Abstract
Objective
To determine whether a clinically based score predicts cryptogenic new-onset refractory status epilepticus (C-NORSE) at the early stage of status epilepticus (SE) with prominent motor symptoms (SE-M) of unclear etiology.
Methods
The score (range 0–6) included 6 clinical features: highly refractoriness to antiseizure drugs, previously healthy individual, presence of prodromal fever, absence of prodromal psychobehavioral or memory alterations, absence of dyskinesias, and symmetric brain MRI abnormalities (the first 2 mandatory). We retrospectively assessed the usefulness of a high scale score (≥5) in predicting C-NORSE in 83 patients with SE-M of unclear etiology, who underwent testing for neuronal surface antibodies (NS-Abs) between January 2007, and December 2019.
Results
Thirty-one (37.3%) patients had a high score. Patients with a high score had more frequent prodromal fever (28/31 vs 24/52), mechanical ventilatory support (31/31 vs 36/52), and symmetric MRI abnormalities (26/31 vs 12/52), had less frequent involuntary movements (2/31 vs 30/52), and had absent prodromal psychobehavioral alterations (0/31 vs 27/52), CSF oligoclonal band detection (0/27 vs 11/38), tumor association (0/31 vs 13/52), or NS-Abs (0/31 vs 29/52) than those with a low score (<5). Thirty-three patients (median age, 27 years; 18 [54.5%] female) were finally regarded as C-NORSE. The sensitivity and specificity of a high score for predicting C-NORSE were 93.9% (95% CI 0.87–0.94) and 100% (95% CI 0.95–1.00), respectively.
Conclusions
Patients with a high score in the indicated scale are more likely to have C-NORSE, making it a useful diagnostic tool at the early stage of SE-M before antibody test results become available.
New-onset refractory status epilepticus (NORSE) is a severe neurologic emergency condition characterized by refractory status epilepticus (SE) without readily identifiable cause in otherwise healthy individuals.1,2 The term NORSE is now defined as a clinical presentation, not a specific diagnosis.3 When the cause remains unknown despite the extensive workup, it is called cryptogenic NORSE (C-NORSE).2–4
According to the consensus definition, NORSE includes patients with viral, paraneoplastic, or autoimmune etiologies3; however, it is crucial in clinical practice to differentiate C-NORSE from secondary NORSE with neuronal surface antibodies (NS-Abs) or classical paraneoplastic antineuronal antibodies because treatment strategy and outcome could be different.5 A large cohort study reported that a half of 130 patients with NORSE remained cryptogenic, but 37% were immune mediated; among those, the most common etiology was anti-NMDA receptor (NMDAR) encephalitis.2
Although antibody tests are important to determine the etiology, in an emergency condition, it is often difficult to get the antibody test results in appropriate time. Therefore, we previously developed a clinically based score (range 0–6) based on 6 clinical features to predict C-NORSE at the early stage of convulsive SE, which is currently classified into SE with prominent motor symptoms (SE-M) according to the 2015 International League Against Epilepsy (ILAE) criteria for SE.6 However, the scale score has not been validated yet.5
Here we report the sensitivity and specificity of the high scale score (≥5) in predicting C-NORSE at the early stage of SE-M of unclear etiology (before NS-Ab test results are known).
Methods
Patients selection and antibody assays (study profile)
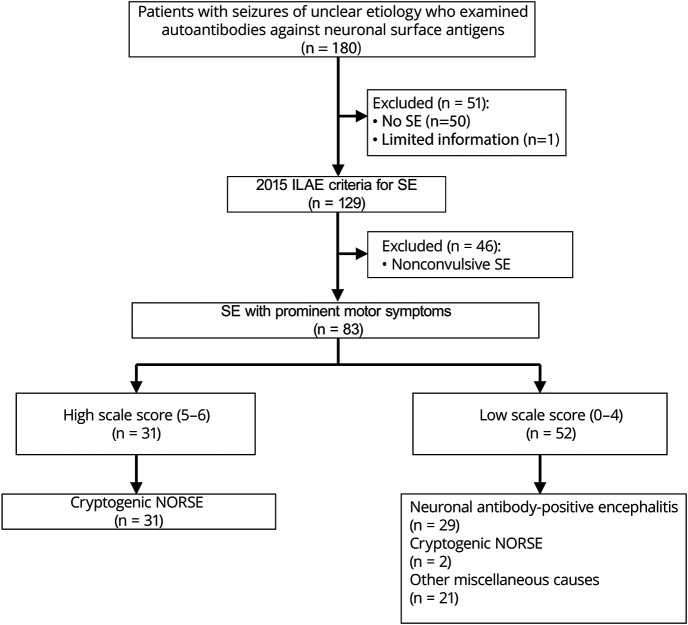
We first reviewed the clinical information of 180 patients with seizures of unclear etiology on admission or early stage of seizures, in whom NS-Abs were examined to investigate potential immune-mediated etiologies between January 1, 2007, and December 31, 2019 (figure 1). These patients were admitted to Kitasato University Hospital or other associated hospitals between January 1, 1999, and December 31, 2019; in 7 patients who were admitted before January 1, 2007, archived serum/CSF samples obtained at onset of disease were used for antibody assays.
Figure 1. Study profile.
The sensitivity and specificity of the clinically based scale score indicated in the text were assessed among 83 patients with SE with prominent motor symptoms. ILAE = International League Against Epilepsy; NORSE = new-onset refractory status epilepticus; SE = status epilepticus.
Then, we selected 129 patients who fulfilled the 2015 ILAE criteria for SE.6 Of those, 46 patients with nonconvulsive SE (NCSE) were excluded because the scale score was originally developed to estimate antibody status in patients with convulsive SE. In this study, we included all patients who developed SE-M regardless of refractoriness to conventional antiseizure drug (ASD) treatment. We assessed the sensitivity and specificity of the high scale score (≥5) in 83 patients with SE-M of unclear etiology during the early stage.
NS-Abs were measured at the laboratory of Josep Dalmau (University of Barcelona) using both a rat brain immunohistochemistry and cell-based assay (CBA)7–13; they included antibodies against the NMDAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), γ-aminobutyric acid B receptor (GABAbR), γ-aminobutyric acid A receptor (GABAaR), metabotropic glutamate receptor 5, dipeptidyl peptidase-like protein 6, contactin-associated protein-like 2, leucine-rich glioma-inactivated 1 (LGI1), and neurexin 3. Both serum and CSF were examined in all patients except 4 (only CSF [n = 2] or serum [n = 2] was available). In addition to NS-Abs, myelin oligodendrocyte glycoprotein (MOG) and aquaporin-4 (AQP4) antibodies were examined with CBA in patients with overlapping encephalitis and demyelinating syndrome.14 Antibodies against classical paraneoplastic intracellular antigens (CV2/CRMP5, Ma2, Ri, Yo, Hu, GAD65, and amphiphysin) were measured in serum at Kitasato University with EUROLINE (Euroimmun AG) in patients when associated tumor was suspected or those with NORSE criteria.
Criteria for C-NORSE
Although C-NORSE is not a specific diagnosis, patients were classified into C-NORSE as a subgroup of cryptogenic epileptic syndrome in this study if those fulfilled the following 3 criteria: (1) new-onset refractory SE in previously healthy individual, (2) refractoriness to conventional ASD treatment, and (3) no etiology identified throughout the course of the disease. If the etiology of SE was identified, patients were diagnosed with etiology-based specific diagnosis (e.g., anti-NMDAR encephalitis and anti-LGI1 encephalitis). SE was considered as refractory when it continued longer than 60 minutes despite adequate administration of benzodiazepines and adequate loading of standard IV ASDs.2,6,15,16 The etiology of NORSE was extensively investigated with CSF examination, malignancy survey, and serologic testing including autoantibodies against neuronal surface and classical paraneoplastic intracellular antigens.
C-NORSE score
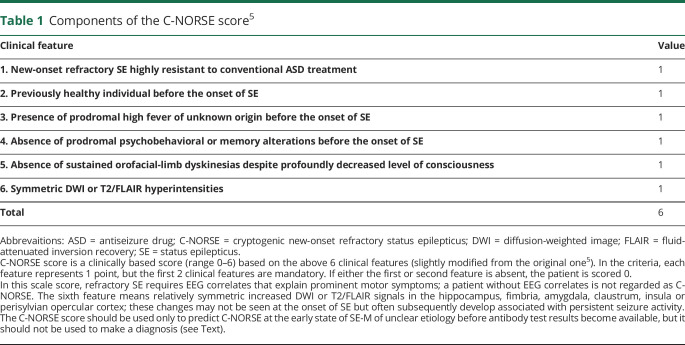
C-NORSE score is a clinically based score (range 0–6) based on the following 6 clinical features5 usually obtained within 14 days after admission in general hospital: (1) NORSE highly resistant to conventional ASD treatment, (2) previously healthy individual before the onset of SE, (3) presence of prodromal high fever of unknown origin before the onset of SE, (4) absence of prodromal psychobehavioral or memory alterations before the onset of SE, (5) absence of sustained orofacial-limb dyskinesias despite a profoundly decreased level of consciousness, and (6) symmetric brain MRI abnormalities (table 1).
Table 1.
Components of the C-NORSE score5
In the criteria, we previously defined that each feature represents 1 point, but the first 2 clinical features are mandatory.5 Accordingly, if either the first or second feature is absent, the patient is scored 0. We applied 2015 ILAE criteria for SE6 to include patients with SE-M, and all patients underwent EEG and MRI repeatedly during their hospitalization. However, only patients who had electroencephalographic correlates (such as spikes and waves or periodic discharges that explain prominent motor symptoms) were regarded to meet the first clinical feature of the score. Accordingly, patients without apparent electroencephalographic correlates despite convulsive SE or epilepsia partialis continua were scored 0. Symmetric brain MRI abnormalities imply relatively symmetric increased diffusion-weighted image (DWI) or T2/fluid-attenuated inversion recovery (FLAIR) signals in the hippocampus, fimbria, amygdala, claustrum, insula, or perisylvian opercular cortex; these changes may not be seen at the onset of SE-M but often subsequently develop associated with persistent seizure activity.5
Clinical assessments
We assessed the clinical features between patients with a high scale score (≥5) and those with a low scale score (≤4), including sex, age at onset of SE-M, prodromal fever, prodromal psychobehavioral or memory alterations, involuntary movements, mechanical ventilatory support, CSF and MRI findings, and presence of tumor. We reviewed the final diagnosis of these patients after extensive workup and finally determined the sensitivity and specificity of the indicated high scale score. In this study, to focus on the C-NORSE score, we did not assess the efficacy of treatment, such as immunotherapy, or long-term outcome in these patients.
Standard protocol approvals, registrations, and patient consents
The study was approved by Institutional Review Boards of Kitasato University (B18-193). Written informed consent was obtained from the patients or their family members. Information on symptoms, CSF, MRI, EEG, and treatments was obtained from the authors or referring physicians.
Statistical analysis
The Fisher exact test was performed for comparison of categorical variables, and the Mann-Whitney test was used for continuous variables. The statistical significance was set at p < 0.05. The sensitivity and specificity of the high C-NORSE score were determined with 2-way contingency table analysis. We used JMP, version 14 (SAS Institute Inc.), for statistical analyses.
Data availability
Any data not published within the article are available and will be shared anonymously by request from any qualified investigator.
Results
Clinical features in patients with a high score and those with a low score
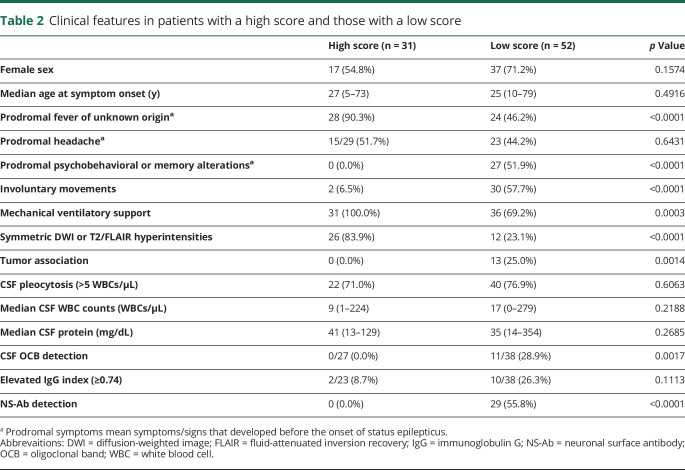
Of 83 patients, 31 (37.3%) had a high score (5–6); 17 patients (54.8%) were female; median age at symptom onset was 27 years (range 5–73 years) (table 2). The remaining 52 patients (62.7%) had a low score (0–4); 37 patients (71.2%) were female; median age at symptom onset was 25 years (range 10–79 years). Other clinical information is shown in table 2. There was no difference between patients with a high score and low score in female sex and median age at onset. However, patients with a high score had more frequent prodromal fever (28/31 vs 24/52), mechanical ventilatory support (31/31 vs 36/52), and symmetric DWI or T2/FLAIR hyperintensities (26/31 vs 12/52) than those with a low score. By contrast, they had less frequent involuntary movements (2/31 vs 30/52) and absent prodromal psychobehavioral alterations (0/31 vs 27/52), CSF oligoclonal band (OCB) detection (0/27 vs 11/38), tumor association (0/31 vs 13/52), or NS-Abs (0/31 vs 29/52) than those with a low score. There was no difference in prodromal headache before the onset of SE, CSF pleocytosis, white blood cell (WBC) counts in CSF, CSF protein levels, or elevated immunoglobulin G (IgG) index.
Table 2.
Clinical features in patients with a high score and those with a low score
Final diagnosis
Of 83 patients with 2015 ILAE criteria for SE-M6 of unclear etiology on admission or early stage of SE, 29 (34.9%) patients were positive for NS-Abs, NMDAR in 26 patients (1 with concurrent AQP4 and 1 with MOG), LGI1 in 1, GABAbR in 1, and unknown antigens (not characterized yet) in 1. No AMPAR or GABAaR antibodies were identified. All antibody-positive patients had a low C-NORSE score: 24 patients had 0, and 5 patients had 3. The remaining 54 patients (65.1%) were negative for NS-Abs; 21 patients were diagnosed with miscellaneous disorders or syndrome including possible autoimmune encephalitis (AE)17 (n = 11), autoantibody-negative but probable AE17 (n = 5), antibody-negative autoimmune limbic encephalitis17 (n = 1), encephalitis associated with systemic lupus erythematosus (n = 2), and nonautoimmune neurologic disorders (n = 2). The remaining 33 patients were finally regarded as C-NORSE based on the above criteria (figure 1).
Clinical features of C-NORSE
Eighteen of 33 patients (54.5%) were female; median age at onset was 27 years (range 5–73 years). Thirty-one patients (93.9%) had a high score; 23 patients had 6, and 8 patients had 5, but 2 patients had a low score (both 4). Of interest, 7 of the 33 patients (21.2%) had a past medical history (PMH) of febrile convulsion (FC), family history of FC or epilepsy, or both; 3 patients had a PMH of FC (one of them had a family history of FC); 4 patients had no PMH of FC but had a family history of FC (n = 1) or epilepsy (n = 3).
Prodromal symptoms developed before the onset of SE in 31 of 33 patients (93.9%), fever in 28 of 33 patients (84.8%), and headache in 15 of 31 patients (2 unknown). Only 1 patient (3.0%) developed psychobehavioral alterations before the onset of SE, whereas 3 patients (9.1%) showed involuntary movements during the course of the disease, but only 1 patient developed sustained dyskinesias mimicking orofacial-limb dyskinesias. All patients required mechanical ventilatory support due to refractory SE.
NS-Abs were not detected in either serum or CSF. Classical paraneoplastic antineuronal antibodies measured in serum in 28 patients were negative but not measured in 5 (no serum was available for examination). CSF examination revealed a median of 9 WBCs/μL (range 0–224 WBCs/μL) and a median protein level of 41 mg/dL (range 13–129 mg/dL). No CSF-restricted OCBs were detected in 29 examined patients, whereas the IgG index was elevated in 2 of 25 examined patients (8.0%). Ten patients (30.3%) had no pleocytosis (>5 WBCs/μL). Initial brain MRI was unremarkable in 15 patients (45.5%), but follow-up MRIs showed abnormal findings in 30 patients (90.9%); in 27 patients (81.8%), brain MRIs showed symmetric DWI or T2/FLAIR hyperintensities in the medial temporal lobes, basal ganglia, fimbria, claustrum, or perisylvian opercular cortex (figure 2). None of these patients had a tumor identified during the course of the disease.
Figure 2. Brain MRIs finding obtained from 3 patients with C-NORSE.
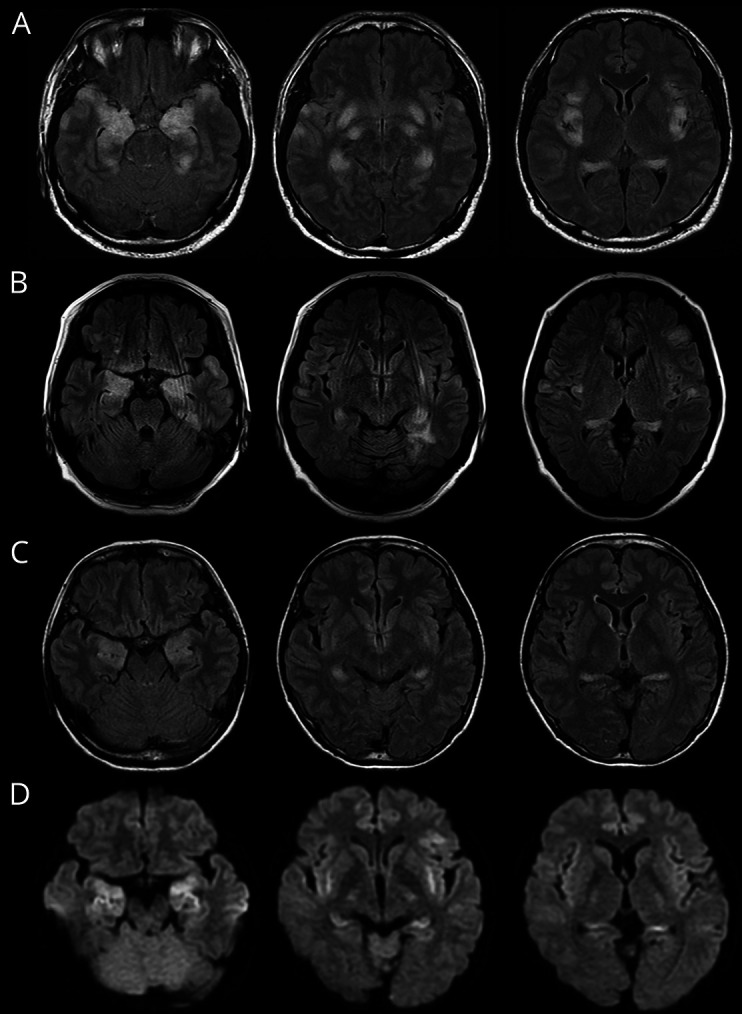
Brain MRIs show symmetric increased DWI or FLAIR signals in the amygdala, hippocampus, fimbria, claustrum, insula, and frontotemporal cortex. Basal ganglia and perisylvian opercular cortex are also involved in patients with C-NORSE (not shown). (A) A 37-year-old man; (B) a 49-year-old woman; (C and D) a 39-year-old woman; (A–C) FLAIR, (D) DWI. C-NORSE = cryptogenic new-onset refractory status epilepticus; DWI = diffusion-weighted image; FLAIR = fluid-attenuated inversion recovery.
The sensitivity and specificity of the high C-NORSE score
The sensitivity and specificity of the high score (≥5) for predicting C-NORSE were 93.9% (95% CI 0.87–0.94) and 100% (95% CI 0.95–1.00), respectively.
Discussion
This study shows that (1) patients with the high score are more likely to have C-NORSE, (2) the clinically based score C-NORSE score has high sensitivity and specificity for predicting the C-NORSE, and (3) patients with C-NORSE had distinctive clinical features.
In clinical practice, it is important to estimate antibody status in patients with SE of unclear etiology and identify patients with C-NORSE as early as possible because patients with C-NORSE are usually less responsive to first-line immunotherapy4,5 and more likely to have poor long-term outcome with cognitive deficits and refractory partial seizures.5
This scale score was originally developed based on our previous preliminary study5 that compared the clinical features of 11 adult patients with C-NORSE (aged ≥17 years) with those of 32 patients with anti-NMDAR encephalitis. We previously reported that the C-NORSE score was higher in patients with C-NORSE than those with anti-NMDAR encephalitis; however, the sensitivity and specificity were not determined. After that, we had recruited additional patients since September 2016. In the meantime, the international consensus definition of NORSE was proposed in 20183; hence, the concept of C-NORSE was much more clearly defined than before. In this study, we adopted the concept of C-NORSE and included pediatric cases as well as newly identified adult cases. Accordingly, we increased the number of patients with C-NORSE from 11 to 33.
In this study, we assessed the sensitivity and specificity of the high score (≥5) in 83 patients with SE-M. In this cohort, the sensitivity and specificity for predicting C-NORSE were 93.9% and 100%, respectively, making it a useful diagnostic tool at the early stage of SE-M of unclear etiology before antibody test results become available.
C-NORSE is a devastating epileptic syndrome of unknown causes, probably of diverse etiologies1–5 including autoimmunity, neuroinflammation, or individual susceptibility to seizure. This study highlighted distinctive clinical features of C-NORSE phenotypically different from antibody-positive AE, such as anti-NMDAR, anti-LGI1, or anti-GABAaR encephalitis. Patients with C-NORSE often present with high fever of unknown cause, followed by sudden onset of mainly convulsive seizures, leading to refractory SE (occasionally super-refractory SE) requiring a mechanical ventilatory support and continuous infusion of sedative drugs. Early brain MRI is often normal or may show symmetric DWI or T2/FLAIR hyperintensities in the medial temporal lobes,5 mimicking autoimmune limbic encephalitis. CSF examination often shows nonspecific mild pleocytosis; however, none of these patients had CSF-restricted OCBs, and the IgG index was not elevated in most of them. Of interest, prodromal psychobehavioral or memory alterations usually did not develop before the onset of SE or decreased level of consciousness. This is highly contrast to those with anti-NMDAR encephalitis5,6,17–19 or autoimmune limbic encephalitis,17 in whom these symptoms usually develop in the early course of the disease, and often predominant. Thus, the lack of prodromal psychobehavioral or memory alterations is an important feature in discrimination of C-NORSE from anti-NMDAR encephalitis or limbic encephalitis. The follow-up brain MRIs often show symmetric brain lesions involving the hippocampus, amygdala, fimbria, claustrum, basal ganglia, insular cortex, and perisylvian opercular cortex presumably associated with persistent seizure activity.5,20–22 Such neuroimaging pattern is quite different from autoimmune limbic encephalitis with highly restricted to bilateral medial temporal lobes17 or anti-GABAaR encephalitis with multiple corticosubcortical lesions.13,23 Involuntary movements may develop in patients with C-NORSE due to secondary basal ganglia lesions, but not like NMDAR-associated orofacial-limb dyskinesias18 or movement disorders,24 or LGI1-associated faciobrachial dystonic seizures.25
The etiology of C-NORSE remains unknown.1–5 It is also controversial whether it is of autoimmune origin.5 One might argue that C-NORSE is an epileptic syndrome and should not be confused with AE; randomized controlled trial with immunotherapy has not been conducted yet; therefore, little information is available on the adequate dosage of other immune treatments to formulate any recommendation.3 However, it is not easy in clinical practice to exclude a possibility of C-NORSE or antibody-positive AE particularly at the early stage of SE before antibody test results become available; therefore, many patients with NORSE may have been treated with immunotherapy,5,26 although the first-line immunotherapy is presumed to be less effective. However, if the C-NORSE score is high (≥5) on referral from other hospital, it is suggested that the patient is more likely to be negative for neuronal antibodies, thus more likely to be less responsive to first-line immunotherapy and have poor outcome. This scoring strategy might help physicians to identify patients with C-NORSE and their decision making in a patient with the high score.
Although the underlying mechanism of C-NORSE is entirely unknown, inflammation-mediated epileptogenesis has been proposed,27 in which a vicious cycle that involves inflammation and seizure activity is assumed to lead to cell death and network reorganization, ultimately causing refractory seizure. One previous study reported high levels of cytokines (interleukin-6 [IL-6]) or chemokines (CXCL10 and IL-8) in serum and CSF in pediatric cases of febrile infection-related epilepsy syndrome (FIRES),28 which is currently regarded as a subcategory of NORSE.3 Among those, proinflammatory cytokines, such as IL-1β and IL-6, have received attention as potential key molecules in C-NORSE. IL-1β has been implicated in seizure-induced neuronal cell death,29 SE,30 and posttraumatic epilepsy.31 Anakinra, IL-1 receptor antagonist, has been reported to be effective in patients with FIRES.32,33 IL-6 secreted from macrophages is also important mediator of fever and its deregulated expression is responsible for development of a variety of autoimmune inflammatory diseases.34 The efficacy of tocilizumab, IL-6 receptor antagonist, has also been reported in patients with NORSE.35 Therefore, elevated CSF levels of proinflammatory cytokines may play an important role in neuroinflammation, leading to development of refractory partial seizures in NORSE or FIRES. In our cohort of patients with C-NORSE, none of them had autoantibodies binding to the neuronal surface membrane with a rat brain immunohistochemistry in either CSF or serum, indicating that autoantibodies may not play an important role in C-NORSE or FIRES, but rather innate immunity may be more important than adaptive immunity as previously described.5
Of interest, 21.2% of patients with C-NORSE had a PMH of FC, family history of FC, or both. In a small group of patients, some genetic predisposition to epileptic seizure might contribute to development of NORSE following fever. Further research is required to determine a role of genomic susceptibility to NORSE.
This study has limitations of being retrospective studies and based on the small number of patients included. Genomic studies have not been performed yet in our cohort. Classical paraneoplastic antineuronal antibodies were not examined in all patients. Cytokine or chemokines were not examined in either case. In an emergency situation, some of the components of the score may be difficult to assess historically due to a variety of individual factors. A brain MRI is often difficult to obtain in a ventilated patient with SE-M or cannot be performed on a patient with contraindication (e.g., implanted pacemakers, intracranial aneurysm clips, and iron-based metal implants). When early brain MRI is unremarkable, repeated studies are required to see symmetric MRI abnormalities. However, a brain MRI within the first 24 hours is currently included in the diagnostic checklist for etiology of NORSE,36 and follow-up MRI is also important in exclusion of alternative diagnosis (multifocal corticosubcortical lesions may appear in the course of the disease in anti-GABAaR encephalitis). It is important to keep in mind that this score was developed in patients with SE-M of unclear etiology. Thus, the results should not be generalized for patients with NCSE.
Despite these limitations, this study demonstrated that the clinically based score is useful for early identification of patients with C-NORSE. However, this score should not be used to make the diagnosis of C-NORSE because NORSE is not a specific diagnosis and exclusion of alternative diagnosis is mandatory. In patients with C-NORSE, irreversible brain damage is expected to occur quickly; thus, early recognition of C-NORSE is crucial. In addition to ASD treatment, we hope that this scoring strategy improves their functional outcome through facilitating early intervention with potential effective drugs that break a vicious cycle of neuroinflammation-induced neuronal damage that consequently increases seizure susceptibility.
Acknowledgment
The authors particularly thank Dr. Josep Dalmau (Service of Neurology, IDIBAPS Hospital Clínic, University of Barcelona, Barcelona, Spain) for examining antibodies against neuronal surface antigens and critical comments on this study. They also thank Drs. Hiroki Asari (Shizuoka City Shimizu Hospital), Junya Kaneko (Nippon Medical School Tama Nagayama Hospital), Kenji Yoshida, Kasumi Hattori, and Yuya Itagaki (Fukushima Medical University Hospital), Arifumi Kosakai (Keiyu Hospital), Masashi Watanabe (Ehime Prefectural Central Hospital), Takeo Shishido (Hiroshima University Hospital), Hiroya Ohara (South Nara General Medical Center), Yasushi Hosoi (Hamamatsu University Hospital), Mizuki Ayano (Kyorin University Hospital), Yuka Terasawa (The Jikei University Hospital), Keisuke Imai (Japanese Red Cross Kyoto Daiichi Hospital), and Masamune Sakamoto (Yokohama City University Medical Center) for providing clinical information. They are grateful to all participants and physicians for their contribution to this study.
Glossary
- AE
autoimmune encephalitis
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- AQP4
aquaporin-4
- ASD
antiseizure drug
- CBA
cell-based assay
- C-NORSE
cryptogenic NORSE
- DWI
diffusion-weighted image
- FC
febrile convulsion
- FIRES
febrile infection-related epilepsy syndrome
- FLAIR
fluid-attenuated inversion recovery
- GABAaR
γ-aminobutyric acid A receptor
- GABAbR
γ-aminobutyric acid B receptor
- IgG
immunoglobulin G
- IL-6
interleukin-6
- ILAE
International League Against Epilepsy
- LGI1
leucine-rich glioma-inactivated 1
- MOG
myelin oligodendrocyte glycoprotein
- NCSE
nonconvulsive SE
- NMDAR
NMDA receptor
- NORSE
new-onset refractory status epilepticus
- NS-Abs
neuronal surface antibodies
- OCB
oligoclonal band
- PMH
past medical history
- SE
status epilepticus
- SE-M
SE with prominent motor symptoms
- WBC
white blood cell
Appendix. Authors
Study funding
This study was supported in part by a grant from the Japan Epilepsy Research Foundation (JERFTENKAN 17002, TI).
Disclosure
A. Yanagida, N. Kanazawa, J. Kaneko, A. Kaneko, R. Iwase, H. Suga, Y. Nonoda, Y. Onozawa, and E. Kitamura report no disclosure. K. Nishiyama received research support from Daiichi Sankyo Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., and Eisai Co., Ltd. T. Iizuka received a grant from The Japan Epilepsy Research Foundation and research support from Astellas Pharma Inc. Go to Neurology.org/NN for full disclosures.
References
- 1.Wilder-Smith EP, Lim EC, Teoh HL, et al. . The NORSE (new-onset refractory status epilepticus) syndrome: defining a disease entity. Ann Acad Med Singapore 2005;34:417–420. [PubMed] [Google Scholar]
- 2.Gaspard N, Foreman BP, Alvarez V, et al. . New-onset refractory status epilepticus: etiology, clinical features, and outcome. Neurology 2015;85:1604–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirsch LJ, Gaspard N, van Baalen A, et al. . Proposed consensus definitions for new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related conditions. Epilepsia 2018;59:739–744. [DOI] [PubMed] [Google Scholar]
- 4.Gaspard N, Hirsch LJ, Sculier C, et al. . New-onset refractory status epilepticus (NORSE) and febrile-infection-related epilepsy syndrome (FIRES): state of the art and perspectives. Epilepsia 2018;59:745–752. [DOI] [PubMed] [Google Scholar]
- 5.Iizuka T, Kanazawa N, Kaneko J, et al. . Cryptogenic NORSE: its distinctive clinical features and response to immunotherapy. Neurol Neuroimmunol Neuroinflamm 2017;4e:e396 doi: 10.1212/NXI.0000000000000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trinka E, Cock H, Hesdorffer D, et al. . A definition and classification of status epilepticus-report of the ILAE task force on classification of status epilepticus. Epilepsia 2015;56:1515–1523. [DOI] [PubMed] [Google Scholar]
- 7.Dalmau J, Tüzün E, Wu HY, et al. . Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 2007;61:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai M, Hughes EG, Peng X, et al. . AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol 2009;65:424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lancaster E, Lai M, Peng X, et al. . Antibodies to the GABA (B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol 2010;9:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai M, Huijbers MG, Lancaster E, et al. . Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol 2010;9:776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lancaster E, Huijbers MG, Bar V, et al. . Investigations of caspr2, an autoantigen of encephalitis and neuromyotonia. Ann Neurol 2011;69:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boronat A, Gelfand JM, Gresa-Arribas N, et al. . Encephalitis and antibodies to dipeptidyl-peptidase-like protein-6, a subunit of Kv4.2 potassium channels. Ann Neurol 2013;73:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petit-Pedrol M, Armangue T, Peng X, et al. . Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol 2014;13:276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Titulaer MJ, Höftberger R, Iizuka T, et al. . Overlapping demyelinating syndromes and anti–N-methyl-D-aspartate receptor encephalitis. Ann Neurol 2014;75:411–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayer SA, Claassen J, Lokin J, Mendelsohn F, Dennis LJ, Fitzsimmons BF. Refractory status epilepticus: frequency, risk factors, and impact on outcome. Arch Neurol 2002;59:205–210. [DOI] [PubMed] [Google Scholar]
- 16.Brophy GM, Bell R, Claassen J, et al. . Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012;17:3–23. [DOI] [PubMed] [Google Scholar]
- 17.Graus F, Titulaer JM, Balu R, et al. . Clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 2016;15:391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iizuka T, Sakai F, Ide T, et al. . Anti-NMDA receptor encephalitis in Japan: long-term outcome without tumor removal. Neurology 2008;70:504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalmau J, Gleichman AJ, Hughes EG, et al. . Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008;7:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chevret L, Husson B, Nguefack S, Nehlig A, Bouilleret V. Prolonged refractory status epilepticus with early and persistent restricted hippocampal signal MRI abnormality. J Neurol 2008;255:112–116. [DOI] [PubMed] [Google Scholar]
- 21.Chatzikonstantinou A, Gass A, Förster A, Hennerici MG, Szabo K. Features of acute DWI abnormalities related to status epilepticus. Epilepsy Res 2011;97:45–51. [DOI] [PubMed] [Google Scholar]
- 22.Meletti S, Slonkova J, Mareckova I, et al. . Claustrum damage and refractory status epilepticus following febrile illness. Neurology 2015;85:1224–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spatola M, Petit-Pedrol M, Simabukuro MM, et al. . Investigations in GABAA receptor antibody-associated encephalitis. Neurology 2017;88:1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varley JA, Webb AJS, Balint B, et al. . The movement disorder associated with NMDAR antibody-encephalitis is complex and characteristic: an expert video-rating study. J Neurol Neurosurg Psychiatry 2019;90:724–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irani SR, Michell AW, Lang B, et al. . Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol 2011;69:892–900. [DOI] [PubMed] [Google Scholar]
- 26.Gugger JJ, Husari K, Probasco JC, Cervenka MC. New-onset refractory status epilepticus: a retrospective cohort study. Seizure 2020;74:41–48. [DOI] [PubMed] [Google Scholar]
- 27.Nabbout R, Vezzani A, Dulac O, Chiron C. Acute encephalopathy with inflammation-mediated status epilepticus. Lancet Neurol 2011;10:99–108. [DOI] [PubMed] [Google Scholar]
- 28.Sakuma H, Tanuma N, Kuki I, Takahashi Y, Shiomi M, Hayashi M. Intrathecal overproduction of proinflammatory cytokines and chemokines in febrile infection-related refractory status epilepticus. J Neurol Neurosurg Psychiatry 2015;86:820–822. [DOI] [PubMed] [Google Scholar]
- 29.Medel-Matus JS, Álvarez-Croda DM, Martínez-Quiroz J, Beltrán-Parrazal L, Morgado-Valle C, López-Meraz ML. IL-1β increases necrotic neuronal cell death in the developing rat hippocampus after status epilepticus by activating type I IL-1 receptor (IL-1RI). Int J Dev Neurosci 2014;38:232–240. [DOI] [PubMed] [Google Scholar]
- 30.Tian DS, Peng J, Murugan M, et al. . Chemokine CCL2-CCR2 signaling induces neuronal cell death via STAT3 activation and IL-1β production after status epilepticus. J Neurosci 2017;37:7878–7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma R, Leung WL, Zamani A, O'Brien TJ, Casillas Espinosa PM, Semple BD. Neuroinflammation in post-traumatic epilepsy: pathophysiology and tractable therapeutic targets. Brain Sci 2019;9:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kenney-Jung DL, Vezzani A, Kahoud RJ, et al. . Febrile infection-related epilepsy syndrome treated with anakinra. Ann Neurol 2016;80:939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Westbrook C, Subramaniam T, Seagren RM, et al. . Febrile infection-related epilepsy syndrome treated successfully with anakinra in a 21-year-old woman. WMJ 2019;118:135–139. [PMC free article] [PubMed] [Google Scholar]
- 34.Kishimoto T, Kang S, Tanaka T. IL-6: a new era for the treatment of autoimmune inflammatory diseases. In: Nakao K, Minato N, Uemoto S, editors. Innovative Medicine: Basic Research and Development [online]. Tokyo: Springer; 2015. [PubMed] [Google Scholar]
- 35.Jun JS, Lee ST, Kim R, Chu K, Lee SK. Tocilizumab treatment for new onset refractory status epilepticus. Ann Neurol 2018;84:940–945. [DOI] [PubMed] [Google Scholar]
- 36.Gofton TE, Gaspard N, Hocker SE, Loddenkemper T, Hirsch LJ. New onset refractory status epilepticus research: what is on the horizon? Neurology 2019;92:802–810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any data not published within the article are available and will be shared anonymously by request from any qualified investigator.