Abstract
RNA silencing is a conserved mechanism in eukaryotic organisms to regulate gene expression. Argonaute (AGO), Dicer-like (DCL) and RNA-dependent RNA polymerase (RDR) proteins are critical components of RNA silencing, but how these gene families’ functions in sugarcane were largely unknown. Most stress-resistance genes in modern sugarcane cultivars (Saccharum spp.) were originated from wild species of Saccharum, for example S. spontaneum. Here, we used genome-wide analysis and a phylogenetic approach to identify four DCL, 21 AGO and 11 RDR genes in the S. spontaneum genome (termed SsDCL, SsAGO and SsRDR, respectively). Several genes, particularly some of the SsAGOs, appeared to have undergone tandem or segmental duplications events. RNA-sequencing data revealed that four SsAGO genes (SsAGO18c, SsAGO18b, SsAGO10e and SsAGO6b) and three SsRDR genes (SsRDR2b, SsRDR2d and SsRDR3) tended to have preferential expression in stem tissue, while SsRDR5 was preferentially expressed in leaves. qRT-PCR analysis showed that SsAGO10c, SsDCL2 and SsRDR6b expressions were strongly upregulated, whereas that of SsAGO18b, SsRDR1a, SsRDR2b/2d and SsRDR5 was significantly depressed in S. spontaneum plants exposed to PEG-induced dehydration stress or infected with Xanthomonas albilineans, causal agent of leaf scald disease of sugarcane, suggesting that these genes play important roles in responses of S. spontaneum to biotic and abiotic stresses.
Subject terms: Plant stress responses, Functional genomics
Introduction
RNA silencing, also known as RNA interference (RNAi), plays an important role in multiple processes in plants, including growth and development, epigenetic modifications and responses to and defenses against abiotic and biotic stresses1–3. Several essential steps and core components of RNA silencing pathways are well-characterized. RNA silencing is initially triggered by the formation of double stranded RNAs (dsRNAs) that are subsequently cleaved by the RNase III-type DICER-LIKE proteins (DCL) into small RNA duplexes (sRNAs) of 21–24 nucleotides that include short-interfering RNAs (siRNAs) and microRNAs (miRNAs)4,5. Diverse sRNAs are denatured and incorporated into the multi-component RNA-induced silencing complex (RISC) having an Argonaute (AGO) protein at its catalytic core6,7. The RISC binds complementary mRNAs guided by single-stranded sRNAs to mediate processes such as translational inhibition, RNA degradation or chromosome modification8,9. These sRNAs are amplified from the targeted RNA by cellular host RNA-dependent RNA polymerases (RDRs) to produce additional dsRNAs that will be processed into secondary siRNAs that amplify the silencing signal10,11. Notably, RNA silencing-based immunity is also integrated with R gene-mediated immunity in plants for defense against pathogens12–14.
The proteins encoded by DCL, AGO, and RDR gene families are core components of the RNA silencing process5. DCLs contain a DEAD domain, a helicase conserved C-terminal (Helicase C) domain, a Dicer dimerization domain (Dicer dimer), a PAZ domain (PAZ), a Ribonuclease III domain (Ribonuclease 3), and a double-stranded RNA-binding domain (DSRM)15,16. AGOs have four functional domains, i.e., variable MID and N-terminal domains, and conserved PAZ and PIWI domains7,17. The PAZ domain can anchor sRNA duplexes with a two-nucleotide 3′ overhang via the specific binding pocket and the PIWI domain that has a similar fold to RNase H and exhibits endonuclease activity, thus playing an important role in target RNA cleavage18. RDRs share a special conserved RNA-dependent RNA polymerase (RdRP) catalytic domain19. The DCL, AGO, and RDR gene families in plants have species-dependent differences in gene numbers, which range from 20 genes in Arabidopsis20 to 51 genes in Brassica species21. Notably, different members of DCL, AGO, and RDR families play different roles in RNA silencing in plants, but they also share partially redundant functions20. Currently, there is limited information about DCL, AGO, and RDR gene families in sugarcane.
Sugarcane is an important sugar crop that accounts for 80% of sugar production worldwide and also is one of the most sustainable energy crops that can serve as a biofuel source22,23. Modern sugarcane hybrids originated from crosses between S. officinarum and one or more other Saccharum species and their progenies were progressively backcrossed with different S. officinarum clones24–26. This process of recurrent introgressive hybridization contributed to commercial hybrids that are highly outcrossed, heterozygous polyploids26. Modern sugarcane cultivars offer high sugar content that can primarily be attributed to S. officinarum, whereas other traits (e.g., growth vigor, stress resistance, and ratooning) mainly arose from S. spontaneum27–29. Recently, a genome sequence of the sugarcane wild species clone AP85-441, a haploid S. spontaneum, was determined and assembled into 32 pseudo-chromosomes comprising eight homologous groups of four members each 28. In addition, a BAC (bacterial artificial chromosome)-based monoploid genome sequence of cultivar R57027 and a polyploid genome sequence of cultivar SP80-328030 were also sequenced and assembled. These sugarcane genomic sequences provide valuable reference sequences in the post-genomics era23.
Sugarcane often suffers diverse biotic (e.g. pathogenic microorganisms) and abiotic (e.g., drought, cold and high salinity) stresses22. Drought stress is one of the most important abiotic stress factors in sugarcane growth and yield worldwide, including in China31,32. Additionally, leaf scald caused by Xanthomonas albilineans is one of three main sugarcane bacterial diseases, and is responsible for significant loss in cane yield and juice quality33. Leaf scald exists in some sugarcane-producing areas in China, where it represents a potential threat to the sugar industry34,35. In this study we identified and classified protein members of the DCL, AGO and RDR gene families in the S. spontaneum genome, and analyzed the functions of these genes to understand their roles in responses to polyethylene glycol (PEG)-induced dehydration stress and X. albilineans infection. Our findings will provide important information for exploration of molecular resistance mechanisms in S. spontaneum.
Methods
Identification of putative DCL, AGO, and RDR genes in S. spontaneum
Hidden Markov Model (HMM) profiles of the characterized and conserved domains of DCL, AGO and RDR families were retrieved from the protein family database (Pfam, https://pfam.xfam.org/)36 to search for these gene families in the S. spontaneum genome database (https://www.life.illinois.edu/ming/downloads/Spontaneum_genome/)28. DCL, AGO, and RDR protein sequences were identified based on the HMM profiles using HMMER software with default parameters37 and a cut-off value of 0.0138. To ensure the complete identification of the three gene families, we further Blasted DCLs, AGOs and RDRs from maize genome sequences39 against the S. spontaneum genome database. Conserved domains in all candidate genes were examined using the Pfam and Simple Modular Architecture Research Tool (SMART, https://smart.embl-heidelberg.de/) program. Sequence length, molecular weight and the isoelectric point of DCL, AGO and RDR proteins were predicted using tools at the ExPasy website (https://web.expasy.org/protparam/).
In-silico analysis of gene structure, promoter cis-acting elements and protein-protein interactions
Information concerning conserved domains among DCL, AGO and RDR genes in S. spontaneum, termed SsDCL, SsAGO and SsRDR, including the domain name and position were obtained by SMART (https://smart.embl-heidelberg.de/). The domain structure and exon–intron organization of the three gene families were analyzed using the online program Gene Structure Display Server (GSDS2.0: https://gsds.cbi.pku.edu.cn)40. One kilobase (kb) upstream region from the initial codon of each candidate gene in the three gene families was used to search cis-elements by the PlantCARE program (https://bioinformatics.psb.ugent.be/webtools/plantcare/html/). Network analysis of protein–protein interactions (PPIs) among all identified SsDCLs, SsAGOs and SsRDRs was performed using STRING v11.0 (https://string-db.org/) and corresponding maize proteins as reference sequences 41. The minimum required interaction score was 0.400, corresponding to medium confidence.
Chromosomal localization and phylogenetic analysis
The physical locations of SsDCL, SsAGO, and SsRDR genes were determined from the S. spontaneum genome database. The chromosomal positions of the three gene families were mapped using Circos software42. Gene duplication events were analyzed using the Multiple Collinearity Scan toolkit (MCScanX) with default parameters43. Multiple sequence alignments with the respective protein family from Arabidopsis21, rice44, maize39 and S. spontaneum were performed using the ClustalW program in MEGA 7.0 software45. Phylogenetic trees were constructed using a Neighbor-Joining (NJ) method with 1,000 bootstrap replications.
Plant materials and experimental treatments
Cuttings of S. spontaneum clone SES208 were provided by the Center for Genomics and Biotechnology, Fujian Agriculture and Forestry University (Fuzhou, China). Clone SES208 is an octoploid donor used to generate the haploid AP85-441 clone through anther cultures26. The cuttings were grown in a plant growth chamber under a 16 h/8 h light/dark period at 30 °C and 70% relative humidity (RH). Four-week-old plants (three leaves fully expanded) of clone SES208 were used in two experimental treatments: drought stress and bacterial stress. Roots of 24 plants were immersed in a 25% PEG-6000 solution for 0, 3, 6, and 12 h and respective top young leaf samples were collected. Leaves from another 24 plants were inoculated with X. albilineans strain Xa-FJ1 following the protocol described by Lin et al.34 and inoculated leaves were collected at 0, 24, 48, and 72 h post inoculation (hpi). All samples were frozen in liquid nitrogen and stored at − 80 °C until total RNA was extracted.
Quantitative real-time PCR (qRT-PCR)
Total RNA from leaf tissues was extracted by TRIzol reagent (Invitrogen/Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. RNA quality was analyzed by electrophoresis on a 1% agarose gel and RNA amounts were quantified using a Synergy™ H1 hybrid multimode reader (BioTek, Winooski, VT, USA). All RNA samples were diluted to a working concentration of 1.0 µg/µL with RNase-free H2O for further analysis. Total RNA (1.0 µg) was used in reverse transcription (RT) reactions to generate the first strand cDNA by HiScript II RT (Hongfeng Science and Technology, Nanjing, China) with random primers following the manufacturer’s directions. Because of the high homology in some gene pairs, we could not design primers with high specificity for every gene members, thus nine, three, and six candidate genes from SsAGOs, SsDCLs, and SsRDRs, respectively, were chosen to represent the different sub-families for quantitative real time PCR (qRT-PCR) transcriptional expression analyses. These genes primer pairs were designed with the GenScript Real-time PCR (TaqMan) Primer Design tool (https://www.genscript.com/tools/real-time-pcr-taqman-primer-design-tool) (Table S1). The qRT-PCR reactions were performed using 94 °C for 30 s followed by 40 cycles of 95 °C for 10 s and 60 °C for 30 s. The housekeeping gene, glyceraldehyde 3-phoshate dehydrogenase (GAPDH), was used as an internal control for normalization. The qRT-PCR results were analyzed by the 2-∆∆Ct quantitative method to determine differences in gene expression44. Three biological and three technical replicates were carried out for each sample.
Data analysis
Relative expression levels determined from qRT-PCR data at different time points for each cultivar were analyzed using one-way ANOVA. Multiple comparisons of the means were conducted by the SNK (Student–Newman–Keuls) Test. All statistical analyses were carried using SAS version 8.1 (SAS Institute, Cary, NC, USA).
Results
Identification and structural analysis of SsAGO, SsDCL, and SsRDR genes
To identify the AGO, DCL, and RDR gene families in S. spontaneum, we gathered the previously characterized and conserved domains of the three gene families and used HMMER software to search for corresponding domains in the S. spontaneum genome database. In addition, members of the three gene families from maize genome sequences were blasted against the S. spontaneum genome database. After evaluation of the structural integrity of conserved domains and elimination of redundant sequences, four genes encoding DCL proteins (SsDCLs), 21 genes encoding AGO proteins (SsAGOs) and 11 genes encoding RDR proteins (SsRDRs) were identified in S. spontaneum (Table S2). The detailed characteristics of all genes identified in this study, including chromosomal location and protein properties (e.g., open reading frame (ORF) length, protein length (amino acid, aa), molecular weight (MW), and isoelectric point (IP)) are listed in Table 1.
Table 1.
Structural characteristics and physio-chemical properties of dicer-like (DCL), argonaute (AGO) and RNA dependent RNA polymerase (RDR) genes from S. spontaneum.
| Gene name | Gene ID | Location | Protein | |||||
|---|---|---|---|---|---|---|---|---|
| Chromosome | Start | End | CDS (bp) | Length (aa) | Mw (Da) | PI | ||
| Dicer-like (DCL) | ||||||||
| SsDCL1a | Sspon.01G0001230-3D | Chr1D | 3,692,925 | 3,701,074 | 4,377 | 1,458 | 163,204.71 | 6.05 |
| SsDCL1b | Sspon.01G0001230-1A | Chr1A | 3,976,165 | 3,983,525 | 4,770 | 1,590 | 178,065.62 | 6.05 |
| SsDCL2 | Sspon.01G0022370-1A | Chr1A | 81,960,057 | 81,976,273 | 3,699 | 1,233 | 139,512.97 | 7.06 |
| SsDCL3 | Sspon.01G0019860-4D | Chr1D | 71,661,374 | 71,672,007 | 4,752 | 1584 | 179,009.05 | 6.24 |
| Argonaute (AGO) | ||||||||
| SsAGO2a | Sspon.05G0030950-2D | Chr5D | 11,880,179 | 11,884,678 | 3,066 | 1,021 | 111,963.65 | 8.73 |
| SsAGO2b | Sspon.05G0037340-1D | Chr5D | 11,924,233 | 11,928,734 | 2,763 | 921 | 100,646.66 | 7.81 |
| SsAGO3a | Sspon.05G0030960-1C | Chr5C | 2,396,093 | 2,401,132 | 3,048 | 1,015 | 110,049.24 | 9.30 |
| SsAGO3b | Sspon.05G0030950-1C | Chr5C | 2,386,282 | 2,391,173 | 2,904 | 967 | 104,620.95 | 9.27 |
| SsAGO3c | Sspon.05G0030950-1P | Chr5D | 12,513,102 | 12,517,597 | 3,036 | 1,011 | 109,860.1 | 9.35 |
| SsAGO4 | Sspon.02G0016200-2D | Chr3D | 39,536,146 | 39,547,268 | 2,838 | 946 | 105,523.71 | 8.60 |
| SsAGO5a | Sspon.01G0028360-2B | Chr1B | 96,848,274 | 96,854,895 | 3,165 | 1,054 | 115,793.38 | 9.42 |
| SsAGO5b | Sspon.01G0014460-3D | Chr1D | 87,130,069 | 87,135,808 | 2,742 | 913 | 102,643.41 | 9.28 |
| SsAGO5c | Sspon.01G0014460-1A | Chr1A | 41,213,733 | 41,219,461 | 2,418 | 806 | 90,379.42 | 9.55 |
| SsAGO5d | Sspon.01G0014460-2B | Chr1B | 96,914,953 | 96,920,845 | 2,784 | 927 | 103,595.76 | 9.55 |
| SsAGO6a | Sspon.07G0020900-1A | Chr7A | 78,332,899 | 78,336,592 | 1,902 | 634 | 70,983.26 | 9.46 |
| SsAGO6b | Sspon.07G0020900-2D | Chr7D | 60,984,388 | 60,994,488 | 2,070 | 689 | 77,218.74 | 9.34 |
| SsAGO10a | Sspon.08G0006580-1P | Chr8D | 17,872,226 | 17,876,344 | 2,271 | 757 | 83,975.06 | 9.22 |
| SsAGO10b | Sspon.08G0006580-1A | Chr8A | 20,403,262 | 20,409,634 | 2,829 | 942 | 105,347.29 | 9.47 |
| SsAGO10c | Sspon.08G0006580-2B | Chr8B | 18,085,409 | 18,091,645 | 2,394 | 797 | 88,300.59 | 9.67 |
| SsAGO10d | Sspon.08G0006580-4D | Chr8D | 17,765,548 | 17,771,337 | 1,884 | 628 | 71,264.01 | 9.40 |
| SsAGO10e | Sspon.08G0006580-3C | Chr8C | 9,379,029 | 9,385,180 | 2,916 | 971 | 108,586.36 | 9.43 |
| SsAGO18a | Sspon.02G0007830-1A | Chr2A | 22,748,211 | 22,753,686 | 3,099 | 1,032 | 113,076.36 | 9.47 |
| SsAGO18b | Sspon.01G0024300-1A | Chr1A | 87,229,183 | 87,234,462 | 2,382 | 793 | 88,734.95 | 9.29 |
| SsAGO18c | Sspon.01G0024300-3D | Chr1D | 84,919,502 | 84,924,770 | 2,364 | 787 | 88,118.01 | 9.00 |
| SsAGO18d | Sspon.02G0007830-2D | Chr2D | 16,391,380 | 16,396,270 | 2,754 | 918 | 100,666.07 | 9.44 |
| RNA dependent RNA Polymerase (RDR) | ||||||||
| SsRDR1a | Sspon.04G0003980-2D | Chr4D | 12,916,500 | 12,921,015 | 3,324 | 1,107 | 126,692.84 | 7.73 |
| SsRDR1b | Sspon.04G0003980-1A | Chr4A | 12,309,373 | 12,314,472 | 3,348 | 1,115 | 127,601.91 | 7.53 |
| SsRDR2a | Sspon.05G0009620-1A | Chr5A | 27,714,217 | 27,719,638 | 3,414 | 1,137 | 126,512.75 | 6.67 |
| SsRDR2b | Sspon.05G0009620-2B | Chr5B | 22,682,674 | 22,688,038 | 3,315 | 1,104 | 122,789.35 | 6.50 |
| SsRDR2c | Sspon.05G0009620-3C | Chr5C | 19,106,947 | 19,112,258 | 3,414 | 1,137 | 126,557.88 | 6.39 |
| SsRDR2d | Sspon.05G0009620-4D | Chr5D | 30,160,562 | 30,165,865 | 2,967 | 989 | 110,364.29 | 7.29 |
| SsRDR3 | Sspon.05G0014430-3C | Chr5C | 48,023,413 | 48,044,408 | 2076 | 691 | 79,290.78 | 7.29 |
| SsRDR4 | Sspon.05G0014430-4D | Chr5D | 52,146,251 | 52,168,613 | 2049 | 683 | 77,901.79 | 6.25 |
| SsRDR5 | Sspon.03G0023160-2C | Chr3C | 89,299,759 | 89,307,281 | 2,514 | 838 | 96,153.93 | 8.70 |
| SsRDR6a | Sspon.08G0019290-1B | Chr8B | 11,200,927 | 11,205,278 | 3,240 | 1,079 | 120,677.59 | 7.10 |
| SsRDR6b | Sspon.08G0019290-2D | Chr8D | 11,219,834 | 11,222,839 | 3,006 | 1,001 | 111,860.59 | 8.24 |
CDS coding sequence, MW molecular weight, IP isoelectric point.
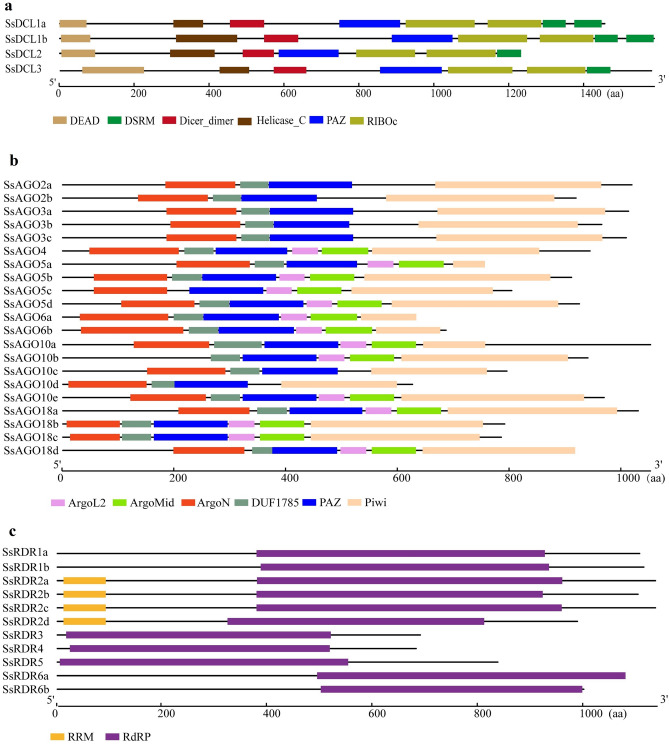
The proteins encoded by the four identified SsDCLs ranged from 1,233 aa (SsDCL2, Sspon.01G0022370-1A) to 1,590 aa (SsDCL1b, Sspon.01G0001230-1A) and contained a Dicer_dimer domain, a PAZ domain, a Helicase_C domain, a DEAD domain, a RIBOc domain and one or two DSRM domains (one for SsDCL2/3, and two for SsDCL1a/b) (Fig. 1A). The 21 identified SsAGOs ranged from 628 aa (SsAGO10d, Sspon.08G0006580-4D) to 1,054 aa (SsAGO5a, Sspon.01G0028360-2B), and shared common domains including a DUF1785 domain, PAZ domain, PIWI domain, and ArgoN domain. ArgoL2 and ArgoMid domains that were present in most of the SsAGOs identified (14/21), but were absent in SsAGO2a/b, SsAGO3a/b/c, and SsAGO10c/d (Fig. 1B). SsRDRs varied from 683 aa for SsRDR4 (Sspon.05G0014430-4D) to 1,137 aa for SsRDR2c (Sspon.05G0009620-3C), which contained the common sequence motif for DNA-dependent RNA polymerases (RdRP) whereas SsRDR2a/b/c/d had another common sequence motif, the RRM domain (Fig. 1C). Comparative structural analysis for exons-introns in the three gene families revealed that the number of introns ranged from two in SsAGO2b/3a to 22 in SsAGO4, from 15 in SsDCL1a to 24 in SsDCL3, and from one in SsRDR6b to 17 in SsRDR3 (Figure S1).
Figure 1.
Structural domains of dicer-like protein (SsDCL) (a), argonaute protein (SsAGO) (b), and RNA-dependent RNA polymerase (SsRDR) (c) from S. spontaneum. Domains are indicated by colored boxes. The scale bars at the bottom represent the length of proteins in aa (amino acid).
Furthermore, pairwise sequence alignment and identity analysis revealed that the conversed motifs among these gene members in each DCL, AGO and RDR families were high discrepancy (Figure S2 and Table S3). The identifies of amino acid sequences in DCL, AGO and RDR gene families were 29.7–85.8%, 12.3–99.3% and 11.3–100%, respectively (Table S3). Among gene pairs of alleles, these sequences shared higher identifies with each other. For example, the gene pairs of alleles in SsDCLs, the amino acid identity was 85.8% between SsDCL1a and SsDCL1b; the gene pairs of alleles in AGOs, the amino acid identities were 91.5% (SsAGO2a and SsAGO2b), 83.6–91.8% (SsAGO3a, SsAGO3b and SsAGO3c), 60.5–89.5% (SsAGO5a, SsAGO5b, SsAGO5c, and SsAGO5d), 93.2% (SsAGO6a and SsAGO6b), 43.5–97.3% (SsAGO10a, SsAGO10b, SsAGO10c, SsAGO10d and SsAGO10e), and 53.0–95.2% (SsAGO18a, SsAGO18b, SsAGO18c and SsAGO18d). The gene pairs of alleles in RDRs, the amino acid identities were 100% (SsRDR1a and SsRDR1b), 77.4–99.6% (SsRDR2a, SsRDR2b, SsRDR2c and SsRDR2d) and 83.7% (SsRDR6a and SsRDR6b).
Prediction of cis-acting elements in the putative gene promoters of SsAGO, SsDCL and SsRDR
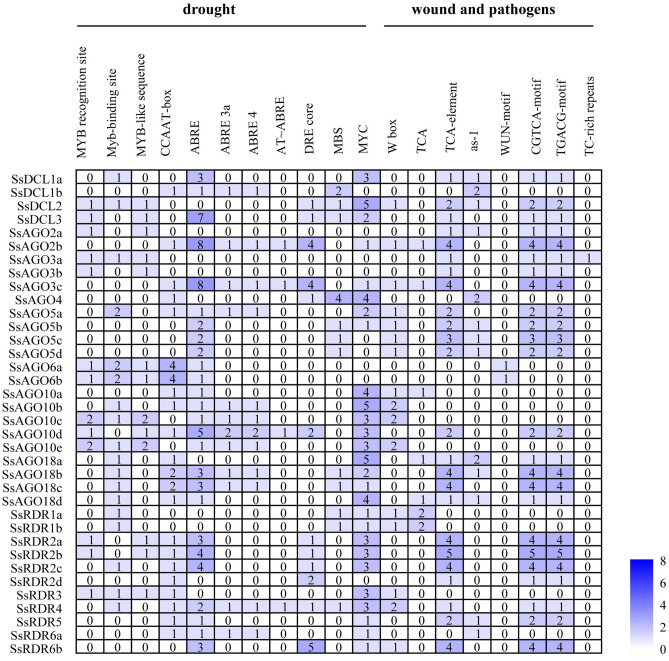
Sequences (1 kb) of upstream of the translation initiation codon for the SsAGO, SsDCL, and SsRDR genes were examined for the presence of cis-acting elements using the PLANTCARE online database. In addition to cis-acting elements that are characteristic of eukaryotic promoters, various cis-acting elements including those associated with plant growth, development, and stress responses were found among SsAGO, SsDCL, and SsRDR family members. The upstream sequences of all SsAGO, SsDCL, and SsRDR genes had the cis-acting element of eukaryotic promoters (CAAT-box) and, except for SsAGO2b/10d and SsRDR2a/2b/6b, had a TATA-box that is another cis-acting element of eukaryotic promoters (Table S4). Numerous cis-acting elements associated with drought/dehydration response including DRE core motifs, MYB recognition or binding sites, MBS, MYC, and ABRE elements were present (Fig. 2). Genes including SsDCL3, SsAGO2b, SsAGO3c, SsAGO10c/d/e, SsAGO18b, SsRDR2b, and SsRDR4 had more than ten cis-elements related to dehydration response. More than three classes of MYC elements were found in SsDCL2, SsAGO3a/b/c, SsAGO18a, SsRDR1a/b, SsRDR2d, and SRDR3, but no MYB elements were predicted in SsAGO5b, SsAGO5d, and SsRDR6b.
Figure 2.
Analysis of putative cis-acting elements related to response to drought or wound and pathogen stresses in S. spontaneum promoter sequences (1 kb) of SsDCL, SsAGO, and SsRDR genes. Numbers of elements present are indicated with darker blue shading representing higher numbers.
Multiple cis-acting elements involved in plant wound and pathogen response were also predicted in these promoters. More than one cis-acting element (W box, TGACG-motif or TCA-element) related to salicylic acid (SA) response were present in all gene family members, except for SsAGO6a/b. Two or more cis-acting element sites (CGTCA motif or TGACG motif) related to Methyl Jasmonate (MeJA) regulation presented in 24 promoters among the three gene families. Notably, the cis-element W box, which is related to responses to plant pathogen invasion, was present in SsDCL2, SsAGO2b, SsAGO3c, SsAGO5a/b/c/d, SsAGO10a/b/c/e, SsRDR1a/b, SsRDR3, SsRDR4, and SsRDR6b. The TC-rich repeat associated with plant defense against pathogen infection was present in SsAGO3a. Meanwhile, the WUN-motif related to mechanical damage was predicted only in the SsAGO6a/b promoter.
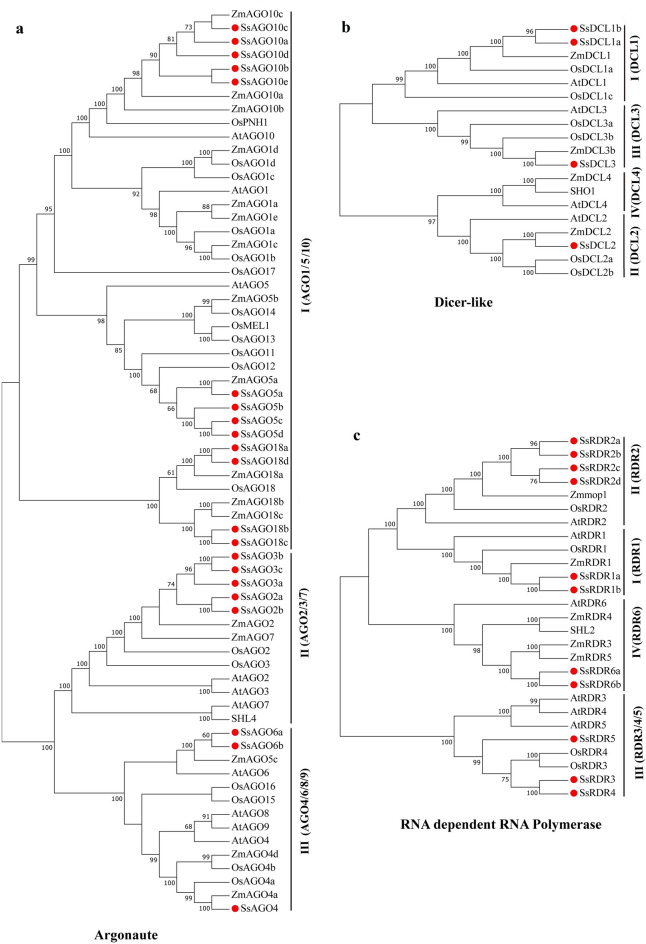
Phylogenetic analysis and chromosomal localization
To demonstrate phylogenetic relationships among identified genes encoding AGO, DCL, and RDR proteins, Neighbor-joining based phylogenetic trees were constructed using MEGA7 software based on the proteins of the three families from Arabidopsis, rice, maize, and S. spontaneum (Fig. 3, Table S2). All 19 DCL genes analyzed were clustered into four Clades, termed Clade I–IV (DCL1-4). Among the four SsDCL genes from S. spontaneum, SsDCL1a/1b genes were in Clade I, whereas SsDCL2 and SsDCL3 were grouped in Clade II and III, respectively. Unexpectedly, no SsDCL gene was found in Clade IV. All 59 AGO genes analyzed were clustered into three major Clades, including Clade I (AGO1/5/10), Clade II (AGO2/3/7), Clade III (AGO4/6/8/9). It is noteworthy that an AGO18 group from rice, maize and S. spontaneum falls into the Clade I. All 27 RDR genes were separated into four Clades, namely Clade I (RDR1), Clade II (RDRII), Clade III (RDR3/4/5), and Clade IV (RDR6). The 11 SsRDR genes from S. spontaneum were present in each of the four Clades.
Figure 3.
Phylogenetic analysis of S. spontaneum SsAGO(a), SsDCL(b), and SsRDR(c) genes. Neighbor-Joining (NJ) trees were constructed using MEGA 7 software based on the protein sequences for each family member. Bootstrap support values from 1,000 replications are indicated above the branches. S. spontaneum genes are indicated by a red circle.
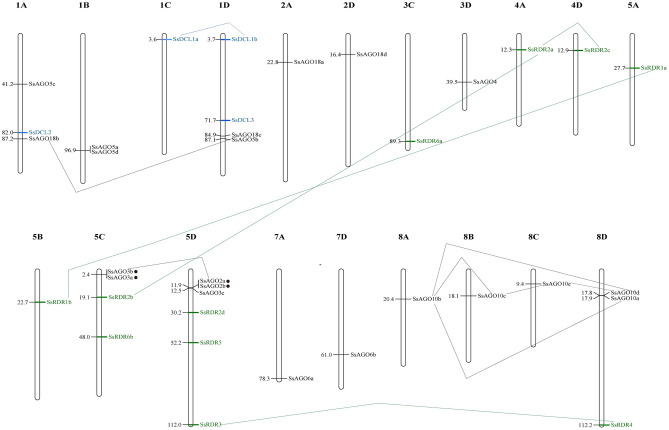
All 36 identified S. spontaneum genes encoding DCLs, AGOs, and RDRs were precisely located on 19 of 32 S. spontaneum chromosomes (comprising 8 homologous groups of 4 members each): six genes on chromosome 5D, four genes on chromosome 1A, 1D and 5C, three genes on chromosome 8D, two genes on chromosome 1B, and one gene on the other remaining chromosomes (1C, 2A, 2D, 3C, 4A, 4D, 5A, 5B, 7A, 7D, 8A, 8B, and 8C (Fig. 4). Of the three gene families, SsAGO genes were widely distributed over thirteen chromosomes, followed by SsRDR genes that were distributed over eight chromosomes (3C, 4A, 4D, 5A, 5B, 5C, 5D, 8B, and 8D), and SsDCL genes over three chromosomes (1A, 1C, and 1D). Meanwhile, duplication events that occurred over the course of S. spontaneum genome evolution were revealed by analysis with the BLASTp tool and MCScanX software. Two pairs of tandem duplicated SsAGO genes (SsAGO2a vs SsAGO2b; SsAGO3a vs SsAGO3b), which localized to chromosomes 5C and 5D, respectively, shared 91.5% and 83.6% identity with each other at an amino acid level. No tandem duplicated genes were seen for SsDCLs and SsRDRs. Twelve pairs of segmental duplicated genes were also found among the three families (one pair in SsDCLs, seven pairs in SsAGOs, and four pairs in SsRDRs), indicating that some of these genes could have been generated by gene duplication and that segmental duplication events play a major role in S. spontaneum genome evolution.
Figure 4.
Chromosome localization of SsDCL (in blue), SsAGO (in black), and SsRDR (in green) genes. The chromosome number is shown at the top of each bar. Horizontal bars represent the gene locations on each chromosome with positions in Mb (megabases) shown. Genes having tandem duplications are indicated by solid circles, whereas segmental duplication genes are joined by blue (SsDCLs), black (SsAGOs), and green lines (SsRDRs).
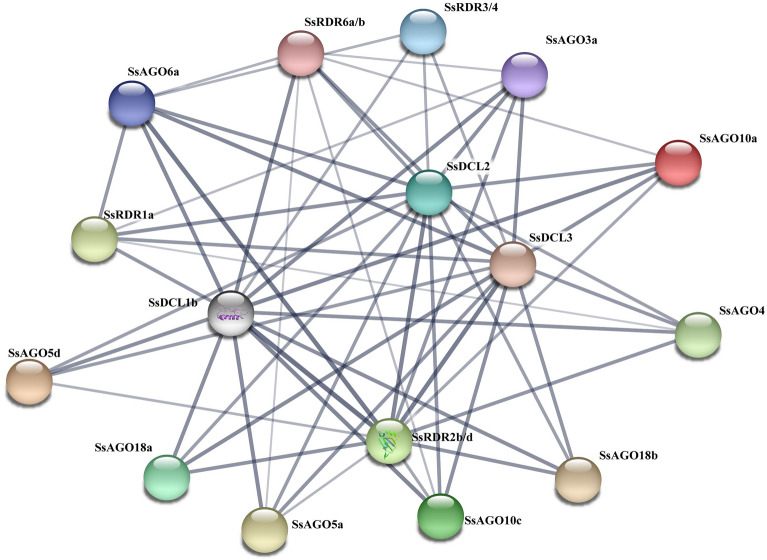
Protein–protein interaction networks for SsAGOs, SsDCLs, and SsRDRs
To investigate protein–protein interactions (PPIs) among the 36 proteins (4 SsDCLs, 21 SsAGOs, and 11 SsRDRs) identified for S. spontaneum, a PPI network was predicted in-silico with the STRING database using maize sequences as queries. PPI network analysis showed that 20 of 36 identified proteins interacted with each other, including SsDCL1b, SsDCL2, SsDCL3, SsAGO3a, SsAGO4, SsAGO5a/d, SsAGO6a/b, SsAGO10a/c, SsAGO18a/b, SsRDR1a, SsRDR2b/d, SsRDR3, SsRDR/4, and SsRDR6a/b (Fig. 5). Some of these proteins (SsDCL1b-SsAGO6a-SsRDR2b/d, SsDCL1b-SsAGO18a-SsRDR2b/d, SsDCL1b-SsAGO6a-SsRDR6a/b, SsDCL1b-SsAGO10a-SsRDR6a/b, SsDCL2-SsAGO3a-SsRDR2b/d, SsDCL3-SsAGO4-SsRDR2b/d, SsDCL3-SsAGO6a-SsRDR2b/d, and SsDCL3-SsAGO18b-SsRDR2b/d) interacted strongly. These results indicated that various combinations of three core components of SsDCLs, SsAGOs, and SsRDRs may participate in different RNA silencing pathways in S. spontaneum.
Figure 5.
Schematic representation of protein–protein interaction (PPI) networks between SsDCLs, SsAGOs, and SsRDRs from S. spontaneum. Nodes having different colors indicate different proteins. Gray lines connect proteins within the PPI networks with darker colors and thicker lines indicating higher core PPI values.
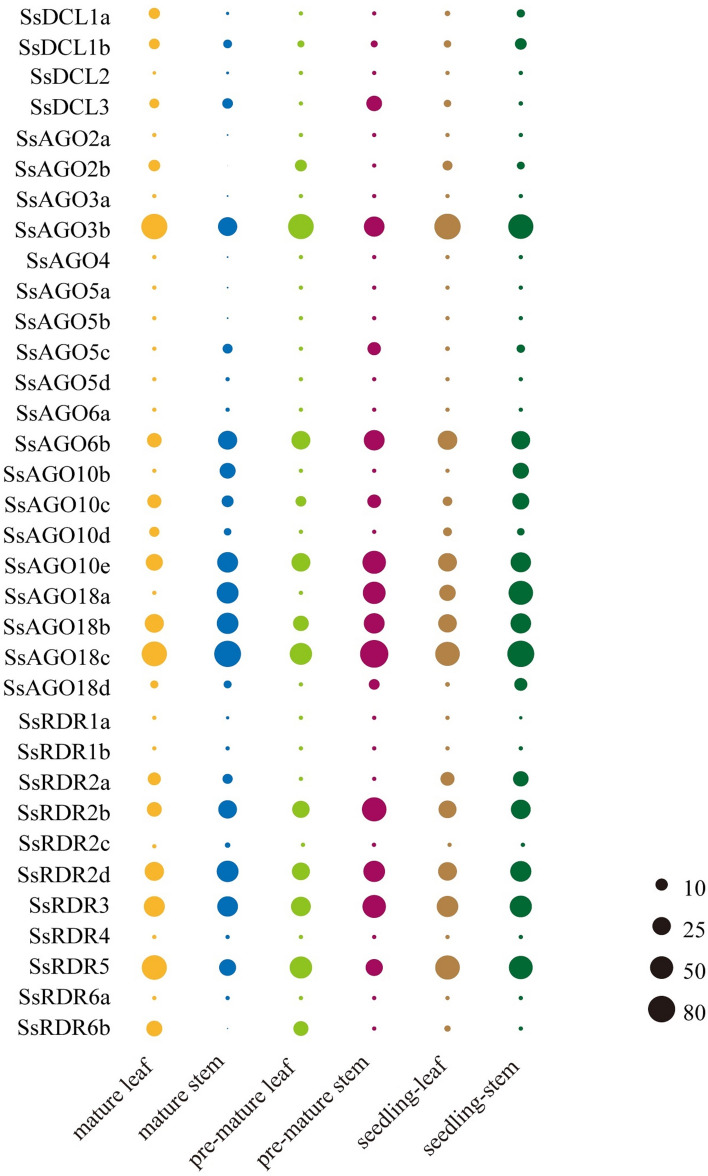
Expression pattern of SsAGOs, SsDCLs and SsRDRs in leaf and stem tissues
To determine the temporal and spatial expression patterns of genes encoding AGO, DCL, and RDR in S. spontaneum, transcriptional expression analysis was performed in leaf and stem samples from seedlings as well as from pre-mature and mature plants using the RNA-sequencing (RNA-seq) database (https://www.life.illinois.edu/ming/downloads/Spontaneum_genome/) (Fig. 6). Overall, no obvious temporal expression pattern was observed among the 36 identified genes, but expression patterns that tended to be specific to some tissues were observed. The four genes encoding SsDCLs exhibited no or very weak expression levels in either leaf or stem tissue. For the SsAGO genes, five genes (SsAGO3b, SsAGO18c, SsAGO18b, SsAGO10e, and SsAGO6b) had high expression levels in both leaf and stem at all growth stages whereas, except for the SsAGO3b, showed higher expression levels in stem tissues relative to leaf tissues. The SsAGO18a gene was also preferentially highly expressed in stem tissues, but not in leaf tissues, particularly mature or pre-mature leaves. The SsAGO10c gene had moderate expression levels, but the others were absent or had very weak expression levels in leaves and stems. Of the SsRDR genes, four (SsRDR2b, SsRDR2d, SsRDR3, and SsRDR5) exhibited higher expression levels in leaves and stems, whereas another five (SsRDR1a/b, SsRDR2c, SsRDR4, and SsRDR6a) had no detectable expression in either tissue type. Notably, of the four high-expression genes, SsRDR5 had higher expression levels in leaves compared to stems. Conversely, SsRDR2b, SsRDR2d, and SsRDR3 genes had higher expression in stems relative to leaves.
Figure 6.
Heat map showing spatiotemporal expression patterns of genes encoding SsDCL, SsAGO, and SsRDR in various S. spontaneum tissues including mature leaf, mature stem, pre-mature leaf, pre-mature stem, seedling leaf, and seedling stem. The size of the circles represents normalized expression level wherein larger circles correspond to higher expression levels.
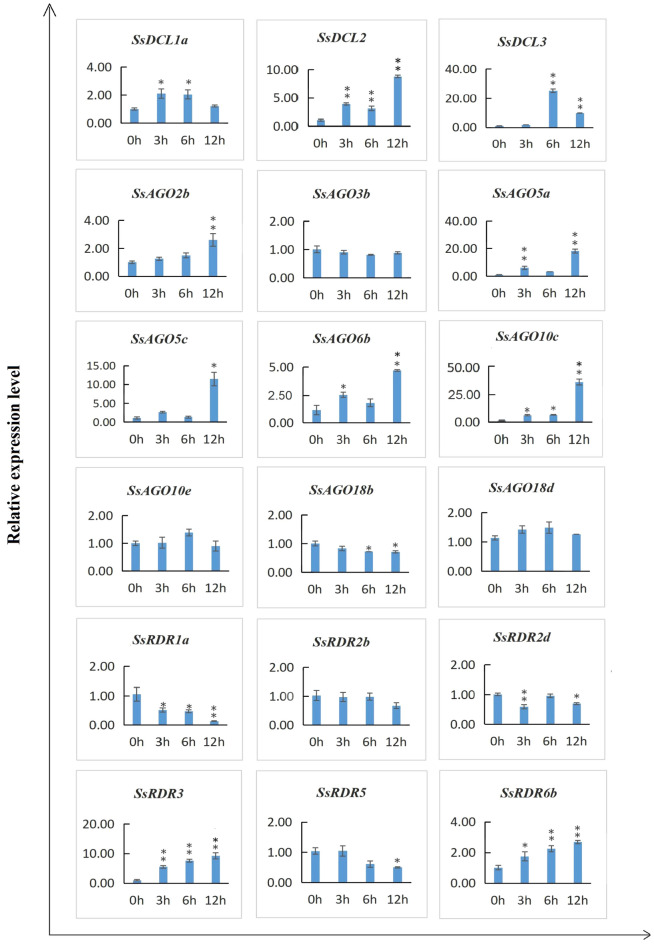
SsAGO, SsDCL, and SsRDR expression patterns induced by polyethylene glycol (PEG)-induced dehydration stress
To measure SsAGO, SsDCL, and SsRDR expression patterns in S. spontaneum under dehydration stress, the transcript expression for 18 candidate genes (9, 3 and 6 SsAGOs, SsDCLs, and SsRDRs, respectively) were analyzed by qRT-PCR upon the young plants of S. spontaneum clone SES208 subjected to PEG6000 treatment for 0–12 h(Fig. 7). The expression levels of five SsAGO genes (SsAGO2b, SsAGO5a, SsAGO5c, SsAGO6b, and SsAGO10c) in top young leaves were significantly upregulated by > 2.5-fold, while SsAGO5a, SsAGO5c, and SsAGO10c had particularly large increases of 18-, 11- and 35-fold, respectively, after 12 h of PEG treatment. Meanwhile, expression of SsAGO18b was significantly downregulated after PEG treatment for 6–12 h. In the SsDCL family, SsDCL1a expression was significantly upregulated by PEG treatment for 3–6 h with an increase of twofold, whereas transcript levels of SsDCL2 and SsDCL3 were dramatically increased by 3–ninefold at 3–12 h and 10–25-fold at 6–12 h, respectively, post PEG treatment. In the RDR family, SsRDR3 and SsRDR6b expression level were highly upregulated with increases of 6–ninefold and 2–threefold, respectively, under dehydration stress (3–12 h). Additionally, SsRDR1a and SsRDR5 expression levels were significantly depressed whereas that of the SsRDR2b gene did not significantly change.
Figure 7.
Quantitative real time PCR (qRT-PCR) analysis of relative transcript expression of 18 candidate genes encoding SsDCL, SsAGO, and SsRDR in S. spontaneum clone SES208 exposed to dehydration treatment (PEG-6000) for 0, 3, 6, and 12 h. The x-axis indicates the time points of PEG-6000 exposure, whereas the y-axis indicates the relative expression level. The top young leaves were sampled and used for qRT-PCR assay. Relative transcript expression values are presented as means ± standard errors based on three biological replicates with three technical replicates. Significant differential expression is indicated by an asterisk (*, p < 0.05; **, p < 0.01).
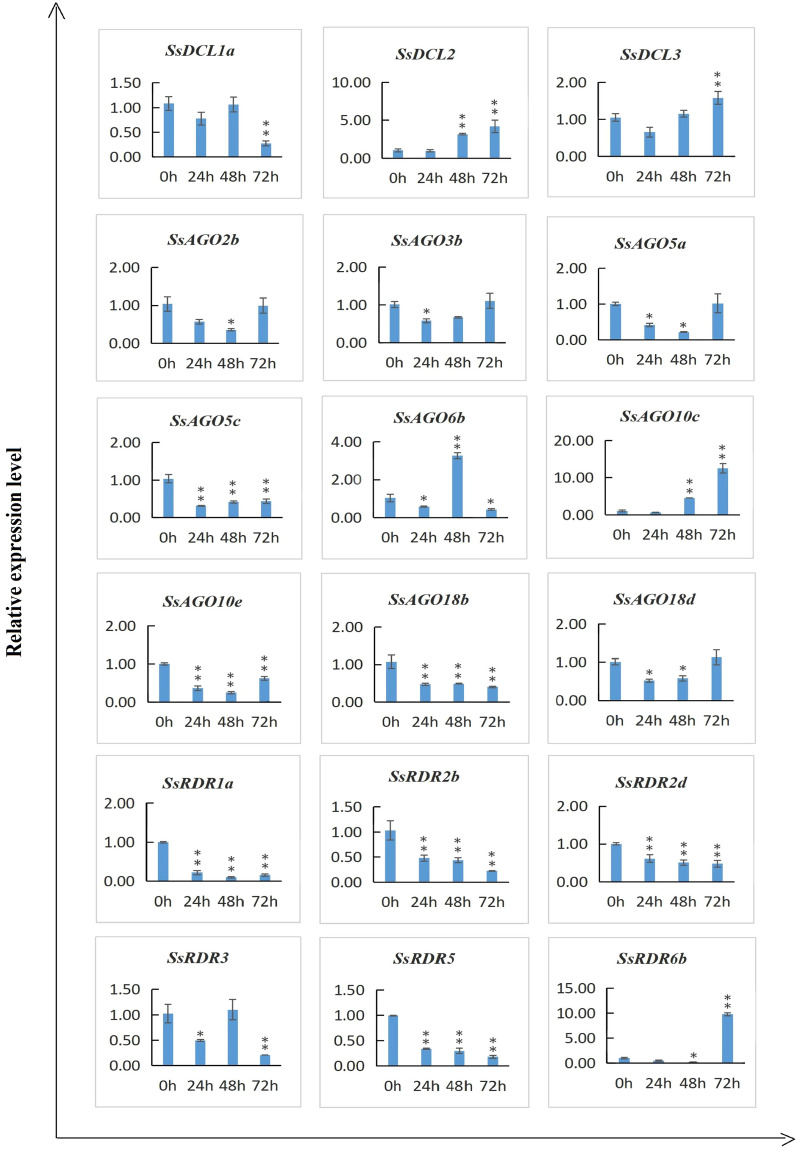
Response of SsAGO, SsDCL and SsRDR expression to Xanthomonas albilineans infection
Expression profiles for the 18 candidate genes were also assessed by qRT-PCR on the young leaves of S. spontaneum clone SES208 inoculated with X. albilineans. Seven of the SsAGO candidate genes had decreased expression levels upon X. albilineans infection, with SsAGO5c, SsAGO10e, and SsAGO18b showing significant down-regulation (Fig. 8). SsAGO10c expression was highly upregulated with increases of 5–13-fold at 48–72 hpi. Expression of the SsAGO6b gene varied, with significant decreases at 24 and 72 hpi but increases at 48 hpi. Of the three SsDCL genes tested, expression of SsDCL1a was dramatically depressed at 72 hpi, whereas that of SsDCL2 and SsDCL3 was highly upregulated with increases of 3–4 folds at 48–72 hpi and ~ twofold at 72 hpi, respectively. Five SsRDR genes were downregulated to some extent by X. albilineans infection, but SsRDR6 was significantly depressed at 48 hpi and highly upregulated (tenfold increase) at 72 hpi.
Figure 8.
Quantitative real time PCR (qRT-PCR) analysis of relative transcript expression of 18 candidate genes encoding SsDCL, SsAGO, and SsRDR in S. spontaneum clone SES208 inoculated with X. albilineans strain Xa-FJ1 at 0, 24, 48, and 72 h post-infection (hpi). The x-axis indicates the time points of experimental treatment, while the y-axis indicates the relative expression level. The top young leaves were sampled and used for qRT-PCR assay. Relative transcript expression values are presented as means ± standard errors based on three biological replicates with three technical replicates. Significant differential expression is indicated by an asterisk (*, p < 0.05; **, p < 0.01).
Discussion
Modern sugarcane cultivars originated from nobilization processes have contributed to worldwide advances in the sugar industry over the last century25. Modern sugarcane cultivars have complex genomes that present major challenges for producing a reference sequence23. Recently three sugarcane genomes, one S. spontaneum clone28 and two modern cultivars27,30 have been sequenced and assembled to provide valuable reference genomes for identification of gene families and functions in sugarcane. RNA silencing in plants plays an important role in regulating gene expression at different levels via sRNAs5. Thus, in this study we performed genome-wide analysis of three gene families involved in RNA silencing, DCL, AGO, and RDR in S. spontaneum, and further analyzed the expression profiles of these genes in response to biotic and abiotic stresses.
Genetic diversity and evolution of DCL, AGO, and RDR gene families in plants
To date, at least 20 plant species have been used to investigate the genetic diversity of the AGO, DCL, and RDR gene families, and in this study we confirmed that members of these families showed obviously species-specific variations (Table S5). The number of genes encoding DCLs varied among plant species from four AtDCLs in Arabidopsis thaliana21 to eight OsDCLs in rice44, millet47, and B. napus21. In S. spontaneum, we identified only three classes of DCL genes (DCL1/2/3) and found no sequences corresponding to the DCL4 gene. Similarly, a DCL4 gene was not identified in B. oleracea21 or A. duranensis48. The number of genes encoding AGOs ranged from seven in cucumber49,50 to 27 in B. napus21. Total 21 SsAGO genes were identified in S. spontaneum. Notably, the monocot plants including rice, maize, and sugarcane have evolved an AGO18 subclade that falls into the AGO1/5/10 clade51,52. The minimum number (5) of RDR gene family members was found in seven plants including rice44, maize39, grapevine53, and three legume crops (chickpea, pigeonpea, and groundnut)48, whereas B. napus had the maximum number (16)21. Our findings revealed that DCL4 and AGO1, which are commonly seen in both monocot and dicot plants, were lost in S. spontaneum, suggesting that the presence of novel DCL4- and AGO1-like genes are substitute for the functions of the two genes in S. spontaneum.
Large segmental duplications and/or tandem duplications might be responsible for evolution of these three gene families, especially for AGOs in rice, giving rise to redundancy or contrasting functions among OsAGO genes44. For example, large segmental duplications occurred in OsDCL2a-2b, OsAGO1a-1b, and OsAGO13-14, which localized on different chromosomes in rice. On the other hand, tandem duplications were present in three OsAGO gene pairs, OsAGO4a-15, OsAGO2-3, and OsAGO11-12, which localized close to each other on chromosomes 1, 4, and 3, respectively. Notably, large segmental duplication events commonly occurred in the AGOs, DCL, and RDR gene families in S. spontaneum, suggesting segmental duplication was an important evolution force for three gene families. However, segmental duplication did not appear in the OsDCL1 or OsRDR genes in rice44. Tandem duplication was also another important evolutionary force during evolution of S. spontaneum AGO genes, but not for OsDCLs or OsRDRs. Similarly, no tandem duplication events occurred among OsDCLs and OsRDRs in rice44.
Different RDR-DCL-AGO combinations are synergistically involved in specialized RNA silencing to control invading nucleic acids from endogenous (mainly transposons) or exogenous (mainly viruses) origins, and are mediated by diverse sRNAs such as miRNA, transacting siRNAs (ta-siRNA), natural-antisense-transcript-siRNA (nat-siRNA) and virus-derived siRNAs (vsiRNA)13,54,55. For instance, DCL1 and AGO1 mainly participate in the miRNA pathway, but the RDR protein is not necessary for miRNA biogenesis56, RDR6, SGS3, AGO1 and DCL4 are the main components of the ta-siRNA pathway at the post-transcriptional level57, RDR2, AGO4, and DCL3 are involved in the RNA-directed DNA methylation (RdDM) pathway58, DCL2/3/4 and RDR6 are common essential components in antiviral RNA silencing medicated by vsiRNA in Arabidopsis and rice, while HSP90-activated AGO1/2/4/5/7/10 is loaded with a vsiRNA in Arabidopsis but AGO18 positively regulates AGO1 binding to vsiRNAs by sequestering miR168 in rice51. In this study, PPI network analysis also indicated that these core component interactions may participate in various RNA silencing pathways. Notably, the SsAGO18s representing a distinct AGO subfamily specific to monocots also actively interacted with two other components.
Response of DCL gene family members to drought and bacterial stresses
Four types of Dicer or DCL proteins are key components in miRNA and siRNA biogenesis pathways and mediate conversion of long double-stranded RNAs into mature small RNAs5,59. These DCLs (DCL1/2/3/4) play different roles in RNA interference-related processes of small RNA biogenesis in A. thaliana: DCL1 produces miRNAs and trigger post-transcriptional gene silencing (PTGS), DCL2 is essential for secondary siRNA-mediated transitive silencing via production of some virus-derived siRNAs; DCL3 produces endogenous RDR2-dependent siRNAs; and DCL4 functions in antiviral defense and development pathways by processing ta-siRNA precursors in the small RNA biogenesis pathway59–61. However, DCL2, DCL3, and DCL4 showed functional redundancy in siRNA and tasiRNA production62,63.
Here, we found that PEG treatment induced substantial upregulation of SsDCL1a, SsDCL2, and SsDCL3, particularly in SsDCL2 and SsDCL3. These findings are similar to those seen for ZmDCL2/3b in maize39,64 and CaDCL1/2/3 in pepper64 that showed moderately and significantly, respectively, upregulated expression levels in response to drought conditions. Meanwhile, in the presence of X. albilineans infection, SsDCL2 and SsDCL3 expression levels were upregulated but SsDCL1a was unchanged or even downregulated. Similarly, in Brassica, the BnDCL1a gene was downregulated to varying degrees at 8 and 16 hpi with Sclerotinia sclerotiorum21. However, previous studies suggested that DCL1-generated sRNAs can positively regulate antibacterial and antifungal immunity13. In addition, a previous study showed that levels of SlDCL1/2a/2c/2d/3 were significantly upregulated in tomato plants infected with Tomato yellow leaf curl virus65. In pepper, CaDCL2/3/4 genes were highly expressed following infection with Cucumber mosaic virus (CMV) and particularly with Potato virus Y (PVY) or Tobacco mosaic virus (TMV)64. These findings suggested that DCL2 and DCL3 play positive roles in the response of plants to pathogen infection and abiotic stress, but the role of DCL1 may vary depending on plant species or the nature of biotic and abiotic stresses.
AGO gene family responses to drought and bacterial stress
AGOs together with small RNAs participate in RNA-induced silencing via cleavage of target mRNAs or blocking their translation66. Of the ten AtAGO family members in Arabidopsis, AGO2 regulates antibacterial immunity by binding miR393b* to modulate exocytosis of antimicrobial PR proteins via a Golgi-localized SNARE gene MEMB1267. AtAGO3 primarily binds 24-nt sRNAs with 5′-terminal adenines68. AtAGO5 can bind both viral RNAs and microRNAs to control plant–microbe interactions and plant physiology such as regulation of systemic resistance of Arabidopsis against Potato virus X69. AtAGO6 is involved in siRNA accumulation, RdDM and transcriptional gene silencing70,71 whereas AtAGO10 promotes miR165/6 degradation via SDN1 and SDN2 exonucleases72. AGO18s is unique to monocots and in rice confers broad-spectrum virus resistance by sequestering host miR168 (microRNA168) or miR528 (microRNA528) following viral infection73,74.
Among the nine SsAGOs genes identified in this study, transcript levels of five genes (SsAGO2b/5a/5c/6b/10c) especially in SsAGO10c were highly increased in S. spontaneum under dehydration stress. Similar results were reported for genes in other plants subjected to drought stress, such as SlAGO6 in tomato65, PtAGO5b gene in poplar75, and CaAGO2 and CaAGO10b in pepper64. Qin et al. (2018) suggested that CaAGO10b might response to osmotic stress of pepper plants by regulating ABA (abscisic acid) responsive genes64. On the other hand, expression levels of all the tested SsAGO genes, expect for SsAGO6b/10c, were significantly depressed to some extent in S. spontaneum plants after X. albilineans infection. Conversely, among the ten AGOs in Arabidopsis, only AGO2 is induced by bacterial infection and AGO2 positively regulates immunity by protein arginine methyltransferase 5 (PRMT5)-mediated dual regulation of this protein as well as associated sRNA levels to ensure appropriate plant immune responses76. A study by Qin et al.64 revealed that expression of CaAGO6, and the CaAGO10b gene in particular, was obviously upregulated in pepper upon inoculation with TMV, CMV, or PVY.
Notably, one gene pair, SsAGO10c/10e, which had a tandem duplication, exhibited different transcript expression patterns for PEG-treatment and X. albilineans infection, indicating that these genes may have evolved by segmental duplication events that were followed by differentiation of expression patterns. Kapoor et al.44 also proposed that the expression patterns of most OsAGO genes differentiated before their evolution by duplication events (tandem or segmental). For the unique class of AGO18 in monocot plants, the expression of SsAGO18b and SsAGO18d was significantly depressed in S. spontaneum exposed to PEG-treatment stress or X. albilineans infection, suggesting these two genes may play a negative role in the response of S. spontaneum to biotic or abiotic stresses. However, a previous study showed that AGO18s confers positive regulation in broad-spectrum virus resistance in rice73,74. This constricting result may be due to the AGO18s from different plants playing different mechanisms, but additional investigation is needed to determine what roles this protein confers in sugarcane resistance to stress. Very little is known about AGO18 gene functions in monocot plants in response to abiotic stress.
Response of the RDR gene family to drought and bacterial stresses
RDRs play an important role in vsRNA biogenesis and vsRNA-mediated antiviral defenses in plants77. Arabidopsis has six AtRDRs, and at least three types act in distinct biological processes such as viral defense and chromatin silencing44. Furthermore, binding of transcription factors to the promoters of RDR1-6 genes may play important roles in how various plant species respond to biotic stresses78. Our data revealed that expression of three RDR genes, SsRDR1a/2d/5, was significantly downregulated, but two (SsRDR3/6b) were upregulated and one gene (SsRDR2b) was not significantly affected by PEG-treatment stress. In contrast to our results, levels of SlRDR1 in tomato65, PtRDR1c/1d in poplar75, and CaRDR1 in pepper64 were strongly increased by PEG-treatment stress. Upon X. albilineans infection, SsRDR1a/2b/5 gene expression was significantly downregulated, but the SsRDR6b gene was strongly upregulated.
Our findings suggested that SsRDR6 plays an important role in defenses against biotic and abiotic stresses in S. spontaneum. Similar results were seen for pepper, in which CaRDR6 gene expression levels were highly increased in the presence of three biotic stresses (CMV, PVY, and TMV inoculation)64. In contrast, Arabidopsis RDR6 acts as a novel negative regulator of pattern-triggered immunity (PTI) and an rdr6 mutant exhibits enhanced basal resistance towards a virulent Pseudomonas syringae strain79. Previous observations showed that RDR6 in Nicotiana attenuata played an important role when plants respond to challenges in their native environments80. In rice, OsRDR6 is responsible for the observed ABA (abscisic acid)-mediated amplification and silencing of RDR6-dependent siRNA transcripts81.
Conclusions
In this study we identified 21 SsAGOs, four SsDCLs and 11 SsRDRs in the S. spontaneum genome. Genes in these three families present characteristic conserved domains and cis-elements as well as distinct expression profiles. Chromosome localization analysis revealed that segmental and/or tandem duplication contributed to the evolution of these genes, particularly for the SsAGO family. RNA-seq data analysis indicated tissue-specific expression patterns for some genes such as SsRDR5, which showed preferential expression in leaves, whereas SsAGO18c, SsAGO18b, SsAGO10e, and SsAGO6b exhibited stem-specific expression. Additionally, qRT-PCR analysis indicated that the expression patterns of some genes in three families differed in S. spontaneum plants exposed to PEG-treatment stress or X. albilineans infection in that SsAGO10c, SsDCL2, and SsRDR6b genes were strongly upregulated and SsAGO18b, SsRDR1a, SsRDR2b/2d, and SsRDR5 were significantly depressed under the two stresses. Our findings can enhance our knowledge of the roles of these genes encoding SsAGOs, SsDCLs, and SsRDRs in biotic and abiotic stress response of sugarcane plants.
Supplementary information
Acknowledgements
This research was funded by the earmarked fund for the China Agricultural Research System (grant no. CARS-170302), and the special fund for science and technology innovation of the Fujian Agriculture and Forestry University, China (grant no. CXZX2018024).
Author contributions
D.L.C carried out analysis of the results and preparation of a draft manuscript; J.Y.M., X.Y.R., and J.J.Y. performed preparation of plant materials and experimental treatments. H.Y.F. and M.T.H. participated in some experiments. Q.Q.Z. and S.J.G. conceived and supervised the project, and writing and finalizing of the manuscript. All authors read and approved the final manuscript.
Data availability
All data, including image files, are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Qing-Qi Zhang, Email: 000q010002@fafu.edu.cn.
San-Ji Gao, Email: gaosanji@fafu.edu.cn.
Supplementary information
is available for this paper at 10.1038/s41598-020-70061-7.
References
- 1.Chen X. Small RNAs and their roles in plant development. Annu. Rev. Cell. Dev. Biol. 2009;25:21–44. doi: 10.1146/annurev.cellbio.042308.113417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez-Gomollon S, Dalmay T. Recent patents in RNA silencing in plants: constructs, methods and applications in plant biotechnology. Recent Pat. DNA Gene Seq. 2010;4:155–166. doi: 10.2174/187221510794751677. [DOI] [PubMed] [Google Scholar]
- 3.Sarkies P, Miska EA. Small RNAs break out: the molecular cell biology of mobile small RNAs. Nat. Rev. Mol. Cell Biol. 2014;15:525–535. doi: 10.1038/nrm3840. [DOI] [PubMed] [Google Scholar]
- 4.Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20:515–524. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- 5.Axtell MJ. Classification and comparison of small RNAs from plants. Annu. Rev. Plant Biol. 2013;64:137–159. doi: 10.1146/annurev-arplant-050312-120043. [DOI] [PubMed] [Google Scholar]
- 6.Hutvagner G, Simard MJ. Argonaute proteins: key players in RNA silencing. Nat. Rev. Mol. Cell Biol. 2008;9:22–32. doi: 10.1038/nrm2321. [DOI] [PubMed] [Google Scholar]
- 7.Carbonell A. Plant ARGONAUTEs: Features, functions, and unknowns. Methods Mol. Biol. 2017;1640:1–21. doi: 10.1007/978-1-4939-7165-7_1. [DOI] [PubMed] [Google Scholar]
- 8.David B. RNA silencing in plants. Nature. 2004;431:356–363. doi: 10.1038/nature02874. [DOI] [PubMed] [Google Scholar]
- 9.Guo Z, Li Y, Ding SW. Small RNA-based antimicrobial immunity. Nat. Rev. Immunol. 2019;19:31–44. doi: 10.1038/s41577-018-0071-x. [DOI] [PubMed] [Google Scholar]
- 10.Voinnet O. Use, tolerance and avoidance of amplified RNA silencing by plants. Trends Plant Sci. 2008;13:317–328. doi: 10.1016/j.tplants.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Hunter LJ, et al. RNA-dependent RNA polymerase 1 in potato (Solanum tuberosum) and its relationship to other plant RNA-dependent RNA polymerases. Sci. Rep. 2016;6:23082. doi: 10.1038/srep23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hua C, Zhao JH, Guo HS. Trans-kingdom RNA silencing in plant-fungal pathogen interactions. Mol. Plant. 2018;11:235–244. doi: 10.1016/j.molp.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Huang CY, Wang H, Hu P, Hamby R, Jin H. Small RNAs big players in plant-microbe interactions. Cell Host Microbe. 2019;26:173–182. doi: 10.1016/j.chom.2019.07.021. [DOI] [PubMed] [Google Scholar]
- 14.Zhu C, Liu T, Chang YN, Duan CG. Small RNA functions as a trafficking effector in plant immunity. Int. J. Mol. Sci. 2019;20:2816. doi: 10.3390/ijms20112816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Q, Feng Y, Zhu Z. Dicer-like (DCL) proteins in plants. Funct. Integr. Genomics. 2009;9:277–286. doi: 10.1007/s10142-009-0111-5. [DOI] [PubMed] [Google Scholar]
- 16.Fukudome A, Fukuhara T. Plant dicer-like proteins: double-stranded RNA-cleaving enzymes for small RNA biogenesis. J. Plant. Res. 2017;130:33–44. doi: 10.1007/s10265-016-0877-1. [DOI] [PubMed] [Google Scholar]
- 17.Tolia NH, Joshua-Tor L. Slicer and the argonautes. Nat. Chem. Biol. 2007;3:36–43. doi: 10.1038/nchembio848. [DOI] [PubMed] [Google Scholar]
- 18.Mallory A, Vaucheret H. Form, function, and regulation of ARGONAUTE proteins. Plant Cell. 2010;22:3879–3889. doi: 10.1105/tpc.110.080671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willmann MR, Endres MW, Cook RT, Gregory BD. The functions of RNA-dependent RNA polymerases in Arabidopsis. Arabidopsis Book. 2011;9:e146. doi: 10.1199/tab.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vazquez F. Arabidopsis endogenous small RNAs: Highways and byways. Trends Plant Sci. 2011;11:460–468. doi: 10.1016/j.tplants.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Cao JY, et al. Genome-wide identification of dicer-Like, Argonaute, and RNA-dependent RNA polymerase gene families in Brassica species and functional analyses of their Arabidopsis homologs in resistance to Sclerotinia sclerotiorum. Front. Plant Sci. 2016;7:1614. doi: 10.3389/fpls.2016.01614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ali, A., Khan, M., Sharif, R., Mujtaba, M. & Gao, S.J. Sugarcane Omics: An update on the current status of research and crop improvement. Plants (Basel)8, 344 (2019). [DOI] [PMC free article] [PubMed]
- 23.Diniz AL, et al. Genomic resources for energy cane breeding in the post genomics era. Comput. Struct. Biotechnol. J. 2019;17:1404–1414. doi: 10.1016/j.csbj.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D'Hont, A. et al. Characterisation of the double genome structure of modern sugarcane cultivars (Saccharum spp.) by molecular cytogenetics. Mol. Gen. Genet.250, 405–413 (1996). [DOI] [PubMed]
- 25.Zhou, M. Sugarcane (S. Officinarum X S. Spontaneum). In: Campos H, Caligari PDS, eds. Genetic Improvement of Tropical Crops. 291–308 (Springer, Cham, 2017).
- 26.Moore PH, Nagai C, Fitch M. Production and evaluation of sugarcane haploids. Proc. Int. Soc. Sugar Cane Technol. 1989;20:599–607. [Google Scholar]
- 27.Garsmeur O, et al. A mosaic monoploid reference sequence for the highly complex genome of sugarcane. Nat. Commun. 2018;9:2638. doi: 10.1038/s41467-018-05051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, et al. Allele-defined genome of the autopolyploid sugarcane Saccharum spontaneum L. Nat. Genet. 2018;50:1565–1573. doi: 10.1038/s41588-018-0237-2. [DOI] [PubMed] [Google Scholar]
- 29.Xu, F. et al. Comparative analysis of two sugarcane ancestors Saccharum officinarum and S. spontaneum based on complete chloroplast genome sequences and photosynthetic ability in cold stress. Int. J. Mol. Sci.20, 3828 (2019). [DOI] [PMC free article] [PubMed]
- 30.Souza GM, et al. Assembly of the 373k gene space of the polyploid sugarcane genome reveals reservoirs of functional diversity in the world's leading biomass crop. Gigascience. 2019;8:12. doi: 10.1093/gigascience/giz129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao, S., Luo, J., Zhang, H., Chen, R. & Lin, Y. Physiological and biochemical indexes of drought resistance of sugarcane (Saccharum spp.). Ying Yong Sheng Tai Xue Bao17, 1051–1054 (2006). [PubMed]
- 32.McQualter R, Dookun-Saumtally A. Expression profiling of abiotic-stress-inducible genes in sugarcane. Proc. Int. Soc. Sugar cane Technol. 2007;29:878–888. [Google Scholar]
- 33.Royer, M., Pieretti, I., Cociancich, S. & Rott, P. Recent progress in understanding three major bacterial diseases of sugarcane: Gumming, leaf scald and ratoon stunting. In: Rott, P., eds. Achieving Sustainable Cultivation of Sugarcane, Breeding, Pests and Diseases (2nd ed.) 311–336 (Burleigh Dodds Science Publishing, Cambridge, 2018).
- 34.Lin L-H. et al. Molecular detection and prevalence of Xanthomonas albilineans, the causal agent of sugarcane leaf scald, in China. Plant Protect.109, 17–23 (2018).
- 35.Ntambo MS, et al. Identification and characterization of Xanthomonas albilineans causing sugarcane leaf scald in China using multilocus sequence analysis. Plant Pathol. 2019;68:269–277. [Google Scholar]
- 36.Finn RD, et al. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res. 2016;44:D279–D285. doi: 10.1093/nar/gkv1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finn RD, Clements J, Eddy SR. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011;39:W29–W37. doi: 10.1093/nar/gkr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie T, et al. Genome-wide investigation of WRKY gene family in pineapple: evolution and expression profiles during development and stress. BMC Genomic. 2018;19:490. doi: 10.1186/s12864-018-4880-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qian Y, et al. Identification and characterization of Dicer-like, Argonaute and RNA-dependent RNA polymerase gene families in maize. Plant Cell Rep. 2011;30:1347–1363. doi: 10.1007/s00299-011-1046-6. [DOI] [PubMed] [Google Scholar]
- 40.Hu B. et al. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics31, 1296–1297 (2015). [DOI] [PMC free article] [PubMed]
- 41.Szklarczyk D, et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krzywinski M, et al. Circos: An information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40:e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kapoor M, et al. Genome-wide identification, organization and phylogenetic analysis of dicer-like, Argonaute and RNA-dependent RNA polymerase gene families and their expression analysis during reproductive development and stress in rice. BMC Genomics. 2008;9:451. doi: 10.1186/1471-2164-9-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 47.Yadav CB, Muthamilarasan M, Pandey G, Prasad M. Identification, characterization and expression profiling of dicer-like, Argonaute and RNA-dependent RNA polymerase gene families in Foxtail millet. Plant Mol. Biol. Rep. 2015;33:43–55. [Google Scholar]
- 48.Garg V, et al. Genome-wide identification, characterization, and expression analysis of small RNA biogenesis purveyors reveal their role in regulation of biotic stress responses in three legume crops. Front. Plant Sci. 2017;8:488. doi: 10.3389/fpls.2017.00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gan, D. et al. Expression analysis of argonaute, dicer-like, and RNA-dependent RNA polymerase genes in cucumber (Cucumis sativus L.) in response to abiotic stress. J. Genet.96, 235–249 (2017). [DOI] [PubMed]
- 50.Gan D. F. et al. Genome-wide identification of the dicer-like, Argonaute, and RNA-dependent RNA polymerase gene families in cucumber (Cucumis sativus L.). J. Plant Growth Regul.35, 135–150 (2016).
- 51.Zhang H, Xia R, Meyers BC, Walbot V. Evolution, functions, and mysteries of plant ARGONAUTE proteins. Curr. Opin. Plant Biol. 2015;27:84–90. doi: 10.1016/j.pbi.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 52.Fang X, Qi Y. RNAi in plants: An argonaute-centered view. Plant Cell. 2016;28:272–285. doi: 10.1105/tpc.15.00920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao H, et al. Comprehensive analysis of dicer-like, Argonaute, and RNA-dependent RNA polymerase gene families in grapevine (Vitis Vinifera) J. Plant Growth Regul. 2014;34:108–121. [Google Scholar]
- 54.Brodersen P, Voinnet O. The diversity of RNA silencing pathways in plants. Trends Genet. 2006;22:268–280. doi: 10.1016/j.tig.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 55.Borges F, Martienssen R. The expanding world of small RNAs in plants. Nat. Rev. Mol. Cell Biol. 2015;16:727–741. doi: 10.1038/nrm4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bologna N, Voinnet O. The diversity, biogenesis, and activities of endogenous ssilencing small RNAs in Arabidopsis. Annu. Rev. Plant. Biol. 2014;65:473–503. doi: 10.1146/annurev-arplant-050213-035728. [DOI] [PubMed] [Google Scholar]
- 57.Skopelitis D, Husbands A, Timmermans M. Plant small RNAs as morphogens. Curr. Opin. Cell Biol. 2011;24:217–224. doi: 10.1016/j.ceb.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 58.Julie A, L. & Steven E, J. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet.11, 204–220 (2010). [DOI] [PMC free article] [PubMed]
- 59.Fukudome A, Fukuhara T. Plant dicer-like proteins: Double-stranded RNA-cleaving enzymes for small RNA biogenesis. J. Plant Res. 2017;130:33–44. doi: 10.1007/s10265-016-0877-1. [DOI] [PubMed] [Google Scholar]
- 60.Kurihara Y, Watanabe Y. Arabidopsis micro-RNA biogenesis through dicer-like 1 protein functions. Proc. Natl. Acad. Sci. USA. 2004;101:12753–12758. doi: 10.1073/pnas.0403115101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xie Z, Allen E, Wilken A, Carrington JC. DICER-LIKE 4 functions in trans-acting small interfering RNA biogenesis and vegetative phase change in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2005;102:12984–12989. doi: 10.1073/pnas.0506426102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gasciolli V, Mallory AC, Bartel DP, Vaucheret H. Partially redundant functions of Arabidopsis DICER-like enzymes and a role for DCL4 in producing trans-acting siRNAs. Curr. Biol. 2005;15:1494–1500. doi: 10.1016/j.cub.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 63.Henderson I, et al. Dissecting Arabidopsis thaliana DICER function in small RNA processing, gene silencing and DNA methylation patterning. Nat. Genet. 2006;38:721–725. doi: 10.1038/ng1804. [DOI] [PubMed] [Google Scholar]
- 64.Qin, L., Mo, N., Muhammad, T. & Liang, Y. Genome-wide analysis of DCL, AGO, and RDR gene families in Pepper (Capsicum Annuum L.). Int. J. Mol. Sci.19, 1038 (2018). [DOI] [PMC free article] [PubMed]
- 65.Bai M, et al. Genome-wide identification of dicer-like, Argonaute and RNA-dependent RNA polymerase gene families and their expression analyses in response to viral infection and abiotic stresses in Solanum lycopersicum. Gene. 2012;501:52–62. doi: 10.1016/j.gene.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 66.Ma Z, Zhang X. Actions of plant Argonautes: Predictable or unpredictable? Curr. Opin. Plant Biol. 2018;45:59–67. doi: 10.1016/j.pbi.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 67.Zhang X, et al. Arabidopsis Argonaute 2 regulates innate immunity via miRNA393*-mediated silencing of a golgi-localized SNARE gene, MEMB12. Mol. Cell. 2011;42:356–366. doi: 10.1016/j.molcel.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Z, Liu X, Guo X, Wang XJ, Zhang X. Arabidopsis AGO3 predominantly recruits 24-nt small RNAs to regulate epigenetic silencing. Nat. Plants. 2016;2:16049. doi: 10.1038/nplants.2016.49. [DOI] [PubMed] [Google Scholar]
- 69.Reyero-Saavedra, M. et al. Gene silencing of Argonaute5 negatively affects the establishment of the legume-rhizobia symbiosis. Genes (Basel)8, 352 (2017). [DOI] [PMC free article] [PubMed]
- 70.Zheng X, Zhu J, Kapoor A, Zhu JK. Role of Arabidopsis AGO6 in siRNA accumulation, DNA methylation and transcriptional gene silencing. EMBO. J. 2007;26:1691–1701. doi: 10.1038/sj.emboj.7601603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duan CG, et al. Specific but interdependent functions for Arabidopsis AGO4 and AGO6 in RNA-directed DNA methylation. EMBO. J. 2015;34:581–592. doi: 10.15252/embj.201489453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu Y, et al. ARGONAUTE10 promotes the degradation of miR165/6 through the SDN1 and SDN2 exonucleases in Arabidopsis. PLoS Biol. 2017;15:e2001272. doi: 10.1371/journal.pbio.2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu J, et al. Viral-inducible Argonaute18 confers broad-spectrum virus resistance in rice by sequestering a host microRNA. Elife. 2015;4:e05733. doi: 10.7554/eLife.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu J, et al. ROS accumulation and antiviral defence control by microRNA528 in rice. Nat. Plants. 2017;3:16203. doi: 10.1038/nplants.2016.203. [DOI] [PubMed] [Google Scholar]
- 75.Zhao K, et al. The dicer-like, Argonaute and RNA-dependent RNA polymerase gene families in Populus trichocarpa: gene structure, gene expression, phylogenetic analysis and evolution. J. Genet. 2015;94:317–321. doi: 10.1007/s12041-015-0508-y. [DOI] [PubMed] [Google Scholar]
- 76.Hu P, et al. Dual regulation of Arabidopsis AGO2 by arginine methylation. Nat. Commun. 2019;10:844. doi: 10.1038/s41467-019-08787-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qi X, Bao FS, Xie Z. Small RNA deep sequencing reveals role for Arabidopsis thaliana RNA-dependent RNA polymerases in viral siRNA biogenesis. PLoS ONE. 2009;4:e4971. doi: 10.1371/journal.pone.0004971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Prakash V, Chakraborty S. Identification of transcription factor binding sites on promoter of RNA dependent RNA polymerases (RDRs) and interacting partners of RDR proteins through In-silico analysis. Physiol. Mol. Biol. Plants. 2019;25:1055–1071. doi: 10.1007/s12298-019-00660-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boccara M, et al. The Arabidopsis miR472-RDR6 silencing pathway modulates PAMP- and effector-triggered immunity through the post-transcriptional control of disease resistance genes. PLoS Pathog. 2014;10:e1003883. doi: 10.1371/journal.ppat.1003883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shree P, Emmanuel G, Klaus G, Ian T. RNA-directed RNA polymerase 3 from Nicotiana Attenuata is required for competitive growth in natural environments. Plant Physiol. 2008;147:1212–1224. doi: 10.1104/pp.108.121319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ji HY, et al. Phytohormone abscisic acid control RNA-dependent RNA polymerase 6 gene expression and post-transcriptional gene silencing in rice cells. Nucleic Acids Res. 2008;36:1220–1226. doi: 10.1093/nar/gkm1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data, including image files, are available from the corresponding author on reasonable request.