Summary:
Necrobiosis Lipoidica (NL) is a rare necrotising disorder of the skin characterized by collagen degeneration, thickening of blood vessels, and granulomatous inflammatory process. Its main clinical features are brownish-red papules and yellowish plaques with atrophic central areas. NL affects 0.3% -1.2% of the diabetic population, mostly women (female/male ratio is 3:1). Management of NL is challenging, especially for large lesions refractory to medical therapy, thus requiring surgical excision as an alternative option. Due to the rare occurrence of this condition no treatment guidelines exist and individualized treatment mostly depends on the severity of the lesion, location and patient's expectations. A case of a 30-year-old diabetic woman with very high aesthetic expectations was succesfully treated with staged resections of a giant NL to the leg and reconstruction with dermal template and full thickness skin grafts. Grafts were taken from the groin region bilaterally and from the lower abdomen after a cosmetic mini-abdominoplasty procedure. This approach allowed for a stable and very satisfactory aesthetic result with no donor site exposed scars.
INTRODUCTION
Necrobiosis lipoidica (NL), first described by Oppenheim1 in 1929, is a rare chronic idiopathic granulomatous disease of collagen degeneration with a risk of ulceration,2 which appears predominantly in lower extremities. This disease is associated with diabetes and affects about 0.3%–1.2% of patients with diabetes.3 However, there is no evidence that uncontrolled glycemia can be correlated with NL manifestations.4 The average onset age is 30 years, and women are mainly affected.5 NL’s lesions typically exhibit 1–3 well-circumscribed asymptomatic papules, slowly aggregating into plaques. These lesions seem violaceous, with a central area that can become yellow-brown discolored. This area is often covered by an atrophic, waxy, and eroded skin. The degeneration of the epidermic collagen leads to the development of telangiectasias. Seventy-five percentage of NL lesions are painless, but the remaining 25% can be painful,6 especially if ulcerated. Up to 35% of patients suffering from NL develop ulcerations after minor traumatic events.7 Although NL is a benign condition, an occasional finding of squamous carcinoma has been reported; in this case, a proper follow-up is recommended.8 We present the case of a patient suffering from NL on the medial surface of the left leg.
CASE REPORT
A 30-year-old woman model presented with a 14-year history of progressive expanding lesion of the medial surface of the left leg (Fig. 1). On examination, we reported a 25 cm × 12 cm lesion covered by yellow-colored, atrophic, and scaly skin, surrounded by a red border with a light edge and smooth surface. A lamellar hyperkeratosis with thickening of the skin was also observed. A dilation of the venous and capillary reticulum, both in the dermis and in the subcutaneous layer, was present. The lesion was painless, and sensitivity was normal. The patient had an 18-year history of type 1 diabetes treated with insulin and a 5-year history of celiac disease. A skin biopsy showed collagen degeneration in the deep dermis, with peripheral infiltration of lymphocytes and histiocytes. Infiltration of lymphocytes and granulocytes was present in the superficial dermis, supporting the diagnosis of NL. After careful evaluation of the patient’s expectations, and discussion with her on the technical treatment options, including skin expansion and free vascularized tissue transfer, the patient received a staged resection of the lesion associated to bilayered dermal matrix (Integra Dermal Regeneration Template; LifeScience Corporation; Princeton, N.J.) and skin grafts. Skin grafts were applied after full template revascularization, which occurred from 14 to 20 days after placement (Fig. 2). As donor sites, we chose the bilateral groin and the inferior abdomen to obtain hidden scars. During the harvesting of the abdominal graft, nodular subcutaneous lesions were found, which were not palpable at preoperatory workout. Histologic examination classified them as amyloidosis. After the healing of the wound, the patient underwent 10 sessions of carbon dioxide laser in association with Dye laser to minimize the visibility of the scars. At 2-year follow-up, the skin grafts appeared stable and the patient was very satisfied with the aesthetic result (Figs. 3–5).
Fig. 1.
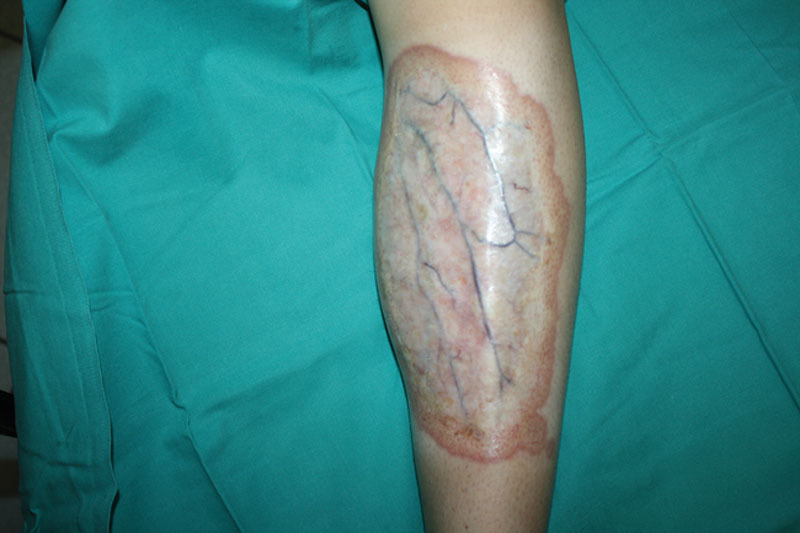
Preoperative view of a large NL lesion involving the left leg.
Fig. 2.
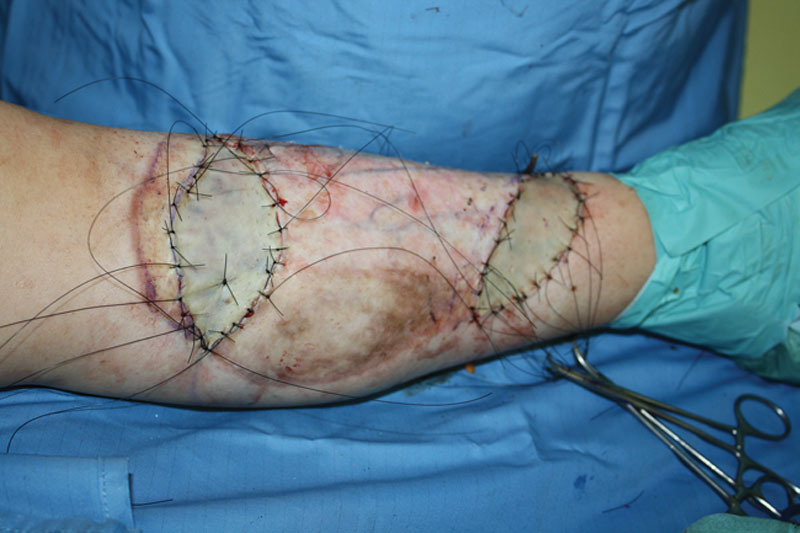
Intraoperative view showing skin grafting after partial resection of the lesion. Skin grafts were harvested from the groin region during this surgical step.
Fig. 3.
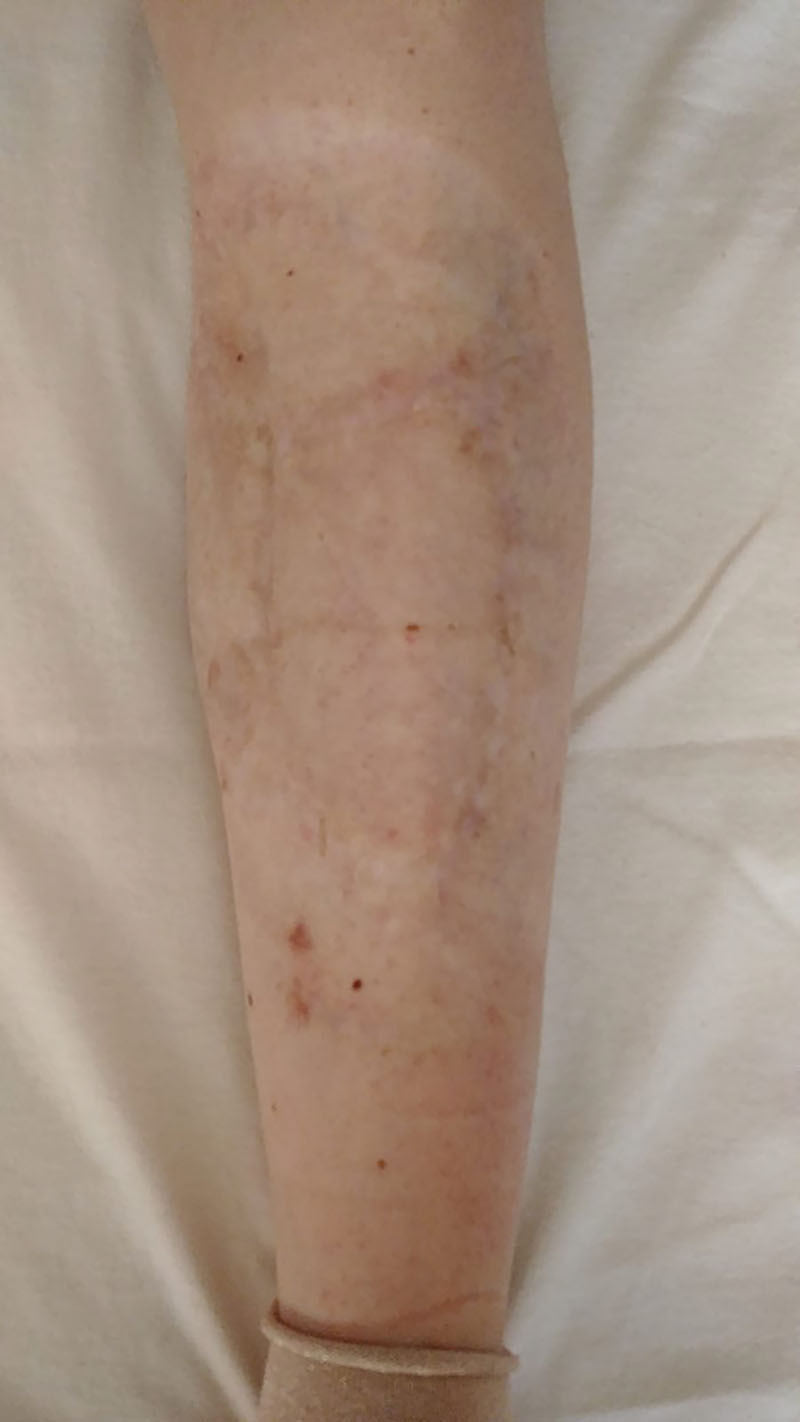
Final outcome at 2-year follow-up.
Fig. 5.
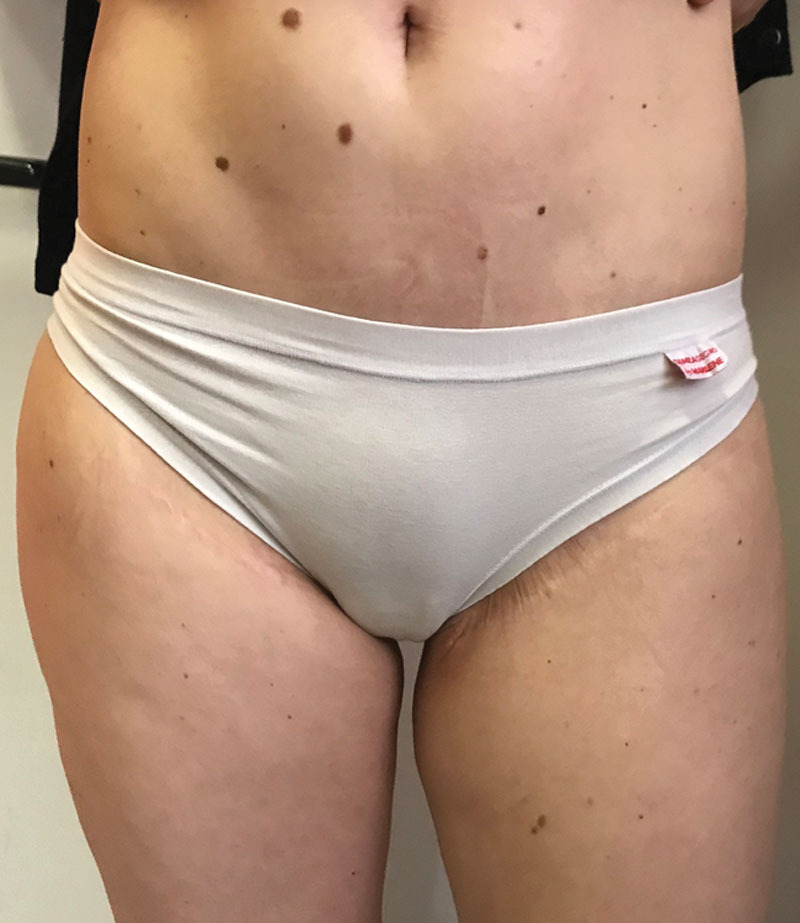
Two-year postoperative view of the patient (wearing underwear) showing hidden abdominoplasty and groin scars.
Fig. 4.

Histologic view of the resected lesion (×40) demonstrating extended NL involving the dermal layer. Histiocytes are present as well as multinucleated giant cells.
DISCUSSION
NL is a rare disease, and there are no sufficient cases to perform prospective clinical trials. This is the reason why most of the literature findings are case reports and small-scale clinical trials.9 There are few comparative studies evaluating different treatments of NL, and it is impossible to draw up a guideline for therapy. The condition may seldom heal on its own (up to 17% cases).10
Peckruhn et al11 delivered a critical assessment of reports on the treatment of NL published up to year 2000. In their work, it can be seen that with an increased number of treated patients, the percentage of successful cases actually decreases. The common practice of starting treatment with topical therapy can be recommended. Other therapeutic options, such as surgical resection and skin grafts, should be considered as well.11 In our case, the patient did not respond to medical treatment. The response’s failure to the medical therapy negatively affected the psychologic life and self-esteem of the patient. Depression and other mood disorders are in fact often associated with this dermatologic condition.12 The surgical approach to this type of lesions is less studied than the medical one in the literature.
The relationship between glycemic control and NL in patients with diabetes mellitus was investigated by Mistry et al.13 In this recent literature review, 622 studies were identified but only 10 met the inclusion criteria (8 case reports and 2 case series). Of the 24 patients with NL, only 13 reported resolution of the lesions after implementing various methods of glycemic control (diet, insulin regimen, and pancreatic transplantation). The authors conclude that glycemic control may have a role in influencing the prognosis of NL, but still there is insufficient evidence to support this claim.
As far as the specific context of our patient is concerned, a radical 1-stage resection of the lesion would have left a too wide a defect to be covered by a single full-thickness skin graft, without tissue prefabrication techniques. As microsurgeons, an alternative option that we have thoughtfully considered for the treatment of this case was the use of free flaps. Free flaps are largely qualified for managing any kind of leg defects. The size of the lesion, however, reduced the options of coverage with respect to the choice of an appropriate free fasciocutaneous flap. Only the anterolateral thigh flap could perhaps meet all the theoretical requirements, including high aesthetic expectations of patients, at the expense of a long scar into her thigh. Van Landuyt et al14 reported the versatility of the deep inferior epigastric artery (DIEP) flap for the reconstruction of inferior limbs, and this technique is shown to be capable of covering large skin defects.15 The DIEP flap appeared to us as a reliable option, but because of the location of perforators, the abdominal scar could not be placed low enough to be hidden. In addition, we had some concerns regarding the thickness of the flap to restore a quite thin defect over the tibia.
At a deeper reflection, the indication for surgery was merely aesthetic. The young patient was psychologically distressed, and complained not being able of wearing skirts or bathing suits, with consequent significant professional implications. Additionally, the free flap technique is not devoid of risks, mostly in a patient with diabetes. Assi et al16 demonstrated that diabetes itself is not a risk factor for the outcome of a free flap, but we must consider that this procedure may entail the sacrifice of one leg’s artery in a patient with a considerable risk of developing a diabetic foot.17
Staged surgical resection and coverage with skin grafts allowed us to achieve full excision of NL. Skin grafts were harvested from the groin region bilaterally. As a last procedure, an aesthetic mini-abdominoplasty was performed, and the resected skin used as the graft to cover the residual defect. By this, we were able to achieve full coverage of the leg without determining visible donor site scars.18
After meticulous postoperatory follow-up and complete graft take, the patient was scheduled for laser treatment to further improve aesthetics.19,20 At 2-year follow-up, the patient was very happy with the results and was back to her profession, as no recurrence of the lesion occurred.
During the last procedure, we found multiple subcutaneous nodules compatible with nodular amyloidosis due to injection of insulin. It is uncommon, like in our patient, that the deposit of amyloid was unpalpable.21 It was not necessary to change the insulin therapy because this condition is supposed to be related to its concentration, and not to the type used. Amyloidosis due to injection of insulin is not correlated to systemic amyloidosis, and it can be avoided by the rotation of the injection site.22,23
CONCLUSIONS
NL is a rare condition related to diabetes. Topical treatment with corticosteroids is usually the first step, and it is possible to see a complete regression in the majority of the patient response. However, there is no consistent guideline for the treatment. It is necessary to engage further studies to standardize the medical and surgical approach.
In our opinion, the conservative surgical procedure we chose yielded excellent aesthetic results in a high-demanding patient. In conclusion, our experience demonstrates that dermal templates and seriates full-thickness skin grafts harvested from hidden donor sites deserve to be carefully considered in the treatment of NL not responding to medical therapy.
Footnotes
Published online 21 July 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Oppenheim M. Necrobiosis lipoidica diabeticorum. Med J Aust. 1934;1:258–258. [Google Scholar]
- 2.Sibbald C, Reid S, Alavi A. Necrobiosis lipoidica. Dermatol Clin. 2015;33:343–360. [DOI] [PubMed] [Google Scholar]
- 3.Özkur E. Atypical presentation of necrobiosis lipoidica in a pediatric patient. Pediatr Dermatol. 2018;36:e31–e33. [DOI] [PubMed] [Google Scholar]
- 4.Mistry BD, Alavi A, Ali S, et al. A systematic review of the relationship between glycemic control and necrobiosis lipoidica diabeticorum in patients with diabetes mellitus. Int J Dermatol. 2017;56:1319–1327. [DOI] [PubMed] [Google Scholar]
- 5.Lause M, Kamboj A, Fernandez Faith E. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reid SD, Ladizinski B, Lee K, et al. Update on necrobiosis lipoidica: a review of etiology, diagnosis, and treatment options. J Am Acad Dermatol. 2013;69:783–791. [DOI] [PubMed] [Google Scholar]
- 7.Muller SA, Winkelmann RK. Necrobiosis lipoidica diabeticorum. A clinical and pathological investigation of 171 cases. Arch Dermatol. 1966;93:272–281. [DOI] [PubMed] [Google Scholar]
- 8.Lefkovits Y, Adler A. Fatal squamous cell carcinoma from necrobiosis lipoidica diabeticorum in a diabetic patient. Endocrinol Diabetes Metab Case Rep. 2019;2019:19-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feily A, Mehraban S. Treatment modalities of necrobiosis lipoidica: a concise systematic review. Dermatol Reports. 2015;7:5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.British Association of Dermatologists. Necrobiosis Lipoidica. Patient information leaflet. 2019.
- 11.Peckruhn M, Tittelbach J, Elsner P. Update: therapie der necrobiosis lipoidica. J Dtsch Dermatol Ges. 2017;15:151–158. [DOI] [PubMed] [Google Scholar]
- 12.Jagmag T, Tirant M, Lotti T. Link between cutaneous infection, stress and depression. J Biol Regul Homeost Agents. 2017;31:1037–1041. [PubMed] [Google Scholar]
- 13.Mistry BD, Alavi A, Ali S, et al. Asystematic review of the relationship between glycemic control and necrobiosis lipoidica diabeticorum in patients with diabetes mellitus. Intern J Dermatol. 2017;56:1319–1327. [DOI] [PubMed] [Google Scholar]
- 14.Van Landuyt K, Blondeel P, Hamdi M, et al. The versatile DIEP flap: its use in lower extremity reconstruction. Br J Plast Surg. 2005;58:2–13. [DOI] [PubMed] [Google Scholar]
- 15.Qing LM, Tang JY. Use of intraflap and extraflap microvascular anastomoses in combination for facilitating bipedicled DIEP/SIEA free flap for reconstruction of circumference soft tissue defect of extremity. Microsurgery. 2019;39:190–191. [DOI] [PubMed] [Google Scholar]
- 16.Assi C, Samaha C, Chamoun Moussa M, et al. A comparative study of the reverse sural fascio-cutaneous flap outcomes in the management of foot and ankle soft tissue defects in diabetic and trauma patients. Foot Ankle Spec. 2019;12:432–438. [DOI] [PubMed] [Google Scholar]
- 17.Schreml S, Berneburg M. The global burden of diabetic wounds. Br J Dermatol. 2017;176:845–846. [DOI] [PubMed] [Google Scholar]
- 18.Osman OF, Emara S. Extended use of full-thickness skin grafts, employing variable donor sites. World J Plast Surg. 2018;7:159–165. [PMC free article] [PubMed] [Google Scholar]
- 19.Ohshiro T, Ohshiro T, Sasaki K. Laser scar management technique. Laser Ther. 2013;22:255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baccarani A, Follmar KE, Erdmann D, et al. Face transplantation surgical options and open problems in cadaveric models: a review article. Microsurgery. 2013;33:239–246. [DOI] [PubMed] [Google Scholar]
- 21.Nagase T, Keiichi I, Yoshiya K, et al. Insulin-derived amyloidosis without a palpable mass at the insulin injection site: a report of two cases. J Diabetes Investig. 2019. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiley J, Bernárdez C, María A. Nodular amyloidosis at the sites of insulin injections. J Cutan Pathol. 2015;42:496–502. [DOI] [PubMed] [Google Scholar]
- 23.Adani R, Delcroix L, Tarallo L, et al. Reconstruction of posttraumatic bone defects of the humerus with vascularized fibular graft. J Shoulder Elbow Surg. 2008;17:578–584. [DOI] [PubMed] [Google Scholar]


