Abstract
Objective:
To examine fertility counseling and fertility preservation (FP) referrals for young women with Turner syndrome (TS) at pediatric centers and identify possible associations with patient demographic and medical characteristics.
Design:
Retrospective medical record review.
Setting:
Pediatric academic medical centers.
Patient(s):
Four hundred and sixty-nine young women with TS (mean age = 14 years, standard deviation 8.5 years; 77% white) who received care between March 2013 and March 2018.
Intervention(s):
None.
Main Outcome Measure(s):
Standardized form to abstract demographics, medical (karyotype; menarchal status; developmental, neuropsychological, and psychological concerns), and treatment characteristics (duration of care, receipt of multidisciplinary care, documentation of fertility/pregnancy counseling, FP specialist referrals) from medical records.
Result(s):
We found that 67% of families had documented fertility counseling, although only 27% of charts documented counseling with patients specifically. Only 10% of patients were referred to a FP specialist; 59% of patients with spontaneous menarche had no referral. Pregnancy risk counseling was documented in 38% of charts. In multivariate analyses, families were more likely to receive counseling if the patients had multidisciplinary care (adjusted odds ratio [AOR] 2.82). Greater duration of care (AOR 1.16); mosaic (AOR 47.94), complex (AOR 14.59), or partial deletions karyotypes (AOR 35.69); spontaneous menarche (AOR 4.65); and multidisciplinary care (AOR 4.02) had increased odds of FP specialist referrals. Patients with developmental concerns (AOR 0.08) had decreased odds of referrals.
Conclusion(s):
Fertility and pregnancy counseling are not routinely documented among patients with TS, and even patients with a limited window of reproductive potential were infrequently referred to FP specialists. Patients seen in multidisciplinary clinics were more likely to receive recommended counseling.
Keywords: Fertility, pediatric, preservation, Turner syndrome
Abstract
Objetivo:
Examinar las derivaciones para el asesoramiento sobre fertilidad y preservación de la fertilidad (PF) para mujeres jóvenes con síndrome de Turner (ST) en centros pediátricos e identificar posibles asociaciones con las características demográficas y médicas de los pacientes.
Diseño:
Revisión retrospectiva de los registros médicos.
Entorno:
Centros médicos académicos pediátricos.
Paciente(s):
Cuatrocientas sesenta y nueve mujeres jóvenes con ST (edad media = 14 años, desviación estándar 8.5 años; 77% blancas) que han sido atendidas entre marzo de 2013 y marzo de 2018.
Intervención(es):
Ninguno.
Variables principales:
Formulario estandarizado para la recogida de datos demográficos, médicos (cariotipo; menarquía; datos sobre el desarrollo neuropsicológico y psicológico), y las características del tratamiento (duración de la atención, recepción de atención multidisciplinaria, documentación de asesoramiento de fertilidad/embarazo, referencias de especialistas en PF) desde los registros médicos.
Resultado(s):
Encontramos que el 67% de las familias habían documentado asesoramiento sobre fertilidad, aunque sólo el 27% de las cartas documentadas con las pacientes específicamente. Sólo el 10% de las pacientes fueron remitidas a un especialista en PF; 59% de las pacientes con menarquía espontánea no habían sido derivadas. El asesoramiento sobre los riesgos del embarazo se documentó en el 38% de las cartas. En los análisis multivariados, las familias eran más propensas a recibir asesoramiento si los pacientes tenían atención multidisciplinaria (relación de probabilidades ajustada [AOR] 2.82). Mayor duración de la atención (AOR 1.16); Mosaico (AOR 47.94), complejo (AOR 14.59) o deleciones parciales en cariotipos (AOR 35.69); menarquía espontánea (AOR 4.65); y cuidados multidisciplinarios (AOR 4.02) habían aumentado las probabilidades de ser derivadas a los especialistas en PF. En las pacientes con problemas de desarrollo (AOR 0.08) se había visto una disminución en sus probabilidades de ser remitidas.
Conclusión(s):
El asesoramiento en fertilidad y embarazo no se documenta de rutina en las pacientes con ST, y sobre todo aquellas con un potencial reproductivo limitado han sido remitidas con poca frecuencia a los especialistas en PF. Las pacientes atendidas en clínicas multidisciplinarias han sido más propensas a recibir el asesoramiento recomendado.
With an estimated prevalence of 25–50 per 100,000 females (1), Turner syndrome (TS) is a condition characterized by chromosome abnormalities and a varying number of associated medical and psychosocial morbidities (2). Turner syndrome is caused by partial or complete loss of an X chromosome leading to haploinsufficiency of genes and contributing to advanced follicular atresia, primary amenorrhea, and infertility in the majority of women (the mechanism of which is not fully understood) (3, 4). Women with TS are also at a higher risk than the general population for mental health conditions (e.g., anxiety) and developmental, social, and neuropsychological concerns (e.g., learning disabilities) (5, 6).
Many of the morbidities associated with TS (e.g., short stature, ovarian insufficiency, developmental concerns, cardiac abnormalities) may negatively impact overall quality of life (7). Specifically, ovarian failure and infertility are rated by patients as the most distressing effect of TS (8, 9) and have been associated with depression (8). These findings are similar to those from studies of other adolescent and young adult populations at risk for infertility, including cancer survivors, which have demonstrated the negative effects of fertility concerns on quality of life and psychosocial functioning (10, 11).
Studies have shown that caregivers of youth with TS worry about when/how to approach discussions about infertility, resulting in delayed discussions about ovarian function, fertility preservation (FP), or coping with potential infertility (12). In addition to the psychosocial implications, the timeliness of fertility-related discussions with caregivers and providers may impact a youth’s reproductive options, as oocyte depletion in TS is progressive. The previous literature has noted that infertility affects 95% to 98% of women with TS, and the majority require hormone therapy to initiate puberty (2, 13, 14); however, up to 30% will experience spontaneous puberty, indicating there may be a window to consider FP (15). Established FP methods include oocyte and embryo cryopreservation, and ovarian tissue cryopreservation remains experimental (16). Individuals with a mosaic karyotype are more likely to experience spontaneous puberty, but premature ovarian insufficiency is highly likely (17). Therefore, especially for individuals who experience spontaneous puberty, timely fertility counseling and exploration of FP options is crucial.
Fertility counseling in TS should be comprehensive (2, 4, 17) and include discussions about the health implications of spontaneous or assisted pregnancy in individuals with TS, alternate options to pregnancy, and other considerations such as prenatal genetic testing. Due to reports of fatal aortic dissection during pregnancy, some professionals have advocated that pregnancy is not safe for anyone with TS, and that a gestational carrier could be considered instead (18). Pregnancies in TS are also associated with higher risks of miscarriage, intrauterine fetal death, and cesarean delivery (19). The international consensus guidelines are supportive of pregnancy in select candidates after ensuring appropriate evaluations and counseling are completed and the pregnancy is closely monitored by multidisciplinary teams with expertise in TS, including a cardiologist and maternal-fetal medicine specialists (1, 15).
The guidelines for TS recommend fertility counseling starting at a young age, with discussions about the risks and potential FP options (1). Specifically, young mosaic TS women with persistent ovarian function should be counseled regarding their options for preservation. Further, management of pregnant women with TS should be undertaken by a multidisciplinary team that includes maternal-fetal medicine specialists and cardiologists (1). Yet more information is needed about how these guidelines are implemented by medical providers caring for patients with TS. It is unknown whether fertility-related counseling is routinely taking place, whether the patients themselves are involved in the counseling, and whether FP and pregnancy-related risks are addressed. Thus, the goal of this multisite study was to evaluate current practices in fertility/pregnancy counseling and FP specialist referrals among young women with TS in pediatric centers and explore their associations with patient demographic characteristics (e.g., insurance type, race), clinical characteristics (e.g., karyotype, spontaneous menarche, developmental concerns), and type of clinical setting.
MATERIALS AND METHODS
Procedures
After receiving institutional review board approval, we conducted a retrospective review of electronic medical records (EMRs) and paper medical charts of individuals with TS from June to December 2018 at two large pediatric academic medical centers: Site A (Midwest) and Site B (West). At Site A, patients with TS are seen by a number of different providers in various disciplines separately (e.g., endocrinology, genetics, cardiology). At Site B approximately one-third of patients with TS are seen in a multidisciplinary clinic, which was established in 2015. The EMRs were queried to identify pheno-typically female patients with a diagnosis of TS (including variants/mosaicism) seen in a TS-related specialty between March 29, 2013, and March 29, 2018. Patients were excluded if they were deceased or had no TS-related care (e.g., patient had only been seen in emergency/urgent care or a specialty unrelated to TS). Per the TS clinical guidelines (20), patients with genital ambiguity or male phenotype were excluded.
Data Collection
A standardized abstraction form, developed by three pediatric endocrinologists and two psychologists, was used by six research staff members to collect the following information from the patients’ EMR: demographics; karyotype (non-mosaic 45,X, mosaic with a 46,XX or 47,XXX cell line, complex karyotypes including ring and isochromosomes, partial deletions of the X chromosome, or 45,X/46,XY); cardiac abnormalities; spontaneous menarche (documented menses in the absence of estrogen/progesterone treatment); developmental concerns (e.g., global developmental delay, autism); neuropsychological concerns/diagnoses (e.g., attention-deficit/hyperactivity disorder, learning disabilities); and psychological concerns/diagnoses (e.g., anxiety, depression). Charts were reviewed for discussion of fertility implications of TS by any provider, including but not limited to the increased risk of infertility, reduced number of oocytes or follicles, measurement of antimüllerian hormone to assess follicle reserve, fertility preservation options, or other counseling directly related to fertility. Discussion of hormone deficiencies or puberty differences alone were not sufficient to qualify as fertility counseling. It was noted if any fertility counseling occurred with the parent and/or the patient herself. Referral ordered or documentation of an offer for a referral to a specialist to discuss FP options was recorded. Finally, documented counseling of pregnancy health risks in TS were recorded as potential risks to the mother including but not limited to aortic dissection or potential risks to the fetus, such as intrauterine growth restriction. If there was no documentation of counseling or referrals in the EMR or paper chart, this was determined to not have occurred. At least 50% of charts were randomly selected and verified by a second investigator. If there was ambiguity in the medical record or aspects that required clinical interpretation, the chart was reviewed by a physician on the study.
Analyses
Primary outcomes were documentation of fertility counseling with the [1] family and [2] patient, [3] referral to a specialist to discuss FP, and [4] counseling of TS-specific pregnancy risks. Explanatory variables were chosen a priori due to potential associations with differences in counseling practices and included patient age, race, and ethnicity; insurance type (as a marker of socioeconomic status); karyotype; spontaneous menarche; cardiac abnormality; and developmental, neuropsychological, or psychological diagnoses. Descriptive statistics were used to summarize demographics; medical, physical, and psychological characteristics; duration of care; care receipt in a TS multidisciplinary clinic; counseling regarding fertility with family and patient; whether the patient was referred to a specialist to discuss FP; and counseling of pregnancy-related risks.
Site differences were evaluated using chi-square or Fisher’s exact tests for categorical variables and Mann-Whitney U tests for continuous variables (age) due to data not being normally distributed. Univariate logistic regression explored associations between patient demographic and clinical characteristics and primary outcomes. Covariates found to be statistically significantly associated with primary outcomes in univariate analyses (P<.10) were entered into multivariate logistic regression models for each outcome after determining that covariates were not collinear. Missing data were managed using pairwise deletion. Analyses were performed using SPSS version 24.0 (Armonk, NY).
RESULTS
Demographic and Medical Characteristics
Of the 469 patients who met the inclusion criteria, 176 were from site A and 293 from site B. Demographic/clinical characteristics for the total sample and each site are presented in Table 1. The mean age at last visit was 13.5 years (standard deviation [SD] = 8.2). The majority of patients were white (77%; n = 359), and approximately half had private/commercial insurance (55%; n = 256). Forty-four percent of patients had a 45,X karyotype (n = 190). Among patients aged 9 years and older at time of last visit, 17% (n = 58) had documented spontaneous menarche. Of the total sample, 44% had documented developmental concerns (n = 205), 53% had documented neuropsychological concerns/diagnoses (n = 246), and 26% had documented psychological concerns/diagnoses (n = 120). Twenty-four percent of patients (n = 110) had been seen in a multidisciplinary TS clinic.
TABLE 1.
Sample demographic/medical characteristics.
| Characteristic | Overall (n = 469) | Site A (n = 176) | Site B (n = 293) | P value |
|---|---|---|---|---|
| Age at last visit (mean, SD) | 13.5 (8.2) | 15.4 (10.3) | 12.4 (6.4) | .02a |
| Duration of care, years (mean, SD) | 5.3 (4.5) | 4.7 (4.0) | 5.6 (4.7) | .06 |
| Race | <.0001a | |||
| White | 359 (76.5) | 133 (75.6) | 226 (77.1) | |
| Asian | 13 (2.8) | 7 (4.0) | 6 (2.0) | |
| Black/African American | 38 (8.1) | 29 (16.5) | 9 (3.1) | |
| American Indian/Alaskan Native | 2 (0.4) | — | 2 (0.7) | |
| More than one race | 30 (6.4) | 7 (4.0) | 23 (7.8) | |
| Unknown/not reported | 27 (5.8) | — | 27 (9.2) | |
| Ethnicity | <.001a | |||
| Hispanic/Latino | 90 (19.2) | 6 (3.4) | 84 (30.1) | |
| Non-Hispanic | 362 (77.2) | 167 (94.9) | 195 (69.9) | |
| Refused/not reported | 17 (3.6) | 3 (1.7) | 14 (4.8) | |
| Insurance type | .06 | |||
| Private/commercial | 256 (54.6) | 88 (50.0) | 168 (57.3) | |
| Medicaid | 205 (43.7) | 88 (50.0) | 117 (39.9) | |
| Unknown | 8 (1.7) | — | 8 (2.7) | |
| Karyotype | .20 | |||
| 45,X | 190 (40.5) | 65 (36.9) | 125 (42.7) | |
| Mosaicb | 87 (18.6) | 26 (14.8) | 61 (20.8) | |
| Complex | 115 (24.5) | 52 (29.5) | 63 (21.5) | |
| Partial deletions | 26 (5.5) | 9 (5.1) | 17 (5.8) | |
| 45,X/46,XY | 18 (3.8) | 6 (3.4) | 12 (4.1) | |
| Unknown | 33 (7.0) | 18 (10.2) | 15 (5.1) | |
| Cardiac abnormalities | .79 | |||
| Yes | 155 (33.0) | 57 (32.4) | 98 (33.4) | |
| No | 314 (67.0) | 119 (67.6) | 195 (66.6) | |
| Spontaneous menarche (patients ≥9 y) | .62 | |||
| Yes | 58 (17.3) | 22 (16.8) | 36 (17.6) | |
| No | 221 (66.0) | 76 (58.0) | 145 (71.1) | |
| Unknown | 53 (16.7) | 33 (25.2) | 23 (11.3) | |
| Concerns/diagnoses | ||||
| Developmental | 205 (43.7) | 82 (46.6) | 123 (42.0) | .33 |
| Neuropsychological | 246 (52.5) | 85 (48.3) | 161 (54.9) | .16 |
| Psychological | 120 (25.6) | 60 (34.1) | 60 (20.5) | .001a |
| Multidisciplinary clinic | 110 (23.5) | — | 110 (37.5) | — |
Note: Values are n (%) unless otherwise indicated. SD = standard deviation.
Significant values.
45,X/46,XX or 45,X/47,XXX.
Fertility Counseling with Families
Sixty-seven percent of families had documented fertility counseling (n = 313) (Fig. 1). In univariate analysis, only age at last visit and receipt of multidisciplinary care were statistically significantly associated with counseling with families (Table 2). In the multivariate model, receipt of multidisciplinary care remained associated with increased odds (adjusted odds ratio [AOR] 2.82; P=.005) of fertility counseling (Table 3).
FIGURE 1.
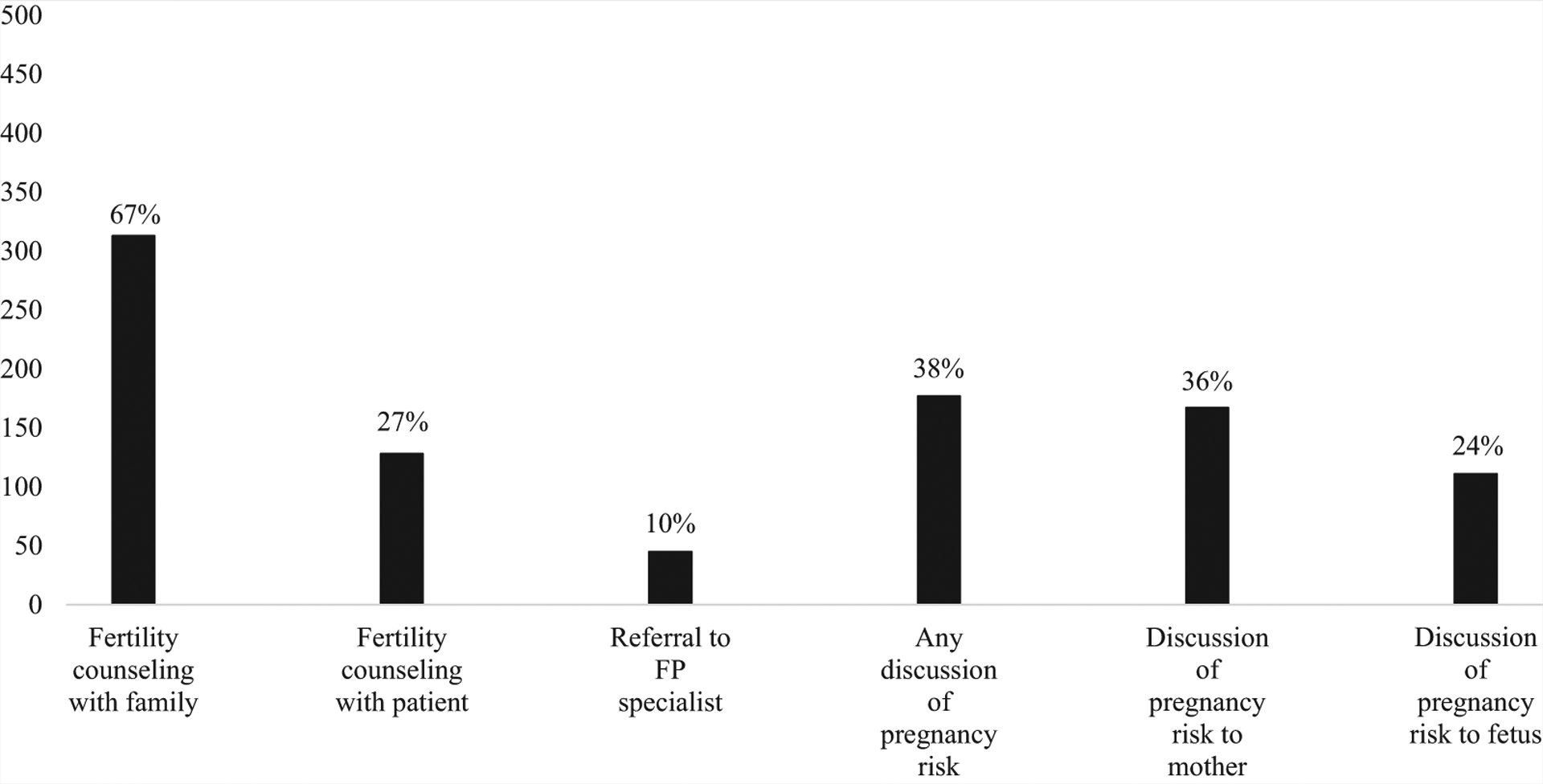
Fertility counseling, referral to a fertility preservation specialist, and pregnancy counseling.
TABLE 2.
Outcomes of interest by patient characteristics.
| Covariate | Fertility counseling with family | Fertility counseling with patient | FP specialist referral | Pregnancy counseling | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Age at last visit, M(SD) | 0.96 (0.94, 0.99) | .002a | 1.14 (1.10, 1.18) | <.001a | 1.03 (1.00, 1.07) | .045a | 1.01 (0.99, 1.03) | .525 |
| Duration of care in years (M, SD) | 0.97 (0.93, 1.01) | .107 | 1.07 (1.02, 1.12) | .003a | 1.07 (1.00, 1.14) | .047a | 0.99 (0.95, 1.04) | .795 |
| Insurance type, Medicaid/self-pay | 1.07 (0.72, 1.58) | .746 | 0.60 (0.39, 0.92) | .018a | 0.51 (0.26, 1.01) | .052a | 0.84 (0.58, 1.23) | .380 |
| Karyotype 45,XO (ref) | — | — | — | — | — | — | — | — |
| Mosaicb | 1.39 (0.78, 2.49) | .264 | 2.88 (1.64, 5.05) | < .001a | 14.92 (5.48, 40.65) | <.001a | 1.31 (0.78, 2.18) | .308 |
| Complex | 0.80 (0.49, 1.31) | .371 | 2.22 (1.31, 3.78) | .003a | 2.77 (0.88, 8.67) | .081a | 0.82 (0.51, 1.32) | .413 |
| Partial deletions | 1.20 (0.48, 3.02) | .695 | 2.27 (0.93, 5.50) | .070a | 8.81 (2.36, 32.95) | .001a | 0.96 (0.41, 2.22) | .921 |
| 45,X/46,XY | 0.89 (0.32, 2.47) | .817 | 1.22 (0.38, 3.93) | .737 | 0.00 | .999 | 0.98 (0.36, 2.63) | .961 |
| Cardiac abnormalities | 0.88 (0.59, 1.32) | .531 | 0.80 (0.52, 1.24) | .324 | 0.63 (0.31, 1.28) | .200 | 1.06 (0.71, 1.57) | .780 |
| Spontaneous menarche | 1.87 (0.96, 3.62) | .065a | 3.65 (1.99, 6.71) | <.001a | 9.27 (4.42, 19.44) | <.001a | 2.03 (1.13, 3.65) | .018a |
| Concerns/diagnoses | ||||||||
| Developmental | 0.95 (0.64, 1.39) | .776 | 0.35 (0.22, 0.54) | < .001a | 0.14 (0.05, 0.36) | <.001a | 0.99 (0.68, 1.45) | .976 |
| Neuropsychological | 1.07 (0.73, 1.58) | .719 | 1.46 (0.97, 2.21) | .071a | 2.13 (1.10, 4.12) | .024 | 1.28 (0.88, 1.87) | .197 |
| Psychological | 0.90 (0.58, 1.39) | .625 | 1.81 (1.16, 2.83) | .009a | 1.06 (0.53, 2.13) | .868 | 1.57 (1.03, 2.39) | .036a |
| Multidisciplinary clinic | 3.43 (1.96, 6.00) | < .001a | 1.75 (1.11, 2.76) | .017a | 2.16 (1.13, 4.12) | .019a | 6.02 (3.77, 9.62) | < .001a |
Note: CI = confidence interval; FP = fertility preservation; OR = odds ratio; ref = reference.
Values that are P< .10.
45,X/46,XX or 45,X/47,XXX.
TABLE 3.
Multivariate models predicting outcomes of interest.
| Covariate | AOR (95% CI) | P value |
|---|---|---|
| Fertility counseling with | ||
| family | ||
| Age at last visit | 0.97 (0.93, 1.00) | .082 |
| Spontaneous menarche | 1.78 (1.04, 4.10) | .105 |
| Multidisciplinary clinic | 2.82 (1.37, 5.82) | .005a |
| Fertility counseling with patient | ||
| Age at last visit | 1.25 (1.15, 1.36) | <.001a |
| Duration of care | 0.97 (0.90, 1.04) | .381 |
| Medicaid insurance | 0.74 (0.40, 1.37) | .338 |
| Karyotype 45,XO (ref) | — | |
| Mosaicb | 3.97 (1.42, 11.10) | .009a |
| Complex | 2.69 (1.27, 5.69) | .010a |
| Partial deletions | 2.46 (0.72, 8.40) | .150 |
| 45,X/46,XY | 0.78 (0.16, 3.85) | .762 |
| Spontaneous menarche | 1.29 (0.51, 3.22) | .592 |
| Developmental concerns/diagnoses | 0.46 (0.24, 0.89) | .023a |
| Neuropsychological concerns/diagnoses | 0.85 (0.43, 1.67) | .641 |
| Psychological concerns/diagnoses | 1.12 (0.55, 2.24) | .760 |
| Multidisciplinary clinic | 7.35 (3.39, 15.94) | <.001a |
| FP specialist referral | ||
| Age at last visit | 0.97 (0.89, 1.05) | .419 |
| Duration of care | 1.16 (1.04, 1.30) | .006a |
| Medicaid insurance | 0.44 (0.16, 1.21) | .112 |
| Karyotype 45,XO (ref) | — | |
| Mosaicb | 47.94 (6.63, 346.55) | <.001a |
| Complex | 14.59 (2.28, 93.28) | .005a |
| Partial deletions | 35.69 (4.21, 302.87) | .001a |
| 45,X/46,XY | 0.00 | .999 |
| Spontaneous menarche | 4.65 (1.48, 14.58) | .008a |
| Developmental concerns/diagnoses | 0.08 (0.02, 0.31) | <.001a |
| Neuropsychological concerns/diagnoses | 1.94 (0.70, 5.38) | .205 |
| Multidisciplinary clinic | 4.02 (1.40, 11.54) | .010a |
| Pregnancy counseling | ||
| Spontaneous menarche | 2.30 (1.24, 4.25) | .008a |
| Psychological concerns/diagnoses | 1.36 (0.80, 2.30) | .254 |
| Multidisciplinary clinic | 6.91 (3.67, 13.01) | <.001a |
Note: Covariates were only included in multivariate analyses if univariate analyses were statistically significant at P< .10 (see Table 2). AOR = adjusted odds ratio; CI = confidence interval; ref = reference.
Values that are P< .05.
45,X/46,XX or 45,X/47,XXX.
Fertility Counseling with Patients
In 27% of cases, fertility counseling was documented specifically with the patient (n = 128) (see Fig. 1). In univariate analyses, counseling with patients was associated with their age at last visit, karyotype, presence of psychological or developmental concerns, insurance type, and history of multidisciplinary care (see Table 2). In the multivariate model, greater patient age at last visit (AOR 1.25; P<.001), mosaic (AOR 3.97; P=.009) and complex (AOR 2.69; P=.01) karyotypes, and receipt of multidisciplinary care (AOR 7.35; P<.001) remained associated with increased odds of fertility counseling. Having developmental concerns (AOR 0.46; P=.02) remained associated with reduced odds of fertility counseling (see Table 3).
Referral for FP
Notably, only 10% of patients (n = 45) were referred to a specialist to discuss FP (see Fig. 1), and only three of these patients pursued FP with oocyte retrieval. Patients 9 years and older who had spontaneous menarche (odds ratio [OR] 9.27; P<.001) were more likely to be referred to a specialist to discuss FP than their peers without spontaneous menarche (see Table 2). However, 59% (n = 34) of patients who had spontaneous menarche had no documented referral to a FP specialist. Age at last visit, duration of care, karyotype, and neuropsychological concerns were statistically significantly associated with FP in univariate analysis (see Table 2). In the multivariate model, patients with developmental concerns (AOR 0.08; P<.001) had reduced odds of referral. Patients with longer duration of care (AOR 1.16; P=.006); mosaic (AOR 47.94; P<.001), complex (AOR 14.59; P=.005), and partial deletion karyotypes (AOR 35.69; P=.001); spontaneous menarche (AOR 4.65; P=.008); and history of multidisciplinary care (AOR 4.02; P=.01) had greater odds of receiving a referral to an FP specialist (see Table 3).
Pregnancy Counseling
Thirty-six percent of families/patients had discussions regarding potential risks of pregnancy to the mother (n = 167), and 24% had discussions about potential risks to the fetus (n = 111) (see Fig. 1). Sixty-two percent of families/patients were not counseled on any potential pregnancy-related risks. As shown in Table 2, spontaneous menarche, psychological diagnoses, and multidisciplinary care were associated with pregnancy counseling in univariate analysis. Patients 9 years and older who had undergone spontaneous menarche (OR 2.03; P=.02), had psychological diagnoses (OR 1.57; P=.04), and received multidisciplinary care (OR 6.02; P<.001) were more likely to be counseled about potential risks of pregnancy than their peers. In the multivariate model, spontaneous menarche (AOR 2.30; P=.008) and multidisciplinary care (AOR 6.91; P<.001) were both associated with increased odds of pregnancy counseling (see Table 3).
DISCUSSION
International TS clinical practice guidelines (1) recommend fertility counseling regarding infertility risk, potential FP options, and pregnancy risks in individuals with TS starting at a young age, yet our study demonstrates that documentation of counseling is not routinely occurring. While two-thirds of families had some fertility counseling, patients themselves (even those who experienced spontaneous menarche) were infrequently counseled regarding fertility and were rarely referred to FP specialists. Additionally, more than half of EMRs reviewed in this study had no documentation of counseling on pregnancy health risks.
Previous literature has found that potential infertility is of major concern for patients with TS and their families (21) and that sharing information about the high likelihood of infertility in patients with TS is particularly challenging for parents (12). Parents often feel they have inadequate knowledge to discuss fertility with their daughters which is compounded by the social stigma of infertility, desire for their daughter to have biological children, and their own loss of having a biological grandchild (12). These challenges make it even more important for medical providers to partner with parents and facilitate fertility-related discussions as part of routine care.
Recent research in other pediatric, adolescent, and young adult populations has shown that uncertainty about infertility causes distress and may have a negative impact on quality of life (10, 11, 22–24). Further, it is also possible patients (if not receiving counseling about fertility potential and/or contraceptive options) may incorrectly assume they are infertile, an assumption supported by recent reports of unplanned pregnancies among women with TS (25). These unplanned pregnancies may be occurring before any discussions about family planning, contraception, and TS-specific health concerns during pregnancy and alternate reproductive options, such as egg donation and surrogacy. Therefore, it is crucial to have timely discussions about fertility and reproductive health risks (particularly with patients who have a higher likelihood of spontaneous pregnancy and those considering FP) to ensure that patients know they would need to follow-up with specialists before/during pregnancy to prevent fatal aortic dissection, miscarriage, and intrauterine fetal death (13, 25).
Fertility counseling is innately challenging, particularly in pediatric populations. These discussions are complex and sensitive, including questions addressing sexual maturity and invasive procedures. Previous research has shown that medical providers desire more guidance and training in this area (26, 27). Specifically, many pediatric endocrinologists believe they are not the best suited or well-trained to facilitate fertility-related discussions (27). Prior research has found provider recommendation to be associated with self-efficacy and successful use of FP, highlighting the importance of more training in this area (28). A recent American Academy of Pediatrics (AAP) clinical report provides guidance regarding fertility and sexual function counseling among a broad range of at-risk pediatric populations at various ages and/or developmental stages (29). In our study, families of younger patients were more likely to have received fertility counseling, suggesting these types of guidelines may be increasing the frequency of these discussions.
Very few patients in our study (even who experienced spontaneous menarche with a potentially limited window to consider FP) were referred to a fertility specialist to discuss FP options. Recent research in adolescents with cancer demonstrated referral to a fertility specialist was a strong predictor of FP use (30); however, pediatric oncologists frequently report barriers to accessing specialists and/or a lack of understanding of how to coordinate such care (31).
Discussions of FP are particularly complicated in TS as a reduced follicle pool further limits FP options (21). Ovarian tissue cryopreservation remains experimental and is not an available option at many institutions for women with TS, including the two medical centers in this study (32). Thus, the only established FP option for individuals with TS is oocyte (or embryo) cryopreservation, requiring the patient to be postmenarchal (21). Guidelines for TS recommend patients with a mosaic karyotype with ovarian function be counseled on oocyte cryopreservation following controlled ovarian stimulation (1). As expected, the patients in our study who had spontaneous menarche, a mosaic karyotype, and absence of developmental concerns were more likely to be referred to a FP specialist.
Additionally, families in our study with private insurance were more likely to be referred to a fertility specialist than those on Medicaid, which is consistent with prior literature in which cost is known to be a barrier to FP (33). Specifically, providers treating patients of lower socioeconomic status may not offer FP, which tends to be expensive and require out-of-pocket costs for families (33). Even among patients with private insurance, the costs may be a barrier to referral for FP services as these visits are often not a covered benefit, and consultation/procedural costs are estimated to range from $1,000 to more than $18,000 per cycle, though this may vary throughout the country (34). Further examination of these practice patterns should be conducted as more states establish mandated insurance coverage for FP among at-risk populations (35).
Notably, patients seen in a TS multidisciplinary clinic in our study were more likely to receive fertility and pregnancy counseling and referrals to FP specialists, suggesting that a multidisciplinary infrastructure may mitigate some of the barriers we have described. Although little evidence exists in TS specifically, multidisciplinary care models have been shown to improve health-care outcomes in multiple other chronic pediatric conditions, and the TS clinical practice guidelines endorse multidisciplinary care (20, 36). The TS multidisciplinary site in this study includes consistent clinicians in genetics, endocrinology, cardiology, gynecology, psychology, and developmental pediatrics who have an interest and expertise in TS, including the fertility implications. In addition to providing counseling in clinic, the team has created a patient handout with information on family planning considerations in TS and has an established relationship with both academic and private practice FP specialists for referrals.
Beyond all of the clinical considerations we have outlined, important ethical challenges may arise in the context of fertility and reproductive health discussions in TS. Ethical dilemmas are common in fertility and reproductive counseling in general, and they are particularly salient for pediatric patients who may not be able to make autonomous decisions for their futures due to young age and/or developmental delays. According to the AAP guidelines, assent of the patient should include ensuring developmental appropriateness to make such decisions as well as awareness of condition (37). Fertility-related decisions are often within the jurisdiction of parents, as they have the right to protect an open future for their children (38). However, patients with significant developmental delays, as sometimes seen in TS, require greater ethical consideration as it may be unclear whether the decision is in fact in the best interest of the child (39).
There are several limitations to these findings. As with any retrospective review of medical records, fertility-related discussions may have occurred but not have been documented; however, a lack of documentation often means that the discussion did not take place (40). On the other hand, due to templates in the EMR, there may be documentation of a discussion when in fact no discussion took place. Clinically, it is important to document detailed discussions because patients with TS are likely seen by multiple medical providers (e.g., endocrinologists, geneticists, cardiologists) and ultimately will undergo transition to adult care. Without clear and specific documentation, providers may make inaccurate assumptions about what has already been discussed with a patient. Additionally, while this study included two sites from two different regions, the majority of patients were white and had private insurance, potentially limiting generalizability.
CONCLUSION
Fertility-related discussions among females with TS are not occurring routinely and are not meeting guidelines of care. Although this study was not designed to identify barriers to these discussions, the multivariate models would suggest there are identifiable barriers to counseling and referrals. In this context, we would recommend the following: [1] more training opportunities for general pediatricians and pediatric subspecialists in the area of fertility; [2] a standardized approach within teams of initiating and documenting these discussions at diagnosis and routinely thereafter, with inclusion of the patient in a developmentally appropriate manner in accordance with recent AAP guidelines (29); [3] development of written materials for patients and families with information about fertility and reproductive health including contraception; and [4] inclusion of psychosocial providers for more comprehensive counseling and support. Specific talking points during these discussions should include risk of infertility, potential preservation options, risks of pregnancy to the mother and fetus, and alternate options for family planning. Counseling is especially necessary for patients who experience spontaneous menarche (more likely among those with a mosaic karyotype), as established FP options currently exist and could be pursued by these individuals before decline of ovarian reserve.
Multidisciplinary clinics should be implemented when possible with discussions about which providers should lead fertility and reproductive health counseling. Further research is needed to determine when and how providers should initiate fertility counseling with patients with TS for the best reproductive outcomes (when applicable) and patient psychosocial well-being and to assess the utility of ovarian tissue cryopreservation. Additionally, future research is needed to examine barriers to fertility counseling, referrals to specialists, and discussions of pregnancy-related risks as well as patient perception and satisfaction with these discussions. Finally, interventions are needed to ensure timely and routine fertility counseling and discussions of reproductive health risks for this population.
Acknowledgments:
The authors thank Athena Erickson, Saakshi Daswani, Anya Taylor, Brooke Palay, and Jennifer Litteral who assisted in reviewing medical charts.
Supported by the Turner Syndrome Global Alliance and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, grant K23HD092588 (to S.D., PI).
Footnotes
T.L.M. has nothing to disclose. H.M.K. has nothing to disclose. C.E.C. has nothing to disclose. J.K. has nothing to disclose. A.T. has nothing to disclose. S.D. has nothing to disclose. L.N. has nothing to disclose.
REFERENCES
- 1.Gravholt CH, Andersen NH, Conway GS, Dekkers OM, Geffner ME, Klein KO, et al. Clinical practice guidelines for the care of girls and women with Turner syndrome: proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur J Endocrinol 2017;177:G1–70. [DOI] [PubMed] [Google Scholar]
- 2.Sybert VP, McCauley E. Turner’s syndrome. N Engl J Med 2004;351: 1227–38. [DOI] [PubMed] [Google Scholar]
- 3.Bouet P-E, Godbout A, El Hachem H, Lefebvre M, B erub e L, Dionne M-D, et al. Fertility and pregnancy in Turner syndrome. J Obstet Gynaecol Can 2016;38:712–8. [DOI] [PubMed] [Google Scholar]
- 4.Jackson-Cook C A hypothesis: Could telomere length and/or epigenetic alterations contribute to infertility in females with Turner syndrome? Am J Med Genet C Semin Med Genet 2019;181:108–16. [DOI] [PubMed] [Google Scholar]
- 5.McCauley E, Feuillan P, Kushner H, Ross JL. Psychosocial development in adolescents with Turner syndrome. J Dev Behav Pediatr 2001;22:360–5. [DOI] [PubMed] [Google Scholar]
- 6.Hutaff-Lee C, Bennett E, Howell S, Tartaglia N. Clinical developmental, neuropsychological, and social-emotional features of Turner syndrome. Am J Med Genet C Semin Med Genet 2019;181:126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saenger P, Wikland KA, Conway GS, Davenport M, Gravholt CH, Hintz R, et al. Recommendations for the diagnosis and management of Turner syndrome. J Clin Endocrinol Metab 2001;86:3061–9. [DOI] [PubMed] [Google Scholar]
- 8.Sylvén L, Magnusson C, Hagenfeldt K, von Schoultz B. Life with Turner’s syndrome—a psychosocial report from 22 middle-aged women. Eur J Endocrinol 1993;129:188–94. [DOI] [PubMed] [Google Scholar]
- 9.Sandberg DE, Singer D, Bugajski B, Gebremariam A, Scerbak T, Dooley Maley KL, et al. Research priorities of people living with Turner syndrome. Am J Med Genet C Semin Med Genet 2019;181:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armuand GM, Wettergren L, Rodriguez-Wallberg KA, Lampic C. Desire for children, difficulties achieving a pregnancy, and infertility distress 3 to 7 years after cancer diagnosis. Support Care Cancer 2014;22:2805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellis SJ, Wakefield CE, McLoone JK, Robertson EG, Cohn RJ. Fertility concerns among child and adolescent cancer survivors and their parents: a qualitative analysis. J Psychosoc Oncol 2016;34:347–62. [DOI] [PubMed] [Google Scholar]
- 12.Sutton EJ, Young J, McInerney-Leo A, Bondy CA, Gollust SE, Biesecker BB. Truth-telling and Turner syndrome: the importance of diagnostic disclosure. J Pediatr 2006;148:102–7. [DOI] [PubMed] [Google Scholar]
- 13.Weiss L Additional evidence of gradual loss of germ cells in the pathogenesis of streak ovaries in Turner’s syndrome. J Med Genet 1971;8:540–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lippe B Turner syndrome. Endocrinol Metab Clin North Am 1991;20: 121–52. [PubMed] [Google Scholar]
- 15.Bondy CA, Group TSCS. Care of girls and women with Turner syndrome: a guideline of the Turner Syndrome Study Group. J Clin Endocrinol Metab 2007;92:10–25. [DOI] [PubMed] [Google Scholar]
- 16.Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2013;31: 2500–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim HH, Kil HR, Koo SH. Incidence, puberty, and fertility in 45, X/47, XXX mosaicism: report of a patient and a literature review. Am J Med Genet 2017;173:1961–4. [DOI] [PubMed] [Google Scholar]
- 18.Karnis MF. Fertility, pregnancy, and medical management of Turner syndrome in the reproductive years. Fertil Steril 2012;98:787–91. [DOI] [PubMed] [Google Scholar]
- 19.Hadnott TN, Gould HN, Gharib AM, Bondy CA. Outcomes of spontaneous and assisted pregnancies in Turner syndrome: the US National Institutes of Health experience. Fertil Steril 2011;95:2251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gravholt CH. Clinical practice in Turner syndrome. Nat Rev Endocrinol 2005; 1:41–52. [DOI] [PubMed] [Google Scholar]
- 21.Grynberg M, Bidet M, Benard J, Poulain M, Sonigo C, Cédrin-Durnerin I, et al. Fertility preservation in Turner syndrome. Fertil Steril 2016;105:13–9. [DOI] [PubMed] [Google Scholar]
- 22.Nilsson J, Jervaeus A, Lampic C, Eriksson L, Widmark C, Armuand G, et al. ‘Will I be able to have a baby?’ Results from online focus group discussions with childhood cancer survivors in Sweden. Hum Reprod 2014;29:2704–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stinson JN, Jibb LA, Greenberg M, Barrera M, Luca S, White ME, et al. A qualitative study of the impact of cancer on romantic relationships, sexual relationships, and fertility: perspectives of Canadian adolescents and parents during and after treatment. J Adolesc Young Adult Oncol 2015;4:84–90. [DOI] [PubMed] [Google Scholar]
- 24.Stein DM, Victorson DE, Choy JT, Waimey KE, Pearman TP, Smith K, et al. Fertility preservation preferences and perspectives among adult male survivors of pediatric cancer and their parents. J Adolesc Young Adult Oncol 2014;3:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernard V, Donadille B, Zenaty D, Courtillot C, Salenave S, Brac de la Perrière A, et al. Spontaneous fertility and pregnancy outcomes amongst 480 women with Turner syndrome. Hum Reprod 2016;31:782–8. [DOI] [PubMed] [Google Scholar]
- 26.Quinn GP, Vadaparampil ST, King L, Miree CA, Wilson C, Raj O, et al. Impact of physicians’ personal discomfort and patient prognosis on discussion of fertility preservation with young cancer patients. Patient Educ Couns 2009;77:338–43. [DOI] [PubMed] [Google Scholar]
- 27.Nahata L, Ziniel SI, Garvey KC, Richard NY, Cohen LE. Fertility and sexual function: a gap in training in pediatric endocrinology. J Pediatr Endocrinol Metab 2017;30:3–10. [DOI] [PubMed] [Google Scholar]
- 28.Klosky JL, Lehmann V, Flynn JS, Su Y, Zhang H, Russell KM, et al. Patient factors associated with sperm cryopreservation among at-risk adolescents newly diagnosed with cancer. Cancer 2018;124:3567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nahata L, Quinn GP, Tishelman AC. Counseling in pediatric populations at risk for infertility and/or sexual function concerns. Pediatrics 2018;142: e20181435. [DOI] [PubMed] [Google Scholar]
- 30.Klosky JL, Wang F, Russell KM, Zhang H, Flynn JS, Huang L, et al. Prevalence and predictors of sperm banking in adolescents newly diagnosed with cancer: examination of adolescent, parent, and provider factors influencing fertility preservation outcomes. J Clin Oncol 2017;35:3830–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quinn GP, Vadaparampil ST, Bell-Ellison BA, Gwede CK, Albrecht TL. Patient-physician communication barriers regarding fertility preservation among newly diagnosed cancer patients. Soc Sci Med 2008;66:784–9. [DOI] [PubMed] [Google Scholar]
- 32.Oktay K, Bedoschi G, Berkowitz K, Bronson R, Kashani B, McGovern P, et al. Fertility preservation in women with turner syndrome: a comprehensive review and practical guidelines. J Pediatr Adolesc Gynecol 2016;29:409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daly C, Micic S, Facey M, Speller B, Yee S, Kennedy ED, et al. A review of factors affecting patient fertility preservation discussions & decision-making from the perspectives of patients and providers. Eur J Cancer Care 2019;28:e12945. [DOI] [PubMed] [Google Scholar]
- 34.Inhorn MC, Birenbaum-Carmeli D, Westphal LM, Doyle J, Gleicher N, Meirow D, et al. Medical egg freezing: how cost and lack of insurance cover impact women and their families. Reprod Biomed Soc Online 2018; 5:82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cardozo ER, Huber WJ, Stuckey AR, Alvero RJ. Mandating coverage for fertility preservation—a step in the right direction. N Engl J Med 2017; 377:1607. [DOI] [PubMed] [Google Scholar]
- 36.Skinner ML, Lee SK, Collaco JM, Lefton-Greif MA, Hoch J, Au Yeung KJ. Financial and health impacts of multidisciplinary aerodigestive care. Otolaryngol Head Neck Surg 2016;154:1064–7. [DOI] [PubMed] [Google Scholar]
- 37.Dudzinski DM. Ethical issues in fertility preservation for adolescent cancer survivors: oocyte and ovarian tissue cryopreservation. J Pediatr Adolesc Gynecol 2004;17:97–102. [DOI] [PubMed] [Google Scholar]
- 38.Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in cancer patients. Fertil Steril 2005;83:1622–8. [DOI] [PubMed] [Google Scholar]
- 39.Quinn GP, Murphy D, Knapp C, Stearsman DK, Bradley-Klug KL, Sawczyn K, et al. Who decides? Decision making and fertility preservation in teens with cancer: a review of the literature. J Adolesc Health 2011;49:337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quinn GP, Block RG, Clayman ML, Kelvin J, Arvey SR, Lee J-H, et al. If you did not document it, it did not happen: rates of documentation of discussion of infertility risk in adolescent and young adult oncology patients’ medical records. J Oncol Pract 2014;11:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]


