Abstract
Context:
Lepra reactions if not managed promptly are an important cause of sudden onset nerve palsy and disability due to leprosy.
Aim:
To evaluate the usefulness of histology in predicting type 1 lepra reaction.
Setting and Design:
After obtaining clearance from institutional research and ethics committees, all histologically proven borderline tuberculoid patients diagnosed at our center from 1.8.2016 to 31.7.2018 were included in this retrospective cross-sectional study.
Method:
Clinical details were collected from patient records. The pathologist who was blinded to clinical evidence of type 1 lepra reaction at the time of biopsy re-evaluated the histopathology slides for evidence of type 1 reaction. The data of individual patient was analyzed to identify those who had a type 1 reaction at the time of the biopsy or who developed a lepra reaction during follow up.
Statistical Analysis Used:
Association between histological evidence of type 1 reaction and clinical manifestation of the same subsequently, was assessed using Pearson's Chi square test.
Results:
Study group comprised of 22 females and 18 males. Clinicohistological concordance was noted in 27 patients (67.5%). Subclinical type 1 reaction was documented in 11 patients (27.5%) based on histopathology evaluation. Five (45.5%) of these 11 patients subsequently developed clinical features of type 1 reaction. This was found to be statistically significant (P value 0.02).
Limitations:
Main limitation was the small sample size.
Conclusions:
Histology could serve as a useful tool in predicting future type 1 lepra reaction.
Keywords: Histopathology, leprosy, type 1 lepra reaction
Introduction
Reactions in leprosy (lepra reactions) assume importance for their ability to produce nerve function impairment (sensory and/or motor) that may lead to disability.[1] Acute onset is the hall mark of nerve palsy associated with lepra reaction, when compared to that produced by leprosy per se.[2]
Though both type 1 (T1R) and type 2 (T2R) lepra reactions can produce nerve damage, more severe neuritis and nerve function impairment are considered as features of T1R.[3] Early detection of T1R and neuritis; and prompt initiation of appropriate therapy can reduce the incidence of nerve palsy.
While describing the histopathology of T1R, Ridley described a prodromal phase that may precede the clinical onset of reaction.[4] He also suggested that all those who manifest this prodromal phase, need not subsequently develop full blown reaction.[4]
In this study, we attempted to assess the usefulness of histopathological analysis in predicting future clinical manifestation of type 1 lepra reaction.
Study design
Retrospective cross-sectional study.
Study subjects
Inclusion criteria: We undertook a cross sectional study of archived biopsy samples of histologically diagnosed borderline tuberculoid (BT) patients at our center from 1.8.2016 to 31.7.2018.
Exclusion criteria: We excluded histology specimens of poor quality (despite preparing fresh slides from paraffin embedded specimens).
Method
Clinical details were collected from records, using a pre-set proforma. Information on patient profile, evolution, and duration of disease, number, morphology, and distribution of skin lesions, thickened peripheral nerves, nerve function impairment, nerve pain/tenderness and skin smear analysis report at the time of biopsy were documented using a pre-set proforma. Clinical diagnosis of BT was made based on morphology, border, and the extent of sensory impairment of skin lesions.[5]
A clinical diagnosis of T1R was made whenever a patient had acute onset of erythema and oedema of skin lesions; with or without neuritis and oedema of the hands, feet, and face.[6,7]
The pathologist who was blinded to whether the patients had clinically evident T1R or not, at the time of biopsy (as per institutional policy, the diagnosis of leprosy is confirmed by biopsy taken from the active border of representative skin lesion/lesion manifesting evidence of TIR whenever there are skin lesions of leprosy or when the skin lesions manifest features of T1R respectively; hematoxylin and eosin, and Wade Fite staining, are carried out in each specimen) re-evaluated the histology slides for features of T1R and the findings were recorded.[7] When the histology slide was of poor quality, fresh slide was prepared from paraffin embedded specimen and restained.
The pathologist evaluated the specimens with a preset proforma documenting data on the appearance of granuloma (compact or diffuse), type of giant cells, inflammatory cells constituting granuloma (abundance of lymphocytes or epithelioid cells), dermal/intragranuloma oedema, vascular dilatation, spaces in dermis or around granuloma, presence of necrosis, arrangement of dermal collagen (whether separated or not), and acid fast bacilli (AFB).
A histological diagnosis of T1R was made when the histology showed at least two of the following features—granulomas with extracellular or intracellular oedema, dilated vascular channels, separation of dermal collagen, intense delayed-type hypersensitivity response manifested as abundance of lymphocytes, epithelioid cells or giant cells or as necrosis within the granuloma.[4,6,7,8,9]
The follow up data (institution documents follow up till completion of fixed duration multidrug treatment or treatment of lepra reactions whichever is later) was carefully analyzed to identify the patients who developed T1R at the time of biopsy and later during multidrug therapy.
Data was entered in Microsoft excel and analyzed by SPSS Inc IBM company version 18. The association between histopathology evidence of T1R and subsequent clinical manifestation of the same was assessed using Pearson's chi square analysis. P value less than 0.05 was considered significant.
Results
Study population comprised of 22 females (55.5%) and 18 males. Age of study group ranged from 11 to 61 years (mean age 33.8 years with standard deviation of 14.2 years).
Time interval between patient noticing the lesions and diagnosis varied from 2 weeks to 84 months in study participants. The mean interval documented was 16.6 months with standard deviation of 17.8 months.
Clinical features of T1R were present in 13 patients (32.5%) at the time of biopsy. The interval between the onset of symptoms suggestive of T1R and time of biopsy varied from 5 days to 3 months in these 13 patients (Mean 43.7 days; standard deviation 35.03 days).
The remaining 27 (67.5%) had no clinical evidence of T1R.
Histopathology findings of T1R were observed in 22 patients (55% of total study subjects). All the 22 patients who manifested histopathological evidence of T1R, had intra-granuloma oedema [Figure 1a and b]. Three of them (13.6%), in addition showed dermal oedema and separation of dermal collagen [Figure 1a and b]. Necrosis within the granuloma was observed in 6/22 (27.3%). Intense inflammation with inflammatory infiltrate predominantly composed of lymphocytes and Langhans giant cells were observed in 14/22 (63.6%) [Figure 2a and b].
Figure 1.
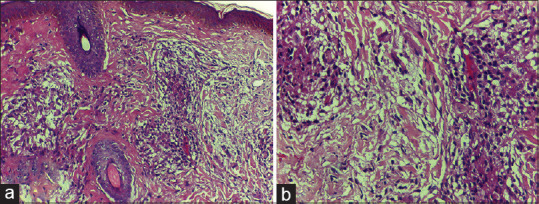
(a) Epithelioid granuloma showing intra-granuloma edema and dermal edema with separation of dermal collagen (H and E, ×200); 1 (b): high power view of Figure 1 (a)(H and E, x400)
Figure 2.
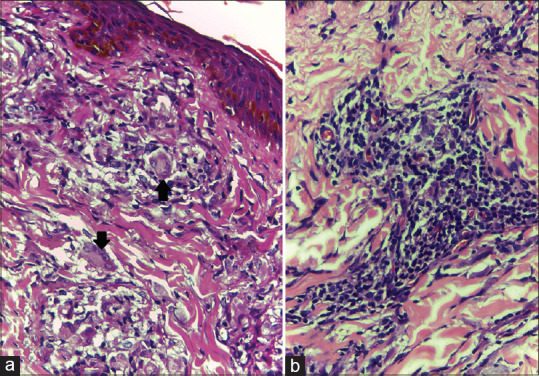
(a) Epithelioid granuloma showing intra-granuloma oedema and Langhans giant cell (H and E, ×400); 2 (b): Biopsy from another patient showing epithelioid granuloma with abundance of lymphocytes (H and E, ×400)
Comparing the clinical and histopathology features in the study group, clinico-histological concordance was noted in 27/40 (67.5%) cases. They included 11/13 (84.6%) with clinical and histology features of T1R and 16/27 (59.3%) who showed neither clinical nor histopathology evidence of reaction [Figure 3].
Figure 3.
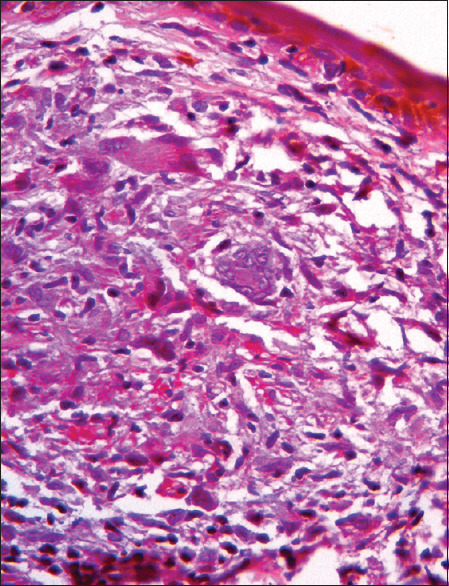
Epithelioid granuloma showing Langhan's giant cells without necrosis or intra-granuloma edema indicating borderline tuberculoid leprosy without any histological evidence of lepra reaction (H and E, ×400)
Thirteen patients (32.5% of total) documented discordant clinico-histological findings and they too could be classified into two groups. One group comprised of two patients who manifested clinical features of TIR without showing concordant histopathology (2/13, 15.4%). These two patients had documented 3-month interval between onset of T1R and skin biopsy (which was the longest interval documented in the study group). The second group of patients manifested histopathology findings suggestive of T1R which was not supported by their clinical features (11/27 (40.7%) who did not manifest clinical evidence of TIR). In other words, 11 cases (27.5% of study group) manifested subclinical T1R.
Follow up data was available for a period ranging from 10 to 18 months after biopsy in study group. At the time of the analysis, all except four patients had completed MDT; and none was receiving steroids.
Six of the twenty-seven (22.2%) patients who did not have any clinical evidence of T1R at the time of biopsy, developed clinical features of the same while on MDT and all of them manifested the reaction within 3 months of biopsy and starting MDT. Five of the six patients (83.3%) had histological evidence of the T1R in pre-treatment biopsy. Two among these five suffered from severe T1R with accompanying neuritis. In short, five of the eleven (45.5%) patients who had subclinical T1R based on histopathology evaluation went on to develop clinical manifestations later whereas only one of the sixteen cases (6.3%) who had no histology evidence of T1R, later developed the same [Table 1]. This was found to be statistically significant (P value 0.02).
Table 1.
Histology findings and subsequent follow up of patients without clinical features of T1R at the time of biopsy
| Histology findings at diagnosis | Subsequent development of clinically evident T1R | No subsequent development of clinically evident T1R | Total |
|---|---|---|---|
| Evidence of T1R | 5 (45.5%) | 6 (54.5%) | 11 |
| No evidence of T1R | 1 (6.3%) | 15 (93.8%) | 16 |
| Total | 6 (22.2%) | 21 (77.8%) | 27 |
T1R=type 1 lepra reaction
Discussion
We limited the study to histologically proven BT patients since it remains the most common spectrum among leprosy patients attending our institution that is at risk for T1R.[10,11] Moreover, sometimes, it can be difficult to differentiate clinically between BT and BT in reaction.[5] Only 50% of study subjects manifesting clinico-histological concordance for T1R in this study, was consistent with previous data.[6,7,8] Our observation of histopathology being a more sensitive method to detect T1R was concordant to one previous study; but was contrary to another earlier study which documented clinical diagnosis to be superior to histopathology analysis in identifying T1R.[8,9] But the latter study did not give any information on the histopathology features suggestive of T1R in patients who lacked clinical features of the same.[9] Hence, information on whether the pathologist would have been able to diagnose subclinical T1R was not available from that study. Their observation of lack of histology evidence to support the clinical manifestation of T1R in some patients was documented in two of our patients also. This may be because dermal or intra-granuloma edema, disappears as the time interval between onset of reaction and biopsy is delayed.[9] This is supported by our observation of 90-day gap between onset of reaction and skin biopsy in the two cases mentioned, compared to the mean interval of 43.7 days.
Necrosis within granuloma as observed in six cases by us was described by Ridley. He opined that tuberculoid reactions with low bacterial load may manifest fibrinoid necrosis involving collagen and this may proceed to secondary tuberculoid leprosy.[4]
The eleven patients who manifested histopathology evidence of T1R, without any clinical findings to suggest the same could be in the prodromal phase of T1R described by Ridley.[4] Subsequent manifestation of full blown T1R in five of the eleven (45.5%) patients was consistent with the natural evolution of reaction.[4] Lockwood et al. have reported subsequent development of clinical manifestations of T1R in 30/74 (40.5%) patients who had subclinical T1R, histologically.[8]
The non-manifestation of clinically evident T1R in six (54.5%) of the eleven patients, who were in the prodromal phase of reaction in the present study, was in concordance with Ridley's observation that all patients manifesting histopathological features of reaction, need not necessarily progress to full blown T1R.[4]
The six patients who developed T1R while on MDT manifesting the same within first 3 months of treatment in our study was as expected since rapid killing of bacilli by the drugs are known to precipitate sudden change in immunity.[3] The previous study reported subsequent development of T1R throughout the follow up period, but the peak incidence was noted within the first 3 months after a consistent histopathology report.[8]
We suggest that a careful analysis of histopathology specimens from clinically non-reactve lesion of leprosy for features of T1R may help us to identify those at risk for developing future clinical evidence of the same. Close monitoring of these patients may help us to intervene early to prevent complications of reactions.
Limitations
Small sample size and dependence on retrospective study design were the major drawbacks of the study.
Despite these limitations, the present study reiterates the usefulness of histology in predicting T1R.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Rao PN, Suneetha S. Current situation of leprosy in India and its future implications. Indian Dermatol Online J. 2018;9:83–9. doi: 10.4103/idoj.IDOJ_282_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harboe M. Over view of host parasite relations. In: Hastings RC, Opromolla DVA, editors. Leprosy. 2nd ed. Edinburgh: Churchill Livingstone; 1994. pp. 87–112. [Google Scholar]
- 3.Kahawita IP, Walker SL, Lockwood DN. Leprosy type 1 reactions and erythema nodosum leprosum. An Bras Dermatol. 2008;83:75–82. [Google Scholar]
- 4.Ridley DS. Reactions. In: Ridley DS, editor. Pathogenesis of Leprosy and Related Diseases. Vol. 15. UK: Butterworth and Co Publishers Ltd; 1988. pp. 118–34. [Google Scholar]
- 5.Kumar B, Dogra S. Case definition and clinical types of leprosy. In: Kumar B, Kar HK, editors. IAL Textbook of Leprosy. 2nd ed. New Delhi: Jaypee Publishers; 2016. pp. 236–53. [Google Scholar]
- 6.Lockwood DN, Nicholls P, Smith WC, Das L, Barkataki P, Van Brakel W, et al. Comparing the clinical and histological diagnosis of leprosy and leprosy reactions in the INFIR cohort of Indian Patients with multibacillary leprosy. PLoS Negl Trop Dis. 2012;6:e1702. doi: 10.1371/journal.pntd.0001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarita S, Muhammed K, Najeeba R, Rajan GN, Anza K, Binitha MP, et al. Astudy on histological features of lepra reactions in patients attending the dermatology department of the government medical college, Calicut, Kerala, India. Lepr Rev. 2013;84:51–64. [PubMed] [Google Scholar]
- 8.Lockwood DN, Lucas SB, Desikan KV, Ebenezer G, Suneetha S, Nicholls P, et al. The histological diagnosis of leprosy type 1 reactions: Identification of key variables and an analysis of the process of histological diagnosis. J Clin Pathol. 2008;61:595–600. doi: 10.1136/jcp.2007.053389. [DOI] [PubMed] [Google Scholar]
- 9.Ridley DS, Radia KB. The histological course of reaction in borderline leprosy and their outcome. Int J Lepr. 1981;49:383–92. [PubMed] [Google Scholar]
- 10.Sasidharanpillai S, Reena Mariyath OK, Riyaz N, Binitha MP, George B, Janardhanan AK, Haridas N. Changing trends in leprosy among patients attending a tertiary care institution. Indian J Dermatol Venereol Leprol. 2014;80:338–4. doi: 10.4103/0378-6323.136909. [DOI] [PubMed] [Google Scholar]
- 11.Premachandran M, George N, Binitha T, Nandakumar V, Jishna P, Sasidharanpillai S, et al. Are children safe from complications of leprosy? A study from North Kerala. J Skin Sex Transm Dis. In press. [Google Scholar]


