Background:
Acellular dermal matrix (ADM) in direct-to-implant breast cancer reconstruction is the standard of care due to superior cosmetic results and decreased capsular contracture, but can be cost prohibitive. Although more economical, using patient’s own dermis (“Autoderm”) instead of ADM has undescribed sterility. Sterility is essential, as bacterial contamination may cause infection and capsular contraction. This study aimed to determine the sterility and optimal decontamination protocol of Autoderm.
Methods:
A prospective controlled study of 140 samples from 20 DIEP (deep inferior epigastric perforator) breast cancer reconstruction patients was performed. Seven de-epithelialized dermal samples (2 × 1 cm) per patient were collected from excess abdominal tissue (6 decontamination protocols and one control). Samples were submerged in povidone-iodine, chlorhexidine, or cefazolin/tobramycin/bacitracin for 15 minutes; half of the samples were agitated (150 rpm) for 15 minutes, and half were not. The control was normal saline without agitation. The solution was removed, and the tissue was sent for aerobic colony count cultures. Patient’s demographic data and complications were also collected.
Results:
Of 140 samples, 3 of 20 non-agitated povidone-iodine and 1 of 20 control samples had aerobic bacterial growth. All of the other 100 samples from 5 experimental groups (povidone-iodine + agitation, chlorhexidine ± agitation, and cefazolin/tobramycin/bacitracin ± agitation) had no aerobic bacterial growth.
Conclusions:
This study suggests povidone-iodine + agitation, chlorhexidine ± agitation, and cefazolin/tobramycin/bacitracin ± agitation are effective at sterilizing de-epithelialized dermis, whereas povidone-iodine without agitation and saline are ineffective. Autoderm with the appropriate decontamination protocol may be a potential sterile alternative to ADM.
INTRODUCTION
Direct-to-implant reconstruction, particularly prepectoral reconstruction, has gained popularity in large part due to the use of acellular dermal matrix (ADM).1,2 It is reported that ADM is used in up to 60% of prosthetic-based breast reconstructions.3,4 Perceived benefits to the use of ADM include improved lower pole expansion, superior cosmetic outcomes, and reduced capsular contracture.4 Although these benefits have increased the popularity of ADM, its significant cost remains a barrier to its widespread use.5–8
Autoderm has been explored as a potential low-cost alternative to ADM, where abdominal tissue from the patient is de-epithelialized and used as a dermal sling rather than using cadaveric ADM.9 Autoderm has been shown to be equally effective and in some cases, superior to ADM for tissue expander and implant breast reconstruction in multiple studies.7–9 The advantages of lower pole support and breast shaping have been matched,7,8 with potentially lower rates of infection and seroma compared with ADM.9
Although the degree of sterility and safety of ADM has been studied, there is a paucity of literature studying the sterility and safety of Autoderm.10–14 This study aimed to develop a safe, sterile, and simple decontamination protocol of Autoderm for further clinical research.
METHODS
Institutional Review Board Approval
The study was reviewed by the Institutional Review Board and approved by the university and hospital site before the study start.
Participants
Participants were patients of ≥18 years of age undergoing unilateral or bilateral autologous breast reconstruction using their abdominal tissue at Health Sciences Center. Patients were excluded if they required neoadjuvant chemotherapy, had a history of abdominal skin infections, were on immunosuppressant drugs, or had insufficient excess abdominal dermis for the study (ie, delayed breast reconstructions).
Subject Recruitment
All patients undergoing autologous breast reconstruction by the 2 senior authors who did not meet exclusion criteria were invited to participate in the study. A total of 20 patients between October 2017 and January 2018 were enrolled in the study. Written consent was obtained by the study coordinator after the initial evaluation with the plastic surgeon to ensure eligibility criteria were met.
Design
This was a blinded, prospective, randomized-controlled, pilot study with a within-subject design. A segment of abdominal skin was harvested from each patient under sterile conditions. This specimen was then de-epithelialized so that only dermis remained, and then divided into 7 equal parts of 2 × 1 cm. Each dermal sample was randomly assigned to 1 of 6 treatment groups, or to the control group. The microbiology laboratory was blinded to the treatment of each sample submitted. This study compared the aerobic bacterial growth of de-epithelialized dermal samples following exposure to different antimicrobial treatments. The decontamination agents used and the use of agitation were decided based on previous research studying autograft decontamination.15–17
Intervention
Patients enrolled in our study underwent no additional interventions that were different from our routine management. All patients received preoperative antibiotics and were prepared with chlorhexidine alcohol 4%. After harvesting the abdominally based autogenous flap and marking the skin paddle, the excess dermis, which would otherwise be discarded, was collected for the study. A total of 7 samples, each measuring 2 × 1 cm, were obtained from each patient and processed. All interaction with the tissue samples was done in the operating room with surgical sterility (prepping, gloves, and gown). A total of 140 de-epithelialized dermal samples were collected.
Samples obtained were prepared and labeled in a sterile container with a unique study identifier. The study had 6 trial arms and 1 control arm.
Control group sodium chloride 0.9%.
Povidone-iodine 10% (without agitation).
Povidone-iodine 10% (with agitation).
Chlorhexidine 2% with 4% alcohol (without agitation).
Chlorhexidine 2% with 4% alcohol (with agitation).
Triple antibiotic solution of 1-g cefazolin/80-mg tobramycin/50,000-IU (International Units) bacitracin in 500 mL of normal saline (without agitation).
Triple antibiotic solution of 1-g cefazolin/80-mg tobramycin/50,000-IU bacitracin in 500 mL of normal saline (with agitation).
Samples were submerged in 30 mL of solution with or without mechanical agitation for 15 minutes. Agitation was at 150 revolutions per minute used by the KJ-201BD Orbital Shaker. The solution was drawn off with sterile syringes, and the remaining sample was transferred in the sterile container to the microbiology laboratory at our institution for aerobic cultures.
Each of the 140 samples was plated on a blood agar plate as a macerated nutrient broth by the microbiology laboratory. Incubation followed for 24 hours at 37°C, and a manual colony count was performed. Colony-forming units (CFUs) counts of zero were considered sterile. Plates with 1–99 CFUs were documented, whereas those with >99 CFUs were recorded as too numerous to count. Specific bacterial identification of colonies was not performed.
Data Collection
Demographic data and patient comorbidities were collected for all patients. Sample culture results were received as standard diagnostic reports with colony counts as prepared by the microbiology laboratory. Review of clinical data and rates of postoperative complications were documented at the standard 6-week postoperative clinic appointment.
Statistical Considerations
Patient data collected included age, smoking status, history of diabetes, antibiotic allergies, methicillin-resistant Staphylococcus aureus (MRSA) status, antibiotics administered pre-/postsurgery, and postoperative abdominal infections. A Mann-Whitney U test was used to look for a relationship between age and culture results. Due to the lack of normality and homogeneity of variances, a parametric test was not appropriate to analyze the culture data. A Fisher exact test using frequency (growth versus no growth) was used. A posthoc power analysis revealed an approximate 80% power.
RESULTS
Of the 20 patients studied, one had diabetes mellitus, one was MRSA positive, and no patients smoked. Postoperative infections were only noted in 1 patient; this patient had negative dermal cultures. The mean age of patients was 55.5 (SD = 8.9), and there was no significant difference in age between patients who had positive or negative culture sample (P = 0.39). None of the patients with diabetes mellitus, MRSA, or postoperative infections had a dermal sample that grew bacteria in any of the treatment limbs.
Culture results are shown in Table 1. No association was found between bacterial culture results and any demographic variable.
Table 1.
Bacterial Growth by Treatment
| Treatment | % Sterile Samples | Negative Cultures | Colony Count of Positive Cultures (CFU/mL) |
|---|---|---|---|
| Control | 95% | 19/20 | TMTC |
| Povidone + still | 85% | 17/20 | 10, 10, 20 |
| Povidone + agitation | 100% | 20/20 | 0 |
| Chlorhexidine + still | 100% | 20/20 | 0 |
| Chlorhexidine + agitation | 100% | 20/20 | 0 |
| Triple antibiotic + still | 100% | 20/20 | 0 |
| Triple antibiotic + agitation | 100% | 20/20 | 0 |
TMTC, too many to count.
DISCUSSION
The decontamination solutions chosen in this study were based from published studies examining autograft decontamination. Bauer et al15 found that chlorhexidine gluconate with mechanical agitation was most effective for decontaminating bone grafts. A systematic review looking at anterior cruciate ligament decontamination had similar results, with chlorhexidine being the most effective and povidone-iodine being the least effective. Polymyxin B-bacitracin antibiotic solution was found to only be effective with agitation.16 Likewise, Schmidt et al18 found chlorhexidine to be the only effective solution to eliminate Staphylococcus epidermidis from biofilms at clinically used concentrations and exposure times. Mann-Salinas et al17 found both chlorhexidine and povidone-iodine to be successful in reducing bacterial contamination of porcine tissue, representing human skin grafts.
Our results were consistent with the findings of other studies despite being the only study to analyze human dermis. Literature supports chlorhexidine as the most effective decontamination agent, whereas povidone-iodine was often ineffective.15–18 Agitation played a key role in the effectiveness of a polymyxin B-bacitracin solution when looking at the decontamination of anterior cruciate ligament grafts.16 Similarly, our results showed povidone was only effective as a sterilizing agent when combined with agitation. Despite the favorable outcomes associated with agitation, accessible equipment, financial means, and time constraints may limit applicability in the operating room.
Both chlorhexidine and triple antibiotic solution provided sterility to all dermal samples with and without agitation (Fig. 1). It remains unclear which will provide better breast reconstruction outcomes. Chlorhexidine-treated cells have been shown to have lower rates of viability following treatment15,19; however, there remains a lack of research on understanding the relation between ADM cellular viability and outcomes in surgical procedures, such as capsule formation.
Fig. 1.
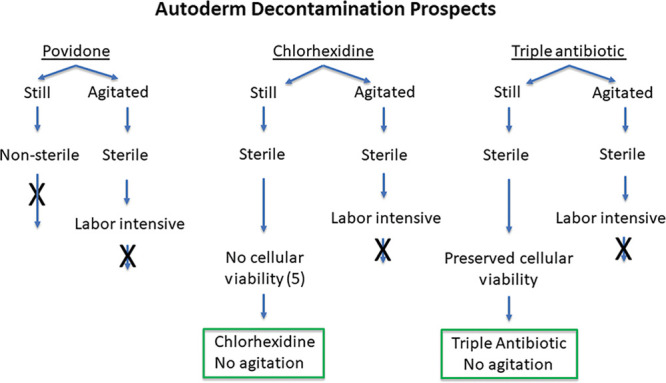
Flow chart showing the efficacy of antimicrobial treatments.
There are multiple strengths to our study. Our patient sample was representative of patients on whom Autoderm would be clinically used. This eliminates many confounders associated with this unique patient population. We chose to only include patients receiving abdominally based autogenous breast reconstruction because this is the dermis that is harvested in a mini-abdominoplasty for Autoderm. We controlled for patients who had received neoadjuvant chemotherapy or were on immunosuppressant drugs to minimize potential confounders. Patients who had a history of abdominal infections were also excluded because an ongoing infection may alter the bacterial flora present and skew results.
An additional strength to this study is its within-subject study design. This allowed for every patient to act as their own control. This mitigates specific patient confounders that could influence bacterial growth.
This study is the first of its kind to explore Autoderm decontamination, a subject area lacking research. Many studies have analyzed ADM and the outcomes associated with sterile versus aseptic processing. However, as Autoderm gains favor in the surgical community as a suitable substitute to ADM, it will be critical to have evidence-based research to guide dermal graft decontamination procedures to maximize the likelihood of favorable postoperative outcomes.
Limitations included low rates of bacterial growth in all treatment groups, which decreased the power of the study to determine statistical significance between treatment groups. However, our results had clinical significance, given that the aim of this study was the identification of a protocol with zero tolerance for bacterial growth.
A second limitation to the study arises from the definition of sterile. Sterility was defined as having zero CFU on objective plating count; however, it is recognized that biofilms and certain bacterial cultures may not be able to adequately grow using this technique. This lowers our confidence that our zero CFU plates were completely sterile; however, rare bacterial species and biofilms were not expected to be present in the dermal samples.
Further research into the efficacy of Autoderm in direct-to-implant breast reconstruction is necessary. Utilization of our findings to suggest appropriate decontamination agents in randomized controlled trials may be critical to allow these studies to commence and compare patient outcomes in procedures involving Autoderm and ADM.
Footnotes
Published online 15 July 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. Funding for the project was provided by the 2016 Surgery Geographical Full-Time (GFT) Research Grant Competition.
REFERENCES
- 1.Sobti N, Ji E, Brown RL, et al. Evaluation of acellular dermal matrix efficacy in prosthesis-based breast reconstruction. Plast Reconstr Surg. 2018;141:541–549. [DOI] [PubMed] [Google Scholar]
- 2.Wazir U, Mokbel K. The evolving role of pre-pectoral ADM-assisted implant-based immediate breast reconstruction following skin-sparing mastectomy. Am J Surg. 2018;216:639–640. [DOI] [PubMed] [Google Scholar]
- 3.Zenn M, Venturi M, Pittman T, et al. Optimizing outcomes of postmastectomy breast reconstruction with acellular dermal matrix: a review of recent clinical data. Eplasty. 2017;17:e18 Available at: http://www.ncbi.nlm.nih.gov/pubmed/28663773. Accessed November 21, 2018. [PMC free article] [PubMed] [Google Scholar]
- 4.JoAnna Nguyen T, Carey JN, Wong AK. Use of human acellular dermal matrix in implant-based breast reconstruction: evaluating the evidence. J Plast Reconstr Aesthet Surg. 2011;64:1553–1561. [DOI] [PubMed] [Google Scholar]
- 5.Andrew Salzberg C, Ashikari AY, Michael Koch R, et al. An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (Alloderm). Plast Reconstr Surg. 2011;127:514. [DOI] [PubMed] [Google Scholar]
- 6.Tran BNN, Fadayomi A, Lin SJ, et al. Cost analysis of postmastectomy reconstruction: a comparison of two staged implant reconstruction using tissue expander and acellular dermal matrix with abdominal-based perforator free flaps. J Surg Oncol. 2017;116:439–447. [DOI] [PubMed] [Google Scholar]
- 7.North WD, Kubajak CS, St Martin B, et al. Dermal autograft using donor breast as alternative to acellular dermal matrices in tissue expander breast reconstruction: a comparative review. Ann Plast Surg. 2017;786S suppl 5S282–S285. [DOI] [PubMed] [Google Scholar]
- 8.Lynch MP, Chung MT, Rinker BD. A comparison of dermal autograft and acellular dermal matrix in tissue expander breast reconstruction. Ann Plast Surg. 2015;74:S214–S217. [DOI] [PubMed] [Google Scholar]
- 9.Selber JC, Clemens MW, Oates S, et al. Autoderm: an alternative bioprosthetic for breast reconstruction. Plast Reconstr Surg. 2013;131:985–987. [DOI] [PubMed] [Google Scholar]
- 10.Parikh RP, Brown G, Sharma K, et al. Immediate implant-based breast reconstruction with acellular dermal matrix. Plast Reconstr Surg. 2018;142:1401–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buseman J, Wong L, Kemper P, et al. Comparison of sterile versus nonsterile acellular dermal matrices for breast reconstruction. Ann Plast Surg. 2013;70:497–499. [DOI] [PubMed] [Google Scholar]
- 12.Lyons DA, Mendenhall SD, Neumeister MW, et al. Aseptic versus sterile acellular dermal matrices in breast reconstruction: an updated review. Plast Reconstr Surg Glob Open. 2016;4:e823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein GM, Nasser AE, Phillips BT, et al. Is sterile better than aseptic? Comparing the microbiology of acellular dermal matrices. Plast Reconstr Surg Glob Open. 2016;4:e761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JH, Park Y, Choi KW, et al. The effect of sterile acellular dermal matrix use on complication rates in implant-based immediate breast reconstructions. Arch Plast Surg. 2016;43:523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauer J, Liu RW, Kean TJ, et al. A comparison of five treatment protocols for contaminated bone grafts in reference to sterility and cell viability. J Bone Joint Surg Am. 2011;93:439–444. [DOI] [PubMed] [Google Scholar]
- 16.Khan M, Rothrauff BB, Merali F, et al. Management of the contaminated anterior cruciate ligament graft. Arthroscopy. 2014;30:236–244. [DOI] [PubMed] [Google Scholar]
- 17.Mann-Salinas EA, Joyner DD, Guymon CH, et al. Comparison of decontamination methods for human skin grafts. J Burn Care Res. 2015;36:636–640. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt K, Estes C, McLaren A, et al. Chlorhexidine antiseptic irrigation eradicates staphylococcus epidermidis from biofilm: an in vitro study. Clin Orthop Relat Res. 2018;476:648–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu JX, Werner J, Kirsch T, et al. Cytotoxicity evaluation of chlorhexidine gluconate on human fibroblasts, myoblasts, and osteoblasts. J Bone Jt Infect. 2018;3:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]


