Erythema nodosum leprosum (ENL), also known as type 2 reaction (T2R) is an immune complex-mediated (type III hypersensitivity) reactional state encountered in patients with borderline lepromatous and lepromatous leprosy (BL and LL) either before, during or after the institution of anti-leprosy treatment (ALT). ENL is characterized by the eruption of evanescent tender subcutaneous nodules and constitutional symptoms (fever, malaise, arthralgia, and myalgia) that may be accompanied by neuritis, uveitis, orchitis, or other systemic organ involvement. Consequences of ENL may be serious, leading to permanent nerve damage and deformities. The overall prevalence of ENL is variable ranging from 25% in Brazil and Thailand to 49.4% in India among lepromatous leprosy patients.[1]
The incidence of ENL is certainly on the rise with the increasing number of multibacillary cases. Many risk factors have been identified for T2R; important being lepromatous leprosy with bacillary index (BI) of >4+, patients <40 years of age, intercurrent infections (bacterial, viral, parasitic), institution of ALT, physical and mental stress, surgical intervention, and pregnancy/parturition.[2]
ENL may be graded as mild and severe. Severe ENL reaction is often recurrent, chronic with frequent systemic involvement and may have atypical morphological presentation such as ENL necroticans, ulcerative/ulceronecrotic, pustular, and vesicobullous [Figures 1 and 2] ENL lesions.[3] Management of ENL continues to be the most challenging aspect of leprosy eradication program as the chronic and recurrent nature of painful skin lesions, neuritis and organ involvement demands prolonged treatment with prednisolone, thalidomide, anti-inflammatory and immunosuppressive drugs which adds to the existing morbidity.
Figure 1.
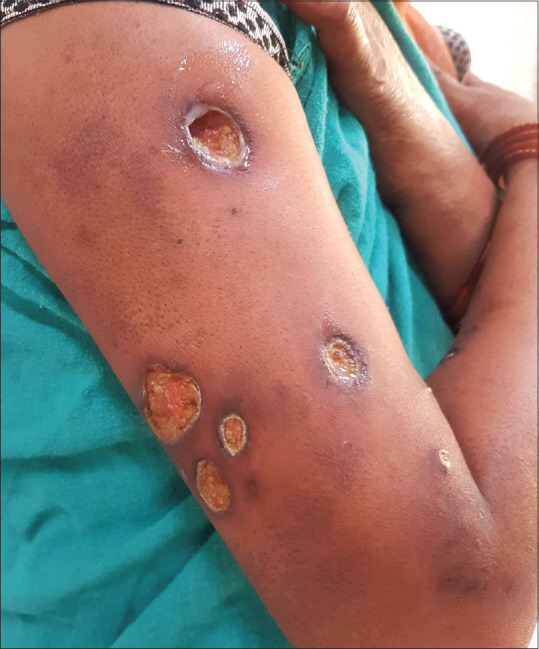
Necroulcerative erythema nodosum leprosum (ENL) lesions in LL patient
Figure 2.
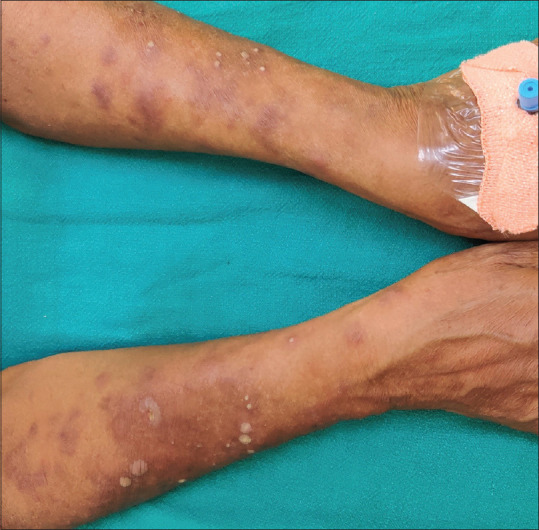
Crop of pustular lesions of ENL in LL patient with chronic ENL; note healed lesions of ENL
Pathogenesis
Though ENL or T2R is considered chiefly to be an immune complex-mediated disease, the exact pathogenesis of ENL is still elusive. Recently there has been tremendous work going on in the field of immunopathogenesis of leprosy reactions specially ENL or T2R, that may translate into improved, targeted and evidence-based management of ENL.[4]
Role of neutrophils
Although ENL is considered a neutrophilic immune-complex mediated condition, the direct role of neutrophils in ENL is not clear. Histology of ENL lesion too has prominent leukocytoclastic vasculitis and neutrophilic infiltrate in the acute stage. However, with the evolution of lesion, neutrophils and eosinophils are gradually replaced by lymphocytes. Recent studies have shown that the neutrophilic population is not homogeneous and has divergent phenotypes (pro- and antitumor profile). These are dynamic subpopulations of neutrophils with distinct phenotypical and functional abilities[5] Moreover, emerging evidence indicates that neutrophils expressing CD64 favor systemic inflammation during ENL. Thus, higher CD64 levels on circulating neutrophils may serve as a marker for ENL and disease severity.
Immune complexes in ENL
The most accepted hypothesis about ENL is that it's an immune complex (IC) mediated disorder that is characterized by the deposition of ICs (immunoglobulins, specific mycobacterial antigens like PGL1 and MCP-1 and complement C3, C5) in the vascular wall, serosa, and glomeruli. Though many studies have confirmed the presence of ICs in the skin using direct immunofluorescence techniques and sera of patients with ENL, their role remains uncertain. There is not enough definitive evidence if ICs are involved in the pathogenesis of ENL or merely an epiphenomenon.[6]
Cytokines
There is substantial evidence about a significant increase in TNF-α and IFN-γ in patients with ENL. In addition, many pro-inflammatory cytokines such as IL-1β, IL-4, IL-6, IL-8 IL-10, Il-12, and cytokine receptors such as sIL2R and sIL6R have been shown to be involved. Therefore, inhibitors of these molecules may be useful in a clinical setting.[6]
B Cells
Amorim et al. studied the differential immunoglobulin and complement levels in leprosy patients presuming that immunoglobulins could have a role in the pathogenesis of ENL. The percentage of circulating B cells increases during ENL but CD21 + B cells decreases. This may be due to the migration of CD21 + B cells to the tissues to secret antibodies. For a similar reason, IgG1 and IC levels are found to be low in patients with ENL.[7]
T cell
T-lymphocytes are part of the adaptive immune response and T cell subsets play an important role in ENL. Many studies have reported an increased percentage of CD4+ T-cells and reduced CD8+ T-cells with an increased CD4+/CD8+ ratio in patients with ENL in both skin and peripheral blood as compared to patients with non-reactional LL. Absolute numbers and proportion of the Treg subset of CD4+ T cells have been shown to be significantly lower during ENL. Tregs are known to downregulate the induction and proliferation of effector T cells. Therefore, lower Tregs may account for the relatively higher proportion of T cells.[8]
Recently, the role of T cells and memory T-cell subset was assessed in LL patients with ENL (n = 35) and LL patients (n = 25) who served as a control, in peripheral blood mononuclear cells (PBMCs) using flow cytometry. It was found that the median percentage of CD3+, CD4+, and CD8+ T-cells expressing activated T-cells were significantly higher in the PBMCs from patients with ENL than from LL patient controls. Likewise, the median percentage of central and activated memory T-cells was also significantly increased in patients with ENL. The study delineates the role of T cell activation in the pathogenesis of ENL. It establishes ENL reaction as a T-cell-mediated pathology so that future research may be targeted at developing treatment options that interfere with T-cell trafficking into tissues and thereby reducing inflammation in these patients.[9]
Management
The goal of treatment of T2R is to control the inflammation. At present we use NSAIDS (Aspirin, clofazimine in increased dosage, minocycline, colchicine, and chloroquine, etc.), and steroids (Prednisolone) for rapid control of neuritis and other inflammatory presentations. Recently Apremilast (oral phosphodiesterase-IV inhibitor) was tried in two patients for its strong anti-inflammatory action.[10] Anti TNF-α agents like thalidomide and pentoxiphylline are in regular use. There are reports of successful use of monoclonal antibodies like infliximab and etanercept in refractory cases. Newer thalidomide analogs, Revlimid, and Actimid may be used in the future. There are anecdotal reports of plasma exchange (to clear ICs) and IVIG for its immunomodulatory action. Many immunosuppressive agents (both T-cells and B-cells) like cyclosporine, methotrexate and azathioprine have been used in refractory and steroid-dependent ENL patients.
Recent work in the field of immunopathogenesis of leprosy reaction, provide additional and newer insights into the long-standing present concept of ENL as an immune complex disease. This will help a great deal to develop a more targeted and focused treatment approach and expand the therapeutic armamentarium for ENL.
Management Challenges in the Current Scenario
There has been no decline in the number of new leprosy patients, both adults and children since elimination in 2005. Unfortunately, more than half of the patients reporting to our institute come from far-flung areas of high endemicity, up to 80% have multibacillary disease and a sizable number presents or develops T2 R/ENL (including ENL necroticans) subsequently. This is due to lack of awareness in both medical fraternity and community, preventing early diagnosis, poor local infrastructure, thus, compelling patients to travel to far off metro cities contributing to high defaulter rate. Another downfall to this is a long incubation period, absent to poor contact/family screening and chemoprophylaxis resulting in a large number of hidden cases.[11] The problem further gets compounded by the advocacy of fixed duration therapy of one-year for all including those with very high baseline bacillary load (BI 6+), leading to premature termination of treatment and thus more reactional states and high potential of drug resistance. It's a challenge to treat ENL in the current scenario as the drugs required to treat reactional states are not provided by the program and therefore lack protocol/standardization. Sadly, the availability of clofazimine, a potent anti-inflammatory and steroid-sparing drug for the treatment of ENL is very erratic and not available outside the blister packs. In addition, unique clofazimine induced skin pigmentation may be a deterrent to its use in many patients, for fear of stigma and social ostracism. Steroid dependency is the major obstacle; multiple adjuvant drugs like thalidomide, immunosuppressive drugs, vaccines and biologics are being tried on case to case basis. These adjuvant drugs are unaffordable by leprosy patients. It will be an overstatement to say that quality control for MDT blister packs has never been done. All these factors singly or collectively are responsible for increased and recalcitrant ENL in India and pose a major therapeutic challenge. The leprosy program needs to be more inclusive and should aim to provide alternative drugs for the management of drug-resistant leprosy and ENL.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Pocaterra L, Jain S, Reddy R, Muzaffarullah S, Torres O, Suneetha S, et al. Clinical course of erythema nodosum leprosum: An 11-year cohort study in Hyderabad, India. A J Trop Med Hyg. 2006;74:868–79. [PubMed] [Google Scholar]
- 2.Manandhar R, LeMaster JW, Roche PW. Risk factors for erythema nodosum leprosum. Int J Lepr. 1999;67:270–8. [PubMed] [Google Scholar]
- 3.Wankhade VH, Debnath P, Singh RP, Sawatkar G, Bhat DM. A retrospective study of the severe and uncommon variants of erythema nodosum leprosum at a Tertiary health center in Central India. Int J Mycobacteriol. 2019;8:29–34. doi: 10.4103/ijmy.ijmy_174_18. [DOI] [PubMed] [Google Scholar]
- 4.Negera E, Bobosha K, Walker SL, Endale B, Howe R, Aseffa A, et al. New insight into the pathogenesis of erythema nodosum leprosum: The role of activated memory T-cells. Front Immunol. 2017;8:1149. doi: 10.3389/fimmu.2017.01149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmitz V, Tavares IF, Pignataro P, Machado AdM, Pacheco FdS, dos Santos JB, et al. Neutrophils in leprosy. Front Immunol. 2019;10:495. doi: 10.3389/fimmu.2019.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polycarpou A, Walker SL, Lockwood DN. A systematic review of immunological studies of erythema nodosum leprosum. Front Immunol. 2017;8:233. doi: 10.3389/fimmu.2017.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amorim FM, Nobre ML, Nascimento LS, Miranda AM, Monteiro GRG, Freire-Neto FP, et al. Differential immunoglobulin and complement levels in leprosy prior to development of reversal reaction and erythema nodosum leprosum. PLoS Negl Trop Dis. 2019;13:e0007089. doi: 10.1371/journal.pntd.0007089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Attia EA, Abdallah M, Saad AA, Afifi A, El Tabbakh A, El-Shennawy D, et al. Circulating CD4+ CD25 high FoxP3+ T cells vary in different clinical forms of leprosy. Int J Dermatol. 2010;49:1152–8. doi: 10.1111/j.1365-4632.2010.04535.x. [DOI] [PubMed] [Google Scholar]
- 9.Negera E, Bobosha K, Walker SL, Endale B, Howe R, Aseffa A, et al. New insight into the pathogenesis of erythema nodosum leprosum: The role of activated memory T-cells. Front Immunol. 2017;8:1149. doi: 10.3389/fimmu.2017.01149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Narang T, Kaushik A, Dogra S. Apremilast in chronic recalcitrant erythema nodosum leprosum: A report of two cases? Br J Dermatol. 2019 doi: 10.1111/bjd.18233. doi: 10.1111/bjd.18233. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Rao PN, Suneetha S. Current situation of leprosy in India and its future implications. Indian Dermatol Online J. 2018;2:83–9. doi: 10.4103/idoj.IDOJ_282_17. [DOI] [PMC free article] [PubMed] [Google Scholar]


