Abstract
Background and Aims:
The ultrasound-guided infraclavicular brachial plexus block (USG ICBPB) is a popular technique for forearm surgeries distal to the elbow. Our study details the ultrasound (US) characteristics of this block and the structures encountered by the needle in four approaches to the infraclavicular area – lateral infraclavicular (LICF), costoclavicular medial to lateral (CML) and lateral to medial (CLM) and retroclavicular (R) by anatomical dissection.
Methods:
USG ICBPB was performed in 10 cadavers—5 on the right side and 5 on the left side by each of four approaches and with an 18 gauge Tuohy needle kept in situ, and US characteristics were noted. Anatomical dissection was done and important structures were described in detail.
Results:
Needle tip and shaft visibility were least with LICF approach and best in R approach. Needle angle correlated with chest and neck circumference in LICF and CML groups. During dissection, in all approaches, neurovascular structures have been observed in the near vicinity of the needle, especially the thoracoacromial artery (TAA) or its branches. In the R approach, the 'blind spot' behind the clavicle is an area where neurovascular structures were present.
Conclusion:
The R approach gives better visibility of needle shaft beyond the clavicle, but the clavicle acts as a 'blind-spot' for the US beam obliterating important neurovascular structures. The various neurovascular structures the needle traverses or in its immediate vicinity, do not necessarily make the CML, CLM or R approach any better than the LICF approach.
Key words: Brachial plexus, cadaver, dissection, nerve block, ultrasound
INTRODUCTION
Infraclavicular brachial plexus block (ICBPB) is a reliable method of providing anesthesia to the upper limb distal to the elbow with a lesser incidence of diaphragmatic paralysis, pneumothorax or Horner's syndrome compared to other methods of brachial plexus block (BPB), and hence safe even in patients with respiratory comorbidities.[1,2] The ultrasound-guided infraclavicular brachial plexus block (USG-ICBPB) is administered by one of four approaches—the traditional (lateral infraclavicular fossa or LICF), costoclavicular medial to lateral (CML), costoclavicular lateral to medial (CLM), and retroclavicular (R), each with their advantages and pitfalls.
The LICF approach has the disadvantage of a steeper trajectory of needle resulting in diminished needle visibility, thereby increasing the procedural time and increased number of passes which in turn increases the risk of needle–nerve contact.[2] The sonoanatomy of the CLM approach was described by Sala-Blanch X et al. as brachial plexus cords clustered lateral to the axillary artery (AA) and with consistent relationship to one another.[3] CLM had better block dynamics as compared to the LICF approach as reported by Li JW et al.[4] Few authors have proposed the successful use of the CML approach for the costoclavicular BPB.[5] The R approach was initially described by Hebbard and Royse and it has been shown to have better visibility of needle due to the parallel-to-US beam angulation of needle as described by Charbonneau et al.[6,7]
Existing evidence describes needle tip vicinity to important neurovascular structures alone, without description about structures in the vicinity of the entire length of the needle. We conducted a cadaver study comparing these four approaches to give us a comprehensive understanding of the US characteristics as well as anatomical structures traversed by or in the vicinity of the block needle throughout its pathway, at the entry point (EP), mid-point (MP) and a target point (TP), which are the brachial plexus cords, to understand which approach would be safest as well as easy to perform in our regional anesthesia practice.
METHODS
After obtaining Institutional Ethics Committee approval, 10 cadavers were studied in the anatomy dissection hall—5 on the right side and 5 on the left side, each of them for all four approaches of USG-ICBPB. Cadavers without infraclavicular fossa deformity, shoulder deformity, or surgical scar were included in the study.
Preparation of cadavers: after thorough cleansing and surface disinfection, the cadavers were preserved by the standard institute policy, infusing a solution containing 10% formalin, isopropyl alcohol, glycerol, phenol, thymol, sodium citrate, sodium borate, and common salt into the femoral artery.
Ultrasound (US) assessment: the cadavers were placed supine and the limb was placed in a neutral position. A SonoSite M-Turbo ultrasound machine (FUJIFILM Sonosite, Inc, Bothell, Washington, USA) with a high-frequency linear probe (5–12 MHz) was used to perform the US assessments. 18 gauge (G) Tuohy needles were used to perform the blocks.
In the LICF approach, the US probe was placed vertically in the infraclavicular area medial to the coracoid process to visualise cords surrounding the axillary artery, deep to the pectoralis major and minor muscles. In CML and CLM approaches, the US probe was placed transversely beneath the clavicle to visualise the cords lateral to the axillary artery, and the needle inserted medial to lateral or lateral to medial as described by the authors.[4,5] In the R approach, the US probe was placed vertically in the infraclavicular area and needle insertion was done from the above clavicle, passing beneath it, with tip reaching the cords beneath the AA.
Cadaver data such as age (at the time of death), height (length), chest circumference (at nipple level), and neck circumference (at thyroid cartilage level) were noted down.
US measurements—visualisation of all three cords in one US frame, depth of target point (TP)—cords from the skin (vertical depth), TP distance from the needle entry point (EP), depth of Tuohy needle inserted, the angle taken by the needle to reach the TP were noted down. Needle tip visibility was given a 5-point Likert's scale as follows: 1 = very poor, 2 = poor, 3 = good, 4 = very good, 5 = excellent. For needle shaft visibility, the 5-point Likert scale given was: 1 = none of shaft visualised, 2= <25% of shaft visualised, 3 = 25-50% of shaft visualised, 4 = 50-75% of shaft visualised, 5 = >75% of shaft visualised.
Statistical analysis was done using Statistical Package for the Social Sciences (SPSS) 21 (IBM, Armonk, NY, USA). US measurements were compared between groups by analysis of variance (ANOVA) with posthoc Bonferroni correction. Each of the US measurements was correlated with cadaver characteristics by using Pearson's correlation analysis. Data are presented as mean ± standard deviation. A P value of <0.05 is considered as significant. Likert scale was analysed using the Kruskal-Wallis test with data presented as median and interquartile range and Spearman's Rho correlation with cadaver characteristics was done.
Dissection of cadavers was done with the needles in situ by two experienced anatomists along with the primary investigator. Skin and subcutaneous tissue were removed and pectoralis major and minor muscles were reflected. The mid-portion of the clavicle was gently removed with a bone saw, making sure the needles are not disturbed.
A systematic assessment of structures encountered at EP, mid-point (MP), and at TP was done. Important neurovascular structures within 1 cm vicinity at each of these points have been noted-caudad, cephalad, lateral, medial, ventral, and dorsal to the needle passage.
RESULTS
US findings: [Refer Tables 1 and 2].
Table 1.
Mean values of variables studied and differences between groups (ANOVA) with posthoc Bonferroni correction (significant values in bold italics). Likert scale analysed with Kruskal-Wallis test and expressed as median and IQR
| Variables | GR1 LICF Mean (SD) | GR2 CML Mean (SD) | GR3 CLM Mean (SD) | GR4 R Mean (SD) | P (<0.05 significant) |
|---|---|---|---|---|---|
| Number Of Cords Visible (Nos) | 2.4 (0.7) | 2.5 (0.7) | 1.9 (0.7) | 2.4 (0.7) | 0.24 |
| Vertical Depth (Cm) | 2.9 (0.6) | 2.6 (0.7) | 2.4 (0.9) | 2.8 (0.6) | 0.37 |
| EP To Target Distance (Cm) | 3.9 (1.1) | 4.0 (0.6) | 3.4 (1.1) | - | 0.40 |
| Needle Angle (Degrees) | 59.3 (3.5) | 21.3 (3.4) | 16.0 (1.5) | 4.5 (1.4) | <0.001 |
| Needle Depth (Cm) | 5.3 (0.9) | 5.8 (1.1) | 4.8 (0.9) | 7.5 (1.4) | <0.001 |
| Median IQR | Median IQR | Median IQR | Median IQR | ||
| Tip Visibility (Likert Scale) | 2.0 (1.0) | 2.0 (2.3) | 2.0 (2.0) | 3.5 (2.3) | 0.02 |
| Shaft Visibility (Likert Scale) | 3.0 (1.3) | 3.0 (2.0) | 3.0 (1.5) | 4.0 (2.0) | 0.01 |
LICF – Lateral infraclavicular fossa approach, CML – Costclavicular medial to lateral approach, CLM – Costoclavicular lateral to medial approach, R – Retroclavicular approach, SD – Standard deviation, EP – Entry point, IQR – Interquartile range
Table 2.
Correlation of each of US measurements with cadaver characteristics in each group (Pearson’s correlation analysis) (significant values in bold italics). Spearman Rho correlation analysis was done for Likert scale
| Variables | Cadaver Characteristics | GR 1LICF | GR 2CML | GR 3CLM | GR 4Retroclavicular | ||||
|---|---|---|---|---|---|---|---|---|---|
| R | P | R | P | R | P | R | P | ||
| Number Of Cords Visible (Nos) | Age | 0.17 | 0.64 | 0.44 | 0.20 | 0.25 | 0.49 | 0.17 | 0.64 |
| Height | -0.15 | 0.69 | 0.04 | 0.92 | 0.14 | 0.71 | -0.15 | 0.69 | |
| Chest Circumf | -0.30 | 0.40 | -0.17 | 0.65 | 0.04 | 0.91 | 0.30 | 0.40 | |
| Neck Circumf | -0.13 | 0.73 | -0.05 | 0.89 | 0.12 | 0.75 | -0.13 | 0.73 | |
| Vertical Depth (Cm) | Age | 0.17 | 0.65 | 0.49 | 0.15 | 0.36 | 0.31 | 0.54 | 0.11 |
| Height | 0.22 | 0.53 | 0.64 | 0.05 | 0.49 | 0.15 | 0.34 | 0.34 | |
| Chest Circumf | 0.62 | 0.06 | 0.65 | 0.04 | 0.36 | 0.31 | 0.70 | 0.03 | |
| Neck Circumf | 0.54 | 0.10 | 0.70 | 0.03 | 0.53 | 0.11 | 0.74 | 0.01 | |
| EP To Target Distance (Cm) | Age | 0.23 | 0.50 | 0.42 | 0.22 | 0.43 | 0.22 | - | - |
| Height | 0.30 | 0.40 | 0.25 | 0.48 | 0.51 | 0.13 | - | - | |
| Chest Circumf | 0.54 | 0.10 | 0.57 | 0.09 | 0.27 | 0.45 | - | - | |
| Neck Circumf | 0.57 | 0.08 | 0.74 | 0.14 | 0.55 | 0.10 | - | - | |
| Needle Angle (Degrees) | Age | 0.42 | 0.23 | 0.56 | 0.88 | 0.13 | 0.72 | -0.02 | 0.96 |
| Height | 0.04 | 0.90 | 010 | 0.78 | 0.22 | 0.54 | -0.04 | 0.90 | |
| Chest Circumf | 0.73 | 0.02 | 0.82 | 0.003 | 0.58 | 0.08 | 0.38 | 0.28 | |
| Neck Circumf | 0.75 | 0.01 | 0.51 | 0.13 | 0.48 | 0.16 | 0.12 | 0.74 | |
| Tip Visibility (Likert Scale) | Age | -0.08 | 0.83 | -0.18 | 0.62 | 0.11 | 0.75 | -0.38 | 0.29 |
| Height | 0.22 | 0.54 | 0.04 | 0.91 | 0.47 | 0.18 | -0.36 | 0.31 | |
| Chest Circumf | -0.04 | 0.92 | 0.06 | 0.87 | 0.12 | 0.75 | 0.27 | 0.46 | |
| Neck Circumf | 0.09 | 0.80 | -0.35 | 0.32 | -0.13 | 0.72 | -0.11 | 0.76 | |
| Shaft Visibility (Likert Scale) | Age | -0.30 | 0.40 | -0.46 | 0.18 | 0.00 | 1.00 | -0.25 | 0.49 |
| Height | 0.01 | 0.97 | -0.09 | 0.81 | 0.42 | 0.22 | -0.19 | 0.60 | |
| Chest Circumf | -0.28 | 0.43 | 0.13 | 0.71 | 0.24 | 0.51 | 0.25 | 0.49 | |
| Neck Circumf | -0.02 | 0.96 | -0.16 | 0.65 | -0.10 | 0.79 | -0.07 | 0.84 | |
| Needle Depth (Cm) | Age | -0.27 | 0.45 | 0.24 | 0.51 | 0.06 | 0.87 | 0.50 | 0.14 |
| Height | -0.40 | 0.26 | 0.02 | 0.96 | 0.09 | 0.81 | 0.51 | 0.13 | |
| Chest Circumf | 0.20 | 0.57 | 0.47 | 0.17 | 0.13 | 0.72 | 0.63 | 0.05 | |
| Neck Circumf | 0.10 | 0.79 | 0.52 | 0.13 | 0.44 | 0.20 | 0.57 | 0.08 | |
LICF – Lateral infraclavicular fossa approach, CML – Costclavicular medial to lateral approach, CLM – Costoclavicular lateral to medial approach, r – Measure of the strength of association between two variables, EP – Entry point, Circumf – Circumference
The mean values of the variables studied and the difference between the groups have been detailed in Table 1. The correlation of each of the US measurements with the cadaver characteristics of all four groups has been enumerated in Table 2.
Dissection findings: [Figure 1a and b].
Figure 1.
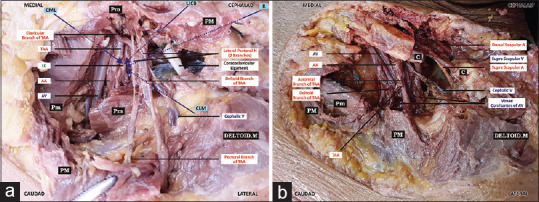
(a and b) Important neurovascular structures in vicinity of the four approaches (the arrows in violet represent the path taken by needles in each of four approaches) of ultrasound-guided infraclavicular brachial plexus block. PM-Pectoralis Major. Pm-Pectoralis Minor. C-Clavicle. TAA-Thoraco-abdominal artery. AA-Axillary Artery. AV-Axillary Vein. LC-Lateral Cord. LICB-Lateral Infra Clavicular Brachial Plexus Block approach. CML-Costoclavicular medial to lateral approach. CLM-Costoclavicular lateral to medial approach. R-Retroclavicular approach
The important structures traversed by, or in the vicinity of block needle in the four approaches have been detailed in Table 3.
Table 3.
Dissection Findings - the important structures traversed by, or in the vicinity of block needle in the four approaches
| Approaches | LICF (Structures traversed) | LICF (Structures in vicinity) | CML (Structures traversed) | CML (Structures in vicinity) | CLM (Structures traversed) | CLM (Structures in vicinity) | R (Structures traversed) | R (Structures in vicinity) |
|---|---|---|---|---|---|---|---|---|
| EP | Cephalic Vein (1) | Dorsal-cephalic vein (3) Suprascapular artery (1) |
None | None | None | Cephalad-Cephalic vein (4) Dorsal-Suprascapular nerve (1) Supraclavicular nerve (1) |
None | None |
| MP | None | Medial - TAA or its branches (3) Ventral-TAA or its branches (5) Dorsal-TAA or its branches (1) Subclavian vein (1) Upper subscapular nerve and thoracodorsal nerve (1) |
None | Medial-TAA origin or its branches (6) Cephalic vein (1) Suprascapular artery and vein (1) Lateral-TAA or its branches (2) Cephalic vein (1) Caudad-Cephalic vein (1) |
None | Medial-TAA or its branches (2) Lateral-TAA or its branches (2) Ventral-TAA or its branches (6) Dorsal-Nerve to subclavius (1) Caudad-TAA or its branches (1) |
Nerve to subclavius (1) Branch of TAA (1) |
Medial-Suprascapular artery and vein (4) Dorsal scapular artery and superficial cervical artery (2) Suprascapular nerve (2) Ventral-Suprascapular artery and vein (3) Nerve to subclavius (4) TAA or its branches (2) Dorsal-TAA or its branches (1) Suprascapular nerve (1) |
| TP | None | Medial-origin of TAA (3) Ventral-Axillary artery and axillary vein (10) TAA branches (2)Venae commitantes to axillary vein (1) |
None | Medial and ventral-TAA or its branches (1) Medial-Axillary artery and vein (5) Lateral-TAA or its branches (2) Dorsal and medial-Dorsal scapular artery (1) |
None | Ventral-Axillary artery and vein (4) Cephalic vein draining into axillary vein (1) Dorsal-Axillary artery and vein (1) Caudad-Axillary artery and vein (1) |
None | Medial-TAA or its branches (1) Lateral-TAA or its branches (1) Ventral-Axillary artery and axillary vein (7) |
The number in brackets ( ) depicts the number of cadavers in which the neurovascular structure was in the vicinity or traversed. LICF – Lateral infraclavicular fossa approach, CML – Costoclavicular medial to lateral approach, CLM – Costoclavicular lateral to medial approach, R – Retroclavicular approach; TAA – Thoracoabdominal artery
In the LICF approach, the needle tip reached the posterior cord (PC) in 3 cadavers and, it reached the lateral cord (LC) in the remaining 7 cadavers. In CML approach, the needle tip reached the LC in all 10 cadavers, whereas, in the CLM approach, it reached the LC in 8 cadavers and the medial cord (MC) and PC in 1 each of cadavers. In R approach, the block needle tip reached the PC in 9 cadavers and reached the LC in 1 cadaver.
DISCUSSION
The number of cords visible in one US frame was similar in all groups [Table 1], though visualisation of all three cords in one frame is rare as described by Du Filippo A et al.[8] In our study, we have found constant cord positions within the groups, unlike some studies which have stated variations in the position of individual cords relative to the axillary artery.[9]
Leurcharusmee et al. have found a higher incidence of vascular breach with the LICF approach and paresthesia with the CLM approach.[9] They opine that the LICF approach may be technically challenging in obese patients and in such a scenario, shoulder abduction would decrease the depth of the three cords. They suggest the CLM approach to be beneficial in patients with altered coagulation status, as the needle tip is in between the cords, rather than perivascular but, our dissection findings suggest otherwise, due to the branches of the TAA criss-crossing the needle pathway, making this approach risky, especially in patients with questionable baseline coagulation status.
Sala-Blanch X et al. have described the disadvantages of having a greater depth of cords and separated from one another in the LICF approach. They have also observed that change in arm position from abduction to adduction does not change the configuration of the cords in relation to the BP in the CLM approach, in contrast to the LICF approach.[3] We too had observed the greatest depth of cords in the LICF approach, but it was not statistically significant though. The CLM approach is attributed to the close proximity of the BP to AA and vein located medial to the BP in a triangular fashion.[10]
The proximity of the acromial branch of TAA in the LICF approach has been reported by Sutton EM et al. and they have advocated that the R approach circumvents this problem, with decreased risk of injury to TAA, LC and cephalic vein, in contrast to our findings.[11]
Li JW et al., in their step-wise approach to sonoanatomy of the costoclavicular space in the CLM approach, have advised to visualise the TAA, before moving to the step 5 of locating the cords lateral to AA.[4] The TAA is a short artery arising from the second part of AA from the ventral aspect and immediately gives off branches—deltoid, clavicular, acromial, and pectoral, which travel in various directions in a flower-shaped pattern. Hence, it may not be precisely cross-sectioned by the US beam.[12] So, trying to find the TAA in color doppler may be erroneous, giving a false sense of reassurance. Apart from this, anatomical variations of the branches directly arising from the AA rather than from a common trunk have also been described.[13,14]
Karmarkar M et al. have acknowledged that the CLM approach has the limitation of being a potential for a vascular and pleural puncture, though they have not encountered such an issue.[15] Our dissection findings prove this potential for vascular breach in the CLM approach.
Nieuwveld D et al. have highlighted the difficulty in needling from lateral to medial direction in the costoclavicular approach, due to the coracoid process being an obstacle, especially in the arm abducted position.[5] They have proposed the CML approach, as needle insertion is away from vascular structures and pleura and unhindered by the coracoid process. We had found either the TAA, or its branches, suprascapular artery, vein or dorsal scapular artery were within 1 cm vicinity of the needle pathway by this approach.
The R approach has been shown to have the best needle tip and shaft visibility due to the parallel to the US beam angle taken by the needle in this approach.[16] Our findings too show that the needle tip and shaft visibility was best seen in the R group as compared to the rest of the three approaches. Nevertheless, the 'blind spot' behind the clavicle should be a major deterrence to this approach. The length of the needle to reach TP was significantly more by this approach, which makes it cumbersome in obese individuals.
Sancheti SF et al. have found that the suprascapular nerve and vein were found either in the pathway or in the vicinity of the needle in their study on cadavers while investigating the anatomical concerns of the R block.[17] In our study, the nerve to subclavius and a branch of TAA were traversed by the needle in this approach apart from going very close to other structures such as a suprascapular artery, vein and nerve, dorsal scapular artery and superficial cervical artery. In none of the cadavers, the needle had touched the posterior aspect of the clavicle, a tendency of this approach as reported by Beh ZY et al.[18] Some authors have stated that by keeping the needle close to the under surface of the clavicle, the block needle passes through only muscle and loose connective tissue and avoids pneumothorax if not directed posteriorly.[2,7] Supraclavicular nerves too may be trespassed by this approach, unlike our dissection findings.[19]
The fullness of the supraclavicular fossa and lack of compressibility of this fullness increases the technical difficulty of the R approach.[7]
The correlation observed between chest and neck circumference with a vertical depth of the cords in the CML and R approaches makes them less feasible in obese patients [Table 2].
The needle entry may be hindered due to the bony coracoid process in the CLM approach and by the clavicle angulation variations in the R approach. The R approach gives better visibility of needle shaft beyond the clavicle, but the clavicle acts as a 'blind-spot' for the US beam obliterating important neurovascular structures. The proximity of the TAA or its branches in these approaches makes these approaches less desirable in patients with questionable baseline coagulation status.
Future research may be directed at a clinical study comparing block dynamics of all these four approaches. US findings of cadavers may not reflect that of the patient population due to differences in tissue texture and visibility.
CONCLUSION
The various neurovascular structures the needle traverses or in the immediate vicinity as observed in our study, do not make the CML, CLM or R approaches any better than the traditional LICF approach. The proximity of neurovascular structures at block needle tip alone should not be a determining factor for the safety of a block, instead, the vicinity of structures throughout the entire length of the needle should be given paramount importance.
Paper presentation at conference
This paper was presented and won 1st prize ISACON Jaipur Award for Acute and Perioperative Pain category at ISACON 2019 National Conference of Indian Society of Anaesthesiologists held at Bangalore, India in November, 2019
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
Dr. Prakash Manivel, Assistant Professor, Department of Preventive and Social Medicine, Indira Gandhi Medical College and Research Institute, Pondicherry, India, for his statistical support. Dr. Sriram Pothapregada, Professor and Head, Department of Pediatrics, Indira Gandhi Medical College and Research Institute, Pondicherry, India, for his editorial assistance and inputs.
REFERENCES
- 1.Sandhu NS, Capan LM. Ultrasound-guided infraclavicular brachial plexus block. Br J Anaesth. 2002;89:254–9. doi: 10.1093/bja/aef186. [DOI] [PubMed] [Google Scholar]
- 2.Luftig J, Mantuani D, Herring AA, Nagdev A. Ultrasound-guided retroclavicular approach infraclavicular brachial plexus block for upper extremity emergency procedures. Am J Emerg Med. 2017;35:773–7. doi: 10.1016/j.ajem.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 3.Sala-Blanch X, Reina MA, Pangthipampai P, Karmakar MK. Anatomic basis for brachial plexus block at the costoclavicular space: A cadaver anatomic study. Reg Anesth Pain Med. 2016;41:387–91. doi: 10.1097/AAP.0000000000000393. [DOI] [PubMed] [Google Scholar]
- 4.Li JW, Songthamwat B, Samy W, Sala-Blanch X, Karmakar MK. Ultrasound-guided costoclavicular brachial plexus block: Sonoanatomy, technique, and block dynamics. Reg Anesth Pain Med. 2017;42:233–40. doi: 10.1097/AAP.0000000000000566. [DOI] [PubMed] [Google Scholar]
- 5.Nieuwveld D, Mojica V, Herrera AE, Pomés J, Prats A, Sala-Blanch X. Medial approach of ultrasound-guided costoclavicular plexus block and its effects on regional perfussion. Rev Esp Anestesiol Reanim. 2017;64:198–205. doi: 10.1016/j.redar.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Hebbard P, Royse C. Ultrasound guided posterior approach to the infraclavicular brachial plexus. Anaesthesia. 2007;62:539. doi: 10.1111/j.1365-2044.2007.05066.x. [DOI] [PubMed] [Google Scholar]
- 7.Charbonneau J, Fréchette Y, Sansoucy Y, Echave P. The ultrasound-guided retroclavicular block: A prospective feasibility study. Reg Anesth Pain Med. 2015;40:605–9. doi: 10.1097/AAP.0000000000000284. [DOI] [PubMed] [Google Scholar]
- 8.Di Filippo A, Orando S, Luna A, Gianesello L, Boccaccini A, Campolo MC, et al. Ultrasound identification of nerve cords in the infraclavicular fossa: A clinical study. Minerva Anestesiol. 2012;78:450–5. [PubMed] [Google Scholar]
- 9.Leurcharusmee P, Elgueta MF, Tiyaprasertkul W, Sotthisopha T, Samerchua A, Gordon A, et al. Arandomized comparison between costoclavicular and paracoracoid ultrasound-guided infraclavicular block for upper limb surgery. Can J Anaesth. 2017;64:617–25. doi: 10.1007/s12630-017-0842-z. [DOI] [PubMed] [Google Scholar]
- 10.Wahal C, Kumar A, Pyati S. Advances in regional anaesthesia: A review of current practice, newer techniques and outcomes. Indian J Anaesth. 2018;62:94–102. doi: 10.4103/ija.IJA_433_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutton EM, Bullock WM, Gadsden J. The retroclavicular brachial plexus block: Additional advantages. Reg Anesth Pain Med. 2015;40:733–4. doi: 10.1097/AAP.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 12.Lambert SM. Shoulder girdle and arm. In: Standring S, editor. Gray’s Anatomy: The Anatomical Basis of Clinical Practice. 41th ed. New York: Elsevier; 2016. p. 828. [Google Scholar]
- 13.Daimi SR, Siddiqui AU, Wabale RN. Variations in the branching pattern of axillary artery with high origin of radial artery. Int J Anat Var. 2010;3:76–7. [Google Scholar]
- 14.Pandey SK, Shukla VK. Anatomical variation in origin and course of the thoracoacromial trunk and its branches. Nepal Med Coll J. 2004;6:88–91. [PubMed] [Google Scholar]
- 15.Karmakar MK, Sala-Blanch X, Songthamwat B, Tsui BC. Benefits of the costoclavicular space for ultrasound-guided infraclavicular brachial plexus block: Description of a costoclavicular approach. Reg Anesth Pain Med. 2015;40:287–8. doi: 10.1097/AAP.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 16.Kavrut ON, Kavakli AS. Comparison of the coracoid and retroclavicular approaches for ultrasound-guided infraclavicular brachial plexus block. J Anesth. 2017;31:572–8. doi: 10.1007/s00540-017-2359-6. [DOI] [PubMed] [Google Scholar]
- 17.Sancheti SF, Uppal V, Sandeski R, Kwofie MK, Szerb JJ. A cadaver study investigating structures encountered by the needle during a retroclavicular approach to infraclavicular brachial plexus block. Reg Anesth Pain Med. 2018;43:752–5. doi: 10.1097/AAP.0000000000000826. [DOI] [PubMed] [Google Scholar]
- 18.Beh ZY, Hasan MS, Lai HY. Ultrasound-guided retroclavicular block (aka posterior approach infraclavicular block): Anatomical variation of the clavicle limits block feasibility. Reg Anesth Pain Med. 2016;41:658–9. doi: 10.1097/AAP.0000000000000456. [DOI] [PubMed] [Google Scholar]
- 19.Nathe T, Tseng S, Yoo B. The anatomy of the supraclavicular nerve during surgical approach to the clavicular shaft. Clin Orthop Relat Res. 2011;469:890–4. doi: 10.1007/s11999-010-1608-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


