Abstract
Context:
Recently, there has been an increase in the number of chronic, recurrent, and recalcitrant dermatophytosis. Many factors implicated are barrier defects, aberrant host immune response, application of steroids or other irrational combination creams, transmission within family, occlusive clothing, poor hygienic conditions, poor compliance, drug resistance and virulence of the infecting strain. Transepidermal water loss (TEWL) is an important index in accessing the barrier function of skin.
Aim:
To ascertain the role of TEWL from the lesional skin and its effect on the cure rate and relapse in patients of tinea cruris.
Materials and Method:
A hospital based prospective comparative study was conducted for 1 year. A total of 200 patients of tinea cruris diagnosed clinically and by KOH examination, were included in the study. TEWL was calculated using Tewameter TM300 open chamber probe of Courage and Khazaka, Cologne, Germany. Patients were classified according to the TEWL values into Group A (patients with abnormal TEWL) and Group B (normal TEWL). Both groups were given oral itraconazole and antihistamines for 4 weeks. The cure rates and recurrence rates of both the groups were analyzed and compared.
Results:
In the Group A, i.e., patients of tinea cruris with abnormal TEWL, only 28% of the patients showed clinical improvement at the end of 1 month. Out of those cured, 78.57% of the cases showed recurrence after 2 months of completion of therapy. In Group B, i.e., patients of tinea cruris with normal TEWL, 69% (n = 69) of the patients showed clinical improvement at the end of 1 month. Out of those cured, only 21.74% of the cases (n = 15) showed recurrence.
Conclusion:
The cases of tinea cruris with abnormal TEWL show significant decrease in cure rates and significant relapse rates among those initially cured.
Keywords: Recalcitrant tinea infections, skin barrier defect, transepidermal water loss
Introduction
Dermatophytosis is a common infection of the skin, hair or nails, i.e., colonization of keratinized tissue caused by dermatophytes, a group of related filamentous fungi. These infections are caused by species of three genera—Trichophyton, Epidermophyton, and Microsporum. Among all fungal infections, infections caused by the dermatophytes are the most frequent forms of human infections, affecting more than 20%–25% of the world's population.[1]
Based on their natural habitat, dermatophytes are classified into three groups—Geophilic, Zoophilic, and Anthrophilic species.
Anthropophilic species include Trichophyton rubrum, Trichophyton schoenleinii, Trichophyton concentricum, Trichophyton tonsurans, Trichophyton mentagrophytes var. interdigitale, Microsporum gypseum, Microsporum audouinii, Microsporum ferrugineum, and Epidermophyton floccosum. These anthropophilic species are responsible for the majority of human infections.
Superficial fungal infections had always been simple to treat with the basket of antifungal agents available; however, recently, an alarming trend of these dermatosis is being observed, with substantial change in the clinical profile of patients associated with an increase in the number of chronic, recurrent, and recalcitrant dermatophytosis.[2,3,4]
Recalcitrant dermatophytosis refers to relapse, recurrences, reinfection, persistence, or chronic infections, and possibly microbiological resistance.[5]
Dermatophytosis is considered to be recurrent when there is recurrence of the disease (lesions) after 4 weeks of completion of approved systemic therapy.[6]
Relapse denotes the occurrence of dermatophytosis (lesions), after a longer period of infection-free interval (6–8 weeks) in a patient who has been cured clinically.[7]
Dermatophytosis is considered to be chronic when the patients who have suffered from the disease for more than 6 months to 1 year, with or without recurrence, in spite of being adequately treated.[7]
Now-a-days, antifungal resistance is also thought to be an important cause for treatment failure in case of dermatophytosis. Other factors such as barrier defects, remains neglected.
Transepidermal water loss (TEWL) measurement is the most widely used objective measurement for assessing the barrier function of the skin.[8]
TEWL represents the diffusion of condensed water through a fixed area of stratum corneum to the skin surface per unit time[9,10] and is measured in grams/m2/hour.
Our study highlights the role of transepidermal water loss from the lesional skin and its effect on the cure rate and relapse in patients of Tinea cruris.
Materials and Method
This was a hospital based prospective comparative study. A total of 200 patients of tinea cruris, attending the dermatology outpatient department of Sawai Mansingh Hospital, Jaipur, not on any topical or systemic treatment previously were included in the study. A written and informed consent was obtained from each patient. The study was conducted over a period of 1 year.
The cases were diagnosed clinically and by KOH examination. Ethical clearance was taken up for the study.
Tewl Measurement
TEWL was calculated in these patients from the lesional skin over the right inguinal region, according to guidelines developed by 5th International Conference on Occupational and Environmental exposure of skin to chemicals (OEESC) using Tewameter TM300 open chamber probe of Courage and Khazaka, Cologne, Germany.
Transepidermal water loss is calculated by measuring the water vapor pressure (VP) gradient at the skin surface, which is considered constant in the absence of external convection currents. In the open-chamber method, the VP gradient is calculated by measuring the difference in VP between two distinct points aligned perpendicularly to the skin surface. VP is calculated as the product of RH (Relative humidity) and saturated VP, which is dependent on temperature.
Prior to measurement of TEWL and/or skin hydration, the study participant were acclimatized to the measurement environment to avoid errors caused by environmental temperature or sweating.
In accordance to OEESC guidelines, the affected area was left open for 20 minutes prior to the test at relative humidity of 50% and ambient temperature of 22°C.
TEWL was measured in grams/m2/hour. A value of >25 grams/m2/hour was considered as abnormal or critical. These patients were divided into 2 groups on the basis of TEWL values.
Group A included patients of tinea cruris with abnormal TEWL, whereas Group B included age and sex matched patients of Tinea cruris with a normal TEWL. Both the groups were given 200 mg Itraconazole OD + oral antihistamines. Although Itraconazole 200 mg single dose is unapproved formulation by USFDA, but the skin department in our institution follows both the regimens, i.e., Itraconazole 200 mg OD as well as 100 mg BD. Little differences are observed in cure rates after both regimens. Cure was defined on the basis of clinicalobservation, and KOH examination. Culture studies could not be performed.
Cure rate of the two groups was compared 1 month later, whereas, recurrence of the two groups was compared 3 months later.
ŸMedcalc 16.4 version software was used to analyze data presented as proportion.
Chi square test was used for analysis and P value <0.05 was taken as significant.
Results
In the Group A (see [Figure 1]) i.e., patients of tinea cruris with abnormal TEWL, only 28% (n = 28) of the patients showed clinical improvement at the end of 1 month. Out of those cured, 78.57% of the cases (n = 22) showed recurrence after 3 months of completion of therapy.
Figure 1.
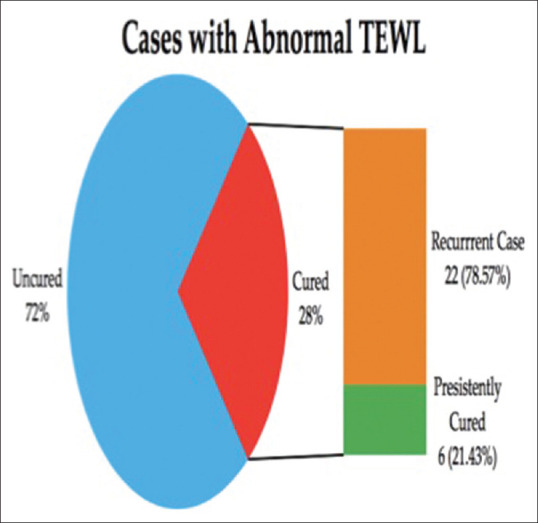
Cure rate and recurrence rate among the cases with abnormal TEWL
In Group B (see [Figure 2]) i.e., patients of tinea cruris with normal TEWL, 69% (n = 69) of the patients showed clinical improvement at the end of 1 month. Out of those cured, only 21.74% of the cases (n = 15) showed recurrence after 3 months of completion of therapy.
Figure 2.
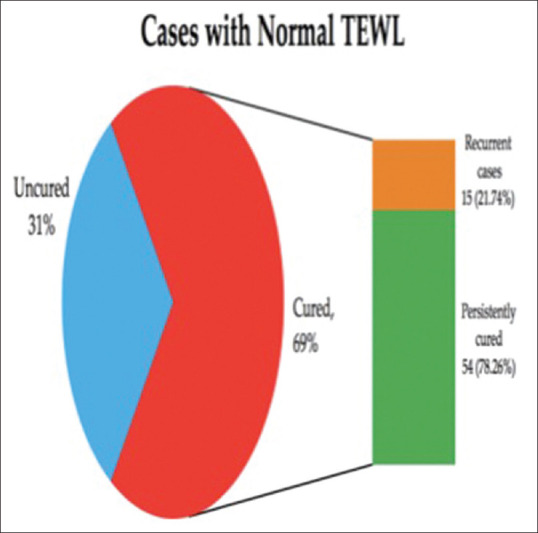
Cure rate and recurrence rate among the cases with normal TEWL
The comparative results of both groups were statistically significant, i.e., P value <0.005.
Therefore, our study concludes that tinea cruris patients with abnormal TEWL have significantly poor cure rate, and significantly higher recurrence rate. This highlights the fact that stratum corneum hydration and TEWL significantly influence the cure rate and recurrence rate of superficial dermatophytosis.
Discussion
In recent years, scenario of dermatophytosis is changing with an alarming rise in the number of difficult to treat chronic and recurrent dermatophytosis in our country. There has been an increasing trend of patients presenting with decreasing cure rates and frequent relapses within a few days to weeks of stopping the treatment with antifungal agents. The cause of such recalcitrant dermatophytosis are multifactorial, listed in Table 1.[6]
Table 1.
Factors implicated in recalcitrant tinea infections
| Inadequate treatment duration with antifungals |
| Treatment with steroid and over the counter formulations |
| Treatment irrational combination agents |
| Local factors—poor hygiene conditions, heat, humidity, occlusive clothing |
| Ping-pong effect—spread of superficial fungal infections within family members |
| Barrier function defects—increased TEWL, decreased stratum corneum hydration |
| Deranged host immune responses. |
| Compliance |
| Microbial factors—virulent species |
| Drug resistance |
A significant but questionable cause of refractory fungal infections is drug resistance.
According to the “90-60 Rule” proposed by Rex and Pfaller, which states that infections caused by fungal isolates that have MICs considered susceptible respond favorably to appropriate therapy approximately 90% of the time, whereas infections caused by the isolates with MICs considered resistant also respond favorably approximately 60% of the time,[11] so factors other than antifungal resistance probably affect the cure rate. A study by Sardana et al. concluded that in vitro resistance to antifungals is not very common and should not be frequently labeled as a cause of treatment failure.[12]
Out of the many causes listed above, the one studied in our study was the role of barrier function defect. The stratum corneum, the main permeability barrier, is formed from extracellular lipids and corneocytes during epidermal differentiation of the skin.[13] The main extracellular stratum corneum lipids are cholesterol, free fatty acids, and ceramides.[14]
The initial step in the dermatophyte infections is stratum corneum penetration and proliferation. A disturbance in barrier function is accompanied or caused by changes in epidermal proliferation and differentiation. The increased epidermal proliferation leads to expression of proliferation associated cytokeratins K6, K16, and K17.[6] Interestingly, filaggrin and involucrin protiens are also downregulated in lesional skin of tinea cruris and therefore these patients have a compromised barrier.
This was supported by a study, where dermatophytoses, except tinea pedis and tinea mannum, showed highly significant increase in TEWL compared with adjacent infection free skin.[15] In the context of our study, either the fungal strain is more invasive resulting into acceleration of the above process and not responding well to treatment, or, the barrier defect may be a primary phenomenon, which needs confirmation by further studies.
It is important to understand that patients with atopic background, have a selective or induced immune deficit for dermatophytic infections in addition to barrier function impairment.[6] We did not segregate our patients according to their atopic background, which is a limitation of our study.
Our study stresses on the fact that barrier defect is an understated cause of recalcitrant superficial fungal infections and therefore should not be neglected. A concomitant or adjuvant application of barrier repair formulations are of great value in both treatment and preventing recurrences of dermatophytosis.
Limitations
There were a few limitations in our study, i.e. we limited our study to tinea cruris only, culture studies for species identification were not done, only itraconazole was used as a prototype antifungal drug in our study and our patients were not segregated according to atopic background.
Conclusion
Our study is an attempt to correlate the barrier function properties of skin with recalcitrant superficial fungal infections. The cases of tinea cruris with abnormal TEWL show significant decrease in cure rates and significant relapse rates among those initially cured.
This finding should urge the researchers to lay focus on factors other than just drug resistance.
Skin barrier dysfunction and increased TEWL is one such factor.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
Dr. Rajeev Yadav, Associate Professor, Department of Preventive and Social Medicine for his help in data analysis.
References
- 1.Havlickova B, Czaika VA, Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses. 2008;51(Suppl 4):2–15. doi: 10.1111/j.1439-0507.2008.01606.x. [DOI] [PubMed] [Google Scholar]
- 2.Panda S, Verma S. The menace of dermatophytosis in India: The evidence that we need. Indian J Dermatol Venereol Leprol. 2017;83:281–84. doi: 10.4103/ijdvl.IJDVL_224_17. [DOI] [PubMed] [Google Scholar]
- 3.Verma S, Madhu R. The great Indian epidemic of superficial dermatophytosis: An appraisal. Indian J Dermatol. 2017;62:227–36. doi: 10.4103/ijd.IJD_206_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dogra S, Uprety S. The menace of chronic and recurrent dermatophytosis in India: Is the problem deeper than we perceive? Indian Dermatol Online J. 2016;7:73–6. doi: 10.4103/2229-5178.178100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahajan K, Sharma S, Sardana K, Gupta A. Superficial fungal infections. In: Sardana K, Mahajan K, Mrig PA, editors. Fungal Infections: Diagnosis and Treatment. 1st ed. New Delhi: CBS; 2017. pp. 52–178. [Google Scholar]
- 6.Sardana K. Overview of causes and treatment of Recalcitrant Dermatophytoses. In: Khurana A, editor. IADVL Manual on Management of Dermatophytoses. 1st ed. New Delhi: CBS Publishers; 2018. pp. 80–104. [Google Scholar]
- 7.Rajagopalan M, Inamadar A, Mittal A, Miskeen AK, Srinivas CR, Sardana K, et al. Expert consensus on the management of dermatophytosis in India. BMC Dermatol. 2018;18:6. doi: 10.1186/s12895-018-0073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fluhr JW, Feingold KR, Elias PM. Transepidermal water loss reflects permeability barrier status: Validation in human and rodent in vivo and ex vivi models. Exp Dermatol. 2006;15:483–92. doi: 10.1111/j.1600-0625.2006.00437.x. [DOI] [PubMed] [Google Scholar]
- 9.Rogiers V. EEMCO guidance for the assessment of transepidermal water loss in cosmetic sciences. Skin Pharmacol Appl Skin Physiol. 2001;14:117–28. doi: 10.1159/000056341. [DOI] [PubMed] [Google Scholar]
- 10.Imhof RE, De Jesus ME, Xiao P, Ciortea LI, Berg EP. Closed-chamber transepidermal water loss measurement: Microclimate, calibration and performance. Int J Cosmet Sci. 2009;31:97–118. doi: 10.1111/j.1468-2494.2008.00476.x. [DOI] [PubMed] [Google Scholar]
- 11.Rex JH, Pfaller MA. Has antifungal susceptibility testing come of age? Clin Infect Dis. 2002;35:982–9. doi: 10.1086/342384. [DOI] [PubMed] [Google Scholar]
- 12.Sardana K, Kaur R, Arora P, Goyal R, Ghunawat S. Is antifungal resistance a cause for treatment failure in dermatophytosis: A study focused on tinea corporis and cruris from a tertiary center? Indian Dermatol Online J. 2018;9:90–5. doi: 10.4103/idoj.IDOJ_137_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elias PM. Epidermal lipids, barrier function and desquamation. J Invest Dermatol. 1983;80:44–7. doi: 10.1038/jid.1983.12. [DOI] [PubMed] [Google Scholar]
- 14.Lampe MA, Burlingame AL, Whitney J, Williams ML, Brown BE, Roitman E, et al. Human stratum corneum lipids: Characterization and regional variations. J Lipid Res. 1983;24:120–30. [PubMed] [Google Scholar]
- 15.Lee WJ, Kim Song CH, Jung HD, Lee SH, Lee SJ, Kim do W. Disrution of barrier function in dermatophytosis and pityriasis versicolor. J Dermatol. 2011;38:1049–53. doi: 10.1111/j.1346-8138.2011.01320.x. [DOI] [PubMed] [Google Scholar]


