Abstract
Background:
In the absence of a standard protocol, several methods and devices have been used for preparing platelet-rich plasma (PRP) with varying platelet concentrations.
Methods:
Venous blood sample from 20 patients was used for preparing PRP using two methods: a manual double-spin method (1st spin at 160 g × 10 min, 2nd spin at 400 g × 10 min), and using a commercially available automated device (DrPRP-Kit®, REMI Laboratory Instruments). Platelet, erythrocyte, and total leukocyte counts were calculated for each PRP sample and compared.
Results:
Platelet count in the PRP prepared with the manual double-spin method (PRPm, 12.51 ± 5.89 × 105/μL) as well as with the automated device (PRPa, 7.25 ± 4.74 × 105/μL) had significantly higher mean platelet count than whole blood (2.58 ± 0.81 × 105/μL, P < 0.001). The mean platelet count in PRPm was statistically significantly higher than PRPa (P < 0.001). The platelet capture efficiency of the manual method (mean 47.11%, median 41.75%) was statistically significantly higher than that of the automated device (mean 31.89%, 29.51%, P = 0.012). Platelet counts in both PRPs were variable, but the counts were more dispersed in PRPa(coefficient of variation 65%) as compared to PRPm(coefficient of variation 47%).
Conclusion:
The manual double-spin method had a higher platelet capture efficiency resulting in a higher platelet concentration as compared to the automated device. Though there was a significant interindividual variation in the platelet yield in the PRPs produced by both methods, results were more consistent with the manual method.
Keywords: Automated device, comparison, manual double-spin, platelet-rich plasma, platelet yield
Introduction
Platelet-rich plasma (PRP) is increasingly becoming popular as a therapeutic option for various dermatological and aesthetic indications. There is no standard method of PRP preparation, and several protocols have been described in the literature with varying platelet yield. PRP can be prepared either manually or using an automated device. Though more convenient to use and quicker as compared to manual methods, automated devices are also more expensive. Comparative studies between different methods or devices of PRP preparation are relatively few in the dermatology literature.[1,2] In this study, we compared the composition of PRP prepared using a manual double-spin method and a commercially available automated device.
Methods
This was a cross-sectional comparative study conducted in the department of dermatology and venereology of All India Institute of Medical Sciences, New Delhi, India between May 2017 and August 2017 after approval from the institute ethics committee. All patients gave written informed consent for participation in the study. Blood samples were collected from 20 consecutive patients scheduled for treatment with PRP for various dermatological indications. Whole venous blood (45 mL) was drawn from each patient under strict aseptic precautions using a 22G needle attached to a 20 mL syringe: 5 mL as control and about 20 mL each for PRP preparation with the manual double-spin method and an automated device. The research personnel preparing PRP for a particular method remained the same for all samples.
PRP preparation using manual double-spin method
Whole venous blood (20 mL) in three 9 mL sterile tubes prefilled with 2 mL acid citrate dextrose solution (VACUETTE® TUBE, Greiner Bio-One, Austria) was centrifuged in a standard laboratory centrifuge (Heraeus Multifuge X1R centrifuge, Thermo Scientific™) at 20°C. The first spin was done for 10 min at 160 g (about 923 rpm, as per standard conversion g = (1.118 × 10−5) R × S2, where “g” is the relative centrifugal force, R is the radius of the rotor in centimeters, and S is the speed of the centrifuge in revolutions per minute). The resulting supernatant plasma was transferred into a sterile conical bottom tube without anticoagulant (Tarsons PS 15 mL centrifuge tube), which was then centrifuged for 10 min at 400 g (about 1460 rpm).[3] The upper 2/3rd cell-poor plasma supernatant was removed and the platelet pellet was suspended in the lower 1/3rd (about 2 mL) of plasma by gently shaking the tube.
PRP preparation using automated device
Whole venous blood (18 mL) was mixed with 2 mL of acid citrate dextrose solution in the commercially available PRP kit (DrPRP-Kit®, REMI Laboratory Instruments) and was centrifuged in the automated centrifuge (REMI PRP Plus Centrifuge, REMI Laboratory Instruments) at 20°C as per manufacturer's instructions ( first spin at 2700 rpm for men/2600 rpm for women for 12 min, followed by second spin at 3200 rpm for 7 min). The supernatant cell-poor plasma was removed to leave behind about 2 mL of PRP [Figure 1].
Figure 1.
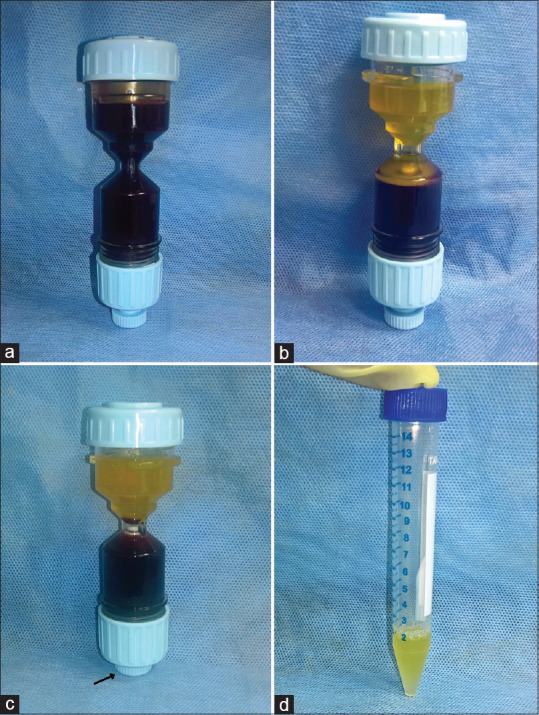
Preparation of platelet-rich plasma using automated device. (a) venous blood (18 ml) and ACD-A solution (2 mL) is collected in DrPRP-Kit®, (b) separation of plasma and buffy coat from RBCs after 1st centrifugation, (c) height of the separated interface is adjusted to include plasma, buffy coat, and superficial layer of RBCs in the upper chamber of the kit by pushing the adjusting knob (arrow) upwards, and 2nd centrifugation is performed, (d) platelet-poor plasma is removed and the platelet pellet is mixed in about 2 mL of plasma in a sterile tube
Estimation of cell counts
Cell counts were estimated using an automated analyzer (COULTER® LH750, Beckman Coulter) in whole venous blood, as well as PRP prepared using the two methods, after 5–10 min of PRP preparation to allow time for uniform dispersion of platelets in the plasma from the pellet.
Statistical analysis
Categorical variables are expressed as frequency and continuous variables as mean (standard deviation, median, and range). Platelet enrichment was calculated as per the formula: (platelet count in PRP—platelet count in whole blood/platelet count in whole blood) × 100, and was expressed as a percentage (%). Platelet concentration was taken as the ratio of platelet count in PRP to platelet count in whole blood. Platelet capture efficiency was calculated as: (PRP volume × platelet count in PRP)/(whole blood volume × platelet count in whole blood) × 100, and was expressed in percentage (%). Correlation of platelet count in PRP with whole blood was tested using the Spearman's correlation coefficient (r). The coefficient of variation (CV) was used as the measure of dispersion of the platelet counts in the PRPs, and was calculated as the ratio of the standard deviation to the mean platelet count. Wilcoxon signed-rank test was used to compare the cell counts, platelet enrichment, and platelet concentration between the paired PRP samples and whole blood. P value ≤0.05 was considered to be statistically significant. Statistical analysis was done using Stata version 14.0 (StataCorp, College Station, TX, U.S.A.).
Results
There were 12 males and 8 females with a mean age of 25.11 ± 5.12 years (range 19–40 years). Ten patients had alopecia areata, nine had androgenetic alopecia, and one had lepromatous leprosy with a nonhealing ulcer on foot. The platelet count, erythrocyte count, and total leukocyte count in whole blood, PRP prepared using the manual double-spin method (PRPm) and the automated DrPRP-Kit® (PRPa) are summarized in Table 1.
Table 1.
Platelet, erythrocyte, and total leucocyte counts in whole blood and the two PRPs
| Whole blood | PRPm | PRPa | |
|---|---|---|---|
| Platelet count (×105/µL) | |||
| Mean±SD | 2.58±0.811 | 12.512±5.897 | 7.254±4.738 |
| Range | 1.06-4.51 | 1.98-22.12 | 1.06-15.62 |
| Percentile | |||
| p25 | 2.01 | 8.23 | 3.16 |
| p50 | 2.38 | 12.18 | 6.27 |
| p75 | 3.05 | 18.21 | 11.15 |
| Erythrocyte count (×106/µL) | |||
| Mean±SD | 4.581±0.886 | 0.045±0.0315 | 0.308±0.331 |
| Range | 2.1-5.74 | 0-0.12 | 0-1.06 |
| Percentile | 3.97 | 0.02 | 0.05 |
| p25 | 4.96 | 0.04 | 0.14 |
| p50 | 5.19 | 0.06 | 0.59 |
| p75 | |||
| Total leucocyte count (×103/µL) | |||
| Mean±SD | 7.94±3.094 | 1.23±1.046 | 7.27±6.904 |
| Range | 4.6-19.3 | 0.2-4.6 | 0.2-19.2 |
| Percentile | 5.65 | 0.6 | 1 |
| p25 | 7.85 | 0.8 | 5.5 |
| p50 | 8.75 | 1.7 | 11.9 |
| p75 |
PRPa: Platelet-rich plasma prepared using automated method; PRPm: Platelet-rich plasma prepared using manual double-spin method; p25, 25thpercentile; p75, 75th percentile
The mean platelet count in PRPm(12.51 ± 5.89, median 12.18 × 105/μL) and PRPa(7.25 ± 4.74, median 6.27 × 105/μL) was higher than that in whole blood (2.58 ± 0.81, median 2.38 × 105/μL), and the difference was statistically significant for both (P < 0.001). The mean platelet count in PRPm was statistically significantly higher than PRPa(P < 0.001). The median platelet enrichment achieved by the manual method (317%) was statistically significantly higher than that attained by the automated device (165%; P < 0.001). Similarly, the median platelet concentration was higher in PRPm(4.17 times, range 0.99–8.29 times, interquartile range [IQR] 3.58–6.37 times) as compared to PRPa(2.65 times, range 0.39–5.42 times, IQR 1.19–4.5 times). The mean platelet capture efficiency of the manual method (47.11 ± 18.4%, median 41.75%) was statistically significantly higher than that of the automated device (31.89 ± 17.9%, median 29.51%, P = 0.012) [Figure 2]. Platelet count in PRPm correlated well with platelet count in whole blood (r = 0.74, P < 0.001), while there was no significant correlation between the platelet count in PRPa and whole blood (r = 0.30, P = 0.194). Platelet count in both the PRPs was variable, but the variability was more with the automated device (CV = 65.32%) than with the manual method (CV = 47.13%).
Figure 2.
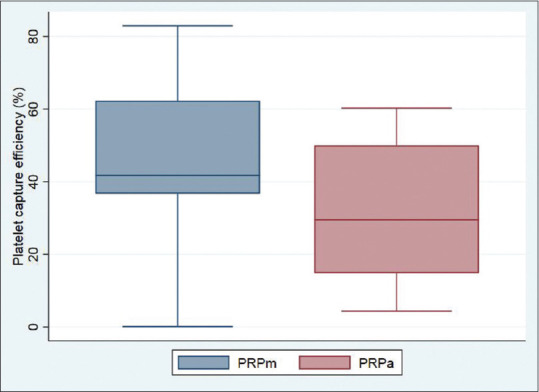
Box and whisker plot showing the platelet capture efficiency of the two PRP preparation methods (PRPa, platelet-rich plasma prepared using automated method; PRPm, platelet-rich plasma prepared using the manual double-spin method)
The mean leukocyte count in PRPm(1.23 ± 1.05 × 103/μL) was statistically significantly lower than in PRPa(7.27 ± 6.90 × 103/μL, P < 0.001) and in whole blood (7.94 ± 3.09 × 103/μL, P < 0.001), while it was comparable between whole blood and PRPa(P = 0.687). The mean erythrocyte count in both the PRPs (PRPm0.045 ± 0.03 × 106/μL, PRPa0.308 ± 0.33 × 106/μL) was significantly lower than whole blood (4.58 ± 0.89 × 106/μL; P < 0.001 for both). Of the two PRPs, PRPm had a significantly lower mean erythrocyte count than in PRPa(P = 0.002).
Discussion
The therapeutic efficacy of a PRP preparation is largely determined by its platelet concentration. Physicians, therefore, should be aware that different PRP preparation protocols can result in different platelet concentrations, which can translate into a difference in clinical response. We found the platelet concentration in PRPs produced by the manual double-spin method (PRPm) as well as the commercially available FDA-approved DrPRP-Kit® (PRPa) to be statistically significantly higher than the baseline whole blood value, however, the platelet yield was significantly more in PRPm. Platelet count in PRPm crossed the proposed therapeutically optimum threshold of 10 × 105/μL[4] more often than in PRPa(n = 11/20 vs 5/20, P = 0.053). However, Mazzucco et al. suggested that a platelet count greater than 2 × 105/μL may be sufficient for therapeutic effect, and this criterion was met by both PRPm as well as PRPa.[5] Barring a single outlier value in one sample (platelet count: whole blood 2 × 105/μL, PRPm1.98 × 105/μL; platelet concentration 0.99 times), manual double-spin method in our study produced a 3–8 (median 4.17)-fold rise in the platelet counts from the whole blood counts, which is similar to the 4–7 times increase reported by Gonshor et al.[3] In contrast, DrPRP-Kit® concentrated the platelets by 0.39–5.4 (median 2.65) times and would be considered a low-yield device.[2] Platelet enrichment achieved by the manual method was 317%, which is comparable to 352% reported by Tamimi et al.[6] using the same centrifugation parameters but much less than the 713% obtained by Gonshor et al.[3] Clearly, apart from the centrifugation parameters (such as centrifugation speed, number of spins), there are other procedural variables as well such as volume and method of drawing blood, size of collecting container, and choice of anticoagulants which can affect the platelet yield. Castillo et al.[7] compared three different commercial PRP preparation systems, with different starting whole blood volumes (18–55 mL) but resulting in comparable volumes of PRP (6–7.5 mL), and found no statistically significant difference in the platelet counts in the PRPs. This was because of the difference in platelet capture efficiency of the three systems; the system which started with lower blood volume had higher platelet capture efficiency. For similar reasons, the efficiency of platelet capture might be a more accurate comparative measure than the platelet count in our study as well, though the difference in the starting blood volume was relatively small (18 vs 20 mL) between the two methods. We found the platelet capture efficiency of the manual double-spin method (median 42%) to be statistically significantly higher than that of the automated device used in our study (median 30%). If known beforehand, the platelet capture efficiency of a PRP protocol can be useful in determining the starting volume of whole blood to produce a requisite PRP volume with the desired platelet count.
We observed a wide inter-subject variability in the platelet concentrations in both PRPm(CV 47%) and PRPa(CV 65%), but the results were more consistent in PRPm. Previous studies have also reported a considerable inter-subject as well as intra-subject variation in the PRP platelet counts.[8,9] Tamimi et al. reported a mean platelet count of 6.30 ± 2.69 × 105/μL translating into a CV of 42.7% using the same manual double-spin centrifugation parameters as ours.[6] Physiological differences between individuals such as hematocrit variability and size of platelets may explain such inter-individual variability in the platelet yield. Another recent study reported an intra-individual variation of 19.7% (range 0.5–56.3%) in the platelet counts obtained by the same method on four different occasions 15 days apart as part of a therapeutic study.[10]
PRPm had a much lower concentration of leukocytes than baseline whole blood values, in contrast to PRPa, which had a comparable leukocyte count as whole blood. This is probably a result of including the leukocyte-rich buffy coat for the second spin in the automated kit. The “buffy coat” protocol (supernatant along with the entire buffy coat is collected) is known to produce leukocyte rich PRP, as opposed to the “PRP” protocol (supernatant and only the superficial portion of the buffy coat is collected) which gives rise to pure PRP.[1,2] The biological role of leukocytes in PRP is as yet unclear. Some authorities believe that leukocytes exert a negative effect by virtue of their inflammatory properties.[11] Contrarily, leukocytes have antimicrobial properties and are known to increase the growth factor levels.[10,11,12,13,14] Orthopedic literature suggests that the significance of leukocytes in PRP may vary with its indication. However, the clinical relevance of leukocytes in PRP for dermatological indications warrants further investigation.
Our study was limited by relatively small sample size. We did not study the viability or ultrastructure of platelets, and growth factors concentrations in the PRPs. The therapeutic relevance of differing platelet counts in PRP merits further evaluation.
To conclude, our study highlights the differences in the PRPs prepared using two methods. There was a notable inter-individual variation in the platelet yield achieved by a given technique. Dermatologists should be aware of these variabilities while analyzing PRP-related literature or choosing the technique of PRP preparation for their patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We wish to acknowledge the assistance of Ms. Anshu Rawat, Medical Laboratory Technologist, Department of Dermatology and Venereology for her help in the laboratory work.
References
- 1.Arshdeep, Kumaran MS. Platelet-rich plasma in dermatology: Boon or a bane? Indian J Dermatol Venereol Leprol. 2014;80:5–14. doi: 10.4103/0378-6323.125467. [DOI] [PubMed] [Google Scholar]
- 2.Dhurat R, Sukesh M. Principles and methods of preparation of platelet-rich plasma: A review and author's perspective. J Cutan Aesthetic Surg. 2014;7:189–97. doi: 10.4103/0974-2077.150734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonshor A. Technique for producing platelet-rich plasma and platelet concentrate: Background and process. Int J Periodontics Restorative Dent. 2002;22:547–57. [PubMed] [Google Scholar]
- 4.Marx RE. Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant Dent. 2001;10:225–8. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Mazzucco L, Balbo V, Cattana E, Guaschino R, Borzini P. Not every PRP-gel is born equal.Evaluation of growth factor availability for tissues through four PRP-gel preparations: Fibrinet, RegenPRP-Kit, Plateltex and one manual procedure. Vox Sang. 2009;97:110–8. doi: 10.1111/j.1423-0410.2009.01188.x. [DOI] [PubMed] [Google Scholar]
- 6.Tamimi FM, Montalvo S, Tresguerres I, Blanco Jerez L. A comparative study of 2 methods for obtaining platelet-rich plasma. J Oral Maxillofac Surg. 2007;65:1084–93. doi: 10.1016/j.joms.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39:266–71. doi: 10.1177/0363546510387517. [DOI] [PubMed] [Google Scholar]
- 8.Mazzocca AD, McCarthy MB, Chowaniec DM, Cote MP, Romeo AA, Bradley JP, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94:308–16. doi: 10.2106/JBJS.K.00430. [DOI] [PubMed] [Google Scholar]
- 9.Russell RP, Apostolakos J, Hirose T, Cote MP, Mazzocca AD. Variability of platelet-rich plasma preparations. Sports Med Arthrosc Rev. 2013;21:186–90. doi: 10.1097/JSA.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 10.Rodrigues BL, Montalvão SA, Cancela RBB, Silva FA, Urban A, Huber SC, et al. Treatment of male pattern alopecia with platelet-rich plasma: A double-blind controlled study with analysis of platelet number and growth factor levels. J Am Acad Dermatol. 2019;80:694–700. doi: 10.1016/j.jaad.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 11.McCarrel TM, Minas T, Fortier LA. Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy. J Bone Joint Surg Am. 2012;94:e143. doi: 10.2106/JBJS.L.00019. 1-8. [DOI] [PubMed] [Google Scholar]
- 12.Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) Trends Biotechnol. 2009;27:158–67. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Moojen DJ, Everts PA, Schure RM, Overdevest EP, van Zundert A, Knape JT, et al. Antimicrobial activity of platelet-leukocyte gel against Staphylococcus aureus. J Orthop Res. 2008;26:404–10. doi: 10.1002/jor.20519. [DOI] [PubMed] [Google Scholar]
- 14.Zimmermann R, Jakubietz R, Jakubietz M, Strasser E, Schlegel A, Wiltfang J, et al. Different preparation methods to obtain platelet components as a source of growth factors for local application. Transfusion (Paris) 2001;41:1217–24. doi: 10.1046/j.1537-2995.2001.41101217.x. [DOI] [PubMed] [Google Scholar]


