Abstract
Klinefelter syndrome (KS) occurs in 1:600 males and is associated with high morbidity and mortality due to diabetes and cardiovascular disease. Up to 50% of men with KS have metabolic syndrome, a cluster of features conferring increased risk for diabetes and cardiovascular disease. These cardiometabolic (CM) risk features have not been studied in adolescents with KS. The objective of this cohort study was to compare CM risk features in adolescents with KS to controls matched for sex, age, and BMI z score. Fifty males with KS (age 10–17 years) were well-matched to male controls (n = 50) for age (14.0 ± 1.7 vs. 14.0 ± 1.5 years) and BMI z score (0.3 ± 1.3 vs. 0.4 ± 1.2). Three CM risk features were present in 30% of adolescents with KS compared to 12% of controls (RR 2.5, 95% CI 1.1–5.9, p = .048). The KS group had significantly lower HDL cholesterol (p = .006), higher triglycerides (p < .001), and greater waist circumference percentile (p < .001). Despite a normal BMI, the prevalence of CM risk features was very high in adolescents with KS, particularly for central adiposity and dyslipidemia. The pathophysiology of this metabolic profile independent of obesity needs further investigation to facilitate prevention of the high morbidity of cardiovascular disease and diabetes in this population. ClinicalTrials.gov identifiers: NCT01585831 and NCT02723305.
Keywords: central adiposity, dyslipidemia, Klinefelter syndrome, metabolic syndrome, sex chromosome aneuploidy
1 |. INTRODUCTION
Klinefelter syndrome (KS) describes males born with an additional X chromosome, most often with the karyotype 47,XXY. KS occurs in approximately one in 600 males and is associated with a variable phenotype, including neurodevelopmental and physical features, though small testes and impaired spermatogenesis seem to be constant across cases (Smyth & Bremner, 1998). Past epidemiological studies from England and Denmark have found that men with KS have a higher morbidity and mortality due to a variety of conditions, among them cardiovascular disease (CVD) and type 2 diabetes (T2D) (Bojesen & Gravholt, 2011; Bojesen, Juul, Birkebaek, & Gravholt, 2004; Bojesen, Juul, Birkebaek, & Gravholt, 2006; Price, Clayton, Collyer, & de Mey, 1985; Swerdlow et al., 2001; Swerdlow, Higgins, Schoemaker, Wright, & Jacobs, 2005; Swerdlow, Schoemaker, Higgins, Wright, & Jacobs, 2005). In a large cohort study of 3,518 men with KS, the standardized mortality ratio for CVD was 1.3 (95% CI, 1.4–1.7) and 5.8 (95% CI, 3.4–9.3) for T2D (Swerdlow, Higgins, et al., 2005). Together these cardiometabolic (CM) conditions contribute a substantial burden on this population.
Metabolic syndrome describes a constellation of features that confer an elevated risk for the development of CVD and T2D (Haffner et al., 1992; Isomaa et al., 2001). Clinically measurable features that are classically identified to contribute to this CM risk include central adiposity, elevated blood glucose, high triglycerides, low high density lipoprotein (HDL) cholesterol, and hypertension (Al-Hamad & Raman, 2017; Bussler et al., 2017; de Ferranti et al., 2004). If at least three of these features are present, metabolic syndrome can be diagnosed, although the meaning of this diagnosis in youth is not defined. Though this unfavorable CM profile is common in the general population, individuals with certain genetic conditions, including KS, seem to be more susceptible. Studies on the prevalence of metabolic syndrome in adults with KS report that up to 50% meet criteria for diagnosis (Bojesen et al., 2006; Bojesen, Juul, et al., 2006; Gravholt, Jensen, Host, & Bojesen, 2011; Ishikawa, Yamaguchi, Kondo, Takenaka, & Fujisawa, 2008; Pasquali et al., 2013). A study of 70 Danish men with KS found that central adiposity was particularly prevalent and strongly associated with metabolic syndrome and other markers of insulin resistance (Bojesen, Kristensen, et al., 2006). However, the controls in this study were not BMI matched with the KS group and many men with KS were obese, raising the question of whether the poor CM profile is unique to KS or simply secondary to obesity.
Hypogonadism is likely a contributor to central adiposity and CM risk in individuals with KS (Bojesen, Kristensen, et al., 2006; Chang, Skakkebaek, & Gravholt, 2015). Hypogonadal men in general have higher rates of metabolic syndrome than eugonadal men, and testosterone replacement therapy (TRT) has been shown to improve some CM outcomes in this population (Isidori et al., 2015; Traish, Haider, Doros, & Saad, 2014). In KS, the literature is more mixed, with several observational studies finding no differences in CM outcomes between men with KS who had received TRT compared to those who had not (Gravholt et al., 2011; O’Connor, Snyder, & Hayes, 2019; Pasquali et al., 2013; Salzano et al., 2018). A recent randomized placebo-controlled cross-over trial of 6 months of exogenous TRT in 13 adult men with KS demonstrated that TRT led to favorable changes in body composition, however there was no change in insulin sensitivity (Host et al., 2019). This could be secondary to chronic inadequately treated hypogonadism or a mechanism independent from hypogonadism, but given that the poor CM profile seems difficult to reverse in adulthood it is necessary to evaluate CM health in KS at a younger age.
Although the literature is more scarce in pediatrics, CM differences seem to begin early in life for boys with KS. Previous work from our group demonstrated that infants with KS have 15% higher adiposity than healthy male controls at 5 months of age (Davis, Reynolds, Dabelea, Zeitler, & Tartaglia, 2019). Ross and colleagues reported a high prevalence of insulin resistance and CM risk features in 89 prepubertal boys with KS, although this was not compared to a control group (Bardsley, Falkner, Kowal, & Ross, 2011). Neither of these studies investigated whether these CM differences were secondary to hypogonadism, obesity, or some other unique mechanism; however, androgen treatment in both these cohorts resulted in measurable differences in CM measures, particularly adiposity (Davis et al., 2017; Davis et al., 2019). These childhood studies suggest that differences in energy metabolism are already measurable in boys with KS at a young age and may be modifiable.
While there has been ample data confirming high CM risk in adults with KS and emerging data in infants and prepubertal children, there has been no previous study of CM risk factors in adolescents with KS. Understanding the evolution of CM dysfunction throughout the lifespan of individuals with KS is necessary to guide development of preventive and early intervention strategies. In addition, further exploration of the role of testosterone in CM health in youth with KS is needed. To fill this gap, we compared prevalence of CM risk features in pubertal adolescents with KS to healthy controls matched for BMI z score, sex, and age. As a secondary objective, we compared these outcomes between individuals with KS on TRT to those not on testosterone.
2 |. METHODS
2.1 |. Ethical considerations
All protocols were approved by the Colorado Multiple Institutional Review Board (#11–0874, #16–0248, #06–0665, #03–713, #07–0988). All parents of participants provided informed consent and participants provided assent prior to any study procedures.
2.2 |. Study design and participants
This is a cross-sectional study comparing CM risk features in adolescents with KS to controls. Participants with KS had confirmed nonmosaic karyotype of 47,XXY and were recruited from the eXtraordinarY Kids Clinic at Children’s Hospital Colorado, national advertisements to clinicians caring for patients with KS, and community advocacy groups including the Association for X and Y Syndromes (AXYS). Data for controls were taken from a database of participants enrolled in other clinical research studies at our institution; although chromosome analysis was not performed for controls, no controls had microorchidism making undiagnosed KS extremely unlikely. These participants were recruited from the local community and underwent the same study procedures. Inclusion criteria for both cases and controls included age 10–18 years and pubic hair Tanner stage of ≥2 (i.e., pubertal). Exclusion criteria included a known diagnosis of diabetes or inability to tolerate the study protocol. Each male with KS was matched to one control based on age (within one year) and BMI z score (within 0.35). For one participant with KS we were unable to find an appropriate control match and therefore he was not included in the analysis.
2.3 |. Study procedures
All participants had a physical exam by a physician for pubertal staging based on Tanner criteria. Height and waist circumference were measured to the nearest 0.1 cm, weight to the nearest 0.1 kg, and blood pressure was measured in the seated position using an automatic sphygmomanometer. Z scores for height, weight, and body mass index (BMI) were calculated from the 2000 CDC growth curves and waist circumference percentiles from NHANES III using the childsds package in R (Kuczmarski et al., 2002; Sharma, Metzger, Daymont, Hadjiyannakis, & Rodd, 2015). Sex-, age-, and height-specific blood pressure percentiles were calculated (Rosner, Cook, Portman, Daniels, & Falkner, 2008). Venipuncture was performed fasting and in the morning whenever possible and included measurement of serum total testosterone, liver enzymes and a cholesterol panel including triglycerides, low density lipoprotein (LDL), HDL, and total cholesterol. In addition, 30 participants from the KS group and all controls had fasting blood glucose and hemoglobin A1c (HbA1c) measured. Assays were performed by the University of Colorado Clinical Translational Research Core Laboratory, Children’s Hospital Colorado clinical laboratory, and the University of Colorado Hospital clinical laboratory using standard clinical platforms.
2.4 |. CM risk definition
Given there is not an agreed upon definition of metabolic syndrome for youth, we elected to apply criteria put forth by de Ferranti et al., which has been used previously for prepubertal youth with KS (Bardsley et al., 2011; de Ferranti et al., 2004). CM risk factors included: elevated triglycerides defined as ≥100 mg/dl; low HDL cholesterol defined as <50 mg/dl for boys <14 years of age and <45 mg/dl for participants 15–19 years; elevated fasting blood glucose defined as ≥110 mg/dl; elevated waist circumference defined as >75th percentile for age and sex; and elevated systolic blood pressure defined as >90th percentile for sex, age, and height (de Ferranti et al., 2004). The presence of these CM risk features was designated as our primary outcome and absolute values for these CM measures were secondary outcomes.
2.5 |. Statistical analysis
Data were examined for normality and outliers. Missing data for the 20 subjects who did not have a fasting glucose measurement were excluded for the variable of hyperglycemia only. The primary outcome was defined as the presence of three or more CM features. Secondary outcomes included the presence or absence of each CM feature as well as the continuous values for each of these variables. Descriptive statistics were used to summarize the sample population. Categorical outcomes were compared between groups using the Fisher exact test and risk ratios with 95% confidence intervals computed using the Koopman asymptotic score are presented. Paired t tests or Wilcoxon matched pairs signed rank test were used to compare continuous variables between groups. A subgroup analysis was then performed between participants with KS who were on TRT and those who were not. An alpha <0.05 was considered statistically significant. Analyses were performed using RStudio Version 1.2.1335 and GraphPad Prism version 8.00, GraphPad Software, La Jolla, CA, www.graphpad.com.
3 |. RESULTS
A total of 50 adolescents with KS and 50 controls with an average age of 14 years were included in this study. Table 1 shows demographic characteristics including age, race, pubertal stage, BMI, and serum testosterone concentrations. The KS and control groups were well matched on age and BMI by design, though the KS group was less racially diverse and further along in puberty based on pubic hair assessment. Within the KS group, 24 (48%) were diagnosed with KS postnatally at a median age of 8.5 years (range 2–17 years) for reasons including developmental delays (n = 13), learning disability and/or behavior concerns in school age children (n = 7), pubertal microorchidism (n = 3), tall stature (n = 1), and epilepsy evaluation (n = 1). None of the boys with KS were diagnosed due to cryptorchidism or micropenis. At the time of the study, 22 (44%) of the boys with KS were on exogenous TRT with heterogenous formulations, dosages, and duration of treatment. Of note, 41 (82%) had participated in randomized controlled trial of topical testosterone in early puberty with 2:1 randomization to treatment, likely contributing to advanced pubic hair development in some participants with KS.
TABLE 1.
Participant characteristics for Klinefelter syndrome (KS) and control groups
| Controls (n = 50) | KS (n = 50) | p value | KS + T (n = 22) | KS − T (n - 28) | p value +T vs. −T | |
|---|---|---|---|---|---|---|
| Age (years) | 14.0 ± 1.5 | 14.0 ± 1.7 | .66 | 14.1 ± 1.8 | 13.9 ± 1.7 | .61 |
| Race/ethnicity, n (%) | .011* | .10 | ||||
| Non-Hispanic white | 27 (54%) | 41 (82%) | 17 | 24 | ||
| Black | 3 (6%) | 3 (6%) | 0 | 3 | ||
| Hispanic | 9 (18%) | 5 (10%) | 4 | 1 | ||
| Other/unknown | 10 (24%) | 1 (2%) | 1 | 0 | ||
| BMI (kg/m2) | 20 (18, 25) | 20 (18, 24) | .16 | 20 (18, 22) | 20 (18, 24) | .74 |
| BMI z score | 0.36 ± 1.2 | 0.31 ± 1.3 | .12 | 0.26 ± 1.11 | 0.35 ± 1.38 | .80 |
| Total testosterone (mg/dl) | 192 (74, 446) | 286 (108, 422) | .20 | 312 (203, 475) | 208 (67, 400) | .047* |
| Pubic hair Tanner stage, n (%) | .012* | .012* | ||||
| 2 | 14 (28%) | 9 (18%) | 1 (5%) | 8 (29%) | ||
| 3 | 16 (32%) | 8 (16%) | 1 (5%) | 7 (25%) | ||
| 4 | 17 (34%) | 19 (38%) | 11 (50%) | 8 (29%) | ||
| 5 | 3 (6%) | 14 (28%) | 9 (40%) | 5 (18%) |
Note: Data are mean ± standard deviation (SD), median (25th, 75th percentile), or n (%).
p < .05.
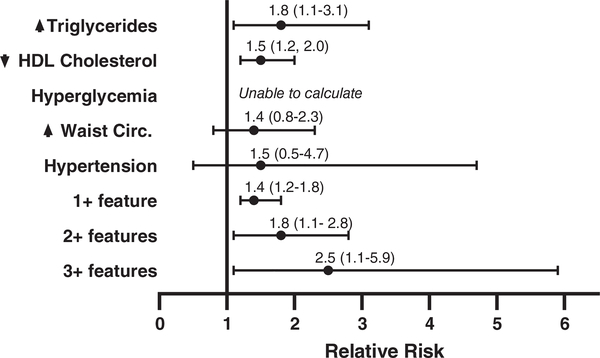
In the KS group, 48 boys (96%) had at least one CM risk feature and 30% of participants with KS had three or more CM risk features, compared to only 12% of controls (RR 2.5, 95% CI 1.1–5.9, p = .048). Adolescents with KS were more likely to have high triglycerides (RR 1.8, 95% CI 1.1–3.1, p = .039) and low HDL (RR 1.5, 95% CI 1.2–2.0, p = .004, Figure 1). Table 2 shows the measures of central tendency for CM risk features compared between the KS and control groups. Triglycerides and waist circumference percentile were significantly higher in the KS group (p < .001 for both), while HDL cholesterol was significantly lower in the KS group (p = .006). Additional clinically applicable CM features not classically included in the definition of metabolic syndrome are also shown in Table 2. Although fasting glucose was not higher in the boys with KS, HbA1c was significantly higher indicative of chronically higher blood glucose. AST was higher in boys with KS; however, ALT was not different.
FIGURE 1.
Risk of meeting cutoff criteria for cardiometabolic (CM) risk features in adolescents with Klinefelter syndrome (KS) compared to sex-, age-, and BMI-matched controls. The point value represents the risk ratio (RR) and the error bars represent the 95% confidence intervals, with values >1 indicating an increased risk in KS. RRs for hyperglycemia could not be calculated as no participant in either group had a fasting blood glucose >110 mg/dl
TABLE 2.
Continuous data for cardiometabolic (CM) risk features in adolescents with Klinefelter syndrome (KS) compared to controls, and by testosterone (T) treatment status
| Controls (n = 50) | All KS (n = 50) | p value KS vs. control | KS + T (n = 22) | KS − T (n = 28) | p value +T vs. T | |
|---|---|---|---|---|---|---|
| Triglycerides (mg/dl) | 81 (49,103) | 99 (75,155) | <.001*** | 85 (67,136) | 108 (84,162) | .09 |
| HDL (mg/dl) | 47 (38, 52) | 41 (35, 46) | .006** | 39 (32, 43) | 42 (38, 49) | .038* |
| LDL (mg/dl) | 82 (71–93) | 83 (73–104) | .14 | 80 (73,106) | 84 (73,104) | .86 |
| Total cholesterol (mg/dl) | 143 (131,157) | 142 (131, 164) | .44 | 138 (126,160) | 151 (138,169) | .14 |
| Waist %ile | 53 ± 33 | 62 ± 29 | <.001*** | 60 ± 27 | 65 ± 30 | .56 |
| SBP %ile | 60 ± 25 | 54 ± 29 | .20 | 45 ± 25 | 62 ± 30 | .033* |
| DBP %ile | 63 ± 24 | 55 ± 24 | .14 | 48 ± 22 | 60 ± 25 | .09 |
| Glucose (mg/dl) | 86 (84, 89) | 86 (81, 91)† | .39 | 88 (84, 93)†† | 84 (79, 90)†† | .17 |
| HbA1c (%) | 5.1 (5.0,5.3) | 5.4 (5.3,5.6)† | <.001*** | 5.5 (5.4,5.7)†† | 5.4(5.2,5.5)†† | .24 |
| ALT (IU/L) | 30 (22, 37) | 31 (28,37) | .23 | 31 (28,37) | 33 (28,35) | .87 |
| AST (IU/L) | 35 (24, 45) | 42 (36,52) | .018* | 43 (39,52) | 41 (34,52) | .44 |
Note: Data are mean ± SD or median (25th, 75th percentile).
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; HbA1c, hemoglobin A1c; HDL, high density lipoprotein; LDL, low density lipoprotein; SBP, systolic blood pressure.
n = 30.
n = 14 treated and 16 untreated.
p < .05
p < .01
p < .001.
Although BMI was within the normal range for the majority of participants, BMI z score was positively correlated with having three or more CM risk features for both KS (r = 0.65, p < .001) and controls (r = 0.49, p < .001). BMI z score also strongly correlated with waist circumference percentile for both groups (KS r = 0.92, p < .001 and controls r = 0.87, p < .001). Within the KS group only, BMI z score was positively correlated with systolic blood pressure percentile (r = 0.50, p < .001) and triglycerides (r = 0.32, p = .03). Fasting glucose correlated with BMI z score for controls only. HDL cholesterol did not correlate with BMI z score in either group.
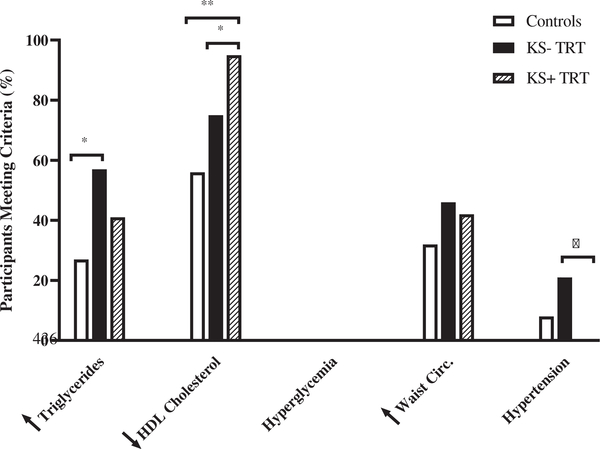
Figure 2 also shows the results of the sub-group analysis of CM risk features in adolescents with KS who had received TRT (n = 22) and those who had not (n = 28). The prevalence of three or more CM risk features in the group who had received TRT was approximately half of the prevalence in those who did not receive TRT (18 vs. 38%), however, this did not reach statistical significance (p = .13). Those who received TRT had significantly lower HDL cholesterol (p = .038) and lower systolic blood pressure percentile (p = .033) than those who were not on TRT (Figure 3). There were no statistical differences by TRT status in other continuous outcomes examined (Table 2).
FIGURE 2.
Prevalence of cardiometabolic (CM) risk features in Klinefelter syndrome (KS) by testosterone replacement therapy (TRT) status and controls
FIGURE 3.
Prevalence of three or more cardiometabolic (CM) risk factors in males with Klinefelter syndrome (KS)
4 |. DISCUSSION
In this study of CM health in adolescents with KS, we found a strikingly high prevalence of CM risk factors in adolescents with KS, with 30% having at least three CM risk factors representing a 2.5 greater risk over their peers with similar BMI. In particular, adolescents with KS had significantly higher fasting triglycerides, lower HDL cholesterol and higher waist circumference than controls, whereas fasting blood glucose and systolic blood pressure did not differ between groups. Although most boys with KS had a BMI within the normal range, many CM features were positively correlated with BMI percentile. Participants with KS on TRT were half as likely to have three or more CM risk factors; however, this subgroup analysis was underpowered to reach conclusions.
Our results are congruent with previous studies on CM health in boys and adult men with KS. A study published in 2011 on prepubertal boys with KS found that 36% met criteria for two CM features using the same definition and 7% met criteria for three or more (Bardsley et al., 2011). Various studies of adults with KS have found dramatically increased rates of metabolic syndrome as high as 50% (Bojesen, Juul, et al., 2006; Bojesen, Kristensen, et al., 2006; Gravholt et al., 2011; Ishikawa et al., 2008; Pasquali et al., 2013). Our results fit well with these previous studies with nearly all adolescents with KS having at least one CM risk feature and 30% having three or more. This suggests the risk of poor CM health begins early in life for boys with KS and increases with age (Figure 3).
Previous studies have shown that central adiposity is especially high in adults with KS and does not differ by TRT status, which is congruent with our findings of higher waist circumference percentiles in adolescents with KS both with and without TRT (Bojesen, Kristensen, et al., 2006; Host et al., 2019). However, waist circumference is only a surrogate for truncal adiposity and rigorous assessments of body composition may be more sensitive to changes with TRT, as recently found in an intervention study of 13 men with KS who had a significant decrease in abdominal fat as measured by both CT and DEXA even though waist circumference did not change (Host et al., 2019). Furthermore, although regional adiposity measures were not obtained, androgen treatment decreased total body fat in two different studies of prepubertal boys with KS (Davis et al., 2017; Davis et al., 2019). Given that abdominal adiposity is hypothesized to lead to insulin resistance via low-grade inflammation and other proposed mechanisms, further investigation into body composition as well as inflammatory measures in youth with KS are needed as targeting adiposity and its sequelae early may decrease the CM related morbidity in this population.
Several previous studies have also described dyslipidemia in adults with KS, which was also true of our adolescents with KS who had higher fasting triglycerides and lower HDL cholesterol compared to controls (Bojesen, Kristensen, et al., 2006; Ishikawa et al., 2008). This increased triglyceride to HDL ratio is a surrogate marker of insulin resistance (Iwani et al., 2017). Dyslipidemia may also be due to differences in dietary intake or in cholesterol metabolism in KS, and further research is needed to elucidate this. Although insulin resistance is widely reported in KS, no one in our study met the criteria for hyperglycemia based on the definition we used (fasting glucose>110 mg/dl). Importantly, we excluded individuals known to have diabetes and we did not evaluate for impaired glucose tolerance, which may be more representative of impaired insulin action than fasting measures. Additional evaluation of glycemia in adolescents with KS is warranted. Finally, this study as well as previous studies have suggested the prevalence of hypertension in KS is similar to controls (Bardsley et al., 2011; Bojesen, Kristensen, et al., 2006).
In this cross-sectional study it is not possible to determine the pathophysiology of CM risk in KS, or speculate on what may improve CM health in this population. Due to the well-appreciated causal association of male hypogonadism and CM dysfunction, relative testosterone deficiency has been proposed as the underlying pathophysiology in KS (Isidori et al., 2015; Traish et al., 2014). However, studies in adults with KS have had conflicting results on the association of testosterone and CM dysfunction. Our study was underpowered to assess the effect of TRT on CM risk factors in adolescents with KS. Importantly, there are no evidence-based recommendations for TRT in adolescents with KS and many of the boys in this study received TRT before clinically initiated as part of a clinical trial. It is also important to note that our control and KS groups had similar serum testosterone concentrations, indicating that hypogonadism does not explain the full picture of poor CM health in boys with KS. Longitudinal assessment of CM health with TRT and other interventions is warranted in adolescents with KS.
The inclusion of a matched control group was a strength of this study and allowed us to determine that the higher prevalence of CM risk features in KS was not simply secondary to obesity. Furthermore, our study has external validity in that our controls had a very similar prevalence of three or more CM risk features to previously published diverse American adolescents (de Ferranti et al., 2004). Unfortunately, the KS group was less ethnically diverse and at a more advanced pubertal stage compared to the control group (likely secondary to exogenous testosterone exposure). We do not believe better matching for these variables would have changed our conclusions given that Hispanic ethnicity and mid-puberty status are associated with lower insulin sensitivity; however, this imbalance remains a limitation (de Ferranti et al., 2004). The other important limitation is that our sample size was determined by the number of subjects available for these analyses, and a post-hoc power calculation concludes our sample size provided only 60% power to detect our primary outcome, although the power was higher for the continuous outcomes. A final note in interpreting these results is the utility of the cutoff criteria we used for CM risk is not universally agreed upon, and while we make the assumption that the difference in CM health we observed between adolescents with KS and controls in this analysis is related to the high mortality from T2D and CVD in adulthood, a long-term longitudinal study would be needed to confirm these assumptions are accurate. Despite these limitations, this study involves a relatively large sample size for a genetic condition in a pediatric population and fills a gap in the current literature for CM risk prevalence in adolescents with KS.
In conclusion, we found a high prevalence of CM risk factors in adolescents with KS compared to sex-, age-, and BMI-matched controls. These data support the importance of counseling for CM risk and evaluation for CM risk factors adolescents with KS, even when BMI is in the normal range. While additional research is needed to determine the underlying mechanisms contributing to this increased risk, healthy lifestyle habits including a well-rounded diet and daily moderate-to-vigorous physical activity should be emphasized for all boys with KS in accordance with recommendations for all children by the American Academy of Pediatrics (“Bright Futures: prevention and health promotion for infants, children, adolescents and their families,”, 2020). Future studies should rigorously examine the pathophysiology of the early CM differences in KS reported here.
ACKNOWLEDGMENTS
The authors would like to thank the study participants and their families for their contribution to this work, as well as AXYS for advertising the studies. Data for this work were collected from research studies supported by the NIH (K23HD092588, K23NS070337, NCRR K23 RR020038, NIH BIRCWH K12 5K12HD057022, R56 DK088971, CTSA UL1TR002535), the American Diabetes Association (ADA 7-11-CD-08, ADA 1-11-JF-23), and the Juvenile Diabetes Research Foundation (#11-2010-343, #1-11-JF-23). Contents are the authors’ sole responsibility and do not necessarily represent official NIH, ADA, or JDRF views. SMD has previously served as a consultant for Antares Pharma, Inc.
Funding information
Juvenile Diabetes Research Foundation, Grant/Award Numbers: 1-11-JF-23, 11-2010-343; American Diabetes Association, Grant/Award Numbers: ADA 1-11-JF-23, ADA 7-11-CD-08; NIH, Grant/Award Numbers: CTSA UL1TR002535, R56 DK088971, NIH BIRCWH K12 5K12HD057022, NCRR K23 RR020038, K23NS070337, K23HD092588
REFERENCES
- Al-Hamad D, & Raman V (2017). Metabolic syndrome in children and adolescents. Translational Pediatrics, 6(4), 397–407. 10.21037/tp.2017.10.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardsley MZ, Falkner B, Kowal K, & Ross JL (2011). Insulin resistance and metabolic syndrome in prepubertal boys with Klinefelter syndrome. Acta Paediatrica, 100(6), 866–870. 10.1111/j.1651-2227.2011.02161.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojesen A, & Gravholt CH (2011). Morbidity and mortality in Klinefelter syndrome (47,XXY). Acta Paediatrica, 100(6), 807–813. 10.1111/j.1651-2227.2011.02274.x [DOI] [PubMed] [Google Scholar]
- Bojesen A, Juul S, Birkebaek N, & Gravholt CH (2004). Increased mortality in Klinefelter syndrome. The Journal of Clinical Endocrinology and Metabolism, 89(8), 3830–3834. [DOI] [PubMed] [Google Scholar]
- Bojesen A, Juul S, Birkebaek NH, & Gravholt CH (2006). Morbidity in Klinefelter syndrome: A Danish register study based on hospital discharge diagnoses. The Journal of Clinical Endocrinology and Metabolism, 91(4), 1254–1260. 10.1210/jc.2005-0697 [DOI] [PubMed] [Google Scholar]
- Bojesen A, Kristensen K, Birkebaek NH, Fedder J, Mosekilde L, Bennett P, … Gravholt CH (2006). The metabolic syndrome is frequent in Klinefelter’s syndrome and is associated with abdominal obesity and hypogonadism. Diabetes Care, 29(7), 1591–1598. [DOI] [PubMed] [Google Scholar]
- Bright Futures: prevention and health promotion for infants, children, adolescents and their families. (2020). Retrieved from https://brightfutures.aap.org/clinical-practice/Pages/default.aspx
- Bussler S, Penke M, Flemming G, Elhassan YS, Kratzsch J, Sergeyev E, … Kiess W (2017). Novel insights in the metabolic syndrome in childhood and adolescence. Hormone Research in Pædiatrics, 88(3–4), 181–193. 10.1159/000479510 [DOI] [PubMed] [Google Scholar]
- Chang S, Skakkebaek A, & Gravholt CH (2015). Klinefelter syndrome and medical treatment: Hypogonadism and beyond. Hormones (Athens), 14(4), 531–548. 10.14310/horm.2002.1622 [DOI] [PubMed] [Google Scholar]
- Davis SM, Cox-Martin MG, Bardsley MZ, Kowal K, Zeitler PS, & Ross JL (2017). Effects of oxandrolone on cardiometabolic health in boys with Klinefelter syndrome: A randomized controlled trial. The Journal of Clinical Endocrinology and Metabolism, 102(1), 176–184. 10.1210/jc.2016-2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SM, Reynolds RM, Dabelea DM, Zeitler PS, & Tartaglia NR (2019). Testosterone treatment in infants with 47,XXY: Effects on body composition. Journal of the Endocrine Society, 3(12), 2276–2285. 10.1210/js.2019-00274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ferranti SD, Gauvreau K, Ludwig DS, Neufeld EJ, Newburger JW, & Rifai N (2004). Prevalence of the metabolic syndrome in American adolescents: Findings from the Third National Health and Nutrition Examination Survey. Circulation, 110(16), 2494–2497. 10.1161/01.CIR.0000145117.40114.C7 [DOI] [PubMed] [Google Scholar]
- Gravholt CH, Jensen AS, Host C, & Bojesen A (2011). Body composition, metabolic syndrome and type 2 diabetes in Klinefelter syndrome. Acta Paediatrica, 100(6), 871–877. 10.1111/j.1651-2227.2011.02233.x [DOI] [PubMed] [Google Scholar]
- Haffner SM, Valdez RA, Hazuda HP, Mitchell BD, Morales PA, & Stern MP (1992). Prospective analysis of the insulin-resistance syndrome (syndrome X). Diabetes, 41(6), 715–722. 10.2337/diab.41.6.715 [DOI] [PubMed] [Google Scholar]
- Host C, Bojesen A, Erlandsen M, Groth K, Kritstensen K, Jurik AG, … Gravholt C (2019). A placebo-controlled randomized study with testosterone in Klinefelter syndrome - beneficial effects on body composition. Endocrine Connections, 8, 1250–1261. 10.1530/ec-19-0323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Yamaguchi K, Kondo Y, Takenaka A, & Fujisawa M (2008). Metabolic syndrome in men with Klinefelter’s syndrome. Urology, 71(6), 1109–1113. 10.1016/j.urology.2008.01.051 [DOI] [PubMed] [Google Scholar]
- Isidori AM, Balercia G, Calogero AE, Corona G, Ferlin A, Francavilla S, … Maggi M (2015). Outcomes of androgen replacement therapy in adult male hypogonadism: Recommendations from the Italian society of endocrinology. Journal of Endocrinological Investigation, 38(1), 103–112. 10.1007/s40618-014-0155-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, … Groop L (2001). Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care, 24(4), 683–689. 10.2337/diacare.24.4.683 [DOI] [PubMed] [Google Scholar]
- Iwani NA, Jalaludin MY, Zin RM, Fuziah MZ, Hong JY, Abqariyah Y, … Wan Nazaimoon WM (2017). Triglyceride to HDL-C ratio is associated with insulin resistance in overweight and obese children. Scientific Reports, 7, 40055 10.1038/srep40055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, … Johnson CL (2002). 2000 CDC growth charts for the United States: Methods and development. Vital and Health Statistics, 11(246), 1–190. [PubMed] [Google Scholar]
- O’Connor MJ, Snyder EA, & Hayes FJ (2019). Klinefelter syndrome and diabetes. Current Diabetes Reports, 19(9), 71 10.1007/s11892-019-1197-3 [DOI] [PubMed] [Google Scholar]
- Pasquali D, Arcopinto M, Renzullo A, Rotondi M, Accardo G, Salzano A, … Cittadini A (2013). Cardiovascular abnormalities in Klinefelter syndrome. International Journal of Cardiology, 168(2), 754–759. 10.1016/j.ijcard.2012.09.215 [DOI] [PubMed] [Google Scholar]
- Price WH, Clayton JF, Collyer S, & de Mey R (1985). Mortality ratios and life expectancy in X chromatin positive males. Journal of Epidemiology and Community Health, 39(1), 33–38. 10.1136/jech.39.1.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner B, Cook N, Portman R, Daniels S, & Falkner B (2008). Determination of blood pressure percentiles in normal-weight children: Some methodological issues. American Journal of Epidemiology, 167(6), 653–666. 10.1093/aje/kwm348 [DOI] [PubMed] [Google Scholar]
- Salzano A, D’Assante R, Heaney LM, Monaco F, Rengo G, Valente P, … Napoli R (2018). Klinefelter syndrome, insulin resistance, metabolic syndrome, and diabetes: Review of literature and clinical perspectives. Endocrine, 61(2), 194–203. 10.1007/s12020-018-1584-6 [DOI] [PubMed] [Google Scholar]
- Sharma AK, Metzger DL, Daymont C, Hadjiyannakis S, & Rodd CJ (2015). LMS tables for waist-circumference and waist-height ratio Z-scores in children aged 5–19 y in NHANES III: Association with cardiometabolic risks. Pediatric Research, 78(6), 723–729. 10.1038/pr.2015.160 [DOI] [PubMed] [Google Scholar]
- Smyth CM, & Bremner WJ (1998). Klinefelter syndrome. Archives of Internal Medicine, 158(12), 1309–1314. 10.1001/archinte.158.12.1309 [DOI] [PubMed] [Google Scholar]
- Swerdlow AJ, Hermon C, Jacobs PA, Alberman E, Beral V, Daker M, … Youings S (2001). Mortality and cancer incidence in persons with numerical sex chromosome abnormalities: A cohort study. Annals of Human Genetics, 65(Pt 2), 177–188. [DOI] [PubMed] [Google Scholar]
- Swerdlow AJ, Higgins CD, Schoemaker MJ, Wright AF, Jacobs PA, & United Kingdom Clinical Cytogenetics, G. (2005). Mortality in patients with Klinefelter syndrome in Britain: A cohort study. The Journal of Clinical Endocrinology and Metabolism, 90(12), 6516–6522. doi: 10.1210/jc.2005-1077 [DOI] [PubMed] [Google Scholar]
- Swerdlow AJ, Schoemaker MJ, Higgins CD, Wright AF, & Jacobs PA (2005). Cancer incidence and mortality in men with Klinefelter syndrome: A cohort study. Journal of the National Cancer Institute, 97(16), 1204–1210. 10.1093/jnci/dji240 [DOI] [PubMed] [Google Scholar]
- Traish AM, Haider A, Doros G, & Saad F (2014). Long-term testosterone therapy in hypogonadal men ameliorates elements of the metabolic syndrome: An observational, long-term registry study. International Journal of Clinical Practice, 68(3), 314–329. 10.1111/ijcp.12319 [DOI] [PMC free article] [PubMed] [Google Scholar]