Abstract
Background and Aims:
Postoperative pain following renal transplantation is moderate to severe. Quadratus lumborum block (QLB) is a new block that can provide effective analgesia following abdominal and retroperitoneal surgeries. This study aimed to evaluate the analgesic efficacy of QLB for postoperative analgesia in patients undergoing renal transplantation.
Methods:
Patients were randomised into two groups of 30 each. In group A (block group), 20 mL of 0.25% bupivacaine and group B (placebo group), 20 mLof normal saline were injected. In the postoperative room, an intravenous patient controlled analgesia (IVPCA) pump with fentanyl was started in both the group. The postoperatively recorded parameters were numerical rating scale (NRS) pain score at rest and on movement and coughing, total fentanyl consumption, sedation score, postoperative nausea vomiting, limb weakness, paralytic ileus, and any other block-related complication. Data were analysed using SPSS software version 22.0. Categorical data were analysed using the Chi-square method. Student t test or Mann–Whitney U test was applied for the continuous data. Numerical data with normal distribution were displayed as mean (standard deviation), abnormal distribution was displayed in the median (interquartile range) values, and as a percentage for categorical variables.
Results:
Fentanyl consumption, numerical rating score, and sedation score were significantly less in group A when compared to group B at 1, 4, 8, 12, and 24 h (P < 0.001).
Conclusion:
Type-1 QLB significantly reduces fentanyl consumption and NRS pain score at 1,4,8,12, and 24 h in the postoperative period in renal transplant recipients.
Key words: Kidney transplantation, patient-controlled analgesia, quadratus lumborum block
INTRODUCTION
Despite advances in both surgical and anaesthesia techniques, postoperative pain remains an important issue in patients undergoing renal transplantation.[1] Options for postoperative analgesia include regional anaesthesia such as epidural analgesia, intrathecal opioids, or interfascial plane block like transverse abdominis plane (TAP) block.[2] In general, peripheral nerve blocks and interfascial plane blocks are considered safe and are associated with a lesser number of complications when compared to the central neuraxial blocks like epidural anesthesia. Performing central neuraxial block like epidural in patients who were on dialysis can be risky due to the use of heparin in dialysis and the presence of platelet dysfunction in patients with end-stage renal disease. Adequate analgesia in the postoperative period is associated with improved patient satisfaction, early mobility, and shorter hospital stay. TAP block has been used to manage postoperative pain in renal transplant recipients. A disadvantage of this block is that it provides only limited dermatome coverage of T10-L1 and does not provide visceral analgesia. Recently, quadratus lumborum block (QLB) has shown promising results in managing postoperative pain following both abdominal and retroperitoneal surgeries. It has been used successfully to provide analgesia in various surgeries such as open hysterectomies, open liver resections, percutaneous nephrolithotomy, cesarean sections, laparoscopic ovarian surgeries, laparotomies, and hip arthroplasties.[3] The advantage of QLB over TAP block is its ability to provide better sensory coverage and visceral analgesia even with a single injection.[4] It provides analgesia by blocking the spinal nerves from T6 to L1. It can be given by various approaches such as posterior, anterior, anterolateral, and intramuscular. The drug spread, dermatome coverage, and duration of analgesia may be different in different approaches. As per our literature search, the efficacy of type 1 QLB in renal transplantation recipients has not been studied. Hence, this study aimed to evaluate the analgesic efficacy of type-1 QLB for postoperative analgesia in patients undergoing renal transplantation under general anesthesia. The primary aim of the study was to compare the postoperative total fentanyl consumption in the first 24 h and the secondary aim was to compare the postoperative pain at rest, pain on movement, and sedation score at 1, 4, 8, 12, and 24 h after the surgery.
METHODS
This prospective, randomised study was conducted after obtaining the institutional ethical committee (IEC) clearance (IEC code: 2017-31-IP-96 dated 26/04/17). This trial was registered with the clinical registry of India (CTRI/2017/06/008924) and was conducted from April 2017 to February 2018. The study was conducted in accordance with the principles of the Declaration of Helsinki. Sixty patients age 18–65 years scheduled for elective renal transplantation under general anaesthesia were included in the study. On the contrary, patients refusing to participate in the study, difficult anticipated anatomy on ultrasound, localised infection at the proposed site of injection, having a psychiatric illness, and known allergy to local anesthetics were excluded from the study. Written informed consent was obtained from all the patients after explaining the procedure. Patients were randomised into two groups of 30 each using block randomisation method by taking block size of 10. Blocks were performed by the attending anesthesiologist not involved in the ultrasound-guided procedure. Patients, investigators, and attending nurses were kept blinded to the block. The final results were handed over to the investigator in a sealed envelope.
In both groups, general anesthesia was given using injection fentanyl 3 μgkg–1 intravenous (i.v.), propofol 1–2 mg kg–1 i.v., and atracurium 0.5 mg kg–1 i.v. to all the patients. Anesthesia was maintained with 6%–7% desflurane and 50% air in oxygen. Inj fentanyl 50 μg i.v. bolus was repeated every 2 h in both the groups. After the surgery was complete and before extubation, a trained and experienced anesthetist performed the block using ultrasound (SonoSite, Bothell, Washington). An anaesthesia resident not involved in the study loaded either 20 mL of 0.25% bupivacaine or an equal volume of saline in a 25-mL syringe. The syringe was then labelled as the “study medicine.” A high-frequency linear ultrasound probe (6–12 Hz) was placed in the anterior axillary line above the iliac crest to visualise the typical triple abdominal layers (external oblique, internal oblique, and transverse abdominis) [Figure 1]. The probe was then moved posteriorly to visualise the tapering of transverse abdominis muscle and the beginning of thoracolumbar fascia (TLF) over quadratus lumborum (QL) muscle. Using an in-plane technique, under all aseptic precautions, a 23 g spinal needle was inserted until the needle tip reached the anterolateral border of the QL muscle at the junction of QL muscle with the transversalis fascia. The position of the needle was confirmed by injecting 5 ml of normal saline. After negative aspiration for blood, 20mL of 0.25% bupivacaine or an equal volume of normal saline was given. All patients were extubated on the table if they met the extubation criteria. Immediately in the postoperative room, intravenous patient-controlled analgesia (IVPCA)(CADD Legacy PCA infusion model 6300 by Smith medicals) pump was started in both the groups. IVPCA pump was set to give boluses of 15 μg fentanyl with the lockout interval of 10 min and a maximum hourly dose of 90 μg fentanyl. There was no continuous background infusion. Injection paracetamol 1 g i.v. four times a day and injection ondansetron 8 mg i.v. two times a day were given to all patients.
Figure 1.
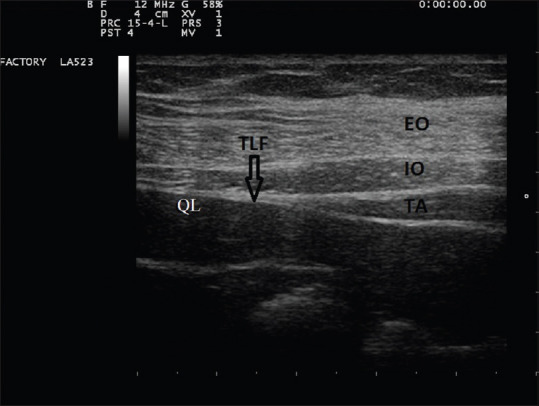
USG guided Quadratus lumborum block, Three layers of anterior abdominal wall. EO = external oblique muscle, IO = internal oblique muscle, TA = transverse abdominis muscle, QL = quadratus lumborum muscle, TLF = thoraco lumbar fascia
The following parameters were recorded postoperatively at 1st, 4th, 8th, 12th, and 24th h by the attending nurse which include numerical rating scale (NRS) pain score at rest and on movement and coughing, total fentanyl consumption, sedation score, postoperative nausea vomiting (PONV), limb weakness, paralytic ileus and any other complication related to the block. The NRS scale is a 11-point numeric scale which ranges from 0 to 10. Zero represents no pain, whereas 10 represents the pain as bad as you can imagine or worst pain imaginable. [0: no pain, 1–4: mild pain, 5–6: moderate pain, and 7–10: severe pain].[5] The level of sedation was assessed using the observer's assessment of alertness/sedation score.[6] (Score1––no response after name is called loudly with mild shaking, Score 2––responds only after name called loudly and after mild shaking of body, Score 3––responds only after name called loudly and/or repeatedly, Score 4––lethargic response to name, spoken in normal tone, Score 5––awake and responds to name, spoken in normal tone).
In the results of a previous 10-patient pilot study, it was found that fentanyl consumption at 24 h in the QLB group and the placebo group was 465 ± 190 (μg) and 660 ± 256 (μg), respectively. The primary outcome measure of this pilot study was a 25% reduction in the total fentanyl consumption at 24 h in the QLB when compared to the placebo group. A sample size of 29 patients in each group was determined with a statistical power of 0.8 and a type-1 error of 0.05. To rule out any dropouts, 30 patients in each group were taken. Data were entered into Microsoft Excel and were analysed using SPSS software version 22.0 (IBM, Armonk, New York). Categorical data were analysed using the Chi-square method. Student t test or Mann–Whitney U test were applied for the continuous data. Numerical data with normal distribution were displayed in a mean (standard deviation);abnormal distribution was displayed in the median (interquartile range) values, and as a percentage for categorical variables. A value of P < 0.05 was considered statistically significant.
RESULTS
The study consort diagram is shown in [Figure 2]. A total of 60 patients were enrolled in the study with 30 patients in each group. Demographic data such as weight, height, body mass index (BMI), age, and duration of surgery were comparable in both the groups [Table 1]. The median (interquartile range Q1, Q3) of static NRS pain score at 1, 4. 8, 12, and 24 h in group A was 3 (2–3), 2 (2–3), 2 (1–2), 2 (1–2) and 1 (0–1.75), and in group B was 4 (3–5), 4 (3–5), 3 (3–4), 3 (2–3) and 2 (2–3), respectively [Table 2]. Similarly, the median (interquartile range Q1, Q3) of dynamic NRS pain score at 1, 4,8 12, and 24 h in group A was 3 (3–4), 3 (2–4), 2 (2–3), 2 (2–3) and 2 (1–3), and in group B was 6 (5–7), 5 (4–6), 5 (4–5), 4 (4–5) and 3 (3–4), respectively [Table 3]. Fentanyl consumption was also significantly less in group A when compared to group B at 1, 4, 8, 12, and 24 h (P < 0.001) [Table 4]. The median (interquartile range Q1, Q3) of sedation score at 1, 4. 8, 12, and 24 h in group A was 3 (3–4), 4 (3–4), 4 (3–4), 5 (4–5) and 5 (4–5), and in group B was 3 (2–3), 3 (2–3), 3 (3–3), 3 (3–4) and 4 (4–5), respectively. The incidence of nausea and vomiting was 10%and 5%, respectively, in the placebo group, whereas in the control group none of the patients had nausea and vomiting. Two patients in the placebo group had abdominal distension due to the paralytic ileus, whereas none of the patients had paralytic ileus in the block group. There was no incidence of any lower limb weakness related to the QLB.
Figure 2.
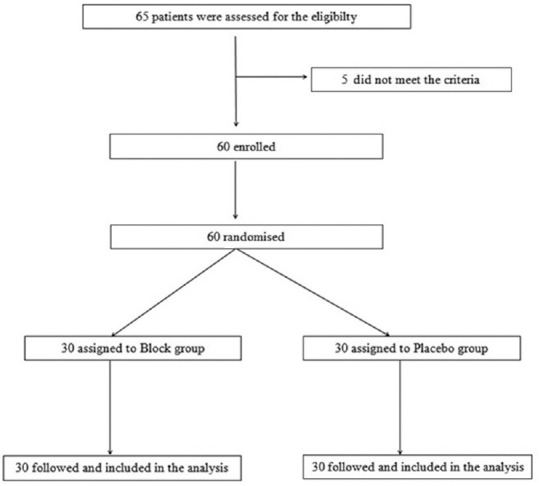
Consort diagram
Table 1.
Demographic variables
| Group A (QLB) | Group B (placebo) | P | |
|---|---|---|---|
| Age (years) | 39.07±9.75 | 37.93±7.55 | 0.62 |
| Sex (n%) | 28 (93.3) | 28 (93.3) | 1.0 |
| Weight (kg) | 49.90±3.88 | 51.40±4.12 | 0.15 |
| Height (cm) | 162±4 | 161±3 | 0.28 |
| BMI | 21±2 | 20±3 | 0.13 |
| Duration of surgery (min) | 353.10±24.23 | 363.10±15.11 | 0.06 |
BMI – Body mass index, QLB – Quadratus lumborum block. Values are expressed as mean±SD
Table 2.
Comparison of NRSR scores at various time points
| Time (h) | Group A (QLB)Median (IQR values) | Group B (placebo)Median (IQR values) | P |
|---|---|---|---|
| 1 | 3 (2-3) | 4 (3-5) | <0.001 |
| 4 | 2 (2-3) | 4 (3-5) | <0.001 |
| 8 | 2 (1-2) | 3 (3-4) | <0.001 |
| 12 | 2 (1-2) | 3 (2-3) | <0.001 |
| 24 | 1 (0-1.75) | 2 (2-3) | <0.001 |
QLB – Quadratus lumborum block, NRSR – Numerical rating score at rest, IQR – Interquartile range
Table 3.
Comparison of NRSD scores at various time points
| Time (h) | Group A (QLB)Median (IQR values) | Group B (placebo)Median (IQR values) | P |
|---|---|---|---|
| 1 | 3 (3-4) | 6 (5-7) | <0.001 |
| 4 | 3 (2-4) | 5 (4-6) | <0.001 |
| 8 | 2 (2-3) | 5 (4-5) | <0.001 |
| 12 | 2 (2-3) | 4 (4-5) | <0.001 |
| 24 | 2 (1-3) | 3 (3-4) | <0.001 |
QLB – Quadratus lumborum block, NRSD – Numerical rating score dynamic, IQR – Interquartile range
Table 4.
Comparison of cumulative fentanyl consumption at various time points
| Time (h) | Group A (QLB) | Group B (placebo) | P |
|---|---|---|---|
| 1 | 17.16±22.19 | 92.16±48.94 | <0.001 |
| 4 | 70.67±44.31 | 234.67±72.42 | <0.001 |
| 8 | 123.50±67.16 | 393.17±94.75 | <0.001 |
| 12 | 167.00±87.17 | 541.00±125.49 | <0.001 |
| 24 | 242±94.87 | 768.67±90.30 | <0.001 |
QLB – Quadratus lumborum block. Values are expressed as mean±SD
DISCUSSION
Recently QLB has been reported to attenuate pain more effectively than TAP block in patients undergoing laparotomy and upper abdominal surgeries. When compared to the transversalis fascial plane block, the QLB site is close to the spine which results in the wider spread of drug and better analgesic effect.[6,7] The promising results make it likely to emerge as a particularly efficient means of pain management, especially in renal transplant recipients. The objective of this study was to find out the effectiveness of type-1 QLB in reducing the requirement of postoperative fentanyl consumption in renal transplant recipients.
Block range can be affected by various factors like the type of QLB given, volume of injected drug, puncture level, and needle direction. Thus, different approaches can be used depending upon the need. At our center, a modified Gibson incision extending from T 10 to L 1 was taken for all the renal transplant recipients, which can easily be covered by this block. Previous studies have shown its spread from T7 to L1.[8,9] To avoid the effect of surgical manipulation on drug spread and to prevent its spillage through the surgical incision we had given type-1 QLB after the surgery was over.
In our study, there was a significant reduction in postoperative fentanyl consumption at 1,4,8,12 and 24 h in the control group when compared to the placebo group. Blanco et al. performed a study in which they had given bilateral QLB to the patients who had undergone cesarean section and found a significant reduction in the morphine consumption in the QLB group at 6,1224, and 48 h.[10] Another study by Blanco et al., where they compared the QLB with TAP block in patients undergoing cesarean section, found significantly less consumption of morphine at 12, 24, and 48 h in QLB group.[11] Anders Krohg et al. performed a randomised controlled trial (RCT) on QLB in patients undergoing cesarean section and found significantly lower pain scores and decreased ketobemidone consumption in the active group when compared to the control group.[12] A study by Yousuf N K showed a similar result of decreased opioid consumption and better pain scores in the QL group when compared to the TAP block group.[4]
In a study by Marcin Mieszko Mieszkowsk et al., they found significantly decreased morphine consumption and low pain scores up to 48 h in the QLB group when compared to the TAP group patients undergoing cesarean section.[13] Our study results are in continuation of the previously published studies.
In our study, we have found significantly decreased NRS pain scores both at rest and on movement and coughing. Similar results were found by Okmen; they found significantly decreased VAS score in the QL block group when compared to the only IVPCA group.[14] In a study by Zhu et al., they had found a significant decrease in the NRS pain score on coughing in the QLB group when compared to the only IVPCA group.[15]
There is still no consensus about the volume of drug required to provide adequate analgesia after QLB in patients undergoing renal transplantation. In our study, we have used 20 mL of 0.25% bupivacaine which was based on the previous study results.[16] The safety of this volume was well established in a study by Murouchi et al., in which they had found that the plasma concentration of local anesthetic was well below the toxic levels when 20 ml of 0.0375% ropivacaine was given in QLB.[17]
In a case report, Wikner reported to have found an unexpected motor weakness of the lower limb following the lateral QLB, which he thought could have resulted from weakness of psoas, iliacus, and quadriceps muscle involvement due to the spread of drug to the lumbar plexus.[18] However, in our study, we did not find any lower limb weakness in any of our patients.
As per our literature search, this is the first RCT establishing the efficacy of type-1 QLB for postoperative analgesia in renal transplant recipients. However, further large studies are required to consolidate the findings of our study.
The limitation of our study was that a few previous studies have documented the duration of analgesia of up to 48 h after single-shot QLB. However, in our study, we followed the patients only up to 24 h. Therefore, from our study, it is difficult to interpret the actual total duration of analgesia after a single-shot QLB.
CONCLUSION
Type-1 QLB significantly reduces fentanyl consumption and NRS pain score at 1,4,8,12 and 24 h in the postoperative period in renal transplantation recipients and it can be used effectively as a part of multimodal analgesia.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Jain PN. Acute pain service: Round the clock vigilance. Indian J Anaesth. 2018;62:491–2. doi: 10.4103/ija.IJA_471_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jun JH, Kim GS, Lee JJ, Ko JS, Kim SJ, Jeon PH. Comparison of intrathecal morphine and surgical-site infusion of ropivacaine as adjuncts to intravenous patient-controlled analgesia in living-donor kidney transplant recipients. Singapore Med J. 2017;58:666–73. doi: 10.11622/smedj.2017077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verma K, Malawat A, Jethava D, Jethava DD. Comparison of transversus abdominis plane block and quadratus lumborum block for post-caesarean section analgesia: A randomised clinical trial. Indian J Anaesth. 2019;63:820–6. doi: 10.4103/ija.IJA_61_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yousef NK. Quadratus lumborum block versus transversus abdominis plane block in patients undergoing total abdominal hysterectomy: A randomized prospective controlled trial. Anesth Essays Res. 2018;12:742–7. doi: 10.4103/aer.AER_108_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagchi D, Mandal MC, Das S, Basu SR, Sarkar S, Das J. Bispectral index score and observer's assessment of awareness/sedation score may manifest divergence during onset of sedation: Study with midazolam and propofol. Indian J Anaesth. 2013;57:351–7. doi: 10.4103/0019-5049.118557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gopal TV. Ultrasound-guided transmuscular quadratus lumborum plane catheters: In the plane or out of it? Indian J Anaesth. 2019;63:609–10. doi: 10.4103/ija.IJA_585_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Öksüz G, Bilal B, Gürkan Y, Urfalioǧlu A, Arslan M, Gişi G, et al. Quadratus lumborum block versus transversus abdominis plane block in children undergoing low abdominal surgery: A randomized controlled trial. Reg Anesth Pain Med. 2017;42:674–9. doi: 10.1097/AAP.0000000000000645. [DOI] [PubMed] [Google Scholar]
- 8.Lu Y, Zhang J, Xu X, Chen W, Zhang S, Zheng H, et al. Sensory assessment and block duration of transmuscular quadratus lumborum block at L2 versus L4 in volunteers: A randomized controlled trial. Minerva Anestesiol. 2019;85:1273–80. doi: 10.23736/S0375-9393.19.13656-5. [DOI] [PubMed] [Google Scholar]
- 9.Sindwani G, Sahu S, Suri A, Saeed Z. Bilateral quadratus lumborum block for postoperative analgesia in a Von Hippel-Lindau syndrome patient undergoing laparoscopic radical nephrectomy. Saudi J Anaesth. 2017;11:513–4. doi: 10.4103/sja.SJA_263_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanco R, Ansari T, Girgis E. Quadratus lumborum block for postoperative pain after caesarean section: A randomised controlled trial. Eur J Anaesthesiol. 2015;32:812–8. doi: 10.1097/EJA.0000000000000299. [DOI] [PubMed] [Google Scholar]
- 11.Blanco R, Ansari T, Riad W, Shetty N. Quadratus lumborum block versus transversus abdominis plane block for postoperative pain after cesarean delivery: A randomized controlled trial. Reg Anesth Pain Med. 2016;41:757–62. doi: 10.1097/AAP.0000000000000495. [DOI] [PubMed] [Google Scholar]
- 12.Krohg A, Ullensvang K, Rosseland LA, Langesæter E, Sauter AR. The analgesic effect of ultrasound-guided quadratus lumborum block after cesarean delivery: A randomized clinical trial. Anesth Analg. 2018;126:559–65. doi: 10.1213/ANE.0000000000002648. [DOI] [PubMed] [Google Scholar]
- 13.Mieszkowski MM, Mayzner-Zawadzka E, Tuyakov B, Mieszkowska M, Żukowski M, Waśniewski T, et al. Evaluation of the effectiveness of the quadratus lumborum block type I using ropivacaine in postoperative analgesia after a cesarean section - a controlled clinical study. Ginekol Pol. 2018;89:89–96. doi: 10.5603/GP.a2018.0015. [DOI] [PubMed] [Google Scholar]
- 14.Ökmen K, Ökmen BM. Ultrasound-guided anterior quadratus lumborum block for postoperative pain after percutaneous nephrolithotomy: A randomized controlled trial. Korean J Anesthesiol. 2020;73:44–50. doi: 10.4097/kja.19175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu Q, Li L, Yang Z, Shen J, Zhu R, Wen Y, et al. Ultrasound guided continuous quadratus lumborum block hastened recovery in patients undergoing open liver resection: A randomized controlled, open-label trial. BMC Anesthesiol. 2019;19:23. doi: 10.1186/s12871-019-0692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carline L, McLeod GA, Lamb C. A cadaver study comparing spread of dye and nerve involvement after three different quadratus lumborum blocks. Br J Anaesth. 2016;117:387–94. doi: 10.1093/bja/aew224. [DOI] [PubMed] [Google Scholar]
- 17.Murouchi T, Iwasaki S, Yamakage M. Quadratus lumborum block: Analgesic effects and chronological ropivacaine concentrations after laparoscopic surgery. Reg Anesth Pain Med. 2016;41:146–50. doi: 10.1097/AAP.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 18.Wikner M. Unexpected motor weakness following quadratus lumborum block for gynaecological laparoscopy. Anaesthesia. 2017;72:230–2. doi: 10.1111/anae.13754. [DOI] [PubMed] [Google Scholar]


