Background:
Dermal regeneration templates such as Integra are effective reconstructive biomaterials used in a variety of soft-tissue defects. Fully understanding the complications associated with their use is paramount to improve outcomes and maximize patient safety. In this study, our purpose is to perform a comprehensive literature review to assess the previously reported infectious complications linked to Integra-based wound closure.
Methods:
We conducted a systematic review of the literature to identify previous articles indexed in PubMed and Ovid for Integra and its synonymous terms. We used these search terms: [Integra OR (dermal regenerative matrix) OR (dermal regeneration matrix) OR (dermal regenerative template) OR (dermal regeneration template) OR (dermal substitute) OR (skin substitute) OR (artificial skin)] AND infection.
Results:
Of the 3508 articles for initial review, 69 reported rates of infection, of which 26 reported ≥1 infection within their cohort. Of these 26 articles, the patients (n = 602) underwent Integra-based reconstruction in 1254 sites and had reported infections in 212 of the sites (16.9%). Among these, we encountered a single report of a fatal case of toxic shock syndrome (TSS) related to the use of Integra in secondary burn reconstruction.
Conclusions:
While Integra offers many benefits, surgeons must be aware that infectious complications are not uncommon. As a result, a careful risk–benefit analysis of its use in reconstruction must be performed, and open discussion with the patient preoperatively regarding infection rate is of utmost importance.
INTRODUCTION
The use of dermal regeneration templates was first described by Burke et al1 in 1981. In its commercial form, the most widely used dermal regeneration template is Integra (Integra LifeScience Corporation, Plainsboro, N.J.), which is a bilayer composed of a matrix of bovine collagen cross-linked with glycosaminoglycans from shark chondroitin sulfate with an overlying protective silicone layer.2 The use of Integra templates in reconstructive surgery has been described in burns,1,3–5 scalp,6,7 limbs,8 abdominal wall,9 degloving injuries,10 keloids and hypertrophic scars,11 purpura fulminans,12 hypospadias,13 diabetic foot ulcers,14 and necrotizing soft-tissue infections15 among other uses.
Although Integra has been shown to be an effective reconstructive tool with excellent functional outcomes, aesthetic results, and high rates of long-term engraftment,3–5 several complications may be associated with its use. The most common complications linked to Integra use are infections.5,16–18 Most of the time, these infections are superficial, are associated with a lower rate of graft take, and can be resolved with antibiotics and negative-pressure therapy.5,19 In this article, we present the results of an extensive literature review of studies reporting infectious complications associated with Integra-based wound closure.
METHODS
We conducted a comprehensive literature search to identify previous articles by indexing PubMed and Ovid. We used these search terms: ([Integra OR (dermal regenerative matrix) OR (dermal regeneration matrix) OR (dermal regenerative template) OR (dermal regeneration template) OR (dermal substitute) OR (skin substitute) OR (artificial skin)] AND skin) AND infection, which generated 3508 articles for initial review.
Eligibility for Inclusion
The article selection criteria were peer-reviewed publications, case reports or case series utilizing Integra for wound repair, and articles reporting infection rate as one of the surgical outcomes. If the data used in one published article had been reported in another study, we included only the article with the most complete and recent data set. Figure 1 is a diagram showing the steps we followed to identify and select articles for this literature review.
Fig. 1.
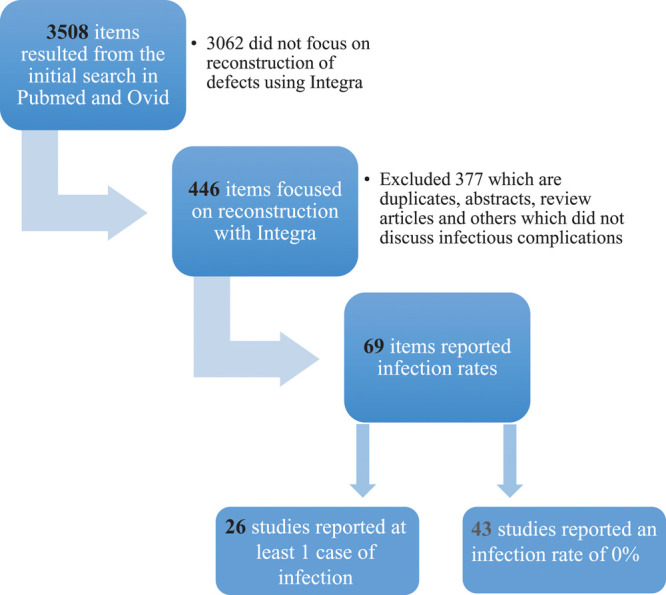
Steps taken to perform the literature review to identify articles who report cases of infections as postoperative outcomes following skin reconstruction with Integra.
RESULTS
Of the 26 articles that were included in the study, we extracted the following data points: type of reconstructive surgery performed (eg, burn, limb, general reconstruction), number of patients in the study, the rates of infection, proportions of superficial versus invasive infection, and would healing outcome. Of 446 articles, only 69 cite the infection rate associated with Integra use, and 43 of these reported no infectious complications. Of the 69 articles, 26 of them reported infections related to the use of Integra.5,8,13,19–41 When grouping the patient population together from these 26 reports, the generalized incidence of infection is 16.9% out of 1254 Integra sites in 602 patients. The results of the systematic review are summarized in Table 1.
Table 1.
Twenty-six Articles That Reported ≥1 Infection with the Use of Integra
| Authors/Year | Reconstruction Type | Patients (#) | Sites (#) | Infections (%) | Superficial (%) | Invasive (%) | Healing Rate (%) |
|---|---|---|---|---|---|---|---|
| Burns: | |||||||
| Heimbach et al5 | Burns | 216 | 758 | 17.3 | 13.2 | 3.1 | NR |
| Shirley et al20 | Burns | 1 | 1 | 100 | 0 | 100 | 0 (death) |
| Dantzer and Braye21 | Burns | 31 | 39 | 12.8 | 80 | 20 | 80 |
| Groos et al22 | Burns | 10 | 22 | 22.7 | — | — | — |
| Lee et al23 | Burns | 7 | 9 | 11.1 | 100 | 0 | 100 |
| Bargues et al24 | Burns | 50 | 71 | 29.6 | 71.4 | 28.6 | NR |
| Yeong et al25 | Burns | 10 | 11 | 9.1 | 100 | 0 | 100 |
| Nessler et al26 | Pediatric burns | 15 | 19 | 21. | 100 | 0 | — |
| Lohana et al27 | Burns | 24 | 37 | 13.5 | 100 | 0 | 100 |
| Huang et al28 | Burns | 5 | 5 | 20 | — | — | — |
| Total | 368 | 972 | 18.1 | ||||
| General reconstruction: | |||||||
| Suzuki et al29 | General reconstruction | 23 | 27 | 3.7 | — | — | — |
| Suzuki et al30 | General reconstruction | 41 | 52 | 13.4 | — | — | — |
| Jeschke et al31 | General reconstruction | 12 | 12 | 25 | 33.3 | 66.7 | NR |
| Unglaub et al32 | General reconstruction | 12 | 19 | 5.21 | 100 | 0 | 100 |
| Total | 88 | 110 | 10.9 | NR | |||
| Limb reconstruction: | |||||||
| Bhavsar and Tenenhaus33 | Hand reconstruction | 4 | 26 | 3.8 | 0 | 100 | 0 |
| Huemer et al34 | Gracilis muscle flap | 20 | 21 | 9.5 | 100 | 0 | 100 |
| Todd et al35 | Self-harm forearm | 6 | 6 | 16.6 | 100 | 0 | 100 |
| Weigert et al36 | Foot and ankle | 21 | 21 | 4.7 | — | — | — |
| Rodriguez Collazo et al8 | Limb reconstruction | 17 | 17 | 23.5 | 0 | 100 | 75 |
| Total | 68 | 91 | 9.8 | ||||
| Pediatric reconstruction: | |||||||
| Martínez et al37 | General reconstruction | 11 | 14 | 14.2 | 100 | 0 | 100 |
| Stiefel et al19 | General reconstruction | 18 | 18 | 16.5 | — | — | — |
| Ghazi and Williams38 | General reconstruction | 8 | 8 | 12.5 | 100 | 0 | 100 |
| Greenhalgh et al39 | Face reconstruction | 23 | 23 | 17.0 | — | — | — |
| Casal-Beloy et al13 | Hypospadias fistula repair | 8 | 8 | 12.5 | 100 | 0 | 0 |
| Total | 68 | 71 | 15.4 | NR | |||
| Others: | |||||||
| Bodmer et al40 | Facial reconstruction after SCC | 6 | 6 | 50 | 0 | 100 | 0 |
| Gonzaga et al41 | Hidradenitis suppurativa | 4 | 4 | 25 | 100 | 0 | 0 |
| Total | 10 | 10 | 40 | ||||
| Total patients | 602 | ||||||
| Total Integra sites | 1254 | ||||||
| Total Integra-site infections | 212 (16.9%) |
NR, not reported; SCC, squamous cell carcinoma.
DISCUSSION
Our literature review demonstrated reports of infection associated with the use of Integra in a variety of wound categories. The highest percentage of infection with Integra use was seen in burn reconstructions. This is well supported by the number of articles (Table 1), including a relatively large study conducted by Heimbach et al,5 which included 13 participating burn centers comprising 216 patients treated with Integra, complicated by infection with an incidence of 16.3% (13.2% superficial and 3.1% invasive). Although the data point to a higher number of infections among the burn reconstruction patient population, patient characteristics, wound pathophysiology, surgeons’ technique, and numerous other confounding variables contribute to the observed differences in infection rate among the studies. There was no statistical difference in infection rate among the different categories relative to burn reconstruction. For example, looking at the incidence of infection alone, the P value of 2-tailed unpaired t test between the burn and non-burn limb reconstruction articles was 0.2316. It is not possible to generate an exact incidence of infection related to Integra use from this review because of the lack of controlled studies and endless confounding variables among the reports. However, Table 1 serves as an organized general overview to the practitioner when discussing with the patient the risks and benefits regarding the use of Integra in wound coverage. Depending on the indications to which Integra is applied, the benefits of its use should routinely surpass its relatively low–moderate rate of infection (13%–15.9%). Most importantly, our literature review identified a single report of a fatal case of toxic shock syndrome related to the use of Integra in burn reconstruction.20
This middle-age patient underwent secondary burn scar revision of neck and axilla with Integra and was readmitted 9 days postoperatively with 2 small (1 cm2) areas of nonadherent graft without purulence, but she succumbed from rapid irreversible sepsis. Culture of debrided Integra grew methicillin-resistant Staphylococcus aureus.
There are various prophylactic measures that may be taken to prevent the development of infection when using dermal regeneration templates such as Integra. Rigid infection control measures must be exercised, including meticulous wound handling techniques to avoid wound contamination during and after surgery, especially with resistant staphylococcal organisms. Preventive dressing options include nanocrystalline silver products such as Acticoat (silver-coated polyethylene; Smith & Nephew, London, United Kingdom)42,43 and silver-coated polyurethane negative-pressure wound therapy sponge.44,45 Antibiotic prophylaxis may also be used.13,16 The use of these prophylactic measures when employing Integra requires prospective investigation.
CONCLUSIONS
While Integra offers many crucial benefits, such as better chance for revascularization than a direct skin graft in certain situations, the surgeon should be aware that infectious complications are not uncommon. As a result, a careful risk–benefit analysis of its use in reconstruction must be performed, and informed consent openly discussing the risk of infection with the patient is paramount. However rare, acknowledging the possibility of toxic shock syndrome as a complication is crucial in early recognition and expedient life-saving surgical and medical intervention.
Footnotes
Published online 15 July 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Burke JF, Yannas IV, Quinby WC, Jr, et al. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann Surg. 1981;194:413–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Integra Dermal Regeneration Template – How INTEGRA Works Ilstraining.com. Available at http://www.ilstraining.com/idrt/idrt/brs_it_04.html. Accessed June 10, 2020.
- 3.Heimbach D, Luterman A, Burke J, et al. Artificial dermis for major burns. A multi-center randomized clinical trial. Ann Surg. 1988;208:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Branski LK, Herndon DN, Pereira C, et al. Longitudinal assessment of Integra in primary burn management: a randomized pediatric clinical trial. Crit Care Med. 2007;35:2615–2623. [DOI] [PubMed] [Google Scholar]
- 5.Heimbach DM, Warden GD, Luterman A, et al. Multicenter postapproval clinical trial of Integra dermal regeneration template for burn treatment. J Burn Care Rehabil. 2003;24:42–48. [DOI] [PubMed] [Google Scholar]
- 6.Corradino B, Di Lorenzo S, Leto Barone AA, et al. Reconstruction of full thickness scalp defects after tumour excision in elderly patients: our experience with Integra dermal regeneration template. J Plast Reconstr Aesthet Surg. 2010;63:e245–e247. [DOI] [PubMed] [Google Scholar]
- 7.Gonyon DL, Jr, Zenn MR. Simple approach to the radiated scalp wound using INTEGRA skin substitute. Ann Plast Surg. 2003;50:315–320. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez Collazo ER, Rathbone CR, Barnes BR. A retrospective look at integrating a novel regenerative medicine approach in plastic limb reconstruction. Plast Reconstr Surg Glob Open. 2017;5:e1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeng JC, Fidler PE, Sokolich JC, et al. Seven years’ experience with Integra as a reconstructive tool. J Burn Care Res. 2007;28:120–126. [DOI] [PubMed] [Google Scholar]
- 10.Herlin C, Louhaem D, Bigorre M, et al. Use of Integra in a paediatric upper extremity degloving injury. J Hand Surg Eur Vol. 2007;32:179–184. [DOI] [PubMed] [Google Scholar]
- 11.Clayman MA, Clayman SM, Mozingo DW. The use of collagen-glycosaminoglycan copolymer (Integra) for the repair of hypertrophic scars and keloids. J Burn Care Res. 2006;27:404–409. [DOI] [PubMed] [Google Scholar]
- 12.Edlich RF, Winters KL, Woodard CR, et al. Massive soft tissue infections: necrotizing fasciitis and purpura fulminans. J Long Term Eff Med Implants. 2005;15:57–65. [DOI] [PubMed] [Google Scholar]
- 13.Casal-Beloy I, Somoza Argibay I, García-González M, et al. Management of recurrent urethrocutaneous fistula after hypospadias surgery in pediatric patients: initial experience with dermal regeneration sheet Integra. Cir Pediatr. 2017;30:207–210. [PubMed] [Google Scholar]
- 14.Driver VR, Lavery LA, Reyzelman AM, et al. A clinical trial of Integra template for diabetic foot ulcer treatment. Wound Repair Regen. 2015;23:891–900. [DOI] [PubMed] [Google Scholar]
- 15.Rashid OM, Nagahashi M, Takabe K. Management of massive soft tissue defects: the use of INTEGRA artificial skin after necrotizing soft tissue infection of the chest. J Thorac Dis. 2012;4:331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muangman P, Deubner H, Honari S, et al. Correlation of clinical outcome of Integra application with microbiologic and pathological biopsies. J Trauma. 2006;61:1212–1217. [DOI] [PubMed] [Google Scholar]
- 17.Wadströ T. Molecular aspects of bacterial adhesion, colonization, and development of infections associated with biomaterials. J Invest Surg. 1989;2:353–360. [DOI] [PubMed] [Google Scholar]
- 18.Yannas IV, Orgill DP, Burke JF. Template for skin regeneration. Plast Reconstr Surg. 2011;127suppl 160S–70S. [DOI] [PubMed] [Google Scholar]
- 19.Stiefel D, Schiestl CM, Meuli M. The positive effect of negative pressure: vacuum-assisted fixation of Integra artificial skin for reconstructive surgery. J Pediatr Surg. 2009;44:575–580. [DOI] [PubMed] [Google Scholar]
- 20.Shirley R, Teare L, Dziewulski P, et al. A fatal case of toxic shock syndrome associated with skin substitute. Burns. 2010;36:e96–e98. [DOI] [PubMed] [Google Scholar]
- 21.Dantzer E, Braye FM. Reconstructive surgery using an artificial dermis (Integra): results with 39 grafts. Br J Plast Surg. 2001;54:659–664. [DOI] [PubMed] [Google Scholar]
- 22.Groos N, Guillot M, Zilliox R, et al. Use of an artificial dermis (Integra®) for the reconstruction of extensive burn scars in children. About 22 grafts. Eur J Pediatr Surg. 2005;15:187–192. [DOI] [PubMed] [Google Scholar]
- 23.Lee LF, Porch JV, Spenler W, et al. Integra in lower extremity reconstruction after burn injury. Plast Reconstr Surg. 2008;121:1256–1262. [DOI] [PubMed] [Google Scholar]
- 24.Bargues L, Boyer S, Leclerc T, et al. Incidence and microbiology of infectious complications with the use of artificial skin Integra® in burns. Ann Chir Plast Esthet. 2009;54:533–539. [DOI] [PubMed] [Google Scholar]
- 25.Yeong EK, Chen SH, Tang YB. The treatment of bone exposure in burns by using artificial dermis. Ann Plast Surg. 2012;69:607–610. [DOI] [PubMed] [Google Scholar]
- 26.Nessler M, Puchala J, Wood FM, et al. Changes in the plasma cytokine and growth factor profile are associated with impaired healing in pediatric patients treated with INTEGRA for reconstructive procedures. Burns. 2013;39:667–673. [DOI] [PubMed] [Google Scholar]
- 27.Lohana P, Hassan S, Watson SB. Integra in burns reconstruction: our experience and report of an unusual immunological reaction. Ann Burns Fire Disasters. 2014;27:17–21. [PMC free article] [PubMed] [Google Scholar]
- 28.Huang SR, Liu JT, Zhang Y, et al. Repair of complex wounds on hands after burns or trauma. Zhonghua Shao Shang Za Zhi. 2019;35:362–366. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki S, Matsuda K, Maruguchi T, et al. Further applications of “bilayer artificial skin.” Br J Plast Surg. 1995;48:222–229. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki S, Kawai K, Ashoori F, et al. Long-term follow-up study of artificial dermis composed of outer silicone layer and inner collagen sponge. Br J Plast Surg. 2000;53:659–666. [DOI] [PubMed] [Google Scholar]
- 31.Jeschke MG, Rose C, Angele P, et al. Development of new reconstructive techniques: use of Integra in combination with fibrin glue and negative-pressure therapy for reconstruction of acute and chronic wounds. Plast Reconstr Surg. 2004;113:525–530. [DOI] [PubMed] [Google Scholar]
- 32.Unglaub F, Ulrich D, Pallua N. Reconstructive surgery using an artificial dermis (Integra): results with 19 grafts. Zentralbl Chir. 2005;130:157–161. [DOI] [PubMed] [Google Scholar]
- 33.Bhavsar D, Tenenhaus M. The use of acellular dermal matrix for coverage u7 exposed joint and extensor mechanism in thermally injured patients with few options. Eplasty. 2008;8:e33. [PMC free article] [PubMed] [Google Scholar]
- 34.Huemer GM, Larcher L, Schoeller T, et al. The free gracilis muscle flap in Achilles tendon coverage and reconstruction. Plast Reconstr Surg. 2012;129:910–919. [DOI] [PubMed] [Google Scholar]
- 35.Todd J, Ud-Din S, Bayat A. Extensive self-harm scarring: successful treatment with simultaneous use of a single layer skin substitute and split-thickness skin graft. Eplasty. 2012;12:e23. [PMC free article] [PubMed] [Google Scholar]
- 36.Weigert R, Leclere FM, Delia G, et al. Long-term patient-reported functional and cosmetic outcomes following severe traumatic foot and ankle wound reconstruction with acellular dermal matrix. J Cosmet Laser Ther. 2015;17:321–329. [DOI] [PubMed] [Google Scholar]
- 37.Martínez L, Ros Z, López-Gutiérrez JC, et al. Integra artificial dermis in pediatric reconstructive surgery. Cir Pediatr. 2002;15:97–100. [PubMed] [Google Scholar]
- 38.Ghazi BH, Williams JK. Use of Integra in complex pediatric wounds. Ann Plast Surg. 2011;66:493–496. [DOI] [PubMed] [Google Scholar]
- 39.Greenhalgh DG, Hinchcliff K, Sen S, et al. A ten-year experience with pediatric face grafts. J Burn Care Res. 2013;34:576–584. [DOI] [PubMed] [Google Scholar]
- 40.Bodmer E, Osinga R, Fritsche E, et al. One-stage reconstruction of facial defects after tumor resection with the Integra system. Handchir Mikrochir Plast Chir. 2012;44:355–359. [DOI] [PubMed] [Google Scholar]
- 41.Gonzaga TA, Endorf FW, Mohr WJ, et al. Novel surgical approach for axillary hidradenitis suppurativa using a bilayer dermal regeneration template: a retrospective case study. J Burn Care & Research. 2013;34:51–57. [DOI] [PubMed] [Google Scholar]
- 42.Khansa I, Schoenbrunner AR, Kraft CT, et al. Silver in wound care—friend or foe? a comprehensive review. Plast Reconstr Surg Glob Open. 2019;7:e2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fong J, Wood F. Nanocrystalline silver dressings in wound management: a review. Int J Nanomedicine. 2006;1:441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sachsenmaier S, Peschel A, Ipach I, et al. Antibacterial potency of V.A.C. Granufoam Silver dressing. Injury. 2013;44:1363–1367. [DOI] [PubMed] [Google Scholar]
- 45.Applewhite A, Chowdhry SA, Desvigne M, et al. Inpatient and outpatient wound treatment recommendations: assessing use of negative pressure wound therapy systems or oxidized regenerated cellulose (ORC)/collagen/silver-ORC dressings. Wounds. 2018;308 supplS19–S35. [PubMed] [Google Scholar]


