvate (Sigma), 1% Glutamax (Life Technologies), 1% non-essential aminoacids (Life Technologies) and 2 mM beta-mercaptoethanol (Life Technologies) (R10 medium), and incubated at 37 °C in 5% CO2 for 3 days before infection. Human induced pluripotent stem cells (iPSC) derived macrophages were differentiated from the human iPSC line OX1-6136 and cultured in DMEM/F12 supplemented with 1% penicillin/streptomycin, Glutamax (2 mM), stabilized Insulin (5 μg/ml), HEPES pH 7.4 (15 mM), M-CSF (100 ng/ml)37.
Reagents
The following reagents were purchased from commercial suppliers: REV-ERB agonist SR9009 (Calbiochem, US); and REV-ERB antagonist SR8278 (Sigma, UK). REV-ERB agonist GSK2667 was synthesized at the University of Birmingham. All drugs were dissolved in dimethyl sulfoxide (DMSO) and their cytotoxicity determined using a lactate dehydrogenase (LDH) assay (Promega, UK).
Flow cytometry
To measure cell viability, control or drug treated cells were stained with Live Dead Aqua (Life Technologies, UK). Cells were fixed with 4% PFA (Santa Cruz, UK) for 10 min at room temperature, then samples were acquired on a Cyan ADP flow cytometer (Beckman Coulter) and data analyzed using FlowJo (TreeStar).
Plasmids
Lenti-shRev-Erbα/β constructs were gifts from Dr. B. Grimaldi, University of Genoa, Italy38. The plasmid encoding HIV NL4.3 R-E- Luciferase was obtained from the NIBSC AIDS Repository. VSV-G expression plasmid was previously reported39. The single-cycle HIV-1 pseudotyped with the VSV-G envelope were generated in 293 T cells using the HIV NL4.3 R-E- Luciferase plasmid. Plasmids encoding full-length infectious molecular clones of NL4.3-Bal were obtained from Drs John Kappes and Christina Ochsenbauer-Jambor (University of Alabama at Birmingham, US). HIV-LTR Luc constructs were previously reported27 and provided by Professor Bill Paxton (University of Liverpool, Liverpool, UK).
HIV LTR E-box analysis and subtypes promoter activity
The HIV-1 LTR sequences deposited in the Los Alamos Database were searched for the E-box motif CACGTG using the Sequence Search Interface program (www.hiv.lanl.gov) and the number and location of matches enumerated. Consensus plot illustrating the conserved nature of the E-box in the HIV-1 LTR based on the 1,263 HIV-1 sequences available in the LANL repository40,41. HIV-1 LTR-Luc plasmids were delivered into Jurkat cells using the Neon Transfection System following the manufacturer's instructions. Luciferase activity was measured using the Promega kit following the manufacturers’ instructions (Promega, UK).
Primers
Oligonucleotide sequences are listed below and were purchased from Life Technologies.
| Forward 5′–3′ | Reverse 5′–3′ | |
|---|---|---|
| HIV RNA | PATH-HIV1 KIT, PRIMER DESIGN LTD. UK | PATH-HIV1 KIT, PRIMER DESIGN LTD. UK |
| E-box deletion | atgcagctctcgggcatgaaatgctaggcg | cgcctagcatttcatgcccgagagctgcat |
| E-box mutant 1 | atgcagctctcgggccatatgatgaaatgctaggcg | cgcctagcatttcatcatatggcccgagagctgcat |
| E-box mutant 2 | ccggatgcagctctcgggctcacggatgaaatgctaggcggctg | cagccgcctagcatttcatccgtgagcccgagagctgcatccgg |
| E-box mutant 3 | gcatttcatcacgtcgcccgagagctgca | tgcagctctcgggcgacgtgatgaaatgc |
Generation and titration of viral stocks
To produce HIV CH058 6 M stocks, plasmid DNA was transfected into 293FT cells using Lipofectamine (Life Technologies, UK) or Fugene 6 (Promega, UK) and virus containing supernatants harvested 3 days later, clarified by centrifugation at 1,400 × g for 10 min and stored at − 80 °C. The infectivity of viral stocks was determined using a colorimetric reverse transcriptase assay (Roche Life Sciences). VSV-G complemented NL4.3 R-E- viral stocks were generated as previously reported39 and RT activity measured using a PCR based method42.
In vitro HIV-1 replication assay
PBMCs from HIV-seronegative donors were depleted of CD8 + T cells using CD8 microbeads (Miltenyi Biotec, Germany). Cells from a single donor or pooled cells from 3 different donors were activated by culturing for 3 days in R10 medium with 50 IU/ml IL-2 and antibodies to CD3 and CD28 as detailed above. Cells were then plated at 200,000 cells/well into 96-well round-bottomed microplates in triplicate, and infected by spinoculation with HIV-1 derived from an infectious molecular clone corresponding to the consensus quasispecies sequence of the virus present in HIV-1 infected patient CH058 at 6 months post-infection (CH058 6 M)43 at a concentration of 0.25 ng reverse transcriptase/106 cells. After a two-hour spinoculation, cells were washed twice before culturing in R10 medium with 50 IU/ml IL-2 with or without SR9009 (20 µM). Supernatants were harvested 90 min later (“day 0”) and at two to three day intervals thereafter for a 10-day period and stored at − 80 °C. Supernatants were subsequently analysed for HIV-1 capsid antigen using a p24 alphaLISA assay (Perkin-Elmer, US). The assay was performed according to the manufacturer’s instructions, and plates were read on FLUOstar Omega plate reader (BMG Labtech, Germany).
Statistical analysis
All experiments were performed at least twice. All data are presented as mean values ± SEM. p values were determined using the unpaired t test (two group comparisons; unpaired data) or paired t test (two group comparison; paired data) using PRISM version 8. In the figures ∗ denotes p < 0.05, ∗∗ denotes p < 0.01, ***denotes p < 0.001, ****denotes p < 0.0001, n.s. denotes non-significant.
Supplementary information
Acknowledgements
We thank Bill Paxton (University of Liverpool) for diverse HIV-LTR Luc plasmids and TZM-bl cells and Xiaoning Xu (Imperial College, London) for Jurkat cells. This work was funded by Wellcome Trust Award IA 200838/Z/16/Z (JAM); MRC Project Grant MR/R022011/1 (JAM) and NIH, NIAID, DAIDS Grants R01 AI 114266 and UM1 AI 126619 (P Borrow). P Borrow is a Jenner Institute Investigator. IP-P and XZ obtained support from a Medical Sciences Internal Pump Priming Fund award from the University of Oxford.
Author contributions
H.B. conducted experiments; R.D. conducted experiments; M.D. conducted experiments; I.P.-P., M.S., A.V.J., A.M. and W.J. provided reagents; P.B. performed data analysis; P Borrow contributed to experimental design, provided critical comments and edited the M.S.; J.A.M. designed study and co-wrote M.S.; X.Z. designed study, conducted experiments and wrote the M.S.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Helene Borrmann and Rhianna Davies.
Supplementary information
is available for this paper at 10.1038/s41598-020-70170-3.
References
- 1.Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017;18(3):164–179. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solt LA, Kojetin DJ, Burris TP. The REV-ERBs and RORs: molecular links between circadian rhythms and lipid homeostasis. Future Med. Chem. 2011;3(5):623–638. doi: 10.4155/fmc.11.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curtis AM, et al. Circadian clock proteins and immunity. Immunity. 2014;40(2):178–186. doi: 10.1016/j.immuni.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Scheiermann C, et al. Clocking in to immunity. Nat. Rev. Immunol. 2018;18(7):423–437. doi: 10.1038/s41577-018-0008-4. [DOI] [PubMed] [Google Scholar]
- 5.Downton P, Early JO, Gibbs JE. Circadian rhythms in adaptive immunity. Immunology. 2019 doi: 10.1111/imm.13167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edgar RS, et al. Cell autonomous regulation of herpes and influenza virus infection by the circadian clock. Proc. Natl. Acad. Sci. U S A. 2016;113(36):10085–10090. doi: 10.1073/pnas.1601895113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long JE, et al. Morning vaccination enhances antibody response over afternoon vaccination: a cluster-randomised trial. Vaccine. 2016;34(24):2679–2685. doi: 10.1016/j.vaccine.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhuang X, et al. Interplay between circadian clock and viral infection. J. Mol. Med. (Berl) 2017;95(12):1283–1289. doi: 10.1007/s00109-017-1592-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazzoccoli G, et al. the circadian clock, the immune system, and viral infections: the intricate relationship between biological time and host-virus interaction. Pathogens. 2020;9(2):83. doi: 10.3390/pathogens9020083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhuang X, et al. Daytime variation in hepatitis C virus replication kinetics following liver transplant. Wellcome Open Res. 2018;3:96. doi: 10.12688/wellcomeopenres.14696.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhuang X, et al. The circadian clock components BMAL1 and REV-ERBalpha regulate flavivirus replication. Nat. Commun. 2019;10(1):377. doi: 10.1038/s41467-019-08299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collier DA, Monit C, Gupta RK. The impact of HIV-1 drug escape on the global treatment landscape. Cell Host Microbe. 2019;26(1):48–60. doi: 10.1016/j.chom.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Bollinger T, et al. Circadian clocks in mouse and human CD4+ T cells. PLoS ONE. 2011;6(12):e29801. doi: 10.1371/journal.pone.0029801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keller M, et al. A circadian clock in macrophages controls inflammatory immune responses. Proc. Natl. Acad. Sci. U S A. 2009;106(50):21407–21412. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitchen GB, et al. The clock gene Bmal1 inhibits macrophage motility, phagocytosis, and impairs defense against pneumonia. Proc. Natl. Acad. Sci. U S A. 2020;117(3):1543–1551. doi: 10.1073/pnas.1915932117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taibi DM. Sleep disturbances in persons living with HIV. J. Assoc. Nurses AIDS Care. 2013;24(1 Suppl):S72–85. doi: 10.1016/j.jana.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baekken M, et al. Association between HIV infection and attenuated diurnal blood pressure rhythm in untreated hypertensive individuals. HIV Med. 2009;10(1):44–52. doi: 10.1111/j.1468-1293.2008.00655.x. [DOI] [PubMed] [Google Scholar]
- 18.Borkum M, et al. Ambulatory blood pressure profiles in a subset of HIV-positive patients pre and post antiretroviral therapy. Cardiovasc. J. Afr. 2014;25(4):153–157. doi: 10.5830/CVJA-2014-029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaczmarek K, Morales A, Henderson AJ. T cell transcription factors and their impact on HIV expression. Virology (Auckl) 2013;2013(4):41–47. doi: 10.4137/VRT.S12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang CC, et al. Variation in cell-associated unspliced HIV RNA on antiretroviral therapy is associated with the circadian regulator brain-and-muscle-ARNT-like-1. AIDS. 2018;32(15):2119–2128. doi: 10.1097/QAD.0000000000001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raghuram S, et al. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBalpha and REV-ERBbeta. Nat. Struct. Mol. Biol. 2007;14(12):1207–1213. doi: 10.1038/nsmb1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng QJ, et al. Ligand modulation of REV-ERBalpha function resets the peripheral circadian clock in a phasic manner. J. Cell Sci. 2008;121(Pt 21):3629–3635. doi: 10.1242/jcs.035048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solt LA, et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature. 2012;485(7396):62–68. doi: 10.1038/nature11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trump RP, et al. Optimized chemical probes for REV-ERBalpha. J. Med. Chem. 2013;56(11):4729–4737. doi: 10.1021/jm400458q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kojetin D, et al. Identification of SR8278, a synthetic antagonist of the nuclear heme receptor REV-ERB. ACS Chem. Biol. 2011;6(2):131–134. doi: 10.1021/cb1002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aguilar-Cordova E, et al. A sensitive reporter cell line for HIV-1 tat activity, HIV-1 inhibitors, and T cell activation effects. AIDS Res. Hum. Retrovir. 1994;10(3):295–301. doi: 10.1089/aid.1994.10.295. [DOI] [PubMed] [Google Scholar]
- 27.Jeeninga RE, et al. Functional differences between the long terminal repeat transcriptional promoters of human immunodeficiency virus type 1 subtypes A through G. J. Virol. 2000;74(8):3740–3751. doi: 10.1128/jvi.74.8.3740-3751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, et al. Structural and functional studies of CCAAT/enhancer binding sites within the human immunodeficiency virus type 1 subtype C LTR. Biomed. Pharmacother. 2010;64(10):672–680. doi: 10.1016/j.biopha.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dickmeis T. Glucocorticoids and the circadian clock. J. Endocrinol. 2009;200(1):3–22. doi: 10.1677/JOE-08-0415. [DOI] [PubMed] [Google Scholar]
- 30.Caratti G, et al. REVERBa couples the circadian clock to hepatic glucocorticoid action. J. Clin. Invest. 2018;128(10):4454–4471. doi: 10.1172/JCI96138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cleret-Buhot A, et al. Identification of novel HIV-1 dependency factors in primary CCR4(+)CCR6(+)Th17 cells via a genome-wide transcriptional approach. Retrovirology. 2015;12:102. doi: 10.1186/s12977-015-0226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dierickx P, et al. SR9009 has REV-ERB-independent effects on cell proliferation and metabolism. Proc. Natl. Acad. Sci. U S A. 2019;116(25):12147–12152. doi: 10.1073/pnas.1904226116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Godfrey C, et al. Global HIV antiretroviral drug resistance: a perspective and report of a national institute of allergy and infectious diseases consultation. J. Infect. Dis. 2017;216(suppl_9):S798–S800. doi: 10.1093/infdis/jix137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deeks SG. HIV: shock and kill. Nature. 2012;487(7408):439–440. doi: 10.1038/487439a. [DOI] [PubMed] [Google Scholar]
- 35.Brown SA, et al. The period length of fibroblast circadian gene expression varies widely among human individuals. PLoS Biol. 2005;3(10):e338. doi: 10.1371/journal.pbio.0030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buchrieser J, et al. RIPK1 is a critical modulator of both tonic and TLR-responsive inflammatory and cell death pathways in human macrophage differentiation. Cell Death Dis. 2018;9(10):973. doi: 10.1038/s41419-018-1053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dafinca R, et al. C9orf72 hexanucleotide expansions are associated with altered endoplasmic reticulum calcium homeostasis and stress granule formation in induced pluripotent stem cell-derived neurons from patients with amyotrophic lateral sclerosis and frontotemporal dementia. Stem Cells. 2016;34(8):2063–2078. doi: 10.1002/stem.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Mei C, et al. Dual inhibition of REV-ERBbeta and autophagy as a novel pharmacological approach to induce cytotoxicity in cancer cells. Oncogene. 2015;34(20):2597–2608. doi: 10.1038/onc.2014.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsu M, et al. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc. Natl. Acad. Sci. U S A. 2003;100(12):7271–7276. doi: 10.1073/pnas.0832180100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crooks GE, et al. WebLogo: a sequence logo generator. Genome Res. 2004;14(6):1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan A, et al. JASPAR 2018: update of the open-access database of transcription factor binding profiles and its web framework. Nucl. Acids Res. 2018;46(D1):D1284. doi: 10.1093/nar/gkx1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vermeire J, et al. Quantification of reverse transcriptase activity by real-time PCR as a fast and accurate method for titration of HIV, lenti- and retroviral vectors. PLoS ONE. 2012;7(12):e50859. doi: 10.1371/journal.pone.0050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fenton-May AE, et al. Relative resistance of HIV-1 founder viruses to control by interferon-alpha. Retrovirology. 2013;10:146. doi: 10.1186/1742-4690-10-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).