The law on human genomics in the United States is currently in transition and under debate. The rapid evolution of the science, burgeoning clinical research, and growing clinical application pose serious challenges for federal and state law. Newer genomic assays, such as gene panels, whole exome and whole genome sequencing that can assess many or all of a patient’s genetic variants are different from former genetic assays that consist of assessing for variants in specific genes that indicate risk for medical conditions and for single-gene Mendelian disorders. Overall, single-gene genetic testing has primarily been used for risk prediction, disease diagnosis, and assessing carrier status, whereas genomic analysis is enabling additional functions, such as disease prognosis and treatment selection. However, these advances in functionality do not come without potential consequences. A pending lawsuit in South Carolina, for example, raises the question of whether genomics laboratories and clinicians are potentially liable for interpreting a genomic variant as a “variant of uncertain significance” (VUS) rather than a “likely pathogenic” variant, given the differences in clinical implications between the two variant classifications, and for failure to recontact the patient with an interpretive update as understanding evolved.1 Another lawsuit pending in Alaska raises the question of whether a direct-to-consumer (DTC) genetics genealogy service is liable for publicly sharing a customer’s genetic information.2
While the federal Food and Drug Administration (FDA) has issued draft guidance on how it plans to regulate next-generation sequencing involved in genomic analysis, it has openly sought advice on regulatory approaches to large-scale genomic sequencing, given the quantity of data generated and enormity of the regulatory challenge.3 The federal Centers for Medicare & Medicaid Services (CMS), which regulates laboratory quality under the Clinical Laboratory Improvement Amendments (CLIA), has similarly struggled to keep up with advancing genomic technology, leading to controversy over return of research results from laboratories lacking CLIA-certification, among other issues.4 CMS also plays a major role in deciding what genomic tests will be reimbursed.
In the face of these questions about the adequacy of federal and state law and the obvious need for greater clarity, the National Human Genome Research Institute (NHGRI) and National Cancer Institute (NCI) at the National Institutes of Health (NIH) funded a 3-year project to conduct empirical research as well as legal and policy analysis in order to map the current law of genomics in the United States, identify areas in need of revision and clarification, and propose solutions. This project, led collaboratively by principal investigators at the University of Minnesota and Vanderbilt University and Medical Center, convened a national Working Group (WG) of legal, scientific, and clinical experts to guide the project and generate consensus recommendations. Work has focused on four large domains of law: liability of genomics researchers, clinicians, laboratories, and institutions; quality of genomic analysis and interpretation; privacy of genomic data and interpretation as well as who has access to both; and the question of what legal frameworks govern blended areas of genomic activity, such as translational genomic research straddling research and clinical care.
In order to guide the project’s effort to generate recommendations for the law governing genomic medicine, the project undertook empirical research to ascertain what key scientific and legal stakeholders regard as the important legal issues and potential solutions that should be addressed to support successful translation of human genomics into clinical practice. A prior study interviewed leaders of genomic sequencing companies and laboratories to elicit their recommendations for policy development.5 Other investigators have elicited the opinions of patients and research participants about topics ranging from consent and privacy to return of results,6 as well as examining what scientists, clinicians, and other experts involved in advancing medical genomics see as the issues arising in research and translation.7 There are also articles that describe stakeholder perspectives and attitudes toward pharmacogenomic testing as well as concerns and usability of the resulting pharmacogenomic reports.8 However, we have found no studies beyond our own that focus on what stakeholders who are involved in genomics as professionals see as key legal concerns and potential solutions.
Empirical work can also inform theoretical analyses of how law should govern rapidly evolving technology. A substantial literature addresses the frequent gap between advancing technology and law, including genomics.9 Similarly, scholars have analyzed judicial decisions, concluding that keeping up with science to appropriately adjudicate litigated cases challenges both lawyers and judges. These challenges led Marchant and Lindor to argue that courts may be increasingly tempted to second-guess genomics clinicians in retrospectively deciding if clinicians have failed to meet the applicable standard of care.10 However, neither the theoretical literature on how law should govern evolving technology nor the analyses of case law have systematically integrated empirical assessment of what key stakeholders see as the most pressing issues and potential solutions. This article presents the results of surveys and interviews of key scientific and legal stakeholders in the field of genomics to help ground identification of the most important legal problems that must be solved to successfully integrate genomics into clinical care.
Methods
Stakeholder opinions were gathered through a mixed-methods approach using an online survey with fixed-response and open-response items as well as in-depth interviews. This approach allowed examination of stakeholder opinions by gathering data from the survey respondents and collection of more nuanced information from the interviewees.11 Survey respondent and interviewee selection as well as analysis techniques are described below. All survey and interview procedures were approved by the University of Minnesota IRB (#1603S85102) and Vanderbilt University Medical Center IRB (#170760).
Survey Development and Administration
The survey was developed based on a modified Delphi process within the Working Group. By utilizing this process, we were able to gather consensus opinions regarding key legal concerns within the WG, engage in detailed examinations and discussion of such concerns, and propose possible solutions that attempt to resolve said concerns.12 The Delphi process began with the WG members responding to open- and fixed-response items brainstormed by the PI team. Those responses were analyzed and developed into a second wave of Delphi questions answered by the WG members. This second survey served as a pilot test of the survey for use outside the WG. The final survey incorporated feedback and data generated by the second wave as well as the expert knowledge within the WG.
The final survey had six parts: (1) general questions about respondents and their work, (2) questions about liability in genomics, (3) questions about the law addressing the quality of genomic analysis and interpretation, (4) questions about the law on privacy and access to data and results, (5) questions about the framework question of when research vs. clinical rules apply, and (6) concluding questions. A copy of a generic version of the survey is posted at https://consortium.umn.edu/lawseq-empirical-data. We invited prospective participants to complete the Qualtrics online survey with both fixed-response and open-response items. All responses were completed anonymously. Fixed-response items were measured on a 5-point Likert scale: 1 (Not important at all), 2 (Slightly important), 3 (Moderately important), 4 (Very important), and 5 (Extremely important). There were no “don’t know” or “not applicable” options, but answering each item was not required. The survey contained 9 open-response items. Two open-response items at the end of each of the four sections (liability, quality, privacy and access, and framework) were used to invite identification of additional legal issues and potential solutions. One final open-response item at the end of the survey asked respondents to name any remaining legal issues thought to be important. The survey had a total of 55 questions and took approximately 20 minutes to complete.
Survey links were distributed by email between April 2017 and February 2018 to different groups to help provide diversity of opinion. The survey collected input from 5 groups of respondents: genomics researchers, genomics clinicians, institutional lawyers, additional lawyers, and industry representatives (Table 1). These are individuals with relevant expertise and perspectives on the law of genomics. We thus elicited a broad range of relevant perspectives on the legal issues, sub-issues, and possible solutions.
Table 1.
Survey groups and contact methods.
| Survey Group Number | Survey Recipients | Contact Method |
|---|---|---|
|
Survey Group 1: NIH Principal Investigators (PIs) & ACMG Key Individuals |
PIs from Electronic Medical Records and Genomics Network (eMERGE), Clinical Sequencing Evidence-Generating Research Consortium (CSER), Pharmacogenomics Research Network (PGRN), Newborn Sequencing in Genomic Medicine and Public Health (NSIGHT) Program, and Implementing Genomics in Practice (IGNITE) Consortium; American College of Medical Genetics and Genomics (ACMG) Board of Directors & ACMG Social, Ethical and Legal Issues (ELSI) Committee | Used the NHGRI website to identify PIs participating in key NIH-funded consortia in translational genomics and used the ACMG website to identify members of the ACMG Board of Directors and the Social, Ethical and Legal Issues Committee. Direct invitations to NIH PIs (65 invitations) and ACMG members (35 invitations) from a project PI (40 responses). |
| Survey Group 2: Institutional Legal Counsel | Legal counsel from Group 1 PIs’ institutions | A list of legal counsel with probable connections to genomics law (i.e., specialty in healthcare and/or genomics) working at each of the institutions with NIH PIs in survey group I was generated using each respective institution’s website. Individual invitations were sent by a project PI to legal counsel with a specialty in healthcare and/or genomics (22 invitations) (3 responses). |
| Survey Group 3: Additional Legal Counsel | American Health Lawyers Association (AHLA) Academic Medical Center Practice Group and National Association of College and University Attorneys (NACUA) | An invitation to participate was posted to the listserv by an existing member. (unknown number of individuals invited) (10 responses). |
| Survey Group 4: Industry Representatives | Biotechnology Industry Organization (BIO) Emerging Companies Section Governing Board (ECSGB), BIO Health Section Governing Board (ECSGB), and Industry Pharmacogenomics Working Group (I-PWWG) | An invitation to participate was posted to the I-PWWG list serve by an existing member and individual invitations were sent to the BIO governing board members. (108 invitations to BIO & unknown number of individuals invited through I-PWWG listserv) (11 responses). |
| Survey Group 5: Two Additional Genomics-related Professional Organizations | National Society of Genetic Counselors (NSGC), American Society of Human Genetics (ASHG) Board of Directors & ASHG Social Issues Committee | An invitation to participate was posted to the NSGC’s listserv by a project PI and a project PI emailed individual invitations to members of the ASHG members (17 invitations) (31 responses). |
Survey Fixed-response Items Analysis
Fixed-response survey items were analyzed by calculating means and standard deviations for each item.
Survey Open-response Items Analysis
Open-response items were analyzed using qualitative techniques that examined concepts and relationships through an inductive, iterative process that identified emerging themes.13 The responses were broken down into meaningful segments; these could be a phrase, a sentence, or several sentences. Each segment was analyzed several times to determine the essence of its meaning. These meanings were then grouped into codes and the codes grouped into conceptual themes.14 If appropriate, segments were included in more than one theme. These themes were developed across questions and respondents, so they were more conceptually oriented than simply listing answers to each question. This means that conceptually similar responses were grouped together regardless of what survey item the response was linked to.
The full research team included a bioethicist lawyer, a lawyer and physician who is also a bioethicist, a genetic counseling graduate student, a law student who is also a doctoral candidate in psychology, and a social science methodologist. Two researchers (F.Y.C. and L.C.) independently analyzed all the responses and coded for emerging themes. They met regularly with a principal investigator (F.L.) to review emergent themes and to ensure consistency. These three researchers (F.Y.C., L.C., and F.L.) discussed the different coding and thematic patterns. These initial themes were then recoded based on similarities and associations and grouped into a potential final thematic structure in a hierarchical fashion with overarching categories, then themes and sub-themes. This structure was then shared with the additional members of the team (E.W.C. and S.M.W.) for feedback and finalized.
Interview Development and Administration
In addition to the survey, we conducted semi-structured interviews.15 Interview participants were invited either because they voluntarily agreed to be interviewed in response to the survey (by identifying themselves and providing their contact information) or were purposefully invited because they were thought to be knowledgeable about the legal issues surrounding genomics. Interviews were conducted between January 2018 and April 2018. Interview length was approximately 30 minutes each. A copy of the interview protocol is posted at https://consortium.umn.edu/lawseq-empirical-data. Non-governmental interviewees were offered a $20 gift card as an incentive for agreeing to and completing the interview.
Potential interviewees were drawn from the following three groups: legal counsel of institutions with NIH-funded principal investigators in genomics research, federal genomics authorities, and state genomics authorities. The legal counsel were selected randomly from the list of legal counsel described in Group 2 of Table 1. A list of recognized federal experts at NIH, CDC, and CMS was developed from existing public lists and feedback from E.W.C., S.M.W., and knowledgeable project members. The state authorities were selected from state public health websites and NBS programs covering six states (NY, CA, TX, MD, TN and MN). The states represented were chosen for geographical diversity and a range of approaches to laboratory regulation (with NY having the most detailed state scheme) from among those states where major state or NIH genomics projects were in operation. No respondent who had identified themselves on the survey was interviewed. It is also not known if any of the interviewees had also completed the survey because the survey was anonymous.
Two of the PIs, E.W.C. and S.M.W, contacted potential participants by email. The email provided a basic overview of the project and asked whether they would be willing to be interviewed. In cases of nonresponse, receipt of the email was verified by a follow-up phone call. Participants who agreed to be interviewed were then sent an e-mail invitation to arrange for an interview time and provide the consent form. E.W.C and S.M.W conducted the telephone interviews using a semi-structured interview guide. The guide was developed by the three PIs to provide information complementary to the survey items but also to allow the interviewees to express their own opinions. All interviews were audio-recorded with permission and transcribed verbatim. The recordings were then erased. All identifiable information was removed prior to data analysis.
Interview Analysis
The interview results were subsequently analyzed using the qualitative techniques described above for the open-ended survey responses.16 Specifically, all the responses were organized into meaningful segments and reviewed by two researchers (F.L. and F.Y.C.). Each researcher coded the responses based on what was said and grouped the responses into the conceptual themes that were identified. The researchers then met to discuss the different coding and thematic patterns and resolve any discrepancies. This resulted in a potential final thematic structure into which all responses were organized which was then shared with the rest of the team for feedback (E.W.C. and S.M.W.).
Combination of Survey Open-response Items and Interviews
As documented above, the analyses of both the open-response survey items and the interview results were conducted independently and in different time frames. To further characterize both the open-response survey item and interview results, two researchers (F.L and F.Y.C) combined the results from the interviews and the open-response survey items. This was accomplished by comparing the categories, themes, underlying data segments, and relationships from each source of data.
Results
Survey Respondent Demographics
A total of 95 survey responses were received. Of the 95 respondents, 56 were females (58%) and 38 were males (40%) with one participant choosing not to disclose their gender. The average respondent age was 48, with a range of 42 to 55. Respondents’ average years of experience in their current role was 14 years, with a range of 9-19. The percentage of work involving genetics or genomics varied dramatically between groups. Group 2 (Institution Legal Counsel) had the lowest average percentage at 25%, while the average for Group 5 (Two Additional Genomics-Related Professional Organizations) was 90%. The response rates for 3 of the groups could not be calculated because invitations were sent to listservs with unknown numbers of people. Of the two groups that could be calculated, Groups 1 and 2 had response rates of 40% and 13.6%. Responses from Group 1 (NIH PIs & ACMG Key Individuals) and Group 5 (Two additional genomics-related Professional Organizations) responses made up a majority of the responses received (74%).
Fixed-response Survey Items Results
The data from all five groups were combined for the analyses. In Table 2, the three highest Mean Scores across all categories are bolded. The three lowest Mean Scores are italicized.
Table 2.
Fixed-response survey item scores (n=95).
| Mean Score (1-5) |
Standard Deviation |
|
|---|---|---|
| LIABILITY | ||
| Q1: How important do you think it is to clarify and improve the law surrounding liability issues in genomics research and clinical care? | 3.83 | 0.91 |
| How important do you think it is to address the following issues? | ||
| Q2: Failure to create appropriate informed consent procedures and documents | 3.73 | 1.01 |
| Q3: Negligent performance of genomic analysis | 3.84 | 0.97 |
| Q4: Negligent interpretation of results | 4.20 | 0.91 |
| Q5: Failure to inform research participants or patients about primary, secondary, or incidental findings | 3.81 | 0.92 |
| Q6: Establishing the standard of care for clinical use of genomics in assessing risk, in diagnosing, and guiding prescribing and other treatment | 4.10 | 0.75 |
| Q7: Defining the duty to re-interpret data due to changes in genomic knowledge | 3.88 | 0.90 |
| Q8: Hospital or other organizational failure to adopt procedures, acquire equipment, or hire personnel for genomic analysis and integration into clinical care | 3.32 | 1.12 |
| Q9: Health insurer/payer failure to pay for genomic testing | 4.02 | 0.99 |
| QUALITY | ||
| Q1: How important do you think it is to clarify and improve the law surrounding quality issues? | 3.82 | 0.97 |
| How important do you think it is to address the following issues? | ||
| Q2: Ensuring adequate validity and reliability of results | 4.08 | 0.90 |
| Q3: Ensuring consistency of interpretation and results across laboratories | 4.04 | 0.93 |
| Q4: Determining when use of a CLIA-certified laboratory is needed | 3.74 | 1.12 |
| Q5: Ensuring adequate quality in biospecimen repositories | 3.48 | 1.09 |
| Q6: Ensuring adequate quality in data archives | 3.54 | 1.01 |
| Q7: Determining the appropriate role of regulatory agencies such as the FDA, CDC, CMS, and NIST |
3.96 | 0.86 |
| Q8: Determining the appropriate role of and standards from professional societies such as CAP, AMP, ACMG, ASHG, and NSGC | 3.78 | 0.95 |
| Q9: Harmonizing international standards | 3.24 | 0.92 |
| PRIVACY AND ACCESS | ||
| Q1: How important do you think it is to clarify and improve the law surrounding privacy and access issues? | 4.01 | 0.95 |
| How important do you think it is to address the following issues? | ||
| Q2: Determining who should have access to raw genomic data (e.g., patient or research participant, family, clinicians, others) | 3.90 | 0.99 |
| Q3: Determining who should have access to interpreted genomic data | 4.01 | 0.94 |
| Q4: Defining how much control individuals should have over how data about them is used--in research, quality control, or public health | 3.81 | 0.96 |
| Q5: Determining how to handle access to genomic data and results after the patient’s death | 3.63 | 0.98 |
| Q6: Determining data sharing rules and practices | 4.05 | 0.89 |
| Q7: Clarifying the law related to using de-identified data and biospecimens in research | 3.65 | 1.06 |
| Q8: Determining how to prevent and penalize re-identification | 3.63 | 1.10 |
| Q9: Addressing employer access to genomic results | 4.09 | 1.00 |
| Q10: Addressing insurer access to genomic results | 4.18 | 0.98 |
| Q11: Controlling potential use of genomic results in other contexts (e.g., to determine parentage, in forensic contexts, in adoption) | 3.63 | 1.11 |
| FRAMEWORK | ||
| Q1: How important do you think it is to clarify and improve the law surrounding the framework question of when clinical rules vs. research rules apply? | 3.91 | 0.93 |
| How important do you think it is to address the following issues? | ||
| Q2: Determining when the laws and norms governing human subjects research should apply in genomics | 3.62 | 1.01 |
| Q3: Determining when laws and norms governing clinical care should apply in genomics (e.g., malpractice liability, robust duty of clinical care) | 3.83 | 1.03 |
| Q4: Determining when genomics researchers have duties to offer return of primary, secondary, or incidental findings | 3.96 | 0.96 |
| Q5: Determining what laws and norms should apply to research with de-identified data or specimens that were not collected for the research | 3.61 | 1.08 |
| Q6: Developing appropriate approaches for translational genomics that combine research and clinical care | 3.97 | 1.01 |
| Q7: Determining when genomics researchers need to seek an investigational device exemption from the FDA | 3.53 | 1.08 |
| Q8: Clarifying the law governing genomic research by private companies | 3.45 | 0.98 |
| Q9: Determining what laws and norms should apply to companies offering direct-to-consumer (DTC) genomic testing services and research | 3.92 | 1.06 |
| Q10: Determining what law applies when research crosses states or countries | 3.36 | 1.15 |
Note:The 3 highest Mean Scores across all categories are bolded. The 3 lowest Mean Scores are italicized.
The three overall highest scoring items across all categories were:
Liability Q4: Negligent interpretation of results (M=4.20, SD=0.91),
Privacy Q10: Addressing insurer access to genomic results (M=4.18, SD=0.98), and
Liability Q6: Establishing the standard of care for clinical use of genomics in assessing risk, in diagnosing, and guiding prescribing and other treatment (M=4.10, SD=0.75).
The three overall lowest scoring items across all of the groups were:
Quality Q9: Harmonizing international standards (M=3.24, SD=0.92),
Liability Q8: Hospital or other organizational failure to adopt procedures, acquire equipment, or hire personnel for genomic analysis and integration into clinical care (M=3.32, SD=1.12), and
Framework Q10: Determining what law applies when research crosses states or countries (M=3.36, SD=1.15).
Open-response Survey Item Results
As described in the methods section, the open-response survey items were analyzed using qualitative techniques which resulted in the development of themes within the data. These themes are presented in Table 3 and will be discussed in more detail in the Combined Open-response Survey Items and Interview Results section.
Table 3.
Comparison of open-ended survey themes and interview themes with final merged themes.
| Overarching Categories |
Survey Themes | Interview Themes | Final Merged Themes |
|---|---|---|---|
| Relationship of Law to Research and Clinical Care |
|
|
|
| Sources and Uses of Genomic Data Outside the Health Care Setting |
|
|
|
| Emerging Field |
|
|
|
| Genomic Data |
|
|
|
| Educational Needs |
|
|
|
Interviewee Demographics
In total, 11 interviews were conducted with 4 participants from Group 1 (Legal counsel of institutions with NIH-funded principal investigators), 4 participants from Group 2 (federal genomics authorities), and 3 participants from Group 3 (state genomics authorities). Seven of the 11 (64%) interviewees were female and 4 of the 11 (36%) interviewees were male.
Interview Results
As described in the Methods section, the interview data were inductively analyzed into themes which are presented in Table 3. These themes are discussed in more detail in the next section.
Combined Open-response Survey Items and Interviews Results
Based on our coding and analysis of the two different sources of qualitative data, we further synthesized our findings to identify potential relationships among the themes. Examination of the open-response survey items and interview results after the independent analyses revealed that the categories, themes, data, and relationships from the two sources were very similar. Therefore, after discussion and careful consideration of all the data, it was possible to combine the results from both data-gathering efforts into a unified set of categories, themes, and relationships with each overarching category made up of themes and subthemes. To accomplish the combination of the two different sets of results, themes from the survey and interviews that were the same were combined (e.g., quotes relating to law encroaching on medicine from each were combined), additional unique themes from either data set were added and items that could be coded in more than one place were used to identify relationships.
The consolidation of the results from the open-response survey items and the interviews is presented in Table 3, which shows the complete list of categories and themes from both the open-ended survey items and the interviews, as well as how they were merged into final themes.
All of the categories, themes and relationships were organized into a single concept map using Inspiration 9 (Inspiration Software, Inc.). This concept-mapping tool allowed us to visually present the categories, themes, and relationships identified through the combination of the interview and open-response survey item data. Figure 1 presents the concept map of the five categories appearing in the comments from the open-ended survey items and the interviews. On the concept map, overarching categories are denoted by rectangles whereas themes are denoted by ovals. Sizes of rectangles and ovals are approximately proportional to the number of times they were each mentioned by respondents in either interviews or surveys as shown by the numbers in parentheses. There is no way to tell if any of the interviewees completed a survey because the survey was anonymous, but it seems unlikely because the groups targeted in the two different data collection efforts are different except for interview group one. Also no one who self-identified in the survey as willing to be interviewed actually ended up being interviewed. Themes are connected to their respective categories by straight arrows and share the same shading/color as other themes under the same category. (Note that the length of arrows has no assigned meaning as their lengths were adjusted to fit the space.) Sub-themes, not mapped in Figure 1, are hexagons and can be seen in Figures 2-4. The concept maps in Figures 2-4 do not show links between themes across different categories because generally only one category is displayed at a time; these connections are presented in the following sections which describe each category.
Figure 1.
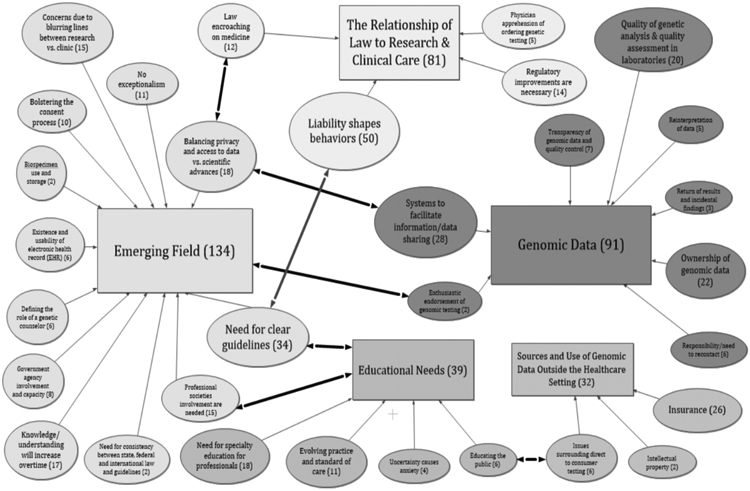
Merged mind map, post-interview. (Overarching categories are depicted as rectangles, themes are ovals, and sub-themes are hexagons.)
Figure 2.
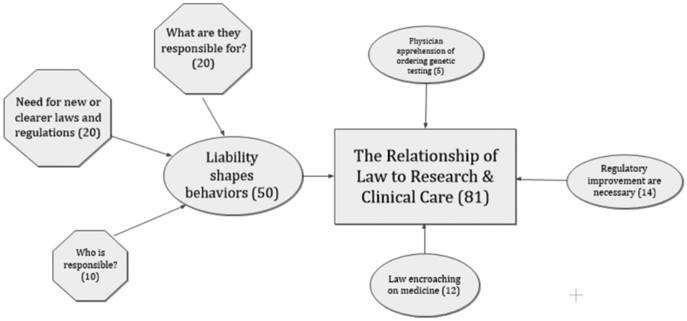
Category of “Relationship of Law to Research & Clinical Care” with themes and sub-themes. (The overarching category is depicted as a rectangle, themes are ovals, and sub-themes are hexagons.)
Figure 4.
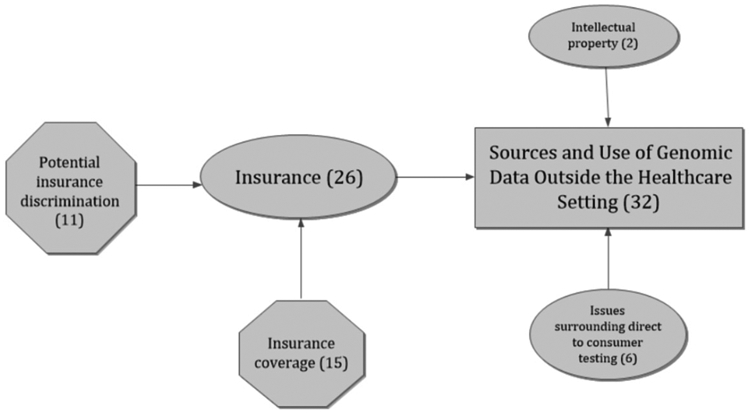
Concept map of themes and sub-themes in the categories of Sources and Use of Genomic Data Outside the Healthcare Setting. (The overarching category is depicted as a rectangle, themes are ovals, and sub-themes are hexagons.)
Three of the five categories could be considered as a related cluster because of the relationships among them, as demonstrated by participant comments that fit in more than one category. These relationships are shown in Figure I by lines linking the following three categories: Emerging Field, Educational Needs, and Genomic Data. For example, comments on the “Quality of genetic analysis” (Genomic Data) are related to, and perhaps even dependent on, what is known about the field (Emerging Field). This is because knowledge about genetics and genomics itself shapes what is considered high or low quality analysis. Additionally, the need for more education and knowledge dissemination (Educational Needs) acknowledges the potential fluidity in what analysis is considered high (vs. low) quality by different practitioners. Moreover, need for education in a field suggests that it’s still emerging. These three categories also had the highest volume of comments from participants.
A second set of comments, which is more distinct and independent from comments in other categories than the three just discussed, fits into the Sources and Use of Genomic Data Outside the Healthcare Setting category. This category highlights the idea of how individuals participate in genomics based on their unique contexts. For example, this category involves questions of how to pay for testing or what the consequences of the testing might be in terms of the results being uniquely meaningful for individuals. The fifth category, Relationship of Law to Research & Clinical Care, has fewer links to other categories, suggesting that considerations under this category are more independent.
RELATIONSHIP OF LAW TO RESEARCH & CLINICAL CARE
In the Relationship of Law to Research & Clinical Care category (Figure 2), an overriding consideration is the idea that liability shapes how clinicians respond in a given situation. Concerns about the risk of liability that would affect how clinicians respond would include consideration of who is responsible for the various aspects of genomics information and procedures and what aspects of these each person is responsible for. This is coupled with a perceived need for new and clearer laws and regulations about genomics, similar to the theme of “Need for clear guidelines” in Emerging Field. Various comments regarding the need for regulation focused on the need to improve rules.
“A big question is where the liability lies - the clinician who orders the test, the laboratory that performs the test and reports a result, the clinician who returns the result to a patient, any clinician who takes part in the care of the patient who has a genetic/genomic result, the healthcare system that holds genomic data in either interpreted/accessible formats or uninterpreted repositories, etc.”
– Survey respondent #4
“If you send your genetic information off to a company that then holds it either as a sample to look at later and you sign consent for that or you sequence it and they hold the results and then subsequently some results are found to be significant, what is the obligation of that company to say we know that you have a risk of lung cancer or brain tumor or whatever?”
– Interviewee #10
Two other themes are related to the intersection of law and medicine. These suggest that rules and regulations are encroaching on the practice of medicine and that physicians may be reluctant to order genomic testing because of liability concerns.
“Trying to define too much by a ‘legal’ standard may not be appropriate and is at risk for overstepping to regulate the practice of medicine.”
– Survey respondent #33
“[There is a need for] mitigating fear within the physician realm to integrate genomics into clinical practice because of liability concerns.”
– Survey respondent #54
“I think a key factor in limiting the use of genetics once it becomes logistically possible and cost-effective to do at scale will be the concern by physicians about potential malpractice cases involving genetic information.”
– Survey respondent #64
EMERGING FIELD
The highest number of respondent comments were aligned with the Emerging Field category, reflecting concerns that genomics is an Emerging Field with an attendant lack of clear understanding and prior experience that might serve as guides for the future (Figure 3). Participants mostly expressed the need for clear guidelines to follow for various situations. They were also concerned about the distinction (or lack thereof) between research and clinical care, and balancing privacy with access to genomic data and scientific advances. Clear guidelines were seen as necessary for moving forward, and it was felt the development of guidelines should include consensus building, consideration of best practices, use of governmental guidelines, and establishing consistency among guidelines. There was also concern that government was underprepared for this task because of the lack of sufficient resources. The quotes below illustrate these ideas.
Figure 3.
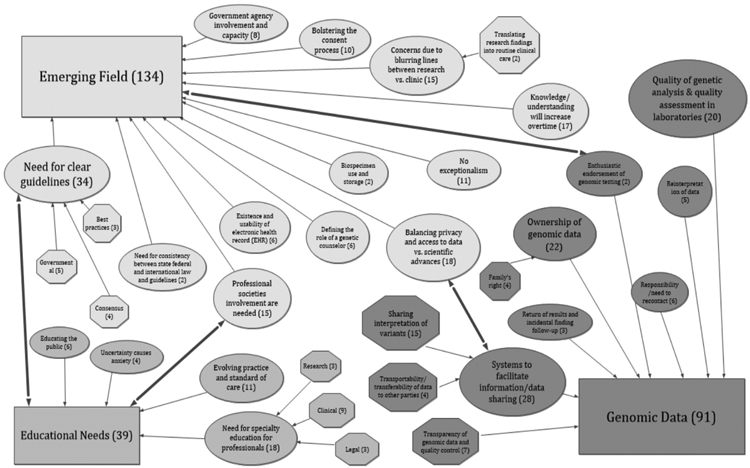
Concept map of themes and sub-themes in the three categories of Emerging Field, Genomic Data, and Educational Needs. (The overarching categories are depicted as rectangles, themes are ovals and sub-themes are hexagons.)
“Developing the standards and criteria for when they [standards of care] need to [be] applied would be a good first step.”
– Survey respondent #21
“The dichotomous view (research vs. care) is somewhat problematic… .Moreover, this dichotomous approach often lulls people into a false sense that research is riskier than care.”
– Survey respondent #66
“Current considerations are very skewed towards traditional Mendelian genetics applications, and those applications are likely to become the least common use of genetic information as genetic data becomes more widely available and we seek and learn more ways to integrate genetics into every facet of medical care.”
– Survey respondent #64
“I think all the agencies appear to be understaffed, under stress and under the current federal regime, I think some of the agencies are just woefully understaffed at this point.”
– Interviewee #1
Participants suggested that professional societies need to be more involved in determining what should be done. They also commented that genomics is no different from other new scientific fields and should not be treated as a special case. They noted that current procedures, practices, and record keeping will inevitably change as our knowledge increases over time.
“Professional society guidance for practitioners and consumers” [is needed].
– Survey respondent #30
“We need to stop treating genetics as a special case.”
– Survey respondent #77
GENOMIC DATA
The category of Genomic Data had several themes (Figure 3). Themes included comments about the need for data sharing and determining how to protect privacy. These themes also included “Ownership of genomic data” and the “Responsibility/Need to recontact” when newer information becomes available, which were related to other themes such as liability. Concerns over quality of the data and its analysis, including reinterpretation, were also prominent in the comments.
“The best thing we can do is to incentivize participation in data sharing (!!!) and provide good resources for unbiased information.”
– Survey respondent #47
“[It needs to be determined] …how organizations share genetic data, the data that has to go with someone’s sequence, the laws don’t quite extend to sharing data to make the research useful.”
– Interviewee #2
“I’m interested to see whether we still treat genomic data as de-identified.... I think it will be considered identifiable at some point soon and we’ll have to treat it differently than we do right now.”
– Interviewee #9
“People should be empowered to take charge of their own data. Moving genomics away from historically paternalistic medical practices, and into a world where people can ask questions and bring their data to new physicians or genetic counselors, would go a long way here.”
– Survey respondent #58
“I think as WES [whole exome sequencing] and WGS [whole genome sequencing] become more and more common, issues of incidental findings will become part of every-day clinical experience rather than isolated to research studies. There needs to be standards across the board of informing patients of incidental findings.”
– Survey respondent #45
“There is the need for support of collaborative efforts like databases for interpretation particularly but also for analytical standards for tests.”
– Interviewee #7
EDUCATIONAL NEEDS
The category of Educational Needs complements Emerging Field in that it highlights that the field hasn’t yet come to grips with the lack of knowledge about genomics (Figure 3). There are strong crosslinks with Emerging Field, in terms of comments that could fit into both the need for professional society involvement and the need for clear guidelines themes included in Emerging Field.
The comments show there is a perceived need for more specialty-related education in legal, clinical, and research areas. However, an evolving standard of care makes education challenging. There were comments indicating that uncertainty could produce anxiety. There was also a perceived need to educate the public, with comments that also fit into the Sources and Use of Genomic Data Outside the Healthcare Setting category, indicating linkages with that category.
“Education of insurer/payer [is important]. Genomic testing can help transform clinical care from an end-of-life treatment to preventive care and precision care, reducing the cost of clinical care while increasing the customer satisfaction.”
– Survey respondent #94
“Education at all levels should be a bigger priority… the education of state and federal legislatures, lawyers, those responsible for regulation in the space of medical tests, devices, and the bodies governing licensing and credentialing of experts. It will be hard even for the best experts to make good decisions in the absence of a better understanding of the issues.”
– Survey respondent #64
“It is so difficult because the landscape is always changing. It may be important to figure out what threshold and measure we are going to use to set standard of care when there is inadequate evidence which is common in emerging areas.”
– Survey respondent #49
SOURCES AND USE OF GENOMIC DATA OUTSIDE THE HEALTHCARE SETTING
The final category of Sources and Use of Genomic Data Outside the Healthcare Setting includes responses that are about differences in the contexts for individuals being tested (Figure 4). The category is meant to indicate that people exist in unique contexts and therefore issues may be particular to each individual. For example, the type of insurance each person has may affect how s/he responds to genomics issues. Many respondents mentioned insurance, addressing insurance coverage and possible genetic discrimination in a variety of ways. Another component of Sources and Use of Genomic Data Outside the Healthcare Setting is direct-to-consumer (DTC) testing, which, as mentioned above, also elicited comments that were related to education of the public about genomic testing. A final theme is about how to manage intellectual property concerns related to genetic testing.
“One of the most important issues is creating some sort of safe haven for freedom from genetic discrimination that people can trust.”
– Survey respondent #2
“We definitely need to clarify rules around genetic testing/medical records and how it relates to insurance. This seems like it could easily turn into people becoming uninsurable due to genetic testing results....”
– Survey respondent #90
“[We need to consider]…the ability to use genomic information in the context of setting insurance premiums, the ability to use genomic information in employment settings and situations, and certainly the ability to get involved in informed decisionmaking.”
– Interviewee #5
“The direct-to-consumer thing is just exploding… it seems to be that there’s got to be this collision between these advancements and algorithms and software… and then you have consumers who are getting the data directly and maybe not even working with their provider, and so I worry a little bit that there will be an overuse or lack of understanding of just how complicated genetics is.”
– Interviewee #2
“Our researchers should be spending their time on the bench reading scientific literature, not reading patent filings.”
– Interviewee #3
Discussion
To our knowledge, this is the first study that has examined the perceived landscape of legal issues raised by key professional stakeholders in the evolving field of genetics and genomics. This mixed methods study provides an in-depth look at that landscape and key insights into the perceived legal problems posed by genomics.
The concept maps, the linkages shown in them, and the relationships discussed indicate the themes, complexity, and interrelatedness of the stakeholder perceptions about how the law does and should address genomics. They reflect generalized anxiety about the field. The stakeholders are concerned and mixed in their opinions about what should happen in the future. The relatively high ratings on the survey of all of the items support the notion that all the issues we raised contributed to the complexity of the situation. The spread between the highest scoring item (Liability Q4; M=4.20, SD=0.91) and the lowest scoring item (Quality Q9; M=3.24, SD=0.92) is only 0.96, which is only slightly higher than their respective standard deviations. All items on the survey received an average score of greater than 3.00, indicating that all items were found to be at least “Moderately Important.” This supports the conclusion that professional stakeholders have important legal concerns about genomics that should be addressed.
We believe that the patterns also suggest a perception of complexity. At the root of the complexity reported is the nature of genomic data itself: how it is gathered, the rapidly evolving but still incomplete understanding of what these data mean for health and disease, and questions concerning how the information should be shared. This leads to understanding genomics as an emerging field based on evolving science and still-developing perceptions of how the science should be conducted and the results implemented clinically, what rules should inform use in research and clinical care, and who should be involved in making these rules. Limited understanding in this emerging field leads to the need for education. The need for public education is particularly pressing, including in DTC testing.
Concerns over liability were prominent, including who would be considered responsible for what. There was uncertainty about whether ordering genomic tests requires that the entire sequence be interpreted and updated with the emergence of new knowledge, or whether it was possible to set limits on what is examined and reported. This concern underlay a call for clear guidelines, even while recognizing that the rapidly changing and still incomplete nature of what is known make formulation of guidelines a challenge. Another challenge is limited access to resources, especially genetics experts, in addition to funds for follow-up care. All of this led to a call for prudence going forward, especially given fears that law would prematurely and inappropriately define practice.
There were also themes about the need to improve CLIA and other regulatory processes. Some concerns related to law also appeared in other themes, in particular the need for clear guidelines and consistency. There was a perceived need to review current policies that were seen as out-of-date. Several of the comments also suggested that there were limited resources available to deal with the need for guidelines and control in this emerging field. There is a definite call for clear guidelines. However, the emerging field theme supports other observations that progress should be evolutionary and that care needs to be taken to more forward prudently, taking into account stakeholder perspectives.
A related concern was that genomic tests would not be deployed properly because of limited knowledge on the part of stakeholders. Genomics is a rapidly changing field. As a result, there is a need for knowledge of various types across different groups of people (i.e., clinicians, patients and research participants, insurance companies, researchers) to decide when genomic information should be obtained and used. Communicating current knowledge and understanding requires development of various types of educational approaches. For example, the incorporation of more genetic counselors who are well-versed in genomics into clinical teams would facilitate patient education.
A number of comments were made about control of genomic data. Respondents noted the importance of promoting data sharing to advance science and the need to balance this with privacy. At the same time, comments were made about the need to define data ownership. Family access was less commonly raised. Other major concerns expressed about genomic data included the need to ensure quality and transparency.
Comparison between the survey’s fixed-responses and the themes generated from the survey’s open-response items and the interviews demonstrated a strong consistency despite the different response formats. The highest-scoring fixed-response survey items were frequently alluded to in the themes, and lowest-scoring fixed-response survey items were rarely, if ever, mentioned. For example, the highest fixed-response items were worries about incorrect interpretation of results, addressing insurer access to genomic results, and establishing the standard of care for clinical use of genomics in assessing risk, in diagnosing, and in guiding prescribing and other treatment. These are directly related to the three themes identified for liability, which are shaping behavior, insurance, and blurring lines for clinical care vs. research. The lowest scoring items – harmonizing international standards; hospital or other organizational failure to adopt procedures, acquire equipment, or hire personnel for genomic analysis and integration into clinical care; and determining what law applies when research crosses states or countries – did not show up in the qualitative responses.
Another comparison of the responses to the closed-ended items and the merged open-ended items and interview responses reveals differences inherent in the two methodologies and differences in interpretation. The closed-ended survey responses show how people rated a given menu of possible responses. The respondents were constrained in responding by the structure provided in the survey. The data show that the respondents perceived high levels of importance for the issues in the four areas of liability, privacy and access, quality, and framework. This could be interpreted to mean that the respondents felt these issues were distinct and important individually. However, although the four categories are logical, there was little indication in the open-ended responses or interviews that the respondents personally viewed genomics law as organized into the four categories. They expressed an interconnected view of the issues. This does not necessarily indicate conflicting beliefs. It is likely that the respondents saw the issues both as separable within the four areas and as connected.
Limitations
This study has several limitations. This was not a random survey of the population and so did not obtain information from all possible groups. We also did not include patients and research participants but focused instead on respondents likely to be more informed about legal issues. Nor did we seek out views of those who are not particularly interested in genomics. Methodologically, the fixed-response survey items may have influenced the responses to the open-response survey items. Additionally, some of the groups included in the survey had a small number of respondents. Interviewing 11 people might be considered a small sample but it was felt that saturation was obtained. Also, because the open-response survey items and the interview results were obtained through different methods, combining them might be considered a limitation.
This study also does not consider how views of the law of genomics may or may not be related to the diversity of our participants. We did not ascertain survey respondents’ and interviewees’ minority ancestries or ethnic backgrounds, whether they were from traditionally marginalized populations or other diversity measures. We used purposeful sampling based on particular stakeholder group membership (e.g., NIH genomics project PIs). It is important to note that the issues that were viewed as important by this group of respondents might change if a group with broader or different representation were studied. Despite these limitations, this study raises prominent issues identified by these stakeholders about genomics law.
Conclusion
The goal of this empirical work was to identify perceived issues and priorities. The Working Group normative process and the resulting consensus articles, three of which are in this volume, provide information on what should be done to address the issues. Further investigation of legal issues raised by genetics and genomics can build on the empirical framework presented here. Our study highlights potential domains to query with other important stakeholders and the public in order to collect broader and more generalizable results. Gathering this type of information should be the goal of future research. The themes developed from this study should be fruitful areas for future research, especially the Relationship of Law to Research & Clinical Care, Educational Needs, and Sources and Use of Genomic Data Outside the Healthcare Setting. Additionally, there are many groups that were not included in this study whose opinions should be gathered and considered. Future research could also focus on how different aspects of respondent diversity might be related to perceived issues of genomics law.
Genomics, now a fundamental tool of research, is rapidly entering clinical care. Yet much remains unknown about this field of knowledge, and current interpretations of data can change over time. This type of transition, exacerbated by scientific uncertainty, inevitably raises a host of legal questions as researchers, health care providers, payers, regulators, and patient/participants use these new tools. A core challenge is to promote research and innovation while protecting patients/participants from harm and clinicians and health care institutions from excessive liability. Recognizing the complexity of the scientific challenges, the respondents in this study identified a wide range of interconnected issues, focusing specifically on the need for clear guidelines about how to use these data, fear of liability for those who use these data as well as those who do not, and the need to protect patients from use of this information particularly by insurers, while endorsing data sharing. Developing legal strategies to support appropriate use of genomics now and in the future clearly will require making trade-offs, taking into account the full complexity of this legal ecosystem.
Acknowledgments
Preparation of this article was funded by National Human Genome Research Institute (NHGRI) and National Cancer Institute (NCI) grant #1R01HG008605 (Wolf, Clayton, and Lawrenz, PIs), for a project on “LawSeq: Building a Sound Legal Foundation for Translating Genomics into Clinical Application.” We are grateful to the project’s Working Group members (listed at https://consortium.umn.edu/sites/consortium.umn.edu/files/may2018_national_working_group_roster_w_photos.pdf) for participation in the modified Delphi process described in the paper and for additional helpful input. This study was approved by the University of Minnesota IRB (#1603S85102) and Vanderbilt University Medical Center IRB (#170760). The views expressed in this article are those of the authors and not necessarily those of the funders.
Each author of this article reports this publication is conducted as part of the National Human Genome Research Institute (NHGRI) and National Cancer Institute (NCI) at the National Institutes of Health (NIH) grant 1R01HG008605 on “LawSeq: Building a Sound Legal Foundation for Translating Genomics into Clinical Application,” during the conduct of the study.
Biography
Susan M. Wolf, She is a Principal Investigator (PI) on NIH/NHGRI/NCI grant 1R01HG008605 on “LawSeq: Building a Sound Legal Foundation for Translating Genomics into Clinical Application.”
Ellen Wright Clayton, She is a Principal Investigator (PI) on NIH/NHGRI/NCI grant 1R01HG008605 on “LawSeq: Building a Sound Legal Foundation for Translating Genomics into Clinical Application.” She is also Co-PI on “GetPreCiSe: The Center for Genetic Privacy and Identity in Community Settings,” an NIH-funded Center of Excellence in ELSI Research.
Frances Lawrenz, Her research focuses on science and mathematics program evaluation, utilizing a variety of techniques and usually involving mixed methodologies. She is a Principal Investigator (PI) on NIH/NHGRI/NCI grant 1R01HG008605 on “LawSeq: Building a Sound Legal Foundation for Translating Genomics into Clinical Application.”
Contributor Information
Fook Yee Cheung, Department of Genetics, Cell Biology & Development in the College of Biological Sciences at the University of Minnesota..
Lauren Clatch, School of Law and the Department of Psychology at the University of Minnesota..
Susan M. Wolf, Consortium on Law and Values in Health, Environment & the Life Sciences, University of Minnesota..
Ellen Wright Clayton, Vanderbilt University Medical Center, Vanderbilt University..
Frances Lawrenz, University of Minnesota..
References
- 1.Sausser L, “‘11 Years After Child’s Death, SC Supreme Court Decides Genetic Lab Is a ‘Health Care Provider,’” Post and Courier, July 29, 2018, available at <https://www.postand-courier.com/health/years-after-child-s-death-sc-supreme-court-decides-genetic/article_58d955da-8083-11e8-82bf-234d4a3fabc6.html> (last visited January 29, 2020). [Google Scholar]
- 2.Wagner JK, “A Constitutional Challenge to Alaska’s Genetic Privacy Statute,” The Privacy Report, July 18, 2017, available at <https://theprivacyreport.com/2017/07/18/a-constitutional-challenge-to-alaskas-genetic-privacy-statute> (last visited January 29, 2020). [Google Scholar]
- 3.Food and Drug Administration (FDA), Considerations for Design, Development, and Analytical Validation of Next Generation Sequencing (NGS) - Based In Vitro Diagnostics (IVDs) Intended to Aid in the Diagnosis of Suspected Germline Diseases: Guidance for Stakeholders and Food and Drug Administration Staff (April 13, 2018): at 13–19, available at <https://www.fda.gov/regulatory-information/search-fda-guidance-documents/considerations-design-development-and-analytical-validation-next-generation-sequencing-ngs-based> (last visited January 29, 2020);; FDA, Use of Public Human Genetic Variant Databases to Support Clinical Validity for Genetic and Genomic-Based In Vitro Diagnostics, Guidance for Stakeholders and Food and Drug Administration Staff (April 13, 2018), available at <https://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm509837.pdf> (last visited January 29, 2020);; see also Press Release, FDA, “FDA Finalizes Guidances to Accelerate the Development of Reliable, Beneficial Next Generation Sequencing-Based Tests,” April 12, 2018, available at <https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm604462.htm> (last visited January 29, 2020).
- 4.Javitt GH and Carner KS, “Regulation of Next Generation Sequencing,” Journal of Law, Medicine & Ethics 42, no. 3 suppl (2014): 9–21. [DOI] [PubMed] [Google Scholar]
- 5.Curnutte MA et al. , “Development of the Clinical Next-Generation Sequencing Industry in a Shifting Policy Climate,” Nature Biotechnology 32, no. 10 (2014): 980–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beskow LM and Smolek SJ, “Prospective Biorepository Participants’ Perspectives on Access to Research Results,” Journal of Empirical Research on Human Research Ethics 4, no. 3 (2009): 99–111; [DOI] [PMC free article] [PubMed] [Google Scholar]; Kaufman DJ et al. , “Public Opinion About the Importance of Privacy in Biobank Research,” American Journal of Human Genetics 85, no. 5 (2009): 642–654; [DOI] [PMC free article] [PubMed] [Google Scholar]; Murphy J et al. , “Public Expectations for Return of Results from Large-Cohort Genetic Research,” American Journal of Bioethics 8, no. 11 (2008): 36–43; [DOI] [PMC free article] [PubMed] [Google Scholar]; Trinidad SB et al. , “Informed Consent in Genome-Scale Research: What Do Prospective Participants Think?” AJOB Primary Research 3, no. 3 (2012): 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curnutte et al. , supra note 5; [Google Scholar]; Downing NR et al. , “Genetics Specialists’ Perspectives on Disclosure of Genomic Incidental Findings in the Clinical Setting,” Patient Education and Counseling 90, no. 1 (2013): 133–138; [DOI] [PMC free article] [PubMed] [Google Scholar]; Miller J et al. , “Professionals’ Attitudes Regarding Large-Scale Genetic Information Generated Through Next Generation Sequencing in Research a Pilot Study,” Journal of Empirical Research on Human Research Ethics 9, no. 3 (2014): 56–58; [DOI] [PubMed] [Google Scholar]; Ramoni RB et al. , “Experiences and Attitudes of Genome Investigators Regarding Return of Individual Genetic Test Results,” Genetics in Medicine 15, no. 11 (2013): 882–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones LK et al. , “Developing Pharmacogenomic Reports: Insights from Patients and Clinicians,” Clinical and Translational Science 11, no. 3 (2018): 289–295; [DOI] [PMC free article] [PubMed] [Google Scholar]; Patel HN et al. , “Stakeholder Views on Pharmacogenomic Testing,” Pharmacotherapy 34, no. 2 (2014): 151–165. [DOI] [PubMed] [Google Scholar]
- 9.See, e.g., Berkman BE and Rothenberg KH, “Teaching Health Law,” Journal of Law, Medicine & Ethics 40, no. 1 (2012): 147–153; [DOI] [PMC free article] [PubMed] [Google Scholar]; Clayton EW and McGuire AL, “The Legal Risks of Returning Results of Genomics Research,” Genetics in Medicine 14, no. 4 (2012): 473–477; [DOI] [PMC free article] [PubMed] [Google Scholar]; Evans BJ, “Minimizing Liability Risks Under the ACMG Recommendations for Reporting Incidental Findings in Clinical Exome and Genome Sequencing,” Genetics in Medicine 15, no. 12 (2013): 915–920; [DOI] [PMC free article] [PubMed] [Google Scholar]; Evans BJ, “The First Amendment Right to Speak About the Human Genome,” University of Pennsylvania Journal of Constitutional Law 16, no. 3 (2014): 549–636; [PMC free article] [PubMed] [Google Scholar]; Gordon MP, “A Legal Duty to Disclose Individual Research Findings to Research Subjects?,” Food and Drug Law Journal 64, no. 1 (2009): 225–260; [PubMed] [Google Scholar]; Marchant GE et al. , “Personalized Medicine and Whole Genome Sequencing in the Era of Big Data: Challenges and Opportunities,” ISPOR Connections 20, no. 4 (2014): 4–6; [Google Scholar]; Marchant GE, Lindor RA, and Campos-Outcalt DE, “Physician Liability: The Next Big Thing for Personalized Medicine?” Personalized Medicine 8, no. 4 (2012): 457–467; [DOI] [PubMed] [Google Scholar]; McGuire AL, Knoppers BM, Zawati MH, and Clayton EW, “Can I be Sued for That? Liability Risk and the Disclosure of Clinically Significant Genetic Research Findings,” Genome Research 24, no. 5 (2014): 719–723; [DOI] [PMC free article] [PubMed] [Google Scholar]; Milstein AC, “Research Malpractice and the Issue of Incidental Findings,” Journal of Law, Medicine & Ethics 36, no. 2 (2008): 356–360; [DOI] [PubMed] [Google Scholar]; Wolf SM, “The Role of Law in the Debate over Return of Research Results and Incidental Findings: The Challenge of Developing Law for Translational Science,” Minnesota Journal of Law, Science & Technology 13, no. 2 (2012): 435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchant GE and Lindor RA, “Genomic Malpractice: An Emerging Tide or Gentle Ripple?” Food and Drug Law Journal 73, no. 1 (2018): 1–37. [Google Scholar]
- 11.Creswell J and Plano Clark V, Designing and Conducting Mixed Methods Research (Los Angeles: Sage Publications, 2018); [Google Scholar]; Fowler FJ, Survey Research Methods (Thousand Oaks: Sage Publications, 2014); [Google Scholar]; Josselson R, Interviewing for Qualitative Inquiry (New York: The Guilford Press, 2013); [Google Scholar]; Seidman I, Interviewing as Qualitative Research (New York: Teachers College Press, 2006); [Google Scholar]; Tashakkori A and Teddlie C, Handbook of Mixed Methods in Social and Behavioral Research (Thousand Oaks: Sage Publications, 2010). [Google Scholar]
- 12.Salkind NJ, Encyclopedia of Research Design (Los Angeles: Sage, 2010), 344–346. [Google Scholar]
- 13.Creswell and Clark, supra note 11; [Google Scholar]; Saldana J, The Coding Manual for Qualitative Researchers (Thousand Oaks: Sage Publications, 2016). [Google Scholar]
- 14.Anfara V, Brown K, and Mangione T, “Qualitative Analysis on Stage: Making the Research Process More Public,” Educational Researcher 31, no. 7 (2002): 28–38; Saldana, supra note 13. [Google Scholar]
- 15.See Josselson R, Interviewing for Qualitative Inquiry (New York: The Guilford Press, 2013); [Google Scholar]; Rubin H and Rubin I, Qualitative Interviewing the Art of Hearing Data (Thousand Oaks: Sage Publications, 2012); [Google Scholar]; Seidman I, Interviewing as Qualitative Research (New York: Teachers College Press, 2006). [Google Scholar]
- 16.See Anfara, et al. , supra note 14;; Creswell and Clark, supra note 11;; Saldana, supra note 13.


