Abstract
Radiation therapy plays a vital role in the treatment of tumours. In particular, the occurrence of the “abscopal effect” brings about a favourable turn for the treatment of patients with advanced metastatic malignant tumours. Because of the abscopal effect, non-irradiated areas are also treated. However, the abscopal effect occurs by chance, not through seeking. Although the abscopal effect has been studied enthusiastically, the desired result does not appear to be achieved. Moreover, its combination with immunotherapy appears to be overwhelming. There is an opinion that abscopal effect is difficult to achieve by irradiation of a single tumour, and irradiation of multiple or total lesions is advocated to increase the possibility of obtaining clinically meaningful outcomes. Obviously, there are still questions about the mechanism, condition and possibility underlying the occurrence of the abscopal effect. Can the abscopal effect truly change the future treatment strategy as the researchers expect? What are the current problems? This article reviewed the research in recent years to explore the progress and controversy surrounding the abscopal effect of radiation therapy.
Keywords: radiation therapy, abscopal effect, immunotherapy, immunomodulation, tumour microenvironment
Following palliative pain relief or systemic treatment, patients with advanced malignant tumours often receive radiation therapy for the purpose of local control of lesions in a single organ or site. However, radiation therapy is often powerless to treat lesions outside the irradiation field. The existence of the “abscopal effect” brings a glimmer of hope. The concept of the abscopal effect originated in 1953. Mole et al found that the irradiation of local tissues induced biological responses in the same or different types of tissues far away from the radiation site and therefore proposed this concept. However, the abscopal effect still “comes by chance, not through seeking”. A melanoma case report by the Memorial Sloan Kettering Cancer Centre in 2012 implied the possibility of immune checkpoint inhibitors to induce the abscopal effect.1 The patient underwent palliative radiation therapy for metastatic thoracic lesions after treatment with immune checkpoint inhibitors. Interestingly, other metastatic lesions also shrank. Doctors examined the changes of immune biomarkers in the peripheral blood to verify the relationship between the abscopal effect and immunity. Similar to throwing a stone into water, this case aroused great enthusiasm for the subsequent study of such phenomena. However, subsequent studies found that the abscopal effect failed to achieve the desired results. Questions remain about the mechanism, condition, and possibility underlying the occurrence of the abscopal effect. This article intends to provide an overview of these questions.
The Potential Mechanisms Underlying the Occurrence of the Abscopal Effect
Radiation-Induced Immune Phenomenon
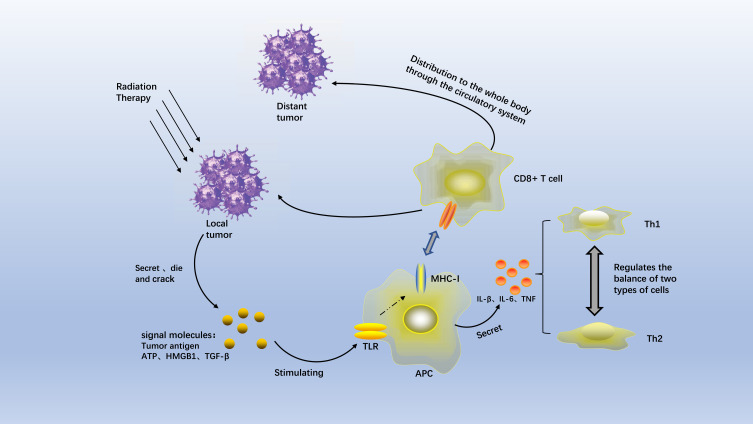
As early as 2004, a study pointed out that the abscopal effect might be mediated by immunity.2 Moreover, cellular immunity might play a more important role than humoural immunity.3 After many animal experiments, some researchers hypothesized that radiation therapy introduces ionizing radiation, resulting in the production of inflammatory signals. Cellular stress or damage causes the dying tumour cells to release adenosine triphosphate (ATP), tumour antigens, and danger signals such as high mobility group box 1 (HMGB1) and calreticulin. Radiation also increases the secretion of transforming growth factor beta (TGF-β) and the inhibition of CD4+ regulatory T cells (Tregs).4 In the context of radiation, the number and diversity of these tumour-associated antigens are significantly increased. The antigens are recognized by Toll-like receptors (TLRs), which activate all components of the immune system5 and stimulate the antigen-presenting cells (APCs) to produce tumour-associated antigens.6,7 Activated APCs enter the tumour-draining lymph nodes, where they activate naive CD8+ T lymphocytes to antagonize the tumour cells presenting these specific antigens.8,9 These newly activated lymphocytes are distributed to the entire body via the circulatory system. They can also extravasate at the unirradiated tumour site, resulting in tumour shrinkage in non-irradiated regions. This phenomenon is known as the abscopal effect.
Cytokine Interactions in the Tumour Microenvironment
The changes in the tumour microenvironment are also the key to the occurrence of the abscopal effect after radiation therapy.10,11 After radiation therapy, the levels of interferon-gamma (IFN-γ), C-X-C motif chemokine ligand 9 (CXCL9), C-X-C motif chemokine ligand 10 (CXCL10), and C-X-C motif chemokine ligand 16 (CXCL16) are increased.12 These radiation-induced key chemokines increase T cell motility and vascular permeability, thereby attracting effector T cells to the tumours.13,14 The factors produced by radiation therapy are very important for tumour treatment. For example, an exogenous increase of type I IFN is sufficient to mimic the tumour-regression effect of radiation therapy.15 Interferon-beta (IFN-β) also plays an important role in the activation of T cells.16 Radiation therapy-induced IFN-β was also related to the development of the abscopal effect in patients with non-small cell lung cancer (NSCLC).17 Shortly after the completion of radiation therapy (day 22), serum IFN-β levels in 7 respondents significantly increase from baseline. IFN-β production is closely related to cytosolic DNA. In regard to cytosolic DNA, the cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) pathway needs to be mentioned. The cGAS-STING pathway is a component of the innate immune system. Once activated, this pathway promotes the production of cytosolic DNA. Therefore, it is deduced that the interferon-induced cGAS/STING pathway promotes the emergence of anti-tumour T cells.18 Other previous clinical trials have verified the relationship between the abscopal effect and p53.19,20 The downstream pathway of p53 is important for triggering the abscopal effect, which, however, was not further elaborated (Figure 1).
Figure 1.
The potential mechanisms underlying the occurrence of the abscopal effect.
The Factors Promoting the Occurrence of the Abscopal Effect
Combination with Immune Factors Increases the Occurrence Probability of the Abscopal Effect
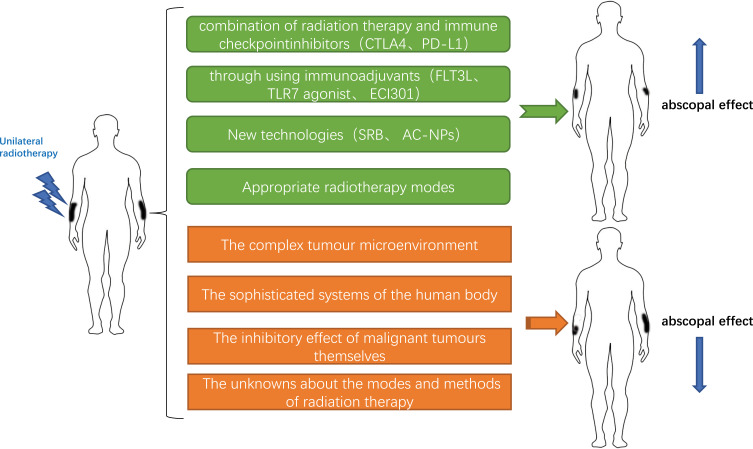
The appearance of “abscopal effect” (Figure 2) indicates that patients can reduce the area of radiotherapy to reduce the side effects of radiotherapy. For this reason, researchers have carried out numerous clinical trials and preclinical studies to explore how to promote the occurrence of the abscopal effect, especially in metastatic tumours. Pfannenstiel et al established two mouse models of metastatic melanoma, representing BRAF mutant and non-mutant tumours.21 The combination of radiation therapy and immunotherapy produced a stronger systemic anti-tumour immune response than did immunotherapy alone, which led to a reduction in tumour growth and an increase in the number of activated CD8+ cytotoxic T cells. In addition, the abscopal effect was observed in unirradiated tumours and was independent of BRAF status. In another melanoma-related study using the combination of radiation therapy and immunotherapy, administration of the inhibitors of the cytotoxic T lymphocyte-associated antigen (CTLA4) and programmed cell death-ligand 1 (PD-L1) increased the incidence of the abscopal effect.22 Golden et al reported the first case of the abscopal effect in a patient with refractory lung cancer who was treated with radiation therapy and ipilimumab. The patient showed no sign of disease progression even at one year after the concurrent radiation therapy and ipilimumab treatment.23 Many clinical and preclinical trials have confirmed that anti-CTLA4 or anti-programmed death-1 (PD1) antibody can be used as immune checkpoint inhibitor to increase T cell activity against tumour cells.24–28 The above data demonstrate that the combination of radiation therapy and immune checkpoint inhibitors induces anti-tumour responses to control local and distant diseases.
Figure 2.
Conditions that induce or limit the occurrence of the abscopal effect.
In addition to combining with immune checkpoint inhibition, the possibility of abscopal effect occurrence can be enhanced through using immunoadjuvants. For example, FMS-like tyrosine kinase 3 ligand (FLT3L) can be used to recruit and stimulate APCs.3 Anti-cluster of differentiation 40 (anti-CD40) antibody can be used to enhance the activation of APCs.29 In a mouse model of pancreatic cancer, anti-CD40 treatment resulted in not only the regression of the untreated contralateral tumours but also the development of long-term immunological memory.30 Administration of the immunocytokine L19-IL2 in combination with radiation therapy (a single dose of 15 Gy) induced the abscopal effect in 20% of the immunocompetent mice with colon tumours.31 Administration of the granulocyte-macrophage colony-stimulating factor also promoted the occurrence of the abscopal effect in patients with metastatic cancer.32 The toll-like receptor 7 (TLR7) agonist imiquimod promoted the radiation therapy-elicited in situ vaccination, thereby enhancing the incidence of the abscopal effect.33 ECI301 enhanced the anti-tumour effect of radiation and induced the occurrence of the abscopal effect in mice.34 New technologies developed in recent years, including the multifunctional smart radiation therapy biomaterials (SRB) loaded with CD40mAb, also enhance the abscopal effect.35 Antigen-capturing nanoparticles (AC-NPs) delivered tumour-specific proteins to APCs and significantly enhanced the efficacy of anti-PD-1 treatment, thereby inducing the abscopal effect.36 In lymphoma,37 renal cell carcinoma,38 breast cancer,39 hepatocellular carcinoma,40 prostate cancer,41 pancreatic cancer,42 and other metastatic solid tumours,32 the abscopal effect also occurred after the combined application of immunotherapy and radiation therapy. In addition to the differences in the immune drugs or adjuvants utilized, another noteworthy point is the mode of administration. Local delivery of immunotherapeutic drugs into the tumours leads to increased local drug concentration, and the odds of abscopal effect occurrence are also increased.43
Table 1.
Clinical trials or cases with abscopal effect
| Years | Tumors | Radiation therapy | Immunotherapy | Sequence of RT and immunotherapy | Reference |
|---|---|---|---|---|---|
| 2014 | Melanoma | RT of brain metastasis or extracranial sites | Anti-CTLA-4 (ipilimumab, 3 mg/kg/3 weeks) | RT after ipilimumab | [105] |
| 2015 | Metastatic solid tumors | 35 Gy/10 fractions | GM-CSF (125 μg/m2/2 weeks) | Concurrent | [32] |
| 2015 | Melanoma | Lung/bone 8 Gy × 2 or 8 Gy × 3 Liver/subcutaneous 6 Gy × 2 or 6 Gy × 3 |
Anti-CTLA-4 (ipilimumab) | RT before ipilimumab | [22] |
| 2016 | Melanoma | SBRT | Anti-CTLA-4 (ipilimumab, 3 mg/kg/3 weeks) | Concurrent and post-radiation | [106] |
| 2017 | Melanoma | 30 Gy/10 fractions | Anti-PD-1 (pembrolizumab, 2 mg/kg/3 weeks or nivolumab, 3 mg/kg/3 weeks) | Concurrent | [107] |
| 2017 | Melanoma | Conventional external beam radiation and stereotactic radiosurgery | Anti-CTLA-4 (ipilimumab, 3 mg/kg/3 weeks) | Concurrent | [108] |
| 2018 | Advanced cancer | Stereotactic ablative RT | DC vaccination and TLR-3 agonist | Concurrent | [109] |
| 2018 | Metastatic breast cancer | 22.5 Gy/3 fractions | Anti-TGFβ (fresolimumab,1 mg/kg/3 weeks or 10 mg/kg/3 weeks) | Concurrent | [110] |
| 2018 | Melanoma | 26 Gy/3–5 fractions | Anti-PD-1 (pembrolizumab, 2 mg/kg/3 weeks or nivolumab, 3 mg/kg/2 weeks) | Concurrent and post-radiation | [111] |
| 2018 | Metastatic NSCLC | Phase I:6 Gy X 5/fractions Phase II :9.5 Gy X 3/fractions |
Anti-CTLA-4 (ipilimumab, 3 mg/kg/3 weeks) | Concurrent | [17] |
| 2018 | Refractory Hodgkin’s Lymphoma | total 40 Gy in 20 fractions, mediastinal nodes | Anti-PD-1 (Nivolumab, 2 doses) | Concurrent and post-radiation | [112] |
| 2018 | Prostate cancer | SABR | Hiltonol intratumoral injections (DC Local ReactionG1) | Concurrent | [109] |
| 2019 | Unresectable stage IIIB/IV bulky `NSCLC | SBRT-PATHY | None | None | [113] |
| 2019 | Renal cell carcinoma | high-dose-rate interstitial brachytherapy (HDR-ISBT) | Anti-PD-1 (Nivolumab infusions of 240 mg /alternate week) | Nivolumab was restarted nine days after HDR-ISBT. | [114] |
Appropriate Radiation Therapy Mode Increases the Occurrence Probability of the Abscopal Effect
The immune response to radiation therapy is inextricably linked to tumour types, immunomodulation, and dose and mode of radiation therapy. Ionizing radiation delivered by radiation therapy induces DNA damage, leading to the apoptosis, senescence, and autophagy of tumour cells.44 Lymphocyte levels are reduced after radiation therapy.45,46 However, low doses of radiation appear to activate macrophage but do not induce cell death, while high doses of radiation appear to induce immunogenic effects.47 In a preclinical trial, low-dose radiation increased the infiltration of T cells into the irradiated area.48 Radiation therapies using higher doses are more likely to cause increased DNA damage and expose more antigens, thereby enhancing the immune response. However, excessive radiation doses are more harmful than beneficial to the patients. Is there a suitable optimal radiation dose that maximally activates the immune response in patients while promoting the occurrence of the abscopal effect? To explore this dose, Professor Poleszczuk established a mathematical model. The model suggested that the optimal radiation doses per fraction were between 10 and 13 Gy, at least under the experimental setting used for model calibration.49 However, in reality, researchers believe that radiation therapy doses ranging from 2 to 20 Gy may trigger immunogenic cell death.50 A pre-clinical trial showed that a combination of low-dose-fractionated radiation therapy and immunotherapy was more likely to trigger the abscopal effect.25 In contrast, high-dose-hypofractionated radiation therapy was more conducive to survival in a tumour mouse model.51 In clinical cases of the abscopal effect, a hypofractionated dose of 30 Gy was delivered in 5 fractions or a total dose of 28.5 Gy was delivered over 3 fractions.1,23 Another study showed that radiation-induced changes in the content of the DNA exonuclease Trex1 (three prime repair exonuclease 1) in various cancer cells.52 Trex1 degrades the DNAs in the cytosol to attenuate their immunogenicity, thereby reducing the immune response.53 Therefore, it is necessary to identify proper radiation doses that will not induce Trex1. Repeated irradiation with these doses will increase the content of cytosolic DNA. Further amplification of interferon-β will lead to the recruitment and activation of Batf3-dependent dendritic cells (DCs), which is also very important for the occurrence of the abscopal effect.
In addition, radiation therapy induces anti-tumour immune response, which usually depends on the immunogenicity of the tumours. Tumour immunogenicity varies greatly among different individuals, different types of cancers, and even the same type of cancers.1,54 High immunogenicity is more potent in stimulating immunity. Moreover, immunogenicity level is related to prognosis. The immunogenicity of tumours induced by different modes of radiation therapy varies considerably. Current combination therapies aim to stimulate and enhance immunogenicity to the greatest extent.55,56 The nature of the radiation ray cannot be ignored. High linear energy transfer (LET) radiation mainly acts directly on the biological macromolecules in the tissues and cells, causing damage. The physical characteristic of high-LET radiation is the presence of Bragg peaks, while the biological characteristics of high-LET radiation include high relative biological effect and low oxygen enhancement ratio. Low-LET radiation mainly acts indirectly. Because organisms are in a high-water environment, low-LET radiation interacts with the water molecules in the biological tissues, generating free radicals. These free radicals then interact with biological macromolecules and damage them. Low-LET radiation can be used to achieve non-discriminatory killing of tumour cells that display heterogeneous radiosensitivity due to the different oxygen content in tumours.57 In a review, Professor Pouget proposed the idea of complex DNA damage leading to immune signal transduction: clustered DNA damage will eventually cause persistent DNA damage, leading to cell aging or cell death (ie, apoptosis), triggering different “dangerous” signals or damage-associated molecular patterns (damps: ATP, short DNAs/RNAs, ROS and others). In terms of targeted radionuclide therapy, Proton/carbon ion radiation therapy and alpha-particles deliver high-LET radiation.58 Low energy γ ray or carbon ion and coradiation elicit the abscopal effect, which is closely related to macrophages.59
Exploration of the Cause That Prevents the Occurrence of the Abscopal Effect
Numerous clinical trials and basic research aim to promote the occurrence of the abscopal effect (Figure 2). In reality, however, the odds of abscopal effect occurrence have not reached expectations.60 Combined administration of radiation therapy and immunotherapy improves the occurrence rate of the abscopal response compared to radiation therapy or immunotherapy alone. However, the overall occurrence rate of the abscopal effect remains unsatisfactory, which indicates its limitations. A systematic review found that there were only 46 documented cases of abscopal effect of radiation therapy between 1969 and 2014.61 A study conducted in Brazil explored the probability of abscopal effect occurring after anti-PD1 therapy and associated radiation therapy. The study examined 16 patients, including 12 patients with metastatic melanoma, 2 patients with metastatic NSCLC, and 2 patients with metastatic renal cell carcinoma. Three patients with melanoma developed the abscopal effect, a rate of 18.7%. In contrast, no patients with NSCLC or renal cancer exhibited the abscopal effect.62 We summarized the reasons behind the unsatisfactory rate of abscopal effect occurrence into the following aspects:
The Complex Tumour Microenvironment
The post-radiotherapeutic changes in the tumour microenvironment are not all beneficial. In fact, it is difficult to overcome the inhibitory effect of the tumour microenvironment even if radiation therapy activates the anti-tumour CD8 + T cells,63 and the infiltration of the anti-tumour effectors to tumour tissues remains weak.64 The tumour microenvironment is not singular and orderly. Radiation therapy leads to the release of a variety of inhibitory factors, including TGF-β. TGF-β is an immunosuppressive factor. It not only inhibits the immune response by reducing the antigen-presenting capacity of DCs and the activation of effector T cells65,66 but also induces radioresistance in tumour cells and decreases their radiosensitivity.67 In addition, the appearance of interleukin 6 (IL-6), interleukin 10 (IL-10) and colony-stimulating factor 1 (CSF-1) also promotes tumour cell proliferation and invasion.68–70 Their appearance not only reduces the occurrence of abscopal effect but also reduces the efficacy of radiation therapy.71 However, radiation therapy induces the release of a variety of cytokines such as interleukin 1 beta (IL-1β), which promote anti-tumour effects.72,73 Radiation therapies using different doses and modes will inevitably yield inconsistent outcomes. These facts strongly confirm the complexity and contradictions of various factors in the tumour microenvironment.
In addition, the tumour microenvironment is equally complex at the cellular level. After irradiation, tumours develop a variety of resistance mechanisms that promote tumour recurrence, including the production of suppressive immune cells capable of inhibiting T cell activation. These suppressive immune cells, including Tregs, bone marrow myeloid-derived suppressor cells (MDSCs), and tumour-associated macrophages (TAMs),74–76 infiltrate more into tumour tissues after radiation therapy.74,77 Tregs are key cells in the maintenance of tumour immune tolerance, and they downregulate immunity and even promote tumour angiogenesis.78 In addition, Tregs not only produce a variety of inhibitory cytokines (including tumour necrosis factor β (TNF-β),79 interleukin 35 (IL-35),80 and IL-1081) but also preempt IL-2 and reduce the activation of cytotoxic T cells.82 MDSCs not only reduce immune activity at the immune level but also promote tumour infiltration and migration.83 TAMs are the main white blood cells that infiltrate solid tumours, comprising up to 50% of the tumour mass. TAMs promote cancer cell proliferation, invasion, metastasis, and angiogenesis through releasing cytokines, growth factors, extracellular matrix-degrading enzymes, the angiogenic factor prokineticin (Bv8), and matrix metalloproteinase 9 (MMP9).76 On the other hand, radiation therapy renders tumour cells more susceptible to immunity. Reits et al found that under high doses of radiation (10–26 Gy), the expression of the major histocompatibility complex I (MHC-I) increased in a dose-dependent manner. Increased MHC-I expression promoted the production of APCs and activated the effector T cells.7 It is evident that both the suppressive and the promotive immune cells change after radiation therapy. At present, there is no definite conclusion about which is the dominant type of immune cell. However, the dual nature of the microenvironment and the difficulty of inducing the abscopal effect are implied. In addition to immune cells, tumor stromal cells not only provide physical support for tumor cells but also drive tumorigenicity. Cancer-associated fibroblasts (CAFs) are one of the most important types.84 The soluble factors secreted by CAFs induce various phenotypes of adjacent tumor epithelial cells and other stromal cells, and then promote the development of tumor.85 Although there is no study on the relationship between CAFs and distant effect, radiation enhances the migration and invasion promoting ability of CAF in vitro and in vivo.86
The Sophisticated Systems of the Human Body
As mentioned in the theory of the mechanism of the abscopal effect, radiation therapy leads to the exposure of tumour antigens and the appearance of a “tumour vaccine”, thereby promoting immune activity. However, in reality, the abscopal effect mechanism is far from simple. The human immune system is a sophisticated and complex system. It is difficult to improve the overall effect through one single point or pathway. Moreover, differences exist among individuals, which undoubtedly render it more difficult to induce the abscopal effect. First, the activation of cytotoxic T cells, the cells that actually function in immunity, requires more than the stimulation imposed by the antigen-MHC complex. Other costimulatory signals such as cluster of differentiation 80 (CD80), CD40 ligand (CD40 L), and cluster of differentiation 28 (CD28) are also essential for the activation of CD8+ T cells. Such facts indicate that radiation-induced DNA damage and exposure of “tumour vaccine” are not sufficient for stimulation of immunity. A preclinical trial of colon cancer found that systemic immune enhancement occurred several weeks (rather than immediately) after radiation therapy and was not long-lasting.51 Poleszczuk et al established a mathematical model.87 Examination of virtual cases using this model revealed that the dissemination of activated T cells among multiple metastatic sites was complex, and not all metastatic sites participated equally in systemic immune surveillance. The above findings demonstrate that the likelihood of promoting systemic immunity is rather low. Moreover, the systemic anti-tumour immunity cannot be maintained in the long term even if the abscopal effect has occurred, which is one reason why new radioimmunotherapy strategies are currently being explored.88 Finally, application of combination therapy to promote abscopal effect has become a new research hotspot since the discovery of immune checkpoints. However, similar to radiation therapy, immune checkpoint inhibitors and immunological adjuvants may cause a variety of side effects, even highly toxic side effects.4,89,90 The combined application of the two will inevitably increase the likelihood of these adverse events.91 However, PACIFIC and LUN14-179 experiments also confirmed that the incidence of side effects, such as pneumonia, in the study group receiving PD-L1 mAb after concurrent radiotherapy and chemotherapy did not increase significantly compared with the control group. More clinical trials need to be conducted to determine how to minimize the additive toxic effect of radiation and immunotherapy4,92 and optimize the order of these two types of treatments. Proper selection of immune checkpoint inhibitors/immunological adjuvants, timing of administration, mode of administration, dosage, and mode of combination are critical to the success of radiation therapy and immunotherapy. However, there is still a lack of definite answers to these questions. These questions further indicate the difficulty of inducing abscopal effect.
The Inhibitory Effect of Malignant Tumours Themselves
Malignant tumour cells exhibit extremely strong mutability, heterogeneity, and atypia, which also increase the difficulty of eliciting the abscopal effect. Studies have shown that the genetic mutations in the primary lesion are not completely consistent with the genetic mutations in the metastatic lesions. Because the local antigen is not necessarily identical to the distant antigen, the immune responses induced by simply targeting local antigens are likely to be ineffective. With the enrichment of the therapeutic approaches aiming to boost immunity, cancer cells have evolved a series of immune-resistance mechanisms that facilitate their evasion of anti-tumour immune responses. This process is known as immunoediting.93,94 Immunoediting downregulates vascular cell adhesion molecule 1 (VCAM1) and intercellular adhesion molecule 1 (ICAM1), resulting in the enhancement of the tumour vasculature capable of inhibiting T cell colonization and migration. Immunoediting also leads to the downregulation of the major histocompatibility complex (MHC) and the promotion of suppressive immune cell infiltration. In addition, hypoxic regions are present in some tumours, which may induce immunosuppression through causing immune cell failure.95 For example, hypoxia alters the antigen-presenting ability of APCs.96 Even if the immune cells that are capable of targeting distant metastases are produced, it is unclear whether these immune cells can smoothly pass through various barriers and successfully reach the targeted area. As demonstrated by studies, the ability of immune cells to accurately reach non-irradiated areas may be one of the rate-limiting steps that trigger the radiation-induced abscopal effect.87 These complex interactions contribute to the development of cancer cells and further limit the effect of immunotherapy.97,98 Even so, the immune system can still recognize and eliminate cancer cells. However, in the cases in which the metastases contain a large number of tumour cells, the limited number of immune cells is a drop in the bucket. Moreover, patients with advanced cancer have more or less impaired immune functions and compensatory ability. The patients often receive concurrent chemotherapy,99 while one of the side effects of chemotherapy is immunosuppression. The integrity of the host immune system determines the sensitivity of the tumours to radiation therapy.100 Therefore, the above factors also limit the occurrence of the abscopal effect.
The Unknowns About the Modes and Methods of Radiation Therapy
Many studies are striving to identify the optimal radiation therapy dose for induction of the abscopal effect. However, fractionated radiation therapy fails to diversify the T cell receptor repertoire in the distal non-irradiated areas.22,101 The above information suggests that the differences in radiation therapy dose, the employed fractionation schemes, and the size and location of the irradiated lesions are also factors influencing the occurrence of the abscopal effect.87,102 To explore the optimal conditions for the induction of the abscopal effect, radiation therapy needs to be standardized.103
A Correct View of the Abscopal Effect
Radiation therapy and immunotherapy both occupy pivotal positions in cancer treatment. Currently, numerous clinical studies have explored the combinations of radiation therapy and immunotherapy. A large number of clinical trials and data show that the combination of the two types of treatments not only alleviates the related symptoms of tumour patients and effectively prolongs survival time but also enhances the possibility of abscopal effect occurrence. Will immunotherapy be overly used? Whether the evaluation of curative effect of the combination of two treatment strategies based on the standard RECIST standard is a little single? More than 90% of these clinical studies attempted to induce clinically significant abscopal effects by irradiating individual tumours for the purpose of controlling all tumours. However, Brooks and Chang proposed that the abscopal effect was difficult to achieve by irradiating single tumours. They advocated irradiating multiple or all lesions to increase the likelihood of obtaining clinically meaningful outcomes.104 There are still questions and controversies surrounding the mechanisms and existence of the abscopal effect. However, the combination of the treatment methods is undoubtedly beneficial to patients. Perhaps there should be more preclinical studies to determine how to better combine radiation therapy and immunity, such as T-cell immunoglobulin and mucin-domain containing-3 (TIM-3) and indoleamine 2,3-dioxygenase (IDO), which suppress immune signals, as well as OX40 and 4–1BB, which activate immune signals. Questions still need to be addressed, such as whether it is possible to combine these signalling pathways with different radiotherapy modes to improve the patients’ survival and quality of life, and how to weigh the benefits and harms elicited by single-site radiotherapy and multi-site/whole body radiotherapy. Because of the unaddressed questions, the occurrence of the abscopal effect is still unclear. We look forward to subsequent studies and better methods to bring about improved and more beneficial treatments.
Disclosure
The authors declare no potential conflicts of interest.
References
- 1.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366(10):925–931. doi: 10.1056/NEJMoa1112824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58(3):862–870. doi: 10.1016/j.ijrobp.2003.09.012 [DOI] [PubMed] [Google Scholar]
- 3.Habets TH, Oth T, Houben AW, et al. Fractionated radiotherapy with 3 x 8 Gy induces systemic anti-tumour responses and abscopal tumour inhibition without modulating the humoral anti-tumour response. PLoS One. 2016;11(7):e0159515. doi: 10.1371/journal.pone.0159515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang C, Wang X, Soh H, et al. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res. 2014;2(9):831–838. doi: 10.1158/2326-6066.CIR-14-0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650. doi: 10.1016/j.immuni.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 6.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31(1):51–72. doi: 10.1146/annurev-immunol-032712-100008 [DOI] [PubMed] [Google Scholar]
- 7.Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259–1271. doi: 10.1084/jem.20052494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grass GD, Krishna N, Kim S. The immune mechanisms of abscopal effect in radiation therapy. Curr Probl Cancer. 2016;40(1):10–24. doi: 10.1016/j.currproblcancer.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 10.Demaria S, Formenti SC. Sensors of ionizing radiation effects on the immunological microenvironment of cancer. Int J Radiat Biol. 2007;83(11–12):819–825. doi: 10.1080/09553000701481816 [DOI] [PubMed] [Google Scholar]
- 11.Rodel F, Frey B, Multhoff G, Gaipl U. Contribution of the immune system to bystander and non-targeted effects of ionizing radiation. Cancer Lett. 2015;356(1):105–113. doi: 10.1016/j.canlet.2013.09.015 [DOI] [PubMed] [Google Scholar]
- 12.Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-induced IFN-γ production within the tumor microenvironment influences antitumor immunity. J Immunol. 2008;180(5):3132–3139. doi: 10.4049/jimmunol.180.5.3132 [DOI] [PubMed] [Google Scholar]
- 13.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174(12):7516–7523. doi: 10.4049/jimmunol.174.12.7516 [DOI] [PubMed] [Google Scholar]
- 14.Matsumura S, Demaria S. Up-regulation of the pro-inflammatory chemokine CXCL16 is a common response of tumor cells to ionizing radiation. Radiat Res. 2010;173(4):418–425. doi: 10.1667/RR1860.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burnette BC, Liang H, Lee Y, et al. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71(7):2488–2496. doi: 10.1158/0008-5472.CAN-10-2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uehara J, Ohkuri T, Kosaka A, et al. Intratumoral injection of IFN-beta induces chemokine production in melanoma and augments the therapeutic efficacy of anti-PD-L1 mAb. Biochem Biophys Res Commun. 2017;490(2):521–527. doi: 10.1016/j.bbrc.2017.06.072 [DOI] [PubMed] [Google Scholar]
- 17.Formenti SC, Rudqvist NP, Golden E, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med. 2018;24(12):1845–1851. doi: 10.1038/s41591-018-0232-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harding SM, Benci JL, Irianto J, Discher DE, Minn AJ, Greenberg RA. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature. 2017;548(7668):466–470. doi: 10.1038/nature23470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camphausen K, Moses MA, Menard C, et al. Radiation abscopal antitumor effect is mediated through p53. Cancer Res. 2003;63(8):1990–1993. [PubMed] [Google Scholar]
- 20.Strigari L, Mancuso M, Ubertini V, et al. Abscopal effect of radiation therapy: interplay between radiation dose and p53 status. Int J Radiat Biol. 2014;90(3):248–255. doi: 10.3109/09553002.2014.874608 [DOI] [PubMed] [Google Scholar]
- 21.Pfannenstiel LW, McNeilly C, Xiang C, et al. Combination PD-1 blockade and irradiation of brain metastasis induces an effective abscopal effect in melanoma. Oncoimmunology. 2019;8(1):e1507669. doi: 10.1080/2162402X.2018.1507669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–377. doi: 10.1038/nature14292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res. 2013;1(6):365–372. doi: 10.1158/2326-6066.CIR-13-0115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687–695. doi: 10.1172/JCI67313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15(17):5379–5388. doi: 10.1158/1078-0432.CCR-09-0265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park SS, Dong H, Liu X, et al. PD-1 restrains radiotherapy-induced abscopal effect. Cancer Immunol Res. 2015;3(6):610–619. doi: 10.1158/2326-6066.CIR-14-0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107(9):4275–4280. doi: 10.1073/pnas.0915174107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hao Y, Yasmin-Karim S, Moreau M, Sinha N, Sajo E, Ngwa W. Enhancing radiotherapy for lung cancer using immunoadjuvants delivered in situ from new design radiotherapy biomaterials: a preclinical study. Phys Med Biol. 2016;61(24):N697–N707. doi: 10.1088/1361-6560/61/24/N697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yasmin-Karim S, Bruck PT, Moreau M, et al. Radiation and local anti-CD40 generate an effective in situ vaccine in preclinical models of pancreatic cancer. Front Immunol. 2018;9:2030. doi: 10.3389/fimmu.2018.02030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rekers NH, Olivo Pimentel V, Yaromina A, et al. The immunocytokine L19-IL2: an interplay between radiotherapy and long-lasting systemic anti-tumour immune responses. Oncoimmunology. 2018;7(4):e1414119. doi: 10.1080/2162402X.2017.1414119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golden EB, Chhabra A, Chachoua A, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol. 2015;16(7):795–803. doi: 10.1016/S1470-2045(15)00054-6 [DOI] [PubMed] [Google Scholar]
- 33.Demaria S, Vanpouille-Box C, Formenti SC, Adams S. The TLR7 agonist imiquimod as an adjuvant for radiotherapy-elicited in situ vaccination against breast cancer. Oncoimmunology. 2013;2(10):e25997. doi: 10.4161/onci.25997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiraishi K, Ishiwata Y, Nakagawa K, et al. Enhancement of antitumor radiation efficacy and consistent induction of the abscopal effect in mice by ECI301, an active variant of macrophage inflammatory protein-1alpha. Clin Cancer Res. 2008;14(4):1159–1166. doi: 10.1158/1078-0432.CCR-07-4485 [DOI] [PubMed] [Google Scholar]
- 35.Moreau M, Yasmin-Karim S, Kunjachan S, et al. Priming the abscopal effect using multifunctional smart radiotherapy biomaterials loaded with immunoadjuvants. Front Oncol. 2018;8:56. doi: 10.3389/fonc.2018.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Min Y, Roche KC, Tian S, et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat Nanotechnol. 2017;12(9):877–882. doi: 10.1038/nnano.2017.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antoniades J, Brady LW, Lightfoot DA. Lymphangiographic demonstration of the abscopal effect in patients with malignant lymphomas. Int J Radiat Oncol Biol Phys. 1977;2(1–2):141–147. doi: 10.1016/0360-3016(77)90020-7 [DOI] [PubMed] [Google Scholar]
- 38.Wersall PJ, Blomgren H, Pisa P, Lax I, Kalkner K-M, Svedman C. Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Acta Oncol. 2006;45(4):493–497. doi: 10.1080/02841860600604611 [DOI] [PubMed] [Google Scholar]
- 39.Leung HW, Wang S-Y, Jin-Jhih H, Chan AL. Abscopal effect of radiation on bone metastases of breast cancer: a case report. Cancer Biol Ther. 2018;19(1):20–24. doi: 10.1080/15384047.2017.1394545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohba K, Omagari K, Nakamura T, et al. Abscopal regression of hepatocellular carcinoma after radiotherapy for bone metastasis. Gut. 1998;43(4):575–577. doi: 10.1136/gut.43.4.575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwee SA, Lim J, Coel MN. Soft tissue response on 18F-fluorocholine PET/CT in metastatic castrate-resistant prostate cancer treated with 223Ra-dichloride: a possible abscopal effect? Clin Nucl Med. 2017;42(11):868–871. doi: 10.1097/RLU.0000000000001807 [DOI] [PubMed] [Google Scholar]
- 42.Shi F, Wang X, Teng F, Kong L, Yu J. Abscopal effect of metastatic pancreatic cancer after local radiotherapy and granulocyte-macrophage colony-stimulating factor therapy. Cancer Biol Ther. 2017;18(3):137–141. doi: 10.1080/15384047.2016.1276133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brody JD, Ai WZ, Czerwinski DK, et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a Phase I/II study. J Clin Oncol. 2010;28(28):4324–4332. doi: 10.1200/JCO.2010.28.9793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eriksson D, Stigbrand T. Radiation-induced cell death mechanisms. Tumour Biol. 2010;31(4):363–372. doi: 10.1007/s13277-010-0042-8 [DOI] [PubMed] [Google Scholar]
- 45.Blomgren H, Glas U, Melen B, Wasserman J. Blood lymphocytes after radiation therapy of mammary carcinoma. Acta Radiol. 1974;13(3):185–200. doi: 10.3109/02841867409129875 [DOI] [PubMed] [Google Scholar]
- 46.Campian JL, Ye X, Brock M, Grossman SA. Treatment-related lymphopenia in patients with stage III non-small-cell lung cancer. Cancer Invest. 2013;31(3):183–188. doi: 10.3109/07357907.2013.767342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de la Cruz-merino L, Illescas-Vacas A, Grueso-Lopez A, Barco-Sanchez A, Miguez-Sanchez C. Cancer immunotherapies Spanish, radiation for awakening the dormant immune system, a promising challenge to be explored. Front Immunol. 2014;5:102. doi: 10.3389/fimmu.2014.00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klug F, Prakash H, Huber PE, et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24(5):589–602. doi: 10.1016/j.ccr.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 49.Poleszczuk J, Enderling H. The optimal radiation dose to induce robust systemic anti-tumor immunity. Int J Mol Sci. 2018;19(11):3377. doi: 10.3390/ijms19113377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Golden EB, Frances D, Pellicciotta I, Demaria S, Helen Barcellos-Hoff M, Formenti SC. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology. 2014;3(4):e28518. doi: 10.4161/onci.28518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Filatenkov A, Baker J, Mueller AM, et al. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin Cancer Res. 2015;21(16):3727–3739. doi: 10.1158/1078-0432.CCR-14-2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8(1):15618. doi: 10.1038/ncomms15618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamazaki T, Galluzzi L. TREX1 cuts down on cancer immunogenicity. Trends Cell Biol. 2017;27(8):543–545. doi: 10.1016/j.tcb.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 54.Blankenstein T, Coulie PG, Gilboa E, Jaffee EM. The determinants of tumour immunogenicity. Nat Rev Cancer. 2012;12(4):307–313. doi: 10.1038/nrc3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17(2):97–111. doi: 10.1038/nri.2016.107 [DOI] [PubMed] [Google Scholar]
- 56.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Reply: immunosuppressive cell death in cancer. Nat Rev Immunol. 2017;17(6):402. doi: 10.1038/nri.2017.48 [DOI] [PubMed] [Google Scholar]
- 57.Tinganelli W, Durante M, Hirayama R, et al. Kill-painting of hypoxic tumours in charged particle therapy. Sci Rep. 2015;5(1):17016. doi: 10.1038/srep17016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gorin JB, Menager J, Gouard S, et al. Antitumor immunity induced after alpha irradiation. Neoplasia. 2014;16(4):319–328. doi: 10.1016/j.neo.2014.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dong C, He M, Tu W, et al. The differential role of human macrophage in triggering secondary bystander effects after either gamma-ray or carbon beam irradiation. Cancer Lett. 2015;363(1):92–100. doi: 10.1016/j.canlet.2015.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wennerberg E, Lhuillier C, Vanpouille-Box C, et al. Barriers to radiation-induced in situ tumor vaccination. Front Immunol. 2017;8:229. doi: 10.3389/fimmu.2017.00229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer. 2016;40(1):25–37. doi: 10.1016/j.currproblcancer.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 62.Ribeiro Gomes J, Schmerling RA, Haddad CK, et al. Analysis of the abscopal effect with anti-PD1 therapy in patients with metastatic solid tumors. J Immunother. 2016;39(9):367–372. doi: 10.1097/CJI.0000000000000141 [DOI] [PubMed] [Google Scholar]
- 63.Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15(7):409–425. doi: 10.1038/nrc3958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–1022. doi: 10.1038/ni.2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vanpouille-Box C, Diamond JM, Pilones KA, et al. TGFbeta is a master regulator of radiation therapy-induced antitumor immunity. Cancer Res. 2015;75(11):2232–2242. doi: 10.1158/0008-5472.CAN-14-3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clin Cancer Res. 2007;13(18):5262–5270. doi: 10.1158/1078-0432.CCR-07-1157 [DOI] [PubMed] [Google Scholar]
- 67.Bouquet F, Pal A, Pilones KA, et al. TGFbeta1 inhibition increases the radiosensitivity of breast cancer cells in vitro and promotes tumor control by radiation in vivo. Clin Cancer Res. 2011;17(21):6754–6765. doi: 10.1158/1078-0432.CCR-11-0544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu J, Escamilla J, Mok S, et al. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73(9):2782–2794. doi: 10.1158/0008-5472.CAN-12-3981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matsuoka Y, Nakayama H, Yoshida R, et al. IL-6 controls resistance to radiation by suppressing oxidative stress via the Nrf2-antioxidant pathway in oral squamous cell carcinoma. Br J Cancer. 2016;115(10):1234–1244. doi: 10.1038/bjc.2016.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Visco C, Vassilakopoulos TP, Kliche KO, et al. Elevated serum levels of IL-10 are associated with inferior progression-free survival in patients with Hodgkin’s disease treated with radiotherapy. Leuk Lymphoma. 2004;45(10):2085–2092. doi: 10.1080/10428190410001712234 [DOI] [PubMed] [Google Scholar]
- 71.Saito H, Tsujitani S, Oka S, et al. An elevated serum level of transforming growth factor-beta 1 (TGF-beta 1) significantly correlated with lymph node metastasis and poor prognosis in patients with gastric carcinoma. Anticancer Res. 2000;20(6B):4489–4493. [PubMed] [Google Scholar]
- 72.Ghiringhelli F, Apetoh L, Tesniere A, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15(10):1170–1178. doi: 10.1038/nm.2028 [DOI] [PubMed] [Google Scholar]
- 73.Calveley VL, Khan MA, Yeung IW, Vandyk J, Hill RP. Partial volume rat lung irradiation: temporal fluctuations of in-field and out-of-field DNA damage and inflammatory cytokines following irradiation. Int J Radiat Biol. 2005;81(12):887–899. doi: 10.1080/09553000600568002 [DOI] [PubMed] [Google Scholar]
- 74.Kachikwu EL, Iwamoto KS, Liao YP, et al. Radiation enhances regulatory T cell representation. Int J Radiat Oncol Biol Phys. 2011;81(4):1128–1135. doi: 10.1016/j.ijrobp.2010.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Du R, Lu KV, Petritsch C, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13(3):206–220. doi: 10.1016/j.ccr.2008.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Laoui D, Van Overmeire E, De Baetselier P, Van Ginderachter JA, Raes G. Functional relationship between tumor-associated macrophages and macrophage colony-stimulating factor as contributors to cancer progression. Front Immunol. 2014;5:489. doi: 10.3389/fimmu.2014.00489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu CY, Yang LH, Yang HY, et al. Enhanced cancer radiotherapy through immunosuppressive stromal cell destruction in tumors. Clin Cancer Res. 2014;20(3):644–657. doi: 10.1158/1078-0432.CCR-13-1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Facciabene A, Motz GT, Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72(9):2162–2171. doi: 10.1158/0008-5472.CAN-11-3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192(2):295–302. doi: 10.1084/jem.192.2.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450(7169):566–569. doi: 10.1038/nature06306 [DOI] [PubMed] [Google Scholar]
- 81.Annacker O, Asseman C, Read S, Powrie F. Interleukin-10 in the regulation of T cell-induced colitis. J Autoimmun. 2003;20(4):277–279. doi: 10.1016/S0896-8411(03)00045-3 [DOI] [PubMed] [Google Scholar]
- 82.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8(12):1353–1362. doi: 10.1038/ni1536 [DOI] [PubMed] [Google Scholar]
- 83.Condamine T, Ramachandran I, Youn JI, Gabrilovich DI. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu Rev Med. 2015;66(1):97–110. doi: 10.1146/annurev-med-051013-052304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Wever O, Van Bockstal M, Mareel M, Hendrix A, Bracke M. Carcinoma-associated fibroblasts provide operational flexibility in metastasis. Semin Cancer Biol. 2014;25:33–46. doi: 10.1016/j.semcancer.2013.12.009 [DOI] [PubMed] [Google Scholar]
- 85.Gascard P, Tlsty TD. Carcinoma-associated fibroblasts: orchestrating the composition of malignancy. Genes Dev. 2016;30(9):1002–1019. doi: 10.1101/gad.279737.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li D, Qu C, Ning Z, et al. Radiation promotes epithelial-to-mesenchymal transition and invasion of pancreatic cancer cell by activating carcinoma-associated fibroblasts. Am J Cancer Res. 2016;6(10):2192–2206. [PMC free article] [PubMed] [Google Scholar]
- 87.Poleszczuk JT, Luddy KA, Prokopiou S, et al. Abscopal benefits of localized radiotherapy depend on activated T-cell trafficking and distribution between metastatic lesions. Cancer Res. 2016;76(5):1009–1018. doi: 10.1158/0008-5472.CAN-15-1423 [DOI] [PubMed] [Google Scholar]
- 88.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–489. doi: 10.1038/nature10673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, Phase 2 trial. Lancet Oncol. 2016;17(11):1558–1568. doi: 10.1016/S1470-2045(16)30366-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Straub BK, Ridder DA, Schad A, Loquai C, Schattenberg JM. Liver injury induced by immune checkpoint inhibitor-therapy: example of an immune-mediated drug side effect. Pathologe. 2018;39(6):556–562. doi: 10.1007/s00292-018-0519-6 [DOI] [PubMed] [Google Scholar]
- 91.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12(4):237–251. doi: 10.1038/nrc3237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vatner RE, Cooper BT, Vanpouille-Box C, Demaria S, Formenti SC. Combinations of immunotherapy and radiation in cancer therapy. Front Oncol. 2014;4:325. doi: 10.3389/fonc.2014.00325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–998. doi: 10.1038/ni1102-991 [DOI] [PubMed] [Google Scholar]
- 94.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486 [DOI] [PubMed] [Google Scholar]
- 95.McNamee EN, Korns Johnson D, Homann D, Clambey ET. Hypoxia and hypoxia-inducible factors as regulators of T cell development, differentiation, and function. Immunol Res. 2013;55(1–3):58–70. doi: 10.1007/s12026-012-8349-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Doedens AL, Stockmann C, Rubinstein MP, et al. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 2010;70(19):7465–7475. doi: 10.1158/0008-5472.CAN-10-1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29(1):235–271. doi: 10.1146/annurev-immunol-031210-101324 [DOI] [PubMed] [Google Scholar]
- 98.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22(1):329–360. doi: 10.1146/annurev.immunol.22.012703.104803 [DOI] [PubMed] [Google Scholar]
- 99.Kang J, Demaria S, Formenti S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J Immunother Cancer. 2016;4(1):51. doi: 10.1186/s40425-016-0156-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stone HB, Peters LJ, Milas L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. J Natl Cancer Inst. 1979;63(5):1229–1235. [PubMed] [Google Scholar]
- 101.Dovedi SJ, Cheadle EJ, Popple AL, et al. Fractionated radiation therapy stimulates antitumor immunity mediated by both resident and infiltrating polyclonal T-cell populations when combined with PD-1 blockade. Clin Cancer Res. 2017;23(18):5514–5526. doi: 10.1158/1078-0432.CCR-16-1673 [DOI] [PubMed] [Google Scholar]
- 102.Demaria S, Formenti SC. Can abscopal effects of local radiotherapy be predicted by modeling T cell trafficking? J Immunother Cancer. 2016;4(1):29. doi: 10.1186/s40425-016-0133-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Durante M, Reppingen N, Held KD. Immunologically augmented cancer treatment using modern radiotherapy. Trends Mol Med. 2013;19(9):565–582. doi: 10.1016/j.molmed.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 104.Brooks ED, Chang JY. Time to abandon single-site irradiation for inducing abscopal effects. Nat Rev Clin Oncol. 2019;16(2):123–135. doi: 10.1038/s41571-018-0119-7 [DOI] [PubMed] [Google Scholar]
- 105.Grimaldi AM, Simeone E, Giannarelli D, et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology. 2014;3:e28780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Theurich S, Rothschild SI, Hoffmann M, et al. Local Tumor Treatment in Combination with Systemic Ipilimumab Immunotherapy Prolongs Overall Survival in Patients with Advanced Malignant Melanoma. Cancer Immunol Res. 2016;4:744–754. [DOI] [PubMed] [Google Scholar]
- 107.Aboudaram A, Modesto A, Chaltiel L, et al. Concurrent radiotherapy for patients with metastatic melanoma and receiving anti-programmed-death 1 therapy: a safe and effective combination. Melanoma Res. 2017;27:485–491. [DOI] [PubMed] [Google Scholar]
- 108.Koller KM, Mackley HB, et al. Improved survival and complete response rates in patients with advanced melanoma treated with concurrent ipilimumab and radiotherapy versus ipilimumab alone. Cancer Biol Ther. 2017;18:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rodriguez-Ruiz ME, Perez-Gracia JL, Rodriguez I, et al. Combined immunotherapy encompassing intratumoral poly-ICLC, dendritic-cell vaccination and radiotherapy in advanced cancer patients. Ann Oncol. 2018;29:1312–1319. [DOI] [PubMed] [Google Scholar]
- 110.Formenti SC, Lee Adams PS, et al. Focal Irradiation and Systemic TGFbeta Blockade in Metastatic Breast Cancer. Clin Cancer Res. 2018;24:2493–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roger A, Finet A, Boru B, et al. Efficacy of combined hypo-fractionated radiotherapy and anti-PD-1 monotherapy in difficult-to-treat advanced melanoma patients. Oncoimmunology. 2018;7:e1442166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Qin Q, Nan X, Miller T, et al. Complete Local and Abscopal Responses from a Combination of Radiation and Nivolumab in Refractory Hodgkin's Lymphoma. Radiat Res. 2018;190:322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tubin S, Khan MK, Salerno G, Mourad WF, Yan W, Jeremic B. Mono-institutional phase 2 study of innovative Stereotactic Body RadioTherapy targeting PArtial Tumor HYpoxic (SBRT-PATHY) clonogenic cells in unresectable bulky non-small cell lung cancer: profound non-targeted effects by sparing peri-tumoral immune microenvironment. Radiat Oncol. 2019;14:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Suzuki G, Masui K, Yamazaki H, et al. Abscopal effect of high-dose-rate brachytherapy on pelvic bone metastases from renal cell carcinoma: a case report. J Contemp Brachytherapy. 2019;11:458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]