Abstract
An additional Y chromosome occurs in ~1 in 1,000 males, resulting in the karyotype 47,XYY. The phenotype includes tall stature, hypotonia, neuropsychiatric comorbidities, and an increased risk of infertility in adulthood. Little is known about testicular function in childhood and adolescence in 47,XYY. This cross-sectional study aimed to assess testicular function serum biomarkers, including total testosterone, inhibin B, and anti-mullerian hormone (AMH), in 82 boys with XYY (11.3 ± 3.8 years) compared with 66 male controls (11.6 ± 3.8 years). The association of testicular hormones with physical features, neuropsychological phenotype, and magnetoencephalography (MEG) was assessed with multiple linear regression models. Results indicate males with XYY have significantly lower inhibin B (median 84 pg/ml vs. 109 pg/ml, p = .004) and higher AMH (median 41 ng/ml vs. 29 ng/ml, p = .011); however, testosterone, testicular volume, and stretched penile length were not different from controls. In the exploratory analysis of relationships between hormone concentrations and phenotypic assessments, higher inhibin B concentrations were positively correlated with lower BMI and better cognitive, academic, and behavioral outcomes in the XYY group. Testosterone concentrations were positively associated with better behavioral outcomes in boys with XYY. Higher testosterone and inhibin B concentrations were also associated with shorter auditory latencies measured using magnetoencephalography (MEG) in XYY. With a few exceptions, testicular hormones were not associated with phenotypic outcomes in controls. In conclusion, there is evidence of subtle impaired testicular function in boys with XYY and a newly described relationship between measures of testicular function and some aspects of the XYY phenotype.
Keywords: infertility, inhibin b, Sertoli cell function, testicular insufficiency, XYY syndrome
1 |. INTRODUCTION
A second Y chromosome occurs in approximately 1 in 1,000 males, resulting in the karyotype 47,XYY, also known as XYY or Jacob syndrome. It is estimated that only 10–15% of males with XYY receive the diagnosis, with a median age of diagnosis of 17 years (Stochholm, Juul, & Gravholt, 2010). Because of the low ascertainment in childhood, comprehensive knowledge of the phenotype in children and adolescents with XYY is limited. Tall stature, hypotonia, mild dysmorphic features, and neuropsychiatric conditions are commonly reported and are assumed to be secondary to increased Y gene dosage from the extra Y chromosome (Bardsley et al., 2013; Joseph et al., 2018).
Historically, biased studies done in prison populations reported men with XYY had higher testosterone concentrations than men without XYY, leading to the terms “super male” or “superman” (Kessler & Moos, 1970; Schiavi, Theilgaard, Owen, & White, 1984; Theilgaard, 1983). Although higher criminality among men with XYY is reported, (Stochholm, Bojesen, Jensen, Juul, & Gravholt, 2012) several studies have failed to show higher testosterone in XYY as a causative mechanism of delinquency (Aksglaede, Skakkebaek, & Juul, 2008; Bardsley et al., 2013; Hudson, Burger, Wiener, Sutherland, & Bartholomew, 1969; Pelzmann & Brodie, 1976; Price & van der Molen, 1970).
Contrary to early reports of robust testicular function, non-obstructive azoospermia and hypogonadism are now more widely appreciated in adults with XYY, and men with 47,XYY are much less likely to father a child than male controls (Borjian Boroujeni et al., 2019; Flannigan & Schlegel, 2017; Kim, Khadilkar, Ko, & Sabanegh, 2013; Stochholm, Juul, & Gravholt, 2012). The number of germ cells may be lower in XYY starting in fetal life, and there are case reports of underdeveloped genitalia with micropenis, cryptorchidism, and hypospadias, suggesting some boys may have impaired testicular function very early (Autio-Harmainen, Rapola, & Aula, 1980; Carakushansky, Neu, & Gardner, 1968; Coerdt, Rehder, Gausmann, Johannisson, & Gropp, 1985; Fryns, Kleczkowska, Kubien, & Van den Berghe, 1995). However, the large majority of males with XYY are born with normal genitalia and experience normal timing and tempo of pubertal development. There are numerous case series of infertility in males with XYY, and the prevalence of XYY among men with azoospermia is at least three times higher than in the general population (Abdel-Razic, Abdel-Hamid, & ElSobky, 2012; Borjian Boroujeni et al., 2019; El-Dahtory & Elsheikha, 2009; Kim, Khadilkar, Ko, & Sabanegh Jr., 2013; Rives et al., 2005). However, the trajectory of testicular development and the prevalence of testicular dysfunction among males with XYY are unknown.
Assessing testicular function across the pediatric lifespan is complex, as the hypothalamic-pituitary-gonadal axis is quiescent in prepubertal boys and hormone concentrations rapidly change when puberty commences. Inhibin B is a hormone produced by the Sertoli cells even prior to pubertal onset, reflecting intact Sertoli cell number and function in prepubertal boys and spermatogenesis in post-pubertal males (Grinspon et al., 2012). Inhibin B is lower in pediatric conditions associated with testicular dysfunction, including cryptorchidism and Klinefelter syndrome (Davis et al., 2016; Hamdi, Almont, Galinier, Mieusset, & Thonneau, 2017; Hildorf et al., 2019). Our group previously reported on testicular function in 42 boys with XYY with a mean age of 9.6 years (range 0.5–36 years) (Bardsley et al., 2013). Serum concentrations of gonadotropins, testosterone, estradiol, and Sertoli cell biomarkers (inhibin B and anti-mullerian hormone (AMH)) were in the normal range in the majority of subjects; however, follicle stimulating hormone (FSH) was elevated in a quarter of the sample, inhibin B was below the reference range in 12%, and there was no comparator group available to determine if relative differences in testicular function were present. Given the potential infertility risk in XYY, inhibin B and other testicular function biomarkers in children and adolescents with XYY may have clinical importance for genetic counseling and proactive fertility preservation, as well as scientific relevance in determining the natural history of testicular dysfunction in the setting of chromosomal aneuploidy. Furthermore, sub-clinical variation in testicular function may have clinical implications for the presenting physical or neurodevelopmental phenotype and therapeutic approaches.
In this study, we aimed to compare testicular function in a large cohort of boys with XYY to boys without XYY, with the hypothesis that inhibin B would be lower in XYY, reflective of early testicular dysfunction. Furthermore, we investigated the relationship of testicular function to physical, neurodevelopmental, and magnetoencephalography (MEG) results, given historic speculation of delinquent behavior being related to higher testosterone.
2 |. METHODS
2.1 |. Overall study design
This is a cross-sectional study comparing males with karyotype 47,XYY (cases) to typical males (controls). The parent study was designed to compare neurodevelopmental and neuroimaging (whole cortex MEG) phenotypes in boys with XYY and male controls. Given the 38% historical prevalence of autism spectrum disorder (ASD) in XYY, controls with ASD were recruited for this study in addition to neurotypical boys (Tartaglia et al., 2017). Since there were no appreciable differences in testicular function between boys in the control group with and without ASD diagnosis (data not shown), both groups were combined into single control group for this analysis.
2.2 |. Setting and participants
The study took place at Nemours Dupont Hospital for Children (AIDHC), Thomas Jefferson University, and the Children’s Hospital of Philadelphia (CHOP). Participants with XYY were recruited through the eXtraordinarY Kids Clinic at Nemours Children’s Network and family support networks for children affected by sex chromosome aneuploidies. Controls were recruited from the AIDHC clinics, postings on AIDHC websites, and referrals from other studies. Boys 4–18 years of age were eligible for participation, with cases having a karyotype of nonmosaic 47,XYY (n = 81) and one with mosaic 47,XYY[94]/46,XY[6]/48,XYYY[2], and controls (n = 66) having no known genetic conditions. No participants had a history of cryptorchidism or any other known condition affecting their hypothalamic-pituitary-gonadal axis. Partial data for 24 of the participants with XYY (29% of the sample) were previously published (Bardsley et al., 2013). The protocol was approved by the AIDHC and CHOP institutional review boards and parental consent and participant assent was obtained prior to any research procedures being performed.
2.3 |. Study procedures
2.3.1 |. Hormone assessments
Venipuncture was performed in the morning and serum was processed and frozen until the time of study completion, when samples were analyzed simultaneously for all controls and 70% of the cases; the other 30% were also measured in batch at a second laboratory. Quantification of inhibin B, anti-mullerian hormone (AMH), and total testosterone were performed with the following assays: Inhibin B was measured by chemiluminescent immunoassay (Quest Nichols Institute, Chantilly, VA) or enzyme-linked immunosorbent assay (AnshLabs, Webster, TX) with intra- and inter-assay CV <5% and < 10% respectively. AMH was measured using dual monoclonal antibodies by chemiluminescent immunoassay with an analytical sensitivity of 0.03 ng/ml (Quest Nichols Institute, Chantilly, VA) or enzyme-linked immunosorbent assay (AnshLabs, Webster, TX) with a quantification limit of 0.03 ng/ml and intra- and inter-assay CV <5% and < 10% respectively. Total testosterone was measured by liquid chromatography tandem mass spectroscopy in both labs with lower limit of quantification of 1.0 ng/dl and inter and intra-assay CV is <10%.
2.3.2 |. Physical assessments
Measurements of height to the nearest tenth of a centimeter (cm), weight to the nearest tenth of a kilogram (kg), waist and hip circumference to the nearest tenth of a cm were obtained by trained study personnel and converted to sex and age normed z-scores per CDC references. Arm span was measured fingertip to fingertip to the nearest cm and converted to sex and age normed z-scores (Gripp, Slavotinek, Hall, & Allanson, 2013). Systolic and diastolic blood pressure (SBP and DBP) were obtained in the seated position by digital sphygmomanometer and converted to percentiles for sex, age, and height (Flynn et al., 2017). Pubic hair was assessed according to Tanner staging by a pediatric endocrinologist (Marshall & Tanner, 1970). Stretched penile length was measured to the nearest cm and converted to age normative data to calculate an age-adjusted z-score (Custer & Rau, 2009). Testicular volume was measured by palpation using the Prader orchidometer and converted to z-scores using the published calculator (Joustra et al., 2015). If testes were different volumes, the average was used in the analysis. A testicular volume ≥ 4 ml was used to define puberty.
2.3.3 |. Auditory magnetoencephalography (MEG)
Latencies of the left and right 50 ms (M50) and 100 ms (M100) components of the auditory evoked response were estimated in a subset of cases and controls using methods described in Bloy et al 2019. Briefly, a total of 530 tones were presented and MEG data were recorded using a whole-head 275 axial gradiometer CTF system (VSM MedTech, Coquitlam, BC). Using BESA 6.0 (BESA®, Gräfelfing, Germany), MEG data were downsampled to 500 Hz and M50 and M100 auditory latencies were determined.
2.3.4 |. Neurodevelopmental assessments
Participants underwent a comprehensive neurodevelopmental evaluation that included standardized measures of cognition, executive function, attention, and motor skills. For all assessments, the raw scores were converted to standard scores with a mean of 100, standard deviation (SD) of 15, and higher scores indicate better results. Differential Ability Scales—Second edition (DAS-II) provides an assessment of intellectual functioning for children 2–17 years including general conceptual ability (GCA, similar to full-scale intelligence quotient), verbal cluster, and nonverbal cluster (Elliot, 1990). Wide Range Achievement Test Fourth Edition (WRAT4) measures academic skills in the areas of reading, math, and spelling. KeyMath Diagnostic Assessment Third Edition (KeyMath-3) is a thorough assessment of mathematical skills. Selected tests from the Woodcock Johnson III (WJ III) Tests of Achievement including letter-word identification to assess reading decoding, word attack to assess phonetic coding, and Basic Reading Skills were administered. Expressive and receptive vocabulary was assessed with the Expressive One-Word Picture Vocabulary Test and the Receptive One-Word Picture Vocabulary Test, respectively. For participants at least 8 years old, verbal fluency was assessed with Delis-Kaplan Executive Function System (D-KEFS) subtests letter fluency and category fluency. The Conners’ Continuous Performance Test (CPT-II) is a computer task to assess attention, impulsivity, and arousal. Assessment of motor and visual-motor skills was done with Bruininks-Oseretsky Test of Motor Proficiency (BOT) and Beery-Buktenica Developmental Test of Visual-Motor Integration (Beery VMI). Finally, the Crovitz-Zener Questionnaire was administered to assess laterality preference for handedness and footedness (Crovitz & Zener, 1962).
2.3.5 |. Psychosocial outcomes
Parents completed the following standardized questionnaires about their son: Child Behavior Checklist (CBCL), revised Conners’ Parent Rating Scale (CPRS), Social Responsiveness Scale second edition (SRS), and the Social Communication Questionnaire (SCQ). Boys completed the following standardized questionnaires: Children’s Depression Inventory (CDI), Revised Children’s Manifest Anxiety Scale Second Edition (RCMAS), Piers-Harris Children’s Self Concept Scale (Piers), Self-Perception Profile for Children (SPP), and the Tennessee Self-Concept Scale (TSCS). For all parent and self-reported questionnaires except the SCQ, raw scores for were converted to standard scores with a mean of 100, SD of 15, with higher scores indicating better results. Higher raw scores on the SCQ indicate more abnormal social behavior, with a score of ≥15 high risk for autism spectrum disorder. Socioeconomic status (SES) was estimated using the Hollingshead 2-Factor Index (Hollingshead & Redlich, 1958).
2.4 |. Statistical analysis
Data were examined for outliers and biologically implausible values and all outcome variables were assessed for normality with visual inspection of plots and the Shapiro-Wilk test. Pairwise deletion was used for any missing data. Within the control group, we compared testicular function by autism status (n = 18 with autism; n = 48 without autism); given there were no differences, controls were pooled together for the remainder of the analyses.
Descriptive statistics were used to summarize data stratified by group with either mean with SD or median with 25th and 75th percentiles, as appropriate. Cases and controls were compared using Welch’s 2-sided independent t-test for normally distributed data, Mann Whitney U for nonnormally distributed data, and chi square test for categorical data. To compare hormone concentrations between cases and controls, both unadjusted analyses and analyses adjusted for age, pubertal status, and batch analysis (binary) to account for two different laboratory batches, using least squares multiple regression were performed. Residuals were plotted to ensure a normal distribution and confirm the assumptions of multiple linear regression were met despite the nonparametric distribution of testosterone concentrations. Due to known clinically significant changes in testicular function at the time of puberty, we further stratified by pubertal status (prepubertal and pubertal). Finally, Fisher exact tests were used to assess group differences in the number of boys with hormone concentrations outside of the normal ranges for age. Results were considered significant at an alpha of .05. For the exploratory objectives to assess the relationship of testicular biomarkers with phenotypic variables, least squares multiple regression was performed with hormone concentration, batch, and age as the independent variables stratified by group. Given the exploratory objective of our analyses, we present unadjusted p-values as well as a delineation for those that remain significant after Bonferroni correction for multiple comparisons. Analyses were conducted in RStudio version 1.2.1335 and Prism Graphpad version 8.3.0.
3 |. RESULTS
3.1 |. Description of the sample
Demographic characteristics for the 82 cases (mean age 11.3 ± 3.8 years) and 66 controls (mean age 11.6 ± 3.8 years) were similar (Table 1). Half of the boys with XYY were diagnosed prenatally, most often as an incidental finding during advanced maternal age screening. Indications for genetic testing postnatally included developmental delay and low muscle tone; one boy was diagnosed due to delayed puberty.
TABLE 1.
Demographic and baseline characteristics
| XYY (n = 82) | Controls (n = 66) | p-value | |
|---|---|---|---|
| Age in years | 11.3 ± 3.8 | 11.6 ± 3.8 | .68 |
| Race/ethnicity n (%) | |||
| Non-Hispanic White | 69 (83%) | 47 (71%) | .06a |
| Black | 1 (1%) | 8 (12%) | |
| Asian | 1 (1%) | 4 (6%) | |
| Hispanic | 3 (4%) | 3 (5%) | |
| Mixed race or other | 8 (10%) | 4 (6%) | |
| Socioeconomic status | 51.9 ± 10.6 | 52.9 ± 9.5 | .53 |
| Pubertal status n (%) | |||
| Prepubertal | 31 (38%) | 31 (47%) | .26 |
| Pubertal | 51 (62%) | 35 (53%) | |
| Handedness, n (%) | |||
| Right dominant | 68 (85%) | 59 (92%) | .35 |
| Left dominant | 9 (11%) | 3(5%) | |
| Mixed dominance | 3 (4%) | 2 (3%) | |
| Footedness, n (%) | |||
| Right dominant | 58 (73%) | 57 (86%) | .042* |
| Left dominant | 22 (28%) | 9 (14%) | |
Note: Data are mean ± SD or median (25th–75th percentile) unless otherwise stated.
Chi square for non-Hispanic White vs. all other race/ethnicities.
p < 0.05
p < 0.01
p < 0.001
As expected, boys with XYY exhibited taller stature; they also had a trend toward a lower BMI but other physical measurements and pubertal status were not different between the groups (Table 3). Overall, handedness did not statistically significantly differ between XYY and controls; however, boys with XYY were approximately twice as likely to be left-hand and left-foot dominant (11% versus 5% controls, 28% versus 14% controls). As previously reported, boys with XYY had significantly worse scores on cognitive, academic, and executive function assessments (Table 4) (Joseph et al., 2018). Mean cognitive standard scores were 10–20 points lower in XYY compared to controls, with the largest discrepancy in the verbal domain. Parents of boys with XYY reporter greater problems in all domains on psychosocial assessments, with particular vulnerabilities in social-related domains with standard scores in the 70’s (Table 5). Boys with XYY did not endorse as severe of problems as their parents (most standard scores in the 90’s); however, they reported significantly more concerns in many domains than their peers.
TABLE 3.
Summary statistics of physical features and motor function with relationship of testicular hormones by group, adjusted for age
| Summary statistics |
Testosterone |
Inhibin B |
AMH |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XYY | Controls | XYY | Controls | XYY | Controls | XYY | Controls | ||||||||
| n = 82 | n = 66 | p-value | Slope | p-value | Slope | p-value | Slope | p-value | Slope | p-value | Slope | p-value | Slope | p-value | |
| Height z | 1.0 ± 1.3 | 0.2 ± 0.8 | <.001* * | .001 | .444 | .001 | .129 | −.001 | .719 | .001 | .462 | −.002 | .507 | −.006 | .066 |
| Weight z | 0.8 ± 1.2 | 0.8 ± 1.1 | .96 | −.001 | .245 | −.002 | .071 | −.005 | .026 | −.002 | .414 | −.000 | .885 | .000 | .992 |
| BMIz | 0.4 ± 1.3 | 0.8 ± 1.1 | .07 | −.002 | .042 | −.003 | .011 | −.007 | .004 | −.003 | .190 | .000 | .970 | .003 | .468 |
| Head Circ z | 0.8 ± 1.1 | 0.6 ± 1.2 | .14 | −.000 | .914 | .001 | .439 | .000 | .985 | .003 | .289 | .001 | .591 | −.004 | .388 |
| SBP %ile | 0.3 ± 0.3 | 0.3 ± 0.3 | .73 | −.000 | .236 | .000 | .783 | −.000 | .626 | −.000 | .722 | .001 | .333 | −.001 | .602 |
| DBP %ile | 0.5 ± 0.2 | 0.4 ± 0.2 | .62 | −.000 | .154 | .000 | .668 | .000 | .805 | .000 | .935 | .000 | .456 | −.000 | .984 |
| Waist z | 0.8 ± 1.0 | 0.4 ± 0.9 | .042 | .000 | .854 | −.001 | .499 | −.001 | .550 | .001 | .802 | .001 | .558 | −.001 | .845 |
| Waist: Hip z | 2.3 ± 0.6 | 2.2 ± 0.6 | .25 | −.001 | .124 | −.001 | .027 | .000 | .781 | −.002 | .080 | −.000 | .853 | .001 | .747 |
| Arm span z | 1.1 ± 1.4 | 0.6 ± 1.1 | .013 | .001 | .936 | .002 | .103 | −.004 | .100 | .002 | .533 | −.004 | .219 | −.008 | .099 |
| Penile length z | −0.5 ± 1.1 | −0.2 ± 1.6 | .34 | .002 | .026 | .002 | .201 | .000 | .905 | .005 | .165 | −.001 | .013 | −.009 | .154 |
| Testis volume | 4.5 (2–15) | 4.0 (2–15) | .87 | .021 | <.001* | .016 | <.001* | .010 | .311 | .032 | .003* | −.011 | .298 | −.040 | .046 |
| Testis volume z | −0.1 ± 1.3 | −0.2 ± 1.2 | .78 | .001 | .438 | .001 | .385 | .005 | .063 | .009 | .002* | −.004 | .140 | −.008 | .099 |
| Beery VMI | 83 ± 14 | 91 ± 14 | <.001* | −.001 | .912 | .004 | .758 | .060 | .031 | −.019 | .535 | −.023 | .483 | −.035 | .511 |
| BOTII | 81 ± 12 | 96 ± 15 | <.001* | −.002 | .859 | −.006 | .694 | .046 | .052 | .032 | .321 | .013 | .647 | −.006 | .924 |
Note: All physical outcomes are normalized to sex and age-appropriate z-scores with a mean of 0 and SD of 1. Beery Visual-Motor Integration (VMI) and Bruininks-Oseretsky Test of Motor Proficiency second edition composite (BOT-II) are standard scores. Summary statistics report means with SDs or median with 25th and 75th percentiles, and unadjusted p-values comparing cases to controls. Results of the multiple linear regression models adjusting for age and batch include the adjusted slope (estimate) and p-value (significance) are presented for each hormone. Bolded p-values indicate significance at the level of 0.05.
p-value remains significant after adjusting for multiple comparisons using the conservative Bonferroni adjustment.
TABLE 4.
Summary statistics of neurocognitive and auditory latencies with relationship of testicular hormones by group, adjusted for age
| Summary statistics |
Testosterone |
Inhibin B |
AMH |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XYY | Controls | XYY | Controls | XYY | Controls | XYY | Controls | ||||||||
| Mean ± SD | p-value | Slope | p-value | Slope | p-value | Slope | p-value | Slope | p-value | Slope | p-value | Slope | p-value | ||
| DAS-II (cognitive) | n = 79 | n = 65 | <.001* | .010 | .425 | .007 | .661 | .049 | .114 | .080 | .056 | .039 | .276 | −.047 | .561 |
| Verbal cluster | 89 ± 18 | 108 ± 18 | <.001* | .003 | .838 | .020 | .194 | .089 | .007 | .017 | .637 | .018 | .646 | .036 | .601 |
| Nonverbal cluster | 94 ± 17 | 106 ± 16 | <.001* | .004 | .748 | .012 | .455 | .065 | .025 | .027 | .485 | .010 | .759 | .034 | .637 |
| General conceptual ability | 90 ± 15 | 108 ± 17 | |||||||||||||
| WRAT4 (academic) | n = 76 | n = 63 | <.001* | .011 | .413 | −.002 | .861 | .062 | .067 | −.002 | .930 | .060 | .139 | .068 | .152 |
| Reading | 94 ± 17 | 111 ± 11 | <.001* | .011 | .411 | −.007 | .635 | .076 | .019 | .006 | .846 | .082 | .035 | .022 | .711 |
| Spelling | 93 ± 16 | 106 ± 14 | <.001* | .006 | .669 | .007 | .694 | .091 | .009 | .021 | .588 | .054 | .201 | .058 | .399 |
| Arithmetic | 88 ± 19 | 102 ± 17 | |||||||||||||
| KeyMath (academic) | n = 71 | n = 62 | <.001* | −.008 | .546 | .001 | .972 | .067 | .030 | −.013 | .699 | .027 | .493 | .006 | .919 |
| Basic concepts | 99 ± 16 | 113 ± 16 | <.001* | −.007 | .607 | −.008 | .652 | .068 | .030 | .008 | .849 | .024 | .542 | .090 | .235 |
| Operations | 92 ± 16 | 105 ± 18 | <.001* | .002 | .885 | −.000 | .982 | .049 | .109 | .001 | .976 | .011 | .782 | .034 | .601 |
| Application | 95 ± 15 | 107 ± 17 | <.001* | −.004 | .788 | −.000 | .982 | .065 | .044 | −.001 | .976 | .022 | .593 | .054 | .454 |
| Total | 95 ± 16 | 108±18 | |||||||||||||
| WJ III (academic) | n = 75 | n = 66 | <.001* | .015 | .240 | −.002 | .878 | .064 | .038 | .015 | .577 | .076 | .038 | −.014 | .778 |
| Letter-word | 96 ± 16 | 109 ± 12 | .001* | .003 | .800 | −.001 | .896 | .071 | .018 | .011 | .627 | .058 | .117 | .001 | .982 |
| Word attack | 99 ± 15 | 106 ± 11 | <.001* | .012 | .308 | −.001 | .895 | .064 | .028 | .014 | .589 | .070 | .048 | −.008 | .867 |
| Basic Reading | 99 ± 15 | 110 ± 12 | |||||||||||||
| PVT (language) | n = 79 | n = 65 | <.001* | −.005 | .679 | −.006 | .672 | .031 | .302 | .011 | .766 | −.004 | .900 | .057 | .368 |
| Expressive one word | 98 ± 15 | 108 ± 15 | <.001* | .002 | .891 | −.008 | .599 | .062 | .020 | .002 | .949 | .056 | .070 | −.020 | .761 |
| Receptive one word | 97 ± 14 | 110 ± 16 | |||||||||||||
| CPT-II (attention) | n = 74 | n = 66 | .002 | −.014 | .447 | .011 | .578 | .095 | .040 | .012 | .804 | .055 | .323 | −.057 | .515 |
| Omissions | 84 ± 24 | 95 ± 21 | .727 | −.021 | .059 | −.014 | .393 | .023 | .390 | −.018 | .643 | .050 | .122 | .041 | .571 |
| Commissions | 97 ± 13 | 98 ± 17 | <.001* | .008 | .586 | .024 | .164 | .010 | .787 | .015 | .713 | −.066 | .136 | −.046 | .526 |
| Hit response time | 85 ± 18 | 97 ±18 | <.001* | −.023 | .066 | .005 | .771 | .073 | .018 | −.029 | .475 | .039 | .311 | .061 | .398 |
| Variability | 85 ± 16 | 96 ± 18 | .887 | −.016 | .370 | −.042 | .063 | .067 | .127 | −.010 | .855 | .092 | .084 | .135 | .171 |
| Perseverations | 90 ± 23 | 90 ± 26 | |||||||||||||
| D-KEFS (executive fxn) | n = 75 | n = 52 | .002 | .026 | .109 | −.004 | .814 | .019 | .635 | .054 | .204 | −.026 | .660 | .045 | .608 |
| Letter fluency | 93 ± 18 | 105 ± 18 | .034 | .030 | .079 | −.008 | .627 | .026 | .537 | .019 | .616 | −.058 | .352 | .008 | .919 |
| Category fluency | 97 ± 19 | 104 ± 16 | |||||||||||||
| Auditory latency (ms) | n = 35 | n = 31 | .203 | −.011 | .464 | .004 | .876 | −.051 | .105 | −.055 | .272 | .018 | .733 | −.021 | .783 |
| Left M50 | 79 ± 15 | 74 ± 17 | .581 | −.025 | .045 | .007 | .733 | −.051 | .051 | −.029 | .523 | .021 | .615 | .091 | .202 |
| Right M50 | 71 ± 12 | 69 ± 15 | .010 | −.059 | .057 | −.002 | .955 | −.138 | .124 | −.065 | .343 | −.0545 | .784 | .487 | <.001* |
| Left M100 | 141 ± 33 | 116 ± 21 | .173 | −.011 | .688 | .016 | .646 | −.112 | .046 | .019 | .751 | −.207 | .021 | −.107 | .544 |
| Right M100 | 120 ± 22 | 112 ± 20 | |||||||||||||
Note: All neurocognitive outcome data have been normalized to standard scores with a population mean of 100, SD of 15, and a normal distribution. Auditory latencies are in milliseconds. Summary statistics report means, SDs, and unadjusted p-values comparing cases to controls. Results of the multiple linear regression models adjusting for age and batch include the adjusted slope (estimate) and p-value (significance) are presented for each hormone. Bolded p-values indicate significance at the level of .05.
Abbreviations: CPT-II, Conners’ Continuous Performance Test Second Edition; DAS-II, Differential Ability Scales Second Edition; D-KEFS, Delis-Kaplan Executive Function System; PVT, Picture Vocabulary Test; WRAT4, Wide-Range Achievement Test 4; WJ III, Woodcock Johnson III Test of Achievement.
p-value remains significant after adjusting for multiple comparisons using the conservative Bonferroni adjustment.
TABLE 5.
Summary statistics of psychosocial measures with relationship of testicular hormones by group, adjusted for age
| Summary statistics |
Testosterone |
Inhibin B |
AMH |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XYY | Controls | XYY | Controls | XYY | Controls | XYY | Controls | ||||||||
| Parent questionnaires | Mean ± SD | p-value | Slope | p−value | Slope | p−value | Slope | p−value | Slope | p−value | Slope | p-value | Slope | p-value | |
| CPRS (SS) | |||||||||||||||
| Cognitive problems | 74 ± 15 | 99 ± 16 | <.001* | .006 | .622 | −.004 | .810 | .052 | .068 | −.036 | .327 | .064 | .052 | .009 | .889 |
| Oppositional | 80 ±22 | 100 ± 14 | <.001* | .045 | .009 | .000 | .975 | .111 | .009 | .034 | .295 | .052 | .305 | −.036 | .536 |
| Hyperactivity-impulsivity | 78 ± 21 | 92 ± 20 | <.001* | .004 | .802 | .009 | .634 | .078 | .058 | .029 | .534 | .074 | .125 | −.012 | .889 |
| Anxious-shy | 83 ±20 | 94 ± 17 | <.001* | .004 | .791 | .031 | .044 | .072 | .069 | .019 | .624 | −.003 | .951 | −.101 | .141 |
| Perfectionism | 94 ± 16 | 100 ± 15 | .045 | .018 | .150 | .032 | .027 | .069 | .026 | .004 | .909 | .025 | .503 | −.052 | .389 |
| Social problems | 70 ± 23 | 91 ± 22 | <.001* | .020 | .278 | .014 | .517 | .108 | .013 | .021 | .680 | .119 | .019 | .002 | .979 |
| Psychosomatic | 84 ± 23 | 99 ± 17 | <.001* | .038 | .041 | .017 | .301 | .067 | .136 | −.008 | .838 | .012 | .824 | −.012 | .872 |
| CBCL Total (SS) | 78 ± 18 | 99 ± 19 | <.001* | .039 | .005 | .018 | .333 | .096 | .004 | .036 | .420 | .035 | .395 | −.008 | .924 |
| Anxious/depressed | 83 ± 17 | 92 ± 13 | <.001* | .035 | .007 | .024 | .051 | .047 | .140 | .033 | .273 | .006 | .878 | −.061 | .256 |
| Withdrawn/depressed | 82 ± 15 | 93 ± 12 | <.001* | .031 | .008 | .005 | .648 | .057 | .045 | .008 | .785 | .015 | .655 | .042 | .368 |
| Somatic complaints | 85 ± 15 | 92 ± 12 | .003 | .024 | .040 | .014 | .235 | .032 | .281 | −.039 | .178 | −.023 | .496 | .028 | .601 |
| Social problems | 74 ± 17 | 92 ± 13 | <.001* | .036 | .006 | .010 | .430 | .036 | .262 | .004 | .900 | .026 | .503 | −.002 | .964 |
| Thought problems | 78 ± 16 | 91 ± 13 | <.001* | .025 | .036 | .010 | .414 | .107 | <.001* | −.005 | 873 | .025 | .482 | −.026 | .600 |
| Attention problems | 73 ± 16 | 91 ± 14 | <.001* | .029 | .023 | .002 | .858 | .058 | .062 | −.011 | .733 | .028 | .438 | −.022 | .705 |
| Rule-breaking behavior | 84 ± 15 | 97 ± 6 | <.001* | .006 | .602 | .004 | .477 | .036 | .210 | .011 | .407 | −.015 | .657 | .010 | .679 |
| Aggressive behavior | 83 ± 18 | 94 ± 11 | <.001* | .029 | .040 | .004 | 731 | .065 | .059 | .009 | .724 | .032 | .439 | .010 | .809 |
| Internalizing behavior | 84 ± 19 | 100 ± 20 | <.001* | .045 | .002 | .030 | .111 | .082 | .021 | .022 | .625 | .024 | .560 | −.063 | .435 |
| Externalizing behavior | 87 ± 21 | 104 ± 17 | <.001* | .036 | .027 | .002 | .916 | .103 | .010 | .045 | .260 | .039 | .406 | .021 | .764 |
| SRS Total (SS) | 72 ± 25 | 95 ± 24 | <.001* | .032 | .127 | .029 | .233 | .152 | .002 | .002 | .977 | .050 | .392 | −.109 | .248 |
| Social awareness | 82 ± 22 | 96 ± 21 | <.001* | .011 | .560 | .021 | .333 | .073 | .096 | .050 | .339 | .025 | .612 | −.136 | .092 |
| Social cognition | 72 ± 26 | 94 ± 24 | <.001* | .017 | .426 | .041 | .088 | .111 | .032 | .029 | .622 | .044 | .473 | −.132 | .168 |
| Social communication | 74 ± 26 | 96 ± 24 | <.001* | .036 | .094 | .023 | .349 | .146 | .004 | −.012 | .838 | .054 | .363 | −.073 | .437 |
| Social motivation | 80 ± 21 | 95 ± 21 | <.001* | .017 | .353 | .009 | .687 | .130 | .002 | −.046 | .359 | .005 | .916 | −.064 | .468 |
| Autistic mannerisms | 73 ± 25 | 96 ± 22 | <.001* | .046 | .024 | .033 | .140 | .161 | <.001* | .013 | .800 | .046 | .442 | −.102 | .229 |
| SCQ (raw score) | 9 (5–20) | 4 (1–9) | <.001* | −.010 | .144 | −.007 | .669 | −.037 | .023 | −.013 | .483 | −.012 | .566 | .009 | .737 |
| Child/self-questionnaires | |||||||||||||||
| CDI Total (SS) | 93 ± 21 | 102 ± 17 | .013 | .038 | .037 | .013 | .463 | .050 | .265 | .050 | .222 | .031 | .631 | −.201 | .006 |
| Negative mood | 96 ± 20 | 101 ± 16 | .169 | .035 | .046 | .026 | .109 | .034 | .442 | .072 | .055 | .025 | .697 | −.104 | .130 |
| Interpersonal problems | 93 ± 20 | 101 ± 17 | .025 | .021 | .236 | −.008 | .653 | .064 | .138 | .036 | .360 | .034 | .591 | −.169 | .015 |
| Ineffectiveness | 93 ± 18 | 103 ± 14 | .002 | .029 | .061 | .008 | .583 | .014 | .719 | .007 | .821 | .062 | .280 | −.132 | .023 |
| Anhedonia | 100 ± 15 | 107 ± 10 | .045 | .035 | .027 | −.009 | .559 | .012 | .802 | .014 | .717 | −.026 | .674 | −.075 | .366 |
| Negative self esteem | 97 ±20 | 102 ± 16 | .134 | .025 | .164 | .010 | .537 | .061 | .151 | .043 | .260 | .023 | .710 | −.218 | .001* * |
| RCMAS Total (SS) | 95 ± 18 | 109 ± 15 | <.001* | .031 | .051 | .008 | .617 | .045 | .241 | −.026 | .494 | −.021 | .707 | −.012 | .861 |
| Physiological anxiety | 99 ± 16 | 110 ± 16 | <.001* | .021 | .142 | −.003 | .874 | .052 | .136 | .014 | .721 | −.008 | .883 | .113 | .121 |
| Worry/oversensitivity | 99 ± 20 | 107 ± 21 | .025 | .016 | .339 | .010 | .633 | .025 | .548 | −.023 | .632 | −.007 | .915 | .150 | .082 |
| Social concerns | 96 ± 18 | 111 ± 17 | <.001* | .023 | .144 | −.002 | .897 | .038 | .322 | −.009 | .829 | .001 | .989 | .159 | .023 |
| SPP global self worth (SS) | 101 ± 16 | 106 ± 18 | .134 | .045 | .001* | .007 | .712 | .087 | .011 | .027 | .539 | .020 | .707 | −.089 | .285 |
| Scholastic competence | 94 ± 16 | 106 ± 16 | <.001* | .015 | .306 | −.026 | .118 | .023 | .514 | −.006 | .876 | .018 | .731 | −.010 | .890 |
| Social competence | 92 ± 19 | 103 ± 17 | .003 | .028 | .099 | .007 | .710 | .094 | .022 | −.037 | .384 | .110 | .070 | −.079 | .334 |
| Athletic competence | 91 ± 16 | 99 ± 18 | .031 | .021 | .123 | −.013 | .507 | .038 | .252 | −.002 | .971 | .057 | .242 | .030 | .723 |
| Physical appearance | 104 ± 15 | 103 ± 16 | .659 | .025 | .060 | −.011 | .511 | .046 | .145 | .028 | .495 | −.031 | .512 | −.041 | .582 |
| Behavioral conduct | 97 ± 19 | 108 ± 23 | .009 | .030 | .074 | .024 | .320 | .053 | .192 | −.011 | .852 | .117 | .054 | −.024 | .817 |
| TSCS (SS) | 99 ± 19 | 109 ± 17 | .003 | .039 | .022 | −.016 | .379 | .105 | .007 | −.018 | .645 | .082 | .160 | −.071 | .333 |
| Physical | 101 ± 18 | 106 ± 16 | .103 | .019 | .259 | −.008 | .638 | .049 | .195 | −.015 | .691 | .048 | .394 | −.101 | .146 |
| Moral | 98 ± 18 | 107 ± 15 | .004 | .036 | .028 | −.168 | .247 | .061 | .112 | −.137 | .662 | .056 | .319 | .182 | .759 |
| Personal | 99 ± 18 | 106 ± 14 | .028 | .034 | .040 | −.017 | .295 | .104 | .006 | −.005 | .880 | .060 | .296 | −.041 | .532 |
| Family | 99 ± 17 | 107 ± 14 | .007 | .046 | .003 | −.030 | .051 | .116 | <.001* | −.033 | .317 | .110 | .035 | −.057 | .351 |
| Social | 102 ± 19 | 109 ± 20 | .066 | .025 | .153 | −.016 | .468 | .091 | .020 | −.022 | .635 | .079 | .179 | −.080 | .351 |
| Academic | 94 ± 16 | 107 ± 13 | <.001* | .022 | .136 | −.021 | .139 | .063 | .068 | −.031 | .312 | .066 | .199 | −.006 | .917 |
| Piers Harris Total (SS) | 95 ± 15 | 108 ± 16 | <.001* | .018 | .174 | −.013 | .432 | .041 | .210 | −.044 | .252 | .031 | .527 | −.097 | .157 |
| Behavior | 97 ± 17 | 107 ± 13 | <.001* | .021 | .175 | .001 | .848 | .064 | .081 | −.019 | .543 | .048 | .381 | −.050 | .380 |
| Intellectual | 95 ± 13 | 105 ± 12 | <.001* | .008 | .517 | −.023 | .062 | .004 | .889 | −.044 | .120 | .022 | .602 | −.019 | .706 |
| Physical | 100 ± 12 | 106 ± 14 | .017 | .010 | .322 | −.011 | .477 | .000 | .993 | −.002 | .948 | .037 | .306 | −.089 | .153 |
| Anxiety | 96 ± 15 | 108 ± 14 | <.001* | .017 | .182 | .007 | .636 | .043 | .172 | −.019 | .555 | −.055 | .237 | −.121 | .032 |
| Popularity | 93 ± 13 | 102 ± 15 | <.001* | .021 | .083 | .015 | .329 | .039 | .177 | −.016 | .654 | .004 | .915 | −.069 | .292 |
| Happiness | 100 ± 14 | 105 ± 11 | .084 | .024 | .048 | −.000 | .986 | .047 | .110 | −.001 | .958 | .024 | .578 | −.059 | .240 |
Note: Psychosocial outcome data have been normalized to standard scores (SS) with a population mean of 100, SD of 15, and higher scores indicated better function. The Social Communication Questionnaire (SCQ) is presented as a raw score with higher numbers indicative of a higher risk of autism spectrum disorder. Summary statistics report means with SDs or median with 25th and 75th percentiles, and unadjusted p-values comparing cases to controls. Results of the multiple linear regression models adjusting for age and batch include the adjusted slope (estimate) and p-value (significance) are presented for each hormone. Bolded p-values indicate significance at the level of .05. Parent-proxy questionnaires include: Child Behavior Checklist (CBCL), revised Conners’ Parent Rating Scale (CPRS), Social Responsiveness Scale second edition (SRS) and SCQ. Child questionnaires include Children’s Depression Inventory (CDI), Revised Children’s Manifest Anxiety Scale Second Edition (RCMAS), Piers-Harris Children’s Self Concept Scale (Piers), Self-Perception Profile for Children (SPP).
p-value remains significant after adjusting for multiple comparisons using the conservative Bonferroni adjustment.
3.2 |. Testicular function
Testicular volume and stretched penile length z-scores were similar in boys with XYY and controls (Table 3). Serum hormone concentrations for cases and controls are presented by age in Figure 1. Testosterone concentrations had a sigmoidal relationship with age for both groups and there were no differences in serum testosterone concentrations between groups, with or without adjusting for age and/or pubertal stage, and the relationship between testosterone and age in XYY was similar to controls. Boys with XYY were no more likely than controls to have a testosterone above the reference range for age (n = 2 for XYY and n = 3 for controls, p = .66). Inhibin B had a linear relationship with age for controls but not for boys with XYY, where there was a lack of the typical pubertal rise (Figure 1 and Table 2, p = .004 for slope difference). Inhibin B concentrations were 30 pg/ml (95% CI 10–50) lower in boys with XYY than controls (adjusted p = .004). Boys with XYY had an eight times greater odds of having an inhibin B below the normal range (p = .043); 17% of boys with XYY >11 years had an inhibin B below the normal range, while no one in the control group did. AMH had an inverse relationship with age in both groups, however, the slope was greater in boys with XYY (p = .010, Figure 1). In the multiple regression model adjusting for age and pubertal status, AMH was 22 ng/ml (95%CI 5–39) higher in XYY compared to controls (adjusted p = .011, Figure 1). When stratified by pubertal status, the higher AMH in boys with XYY was only appreciated prior to pubertal onset (Table 2). There were no statistical differences between cases and controls in the number of boys who had an AMH below (13% vs. 10%) or above (4% vs. 0%) the age-appropriate reference ranges. Finally, a subgroup analysis revealed no differences in testicular function between boys who were diagnosed with XYY prenatally or postnatally (data not shown).
FIGURE 1.
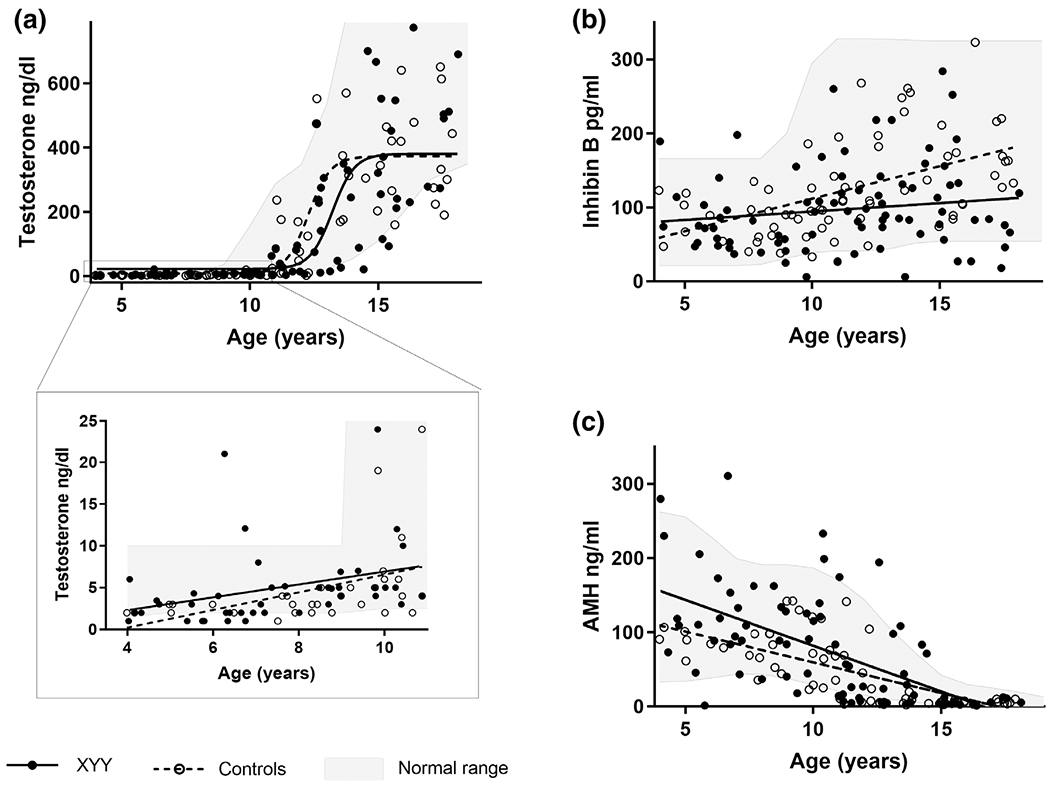
Testicular hormone concentrations by age for individual males with XYY (solid black) and controls (open circles) and the corresponding regression lines. (a) Testosterone had a sigmodal relationship with age that was similar between cases and controls (p = .89). (b) Inhibin B was associated with a pubertal rise in controls but not for males with XYY, resulting in a significant group difference in the relationship of inhibin B and age (p = .004). (c) Anti-mullerian hormone (AMH) declined with age for both cases and controls, and there was a significant difference between XYY and controls for this relationship (p = .010)
TABLE 2.
Testicular hormone concentrations stratified by pubertal status
| Prepubertal |
Pubertal |
|||||
|---|---|---|---|---|---|---|
| XYY (n = 31) | XY (n = 31) | p-value | XYY (n = 51) | XY (n = 35) | p-value | |
| Age (years) | 7.5 ± 2.1 | 8.5 ± 2.2 | .078 | 13.6 ± 2.4 | 14.3 ± 2.6 | .236 |
| Pubic hair stage | 1 (1–1) | 1 (1–1) | — | 3 (1–5) | 3 (2–5) | .971 |
| TT (ng/dl) | 3.0 (2.0–5.0) | 3.0 (2.0–5.0) | .416 | 212 (38–340) | 275 (165–432) | .331 |
| INHB (pg/ml) | 62 (46–95) | 90 (61–104) | .050 | 105 (73–142) | 143 (110–204) | .001** |
| AMH (ng/ml) | 118 (84–168) | 79 (61–98) | .011* | 11 (4.3–47) | 6.9 (5.0–10) | .489 |
| LH (mIU/ml)a | 0.08 (0.03–0.16) | (0.02–0.3) | — | 1.1 (0.9–3.4) | (0.2–7.0) | — |
| FSH (mIU/ml)a | 0.6 (0.4–1.3) | (0.26–3.0) | — | 3.4 (2.2–7.0) | (1.8–9.2) | — |
Data are presented as Median (25th–75th percentile) or Mean ± SD; unadjusted p-values compare XYY and controls; normative values are provided in parentheses in the column for controls.
AMH, anti-mullerian hormone; FSH, follicle stimulating hormone; INHB, inhibin B; LH, luteinizing hormone; TT, total testosterone.
LH and FSH includes only 13 prepubertal and 15 pubertal boys with XYY that were previously published (Bardsley et al., 2013).
p < .05.
p < .01.
p < .001.
3.3 |. Exploration of the relationship between testicular function and phenotypic features
3.3.1 |. Physical assessments
There was a negative association between BMI and testosterone in both cases and controls (p = .042 and .011, respectively), and in the XYY group this relationship was also seen with inhibin B (p = .004, Table 3). Testicular volume was strongly associated with testosterone concentrations in both groups (p < .001), consistent with pubertal changes. In the controls, increasing testicular size was also associated with higher inhibin B (p = .002) and lower AMH (p = .046), as expected. However, the relationship between testicular volume and inhibin B and AMH concentrations were not as strongly related in XYY (p > .2), consistent with testicular volume enlargement without the corresponding expected changes in Sertoli cell biomarkers. Testosterone, inhibin B, and AMH concentrations had minimal relationships with other physical outcomes in either cases or controls after adjusting for age (Table 3).
3.3.2 |. Neurodevelopmental assessments
Inhibin B concentrations had a significant relationship to many neurodevelopmental domains within the XYY group, however, this was not observed in the control group (Table 4). Within the XYY group, there was a positive correlation between relatively higher inhibin B and better cognitive function, which was statistically significant for the nonverbal and general concept ability domains and approached significance in the verbal domain (Figure 2 and Table 4). Inhibin B was also positively correlated with academic achievement assessments, attention, and visual-motor integration within the XYY group when adjusted for age. Testosterone and AMH did not have consistent relationships with cognitive, academic, or motor outcomes in either group.
FIGURE 2.
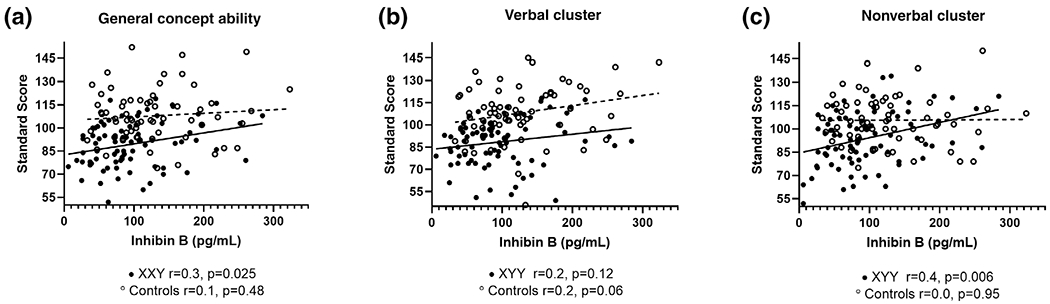
Relationship of Inhibin B with cognition in boys with XYY with adjusted correlation coefficients and p-values. Males with XYY are solid black and controls are open circles with dashed lines. General conceptual ability (a) and nonverbal cluster (c) have a linear relationship with inhibin B in XYY with improved cognitive abilities associated with higher inhibin B, although not significant when applying Bonferroni corrections for multiple comparisons. This relationship did not reach significance in the verbal cluster (b). The relationship of nonverbal cognition and inhibin B was nearly significantly different in XYY compared to controls (p = .064)
3.3.3 |. Auditory magnetoencephalography (MEG)
We measured the latencies of the M50 and M100 components of the auditory evoked response in a subset of the sample (35 cases and 31 controls.) There was a consistent trend for an association between higher inhibin B concentrations and shorter auditory latencies in both hemispheres in boys with XYY, although most of these associations did not reach statistical significance due to the limited sample size (Table 4). In addition, higher testosterone was associated with shorter latencies for M50 on the right (p = .045) and a trend for M100 on the left (p = .057) in boys with XYY. In controls, no relationships were observed between auditory evoked responses and inhibin B or testosterone; however, a surprisingly strong relationship emerged between AMH and M100 response in the left hemisphere (Table 4).
3.3.4 |. Behavioral and psychosocial assessments
There were positive associations with parent-reported behavior and both testosterone and inhibin B in boys with XYY, such that lower hormone concentrations related to more behavioral concerns particularly in internalizing domains even after adjusting for age (Table 5). Importantly, there was no association suggestive of testosterone causing increased aggression or defiance. Lower inhibin B was associated with greater social difficulties and autistic behaviors as evidenced from social domains on multiple assessments. Autistic mannerisms on the SRS and thought problems on the CBCL were strongly associated with lower inhibin B (p < .001 for both). SCQ scores were inversely associated with inhibin B (p = .023), demonstrating an association between lower inhibin B concentrations and an increased risk of autism spectrum disorders. Finally, relationships also emerged between higher inhibin B and testosterone with more positive selfconcept on the self-report questionnaires. There were not consistent relationships between testicular function and behavioral or psychosocial outcomes in controls, with the exception of an association between more immature testicular function (lower inhibin B and testosterone with higher AMH) and self-reported symptoms of anxiety and depression even after adjusting for age (Table 5).
4 |. DISCUSSION
In this cross-sectional study comparing testicular function in boys with and without XYY, the Sertoli cell hormone, inhibin B, was found to be significantly lower in boys with XYY. This is congruent with a known risk of impaired spermatogenesis in adults with XYY. Serum AMH, another product of Sertoli cells, was significantly higher in prepubertal boys, potentially representing lower intratesticular testosterone concentrations. Furthermore, we found a positive relationship between testicular function and neurodevelopmental outcomes, including measures of cognition, academic achievement, motor skills, and behavior. In addition, longer auditory latencies were associated with lower testosterone concentrations and a trend with inhibin B, suggesting a role of testicular hormones in central nervous system maturation. In other words, the testicular hormone profiles in older boys with XYY resembled those of younger control boys. Our results are contrary to early speculations that males with XYY have elevated testosterone levels associated with aggressive or delinquent behaviors. Taken together, the results of this study are an important contribution to the existing literature and dispel myths about testicular function and behavior in XYY syndrome.
X chromosome aneuploidy is well known to affect gonadal function, with both 47,XXY (Klinefelter syndrome) and 45,X (Turner syndrome) being common causes of primary hypogonadism. However, the impact of Y chromosome aneuploidy on gonadal function is less appreciated. The karyotype 47,XYY is identified in 0.4–1% infertile men, which is four to ten times more common than would be expected based on the estimated prevalence of XYY in the general population (Borjian Boroujeni et al., 2019; Hofherr, Wiktor, Kipp, Dawson, & Van Dyke, 2011; Mohammed et al., 2007). The prevalence of gonadal dysfunction among males with XYY is unknown. Several case series of infertile men with XYY have been reported, and about a quarter of these cases had hypergonadotropic hypogonadism in addition to oligospermia or azoospermia (Abdel-Razic et al., 2012; Borjian Boroujeni et al., 2019; El-Dahtory & Elsheikha, 2009). Research suggests that chromosome aneuploidy in general may induce germ cell degradation by epigenetic mechanisms such as altered chromosomal alignment; however, the Y chromosome aneuploidy may also directly affect the Sertoli cells that are necessary to support spermatogenesis (Rives et al., 2005; Sciurano et al., 2019). The exact mechanisms and natural history of testicular dysfunction in Y chromosome aneuploidy are unknown. While this study cannot determine the underlying mechanism of testicular dysfunction in XYY, it does suggest that subtle differences can already be appreciated in childhood.
Puberty in males is associated with sex specific increases in testosterone and inhibin B and decreases in AMH (Grinspon et al., 2012). The blunted pubertal rise in inhibin B and higher prepubertal AMH we observed in boys with XYY is also seen in Klinefelter syndrome (Christiansen, Andersson, & Skakkebaek, 2003; Davis et al., 2016). The peripubertal rise of inhibin B is FSH-independent and thought to be stimulated by the rise in intratesticular testosterone from Leydig cells responding to LH (Crofton et al., 2002). Likewise, the peripubertal fall in AMH is secondary to both increased expression of androgen receptors on Sertoli cells and increased intratesticular testosterone (Grinspon & Rey, 2010). Therefore, both the absence of a rise in inhibin B in early puberty in boys with XYY as well as the higher AMH in the prepubertal cohort may both be explained by lower intratesticular testosterone concentrations or a relative resistance to testosterone. Serum testosterone was not demonstrably different between XYY and control groups before or after puberty, reflecting the lower sensitivity of this measure to assess for subtle differences in testicular function, particularly prepubertally (Grinspon et al., 2012). The finding that differences in testicular function are present in some males with XYY starting in childhood highlights the need for longitudinal research on testicular function and fertility outcomes in this population. There have been significant advancements in fertility preservation opportunities for males with 47,XXY/Klinefelter syndrome as well as pediatric populations that are at risk for reduced fertility, such as boys undergoing cancer treatment (Johnson et al., 2017). The results of this study would suggest that XYY should be included in the efforts for studying male fertility preservation, and future research in this population should include semen analysis to assess sperm count and quality. In addition, counseling families and patients of this potential future risk is warranted.
The positive relationship between higher inhibin B and testosterone and better cognitive, academic, visual-motor, social skills and behavioral assessments was somewhat unanticipated and only observed in the XYY group. Many of these relationships did not maintain significance after Bonferroni correction, although this general trend was nearly globally observed. This suggests a unique relationship may exist between testicular function hormones and the brain in XYY, or alternatively this relationship is also present in typical males but we were unable to observe it in our controls due to less impairment in testicular function and neurodevelopment or too small cohort size. Importantly, we did not observe higher testosterone (or Sertoli cell biomarkers) to be associated with worse externalizing behaviors such as aggression or delinquency in cases or controls. Testosterone acts through neural androgen receptors to influence cell survival, anatomical connectivity, and neurochemical specification producing sex differences in brain structure and function. Changes in concentrations of testosterone in adolescence are associated with altered gray and white matter volume and microstructure, and are likely responsible for post-pubertal sex differences in the brain (Giedd et al., 1999; Herting et al., 2014; Herting et al., 2017; Raznahan et al., 2014; Shaw et al., 2008; Walhovd, Fjell, Giedd, Dale, & Brown, 2017). Like testosterone, inhibin B and AMH receptors are present on neurons throughout the brain, but there is remarkably little research done on the role of these hormones in the central nervous system aside from the hypothalamic-pituitary-gonadal axis (Morgan, Meredith, Kuo, Bilkey, & McLennan, 2011; Wang et al., 2009). Given the increased risk of autism in XYY along with the observed relationship of testosterone and inhibin B with autistic behaviors, future studies of the role of testicular hormones in boys with autism is important. This study generates new hypotheses in the interaction between testicular hormones and the brain, with implications for disorders of male hypogonadism as well as further understanding sex differences.
The positive relationship between higher inhibin B and testosterone and better cognitive, academic, visual-motor, social skills and behavioral assessments was somewhat unanticipated and only observed in the XYY group. Many of these relationships did not maintain significance after Bonferroni correction, although this general trend was nearly globally observed. This suggests a unique relationship may exist between testicular function hormones and the brain in XYY, or alternatively this relationship is also present in typical males but we were unable to observe it in our controls due to less impairment in testicular function and neurodevelopment or too small cohort size. Importantly, we did not observe higher testosterone (or Sertoli cell biomarkers) to be associated with worse externalizing behaviors such as aggression or delinquency in cases or controls. Testosterone acts through neural androgen receptors to influence cell survival, anatomical connectivity, and neurochemical specification producing sex differences in brain structure and function. Changes in concentrations of testosterone in adolescence are associated with altered gray and white matter volume and microstructure, and are likely responsible for post-pubertal sex differences in the brain (Giedd et al., 1999; Herting et al., 2014; Herting et al., 2017; Raznahan et al., 2014; Shaw et al., 2008; Walhovd, Fjell, Giedd, Dale, & Brown, 2017). Like testosterone, inhibin B and AMH receptors are present on neurons throughout the brain, but there is remarkably little research done on the role of these hormones in the central nervous system aside from the hypothalamic-pituitary-gonadal axis (Morgan, Meredith, Kuo, Bilkey, & McLennan, 2011; Wang et al., 2009). Given the increased risk of autism in XYY along with the observed relationship of testosterone and inhibin B with autistic behaviors, future studies of the role of testicular hormones in boys with autism is important. This study generates new hypotheses in the interaction between testicular hormones and the brain, with implications for disorders of male hypogonadism as well as further understanding sex differences.
Our previous study found significant prolongations of the M50 and M100 auditory responses of the left hemisphere in boys with XYY (Bloy, Ross, & Roberts, 2017). Here we found that these longer evoked responses were associated with poorer testicular function. The biological underpinnings of delays in the auditory response are hypothesized to center on atypical maturation of the thalamocortical white-matter pathways and/or atypical synaptic transmission (Roberts et al., 2009; Roberts et al., 2013). Thus, testicular dysfunction may be associated with slower maturation of the central nervous system, at least in XYY. While this relationship was not observed in the control group, the sample size was small and testicular function in the control group was normal, therefore future studies with larger sample sizes and more variability in testicular hormones are warranted. Alternatively, the finding of a strong relationship between higher AMH and prolongation of the left M100 response within the control group was intriguing. A study in kindergarten-age children found a strong negative correlation between AMH and maturity of drawings, suggesting circulating AMH may contribute to slower brain maturation in boys; no associations were found with inhibin B concentrations (Morgan, Ruffman, Bilkey, & McLennan, 2017). Although we did not assess maturation of drawings, higher AMH was associated with longer auditory latencies (i.e., slower maturation) in the left hemisphere in controls, which certainly warrants further investigation.
Insufficient prepubertal testosterone exposure has been associated with altered language organization in the brain, verbal communication, and social anxiety in boys (Alexander, 2014; Friederici et al., 2008; van Rijn, 2018). Geschwind and Galaburda proposed that less right-handedness, as well as altered cerebral dominance for language, may be related to prenatal or early testosterone deficiency (Geschwind & Galaburda, 1985). This alteration in early male hormone exposure may impair the typical maturation of the left hemisphere to gain dominance in language processing. Although we did not appreciate a relationship between current testosterone concentrations and cognitive or language abilities as we did for inhibin B concentrations, inhibin B is a much more sensitive biomarker of testicular function in childhood (Grinspon et al., 2012). Furthermore, in this cohort we noted higher rates of left handedness and footedness in XYY. These findings, together with the well-established deficiencies in language domains in XYY, suggests that altered lateralization in XYY may be secondary to early or chronic hormonal differences. Longitudinal studies assessing the effect of hormone concentrations during the minipuberty period of infancy on left-hemispheric neurocognitive function in XYY and conditions with altered testicular function are also warranted.
While we postulate that the findings of this study represent primary testicular dysfunction directly or indirectly contributing to the variability in the physical or neurodevelopmental phenotype in XYY, we cannot infer causality from this cross-sectional, exploratory study. The observed relationship between lower inhibin B and higher neurodevelopmental risk in this study could be secondary to suppression of the hypothalamic-pituitary-gonadal axis due to stress, depression, or other central nervous system disturbance (i.e., functional hypogonadism) in XYY, however, previous reports of higher gonadotropins in XYY would not support this (Bardsley et al., 2013). Alternatively, our observations may reflect a more severely affected individual secondary to genetic or environmental confounders we cannot control for. Prospective studies that allow longitudinal assessment of testicular function, growth, and neurodevelopment in XYY will be needed to confirm these observed relationships and determine causality. In addition, the sex chromosome trisomy (47,XXY), which is more classically associated with impaired testicular function, would be an important contrast group to study.
Strengths of this study include the large sample size for an underdiagnosed genetic condition and a well-matched comparison group with extensive, rigorously collected outcomes. Although the clinical research team was not blinded to the karyotype of the participants, confirmation bias was minimized as sera for hormone measurements were sent to a clinical laboratory that was unaware of the karyotype of the participants. Important limitations of this study include this was a secondary data analysis and therefore the study was not designed or powered specifically for the analyses conducted; post-hoc power calculations conclude we were sufficiently powered (>80%) to detect clinically significant differences between cases and controls for inhibin B only. Therefore, particularly for our exploratory analyses where most observations did not retain significance after adjustment for multiple comparisons, any relationships presented here should be considered preliminary and additional research will be needed to comprehensively understand the meaning of our findings. Gonadotropin concentrations would have provided additional information on testicular function, especially in pubertal boys, however, were not available to compare with controls. Finally, genetic testing was not completed for all of the controls, therefore there is a small chance that the controls were not 46,XY as presumed. Despite these limitations, these results are an important contribution to the literature for males with XYY, debunking past myths held of XYY, proposing novel insights on the relationships of Inhibin B with brain and behavior, and providing preliminary data to support future investigation in this area.
In summary, this cross-sectional study found evidence of impaired testicular function in a subset of boys with 47,XYY. Furthermore, our exploratory analysis identified a possible relationship of more intact testicular function and better neurodevelopmental outcomes. Testosterone concentrations were not associated with worse externalizing behaviors. These results are important to dispel myths about XYY syndrome, testicular function, and behavior. Additional prospective studies are needed to appreciate the natural history of testicular function in XYY and its relationship with phenotypic features.
ACKNOWLEDGEMENTS
The authors wish to thank the participants and their families. The authors have no conflicts of interest to disclose.
Funding information
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Number: K23HD092588; Fraternal Order of Eagles, Grant/Award Number: Autism Challenge grant; National Institute of Mental Health, Grant/Award Numbers: K01MH108762, R21MH109158; U.S. Department of Defense, Grant/Award Number: AR140197
REFERENCES
- Abdel-Razic MM, Abdel-Hamid IA, & ElSobky ES (2012). Nonmosaic 47,XYY syndrome presenting with male infertility: Case series. Andrologia, 44(3), 200–204. 10.1111/j.1439-0272.2010.01129.x [DOI] [PubMed] [Google Scholar]
- Aksglaede L, Skakkebaek N, & Juul A (2008). Abnormal sex chromosome constitution and longitudinal growth: Serum levels of IGF-1, IGF-BP3, LH, and testosterone in 109 males with 47,XXY, 47,XYY and SRY-positive 46,XX karyotypes. The Journal of Clinical Endocrinology and Metabolism, 93(1), 169–176. [DOI] [PubMed] [Google Scholar]
- Alexander GM (2014). Postnatal testosterone concentrations and male social development. Front Endocrinol (Lausanne), 5, 15 10.3389/fendo.2014.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autio-Harmainen H, Rapola J, & Aula P (1980). Fetal gonadal histology in XXXXY, XYY and XXX syndromes. Clinical Genetics, 18(1), 1–5. [DOI] [PubMed] [Google Scholar]
- Bardsley MZ, Kowal K, Levy C, Gosek A, Ayari N, Tartaglia N,… Ross JL (2013). 47,XYY syndrome: clinical phenotype and timing of ascertainment. The Journal of Pediatrics, 163(4), 1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloy L, Ross RJ and Roberts TP (2017). Structural and functional characteristics of XYY - Relationship to ASD. IMFAR abstracts #25635.
- Borjian Boroujeni P, Sabbaghian M, Vosough Dizaji A, Zarei Moradi S, Almadani N, Mohammadpour Lashkari F,… Mohseni Meybodi A (2019). Clinical aspects of infertile 47,XYY patients: A retrospective study. Human Fertility (Cambridge, England), 22(2), 88–93. 10.1080/14647273.2017.1353143 [DOI] [PubMed] [Google Scholar]
- Carakushansky G, Neu RL, & Gardner LI (1968). XYY with abnormal genitalia. Lancet, 2(7578), 1144 10.1016/s0140-6736(68)91622-x [DOI] [PubMed] [Google Scholar]
- Christiansen P, Andersson AM, & Skakkebaek NE (2003). Longitudinal studies of inhibin B levels in boys and young adults with Klinefelter syndrome. The Journal of Clinical Endocrinology and Metabolism, 88(2), 888–891. 10.1210/jc.2002-021379 [DOI] [PubMed] [Google Scholar]
- Coerdt W, Rehder H, Gausmann I, Johannisson R, & Gropp A (1985). Quantitative histology of human fetal testes in chromosomal disease. Pediatric Pathology, 3(2–4), 245–259. [DOI] [PubMed] [Google Scholar]
- Crofton PM, Evans AE, Groome NP, Taylor MR, Holland CV, & Kelnar CJ (2002). Inhibin B in boys from birth to adulthood: Relationship with age, pubertal stage, FSH and testosterone. Clinical Endocrinology, 56(2), 215–221. 10.1046/j.0300-0664.2001.01448.x [DOI] [PubMed] [Google Scholar]
- Crovitz HF, & Zener K (1962). A group-test for assessing hand- and eye-dominance. The American Journal of Psychology, 75, 271–276. [PubMed] [Google Scholar]
- Custer J, & Rau R (2009). Endocrinology In The Harriet lane handbook (18th ed., pp. 269–300). Elsevier. [Google Scholar]
- Davis S, Lahlou N, Bardsley MZ, Temple CM, Kowal K, Pyle L,… Ross J (2016). Gonadal function is Assocaited with Cardiometabolic health in Prepubertal boys with Klinefelter syndrome. Andrology, 4(6), 1169–1177. 10.1111/andr.12275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Dahtory F, & Elsheikha HM (2009). Male infertility related to an aberrant karyotype, 47,XYY: Four case reports. Cases Journal, 2(1), 28 10.1186/1757-1626-2-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot C (1990). Differential ability scales: Handbook. San Antonio: The Psychological Corporation. [Google Scholar]
- Flannigan R, & Schlegel PN (2017). Genetic diagnostics of male infertile-ity in clinical practice. Best Practice & Research. Clinical Obstetrics & Gynaecology, 44, 26–37. 10.1016/j.bpobgyn.2017.05.002 [DOI] [PubMed] [Google Scholar]
- Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, … Management Of High Blood Pressure In, C. (2017). Clinical practice guideline for screening and Management of High Blood Pressure in children and adolescents. Pediatrics, 140(3), e20171904 10.1542/peds.2017-1904 [DOI] [PubMed] [Google Scholar]
- Friederici AD, Pannekamp A, Partsch CJ, Ulmen U, Oehler K, Schmutzler R, & Hesse V (2008). Sex hormone testosterone affects language organization in the infant brain. Neuroreport, 19(3), 283–286. [DOI] [PubMed] [Google Scholar]
- Fryns JP, Kleczkowska A, Kubien E, & Van den Berghe H (1995). XYY syndrome and other Y chromosome polysomies. Mental status and psychosocial functioning. Genetic Counseling, 6(3), 197–206 [PubMed] [Google Scholar]
- Geschwind N, & Galaburda AM (1985). Cerebral lateralization. Biological mechanisms, associations, and pathology: II. A hypothesis and a program for research. Arch Neurol, 42(6), 521–552. 10.1001/archneur.1985.04060060019009 [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A,… Rapoport JL (1999). Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience, 2(10), 861–863. 10.1038/13158 [DOI] [PubMed] [Google Scholar]
- Grinspon RP, Loreti N, Braslavsky D, Bedecarras P, Ambao V, Gottlieb S,… Rey RA (2012). Sertoli cell markers in the diagnosis of paediatric male hypogonadism. Journal of Pediatric Endocrinology & Metabolism, 25(1–2), 3–11. 10.1515/jpem-2011-0453 [DOI] [PubMed] [Google Scholar]
- Grinspon RP, & Rey RA (2010). Anti-mullerian hormone and sertoli cell function in paediatric male hypogonadism. Hormone Research in Pediatrics, 73(2), 81–92. 10.1159/000277140 [DOI] [PubMed] [Google Scholar]
- Gripp KW, Slavotinek AM, Hall JG, & Allanson JE (2013). Handbook of physical measurements (3rd ed.). Oxford, England: Oxford University Press. [Google Scholar]
- Hamdi SM, Almont T, Galinier P, Mieusset R, & Thonneau P (2017). Altered secretion of Sertoli cells hormones in 2-year-old prepubertal cryptorchid boys: A cross-sectional study. Andrology, 5(4), 783–789. 10.1111/andr.12373 [DOI] [PubMed] [Google Scholar]
- Herting MM, Gautam P, Spielberg JM, Kan E, Dahl RE, & Sowell ER (2014). The role of testosterone and estradiol in brain volume changes across adolescence: A longitudinal structural MRI study. Human Brain Mapping, 35(11), 5633–5645. 10.1002/hbm.22575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Kim R, Uban KA, Kan E, Binley A, & Sowell ER (2017). Longitudinal changes in pubertal maturation and white matter microstructure. Psychoneuroendocrinology, 81, 70–79. 10.1016/j.psyneuen.2017.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildorf S, Dong L, Thorup J, Clasen-Linde E, Yding Andersen C, & Cortes D (2019). Sertoli cell number correlates with serum inhibin B in infant Cryptorchid boys. Sexual Development, 13(2), 74–82. 10.1159/000497374 [DOI] [PubMed] [Google Scholar]
- Hofherr SE, Wiktor AE, Kipp BR, Dawson DB, & Van Dyke DL (2011). Clinical diagnostic testing for the cytogenetic and molecular causes of male infertility: The Mayo Clinic experience. Journal of Assisted Reproduction and Genetics, 28(11), 1091–1098. 10.1007/s10815-011-9633-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A, & Redlich F (1958). Social class and mental illness. New York: John Wiley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson B, Burger H, Wiener S, Sutherland G, & Bartholomew AA (1969). Plasma testosterone and luteinising hormone in XYY men. Lancet, 2(7622), 699 10.1016/s0140-6736(69)90412-7 [DOI] [PubMed] [Google Scholar]
- Johnson EK, Finlayson C, Rowell EE, Gosiengfiao Y, Pavone ME, Lockart B,… Woodruff TK (2017). Fertility preservation for Pediatric patients: Current state and future possibilities. The Journal of Urol0ogy, 198(1), 186–194. 10.1016/j.juro.2016.09.159 [DOI] [PubMed] [Google Scholar]
- Joseph L, Farmer C, Chlebowski C, Henry L, Fish A, Mankiw C, … Raznahan A (2018). Characterization of autism spectrum disorder and neurodevelopmental profiles in youth with XYY syndrome. Journal of Neurodevelopmental Disorders, 10(1), 30 10.1186/s11689-018-9248-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joustra SD, van der Plas EM, Goede J, Oostdijk W, Delemarre-van de Waal HA, Hack WW,… Wit JM (2015). New reference charts for testicular volume in Dutch children and adolescents allow the calculation of standard deviation scores. Acta Paediatrica, 104(6), e271–e278. 10.1111/apa.12972 [DOI] [PubMed] [Google Scholar]
- Kessler S, & Moos RH (1970). The XYY karyotype and criminality: A review. Journal of Psychiatric Research, 7(3), 153–170. 10.1016/0022-3956(70)90003-8 [DOI] [PubMed] [Google Scholar]
- Kim IW, Khadilkar AC, Ko EY, & Sabanegh ES Jr. (2013). 47,XYY syndrome and male infertility. Revista de Urologia, 15(4), 188–196 [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, & Tanner JM (1970). Variations in the pattern of pubertal changes in boys. Archives of Disease in Childhood, 45(239), 13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed F, Al-Yatama F, Al-Bader M, Tayel SM, Gouda S, & Naguib KK (2007). Primary male infertility in Kuwait: A cytogenetic and molecular study of 289 infertile Kuwaiti patients. Andrologia, 39 (3), 87–92. 10.1111/j.1439-0272.2007.00769.x [DOI] [PubMed] [Google Scholar]
- Morgan K, Meredith J, Kuo J-YA, Bilkey DK, & McLennan IS (2011). The sex bias in novelty preference of preadolescent mouse pups may require testicular Mullerian inhibiting substance. Behavioural Brain Research, 221(1), 304–306. 10.1016/j.bbr.2011.02.048 [DOI] [PubMed] [Google Scholar]
- Morgan K, Ruffman T, Bilkey DK, & McLennan IS (2017). Circulating anti-Mullerian hormone (AMH) associates with the maturity of boys’ drawings: Does AMH slow cognitive development in males? Endocrine, 57(3), 528–534. 10.1007/s12020-017-1333-2 [DOI] [PubMed] [Google Scholar]
- Pelzmann KS, & Brodie HK (1976). Circulating plasma testosterone in the XYY male. Life Sciences, 18(11), 1207–1212. 10.1016/0024-3205(76)90195-8 [DOI] [PubMed] [Google Scholar]
- Price WH, & van der Molen HJ (1970). Plasma testosterone levels in males with the 47,XYY karyotype. The Journal of Endocrinology, 47(1), 117–122. 10.1677/joe.0.0470117 [DOI] [PubMed] [Google Scholar]
- Raznahan A, Shaw PW, Lerch JP, Clasen LS, Greenstein D, Berman R, … Giedd JN (2014). Longitudinal four-dimensional mapping of subcortical anatomy in human development. Proceedings of the National Academy of Sciences of the United States of America, 111(4), 1592–1597. 10.1073/pnas.1316911111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rives N, Milazzo JP, Miraux L, North MO, Sibert L, & Mace B (2005). From spermatocytes to spermatozoa in an infertile XYY male. International Journal of Andrology, 28(5), 304–310. 10.1111/j.1365-2605.2005.00540.x [DOI] [PubMed] [Google Scholar]
- Roberts TP, Khan SY, Blaskey L, Dell J, Levy SE, Zarnow DM, & Edgar JC (2009). Developmental correlation of diffusion anisotropy with auditory-evoked response. Neuroreport, 20(18), 1586–1591. 10.1097/WNR.0b013e3283306854 [DOI] [PubMed] [Google Scholar]
- Roberts TP, Lanza MR, Dell J, Qasmieh S, Hines K, Blaskey L,… Berman JI (2013). Maturational differences in thalamocortical white matter microstructure and auditory evoked response latencies in autism spectrum disorders. Brain Research, 1537, 79–85. 10.1016/j.brainres.2013.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavi R, Theilgaard A, Owen D, & White D (1984). Sex chromosome anomalies, hormones, and Aggressivity. Archives of General Psychiatry, 41, 93–99 [DOI] [PubMed] [Google Scholar]
- Sciurano RB, Rahn IM, Gonzalez Arias B, Rey Valzacchi G, Benavente R, & Solari AJ (2019). Selective advantage of euploid spermatocytes I in an azoospermic 47,XYY man with gonadal mosaicism. Human Reproduction, 34(3), 568–573. 10.1093/humrep/dey387 [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, … Wise SP (2008). Neurodevelopmental trajectories of the human cerebral cortex. The Journal of Neuroscience, 28(14), 3586–3594. 10.1523/JNEUROSCI.5309-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stochholm K, Bojesen A, Jensen AS, Juul S, & Gravholt CH (2012). Criminality in men with Klinefelter’s syndrome and XYY syndrome: A cohort study. BMJ Open, 2(1), e000650 10.1136/bmjopen-2011-000650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stochholm K, Juul S, & Gravholt CH (2010). Diagnosis and mortality in 47,XYY persons: A registry study. Orphanet Journal of Rare Diseases, 5, 15 10.1186/1750-1172-5-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stochholm K, Juul S, & Gravholt CH (2012). Socioeconomic factors affect mortality in 47,XYY syndrome-a comparison with the background population and Klinefelter syndrome. American Journal of Medical Genetics. Part A, 158A(10), 2421–2429. 10.1002/ajmg.a.35539 [DOI] [PubMed] [Google Scholar]
- Tartaglia NR, Wilson R, Miller JS, Rafalko J, Cordeiro L, Davis S, … Ross J (2017). Autism Spectrum disorder in males with sex chromosome aneuploidy: XXY/Klinefelter syndrome, XYY, and XXYY. Journal of Developmental and Behavioral Pediatrics, 38(3), 197–207. 10.1097/DBP.0000000000000429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilgaard A (1983). Aggression and the XYY personality. International Journal of Law and Psychiatry, 6(3–4), 413–421. 10.1016/0160-2527(83)90028-6 [DOI] [PubMed] [Google Scholar]
- van Rijn S (2018). Salivary testosterone in relation to social cognition and social anxiety in children and adolescents with 47,XXY (Klinefelter syndrome). PLoS One, 13(7), e0200882 10.1371/journal.pone.0200882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Giedd J, Dale AM, & Brown TT (2017). Through thick and thin: A need to reconcile contradictory results on trajectories in human cortical development. Cerebral Cortex, 27(2), 1472–1481. 10.1093/cercor/bhv301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P-Y, Protheroe A, Clarkson AN, Imhoff F, Koishi K, & McLennan IS (2009). Mullerian inhibiting substance contributes to sex-linked biases in the brain and behavior. Proceedings of the National Academy of Sciences, 106(17), 7203–7208. 10.1073/pnas.0902253106. [DOI] [PMC free article] [PubMed] [Google Scholar]


