Abstract
The nationwide focus on patient safety and the health of residents has increased the demand for educational tools outside the operating room. Simulation is a valuable tool for assessing and developing surgical skills in a controlled and safe environment. The use of simulation as a formal component of training has been increasing in various surgical subspecialties. In general surgery, simulation examinations such as the Fundamentals of Laparoscopic Surgery and Fundamentals of Endoscopic Surgery have become a prerequisite to board certification. Although formal simulation examinations in plastic surgery are not universal, there has been an increase in the use of simulation to increase resident competency in the operating room. For now, we will review the current state of simulation in craniofacial, hand, microvascular, and esthetic surgery and discuss applications for the future. We will also discuss the evolving role of artificial intelligence, virtual reality, and augmented reality in plastic surgery training and testing.
INTRODUCTION
Simulation was first introduced in the field of general surgery, with predominant applications in laparoscopic and endoscopic procedures.1 Its utilization has subsequently been adopted by various surgical specialties.2,3 The term encompasses a broad range of synthetic, animal, cadaver, and virtual models, including both static and dynamic, with a common goal to recreate the operating room environment to allow learners to practice in low stakes situations.4 Simulation allows trainees to develop both physical and cognitive skills through repetition and anatomic representation in a controlled environment that promotes trainee development.4–8 It also mitigates many of the constraints present in the operating room including time, attending surgeon’s teaching approach, and trainee learning style.5,7 Finally, use of simulation has been shown to yield greater increases in knowledge and skill compared to using traditional educational methods, such as self-directed reading and use of digital images.9
In plastic surgery, simulation has been used to objectively assess baseline knowledge, including operative anatomy, as well as to evaluate trainee advancement in surgical skills.10–12 Since numerous factors contribute to the variation in trainee operative experience, simulation standardizes many of these variables. It therefore permits assessment of learners at parallel time points in training, and it has been used to evaluate surgical knowledge, skill, and judgment.13,14 In addition, simulation compliments the qualitative evaluations of performance that take place within the operating room by providing more objective quantitative measures of skill.13 The confirmed efficacy of simulation and increased availability of validated models for use in both training and assessment has allowed plastic surgery to adopt this practice more routinely into residency and fellowship training. This article aims to characterize the different modalities of simulation and review their various applications in the field of plastic surgery and its subspecialties.
SIMULATION MODALITIES
Simulators can be synthetic, animal-derived, cadaver-derived, or virtual, and each modality has its own advantages and disadvantages (Table 1).4 Although synthetic simulators provide basic anatomic representation, they do not fully resemble live, physiologic tissue, and low fidelity models require replacement after use. Animal simulators are heavily used for microsurgery training, as the vessels of anesthetized live animals best reproduce the anastomosis of live human vessels; however, ethical constraints, cost, and availability often limit their use. They also realistically represent a dynamic form of real situation simulation, where the patency of the anastomosis can be assessed following the procedure. Alternatively, cadavers provide a more accurate model of anatomic representation and variation but are unable to emulate bleeding tissues. Finally, computer-based simulators provide high-quality visualizations, but are expensive and rely heavily on repetition for reinforcement of knowledge.2,4
Table 1.
Advantages and Disadvantages of Simulation Modalities
| Simulation Modality | Advantages | Disadvantages | Types of Learning |
|---|---|---|---|
| Synthetic | Basic anatomic representation; least expensive option | Insufficient resemblance of tissue; requires replacement after use | Technique |
| Animal | Behavior similar to human vessels during dissection and anastomosis | Ethical dilemmas; expensive; limited availability | Technique |
| Cadaver | Most accurate real-life anatomic variation and availability | Expensive; does not emulate bleeding of living tissue | Anatomy |
| Virtual reality | Platform for complex operations, anatomy identification | Expensive up-front costs; low-quality haptic feedback | Decision-making |
| Augmented reality | Provides a 3D surgical experience; applications in telesurgery | Decreased software availability; side effects with prolonged use | Decision-making |
3D, 3-dimensional.
The most recent development in simulation is the use of virtual reality (VR), augmented reality (AR), and even artificial intelligence (AI) technologies. VR is touted for its ability to provide rare and complex experiences that are not often encountered in the day-to-day setting.15,16 Many VR programs focus on improving identification of anatomic variation and evaluating procedural knowledge. The drawbacks of VR are its expensive up-front costs and its inability to fully capture the physical aspects of surgery because of its lack of high-quality haptic feedback.15,16
While VR creates an isolated training environment, AR superimposes virtual information, such as 3-dimensional images, to the real-time visual field and has applications not only in medical education but also in telesurgery.17,18 The most popular of these was Google Glass (Google LLC, Mountain View, Calif.).19 AR allows training to occur remotely, extending its range of utility beyond residents’ home institutions.20 The disadvantages of AR include decreased software availability and again the up-front cost.18 These various modalities and their associated qualities make simulators a flexible education tool for use throughout the residency training curriculum in various subspecialties of plastic surgery.
AI uses machine learning to analyze big data, recognize patterns, and predict outcomes.21 In plastic surgery training, machine learning could act as an assessment tool that may not only evaluate performance but also predict trainee outcomes.22 The use of historical data—through various imaging modalities of surgical performance by trainees—can be analyzed by AI programs to recognize trainee-specific patterns, pinpoint strengths and weaknesses, and predict postoperative results.22 It would likely be some time before AI is implemented into training with any regularity (Table 2).
Table 2.
Best Simulation Model for Level of Residency
| Residency Level | Best Simulation Modality |
|---|---|
| Lower level | Synthetic, cadaver |
| Mid-level | Animal, cadaver |
| Upper level | Cadaver, VR, AR |
SIMULATION IN HAND SURGERY
The goal of simulation in hand surgery is to master surgical handling of delicate soft tissues and sturdy bony structures, while understanding the finer anatomy of the hand. Animal models provide accurate representations of the bony anatomy suitable for practicing fracture fixation, bone anchoring, and tendon repair.23 Chicken femurs, specifically, have comparable metacarpal shape, size, and bone density to those of humans, thereby proving useful for training in hand surgery.23 Porcine forelimbs have also been used for practicing flexor tendon repairs due to similarities in their digital flexor tendon system with that of humans.24,25 Despite the translatable anatomic representation of animal models for hand surgery, they are costly, often limited to one use, and require proper disposal. Therefore, newer, high-fidelity synthetic models have been proposed for their low-cost and multiuse qualities. Kempton et al26 developed an impressive synthetic model for endoscopic carpal tunnel surgery. Through a randomized controlled trial, they showed that the model was able to help trainees be more prepared for participating in surgery, particularly those with limited experience.26
Cords with synthetic coating, acrylic “bones” made of rubber, and compact cotton wool have been proposed to represent tendons that can be used for suture training.27–29 Cadavers, specifically fresh-frozen cadavers, have also been used for surgical training to increase trainee confidence and skill during complex procedures with a steep learning curve, such as zone II and IV flexor tendon repairs.30–32 Animal models have been the predominant modality used for training in hand surgery, but cadavers and synthetic models also possess the capability to increase skills needed for these procedures.
The importance of understanding nerve surgery for trauma, nerve compression, amputations, targeted muscle reinnervation, regenerative peripheral nerve interfaces, and even migraine surgery and facial reanimation is becoming more important for broad training in plastic surgery.33 As discussed elsewhere, the gold standard for teaching microsurgery is on live rats, where the femoral bundle including the nerve can easily be identified, divided, and coapted.34 Gul et al35 have successfully developed a synthetic model made from silicone, cotton, and dyes that does a great job of emulating the components of a nerve. They were able to create a model that had a 3-mm “epineurium” and 3 “fascicles.”35 Continued innovations such as this model will help lower the cost of training residents in nerve coaptation.
Hand surgery training using AR can be used to extend a surgeon’s ability to help fellow colleagues. Greenfield et al20 describe an AR program, Proximie (Proximie, London, United Kingdom), that was used in Gaza due to restricted migration into the area. This technology allows specialized plastic surgeons at a remote location to guide local surgeons through overlaid annotations and diagrams during surgical planning and demonstrations of hand gestures throughout the procedure. The ability to provide visual—in addition to verbal—aid allowed local surgeons to perform a complex hand reconstruction of an injured soldier that involved palmar contracture release and forearm flap in setting. AR not only enhances training in a controlled environment but also creates opportunities for telesurgery to have significant impacts on underserved and restricted communities.20
SIMULATION IN CRANIOFACIAL SURGERY
The goals of simulation in craniofacial surgery are important not only to trainees in the United States but also to help supplement the education of surgeons who perform specialized surgeries and treatments (such as cleft care) worldwide. Simulation has been proved to not only increase comfort and decrease operative time but also to decrease serious complications such as poor scarring, fistula formation, and velopharyngeal insufficiency.36,37
Cadaver and synthetic models are widely used for training in craniofacial surgery. Drawing the various lip and palate repairs on paper is a prerequisite for any trainee who enters the operating room; however, the actual dissection and soft tissue layers of the lip and palate are some of the most challenging to teach in plastic surgery. This is further accentuated by the anatomical variability in cleft lip and palate patients. As a result, various high-fidelity synthetic simulators with realistic physical properties and accurate anatomic representation have been validated for training in cleft palate repairs to increase the knowledge and confidence of trainees as they move through residency.38,39 One high-fidelity simulator incorporates the use of the 3-dimensional printing with silicone casting that not only enhances skill development but also has the added benefit of low cost and high portability.36 This model was developed from the computed tomography scan of a patient and adjusted by highly trained cleft surgeons. The tensile strength and feel of tissues were also adjusted based on recommendations by the surgeons. Impressively, the model with simulation requires everything from inserting the Dingman Retractor to raising mucoperichondrial flaps to the final suture closure. Furthermore, it contains a low-cost insertable cartridge that can be easily changed out after each training exercise.38,39
In craniofacial surgical training, VR has been used as early as 2006 and continues to be widely used for training locally and internationally. Smith et al40 presented a virtual atlas of craniofacial anatomy early on. Four years later, Flores et al41,42 presented the first virtual surgical atlas of craniofacial procedures including the various surgical simulators available for training in select topics, including cleft lip and palate care, soft tissue manipulation, and mandibular distraction. Additional procedures available in VR simulators include monobloc, Le Fort III, and fronto-orbital advancements.4 In time, VR will likely represent the best way to supplement training in craniofacial surgery.
Digital simulation for cleft palate repair training has already been shown to yield superior results in knowledge retention and skills performance when compared with textbook education.9 The group out of New York University has developed an online and freely available training simulator for cleft lip repair. In a blinded study, it yielded superior results when compared with those in traditional training.43
SIMULATION IN MICROSURGERY
The goal of simulation in microsurgery is 2-fold: first of all, to train residents in the technique of microvascular anastomosis, and second, to teach residents how to effectively and safely raise flaps. To this end, over 90% of integrated plastic surgery programs have a practice microscope that can be used by the residents (Fig. 1). Around 70% of programs with over 18 residents use nonliving animal models like a chicken thigh for practice, while 70% of programs with less than 18 residents use a living biologic model like a rat.44 There has also been an interest in the ways to evaluate the skill of residents under the microscope. Mcgoldrick et al45 used motion capture analysis to evaluate the components to a good microsurgical repair including efficiency of movement, quality of knot, among others. The program was set to evaluate the movement of the microsurgical instruments and ensure fluidity of motion and the flow of the operation. The results of the program correlated positively with the evaluations of expert surgeons who also scored the blinded videos of residents performing simulated anastomosis.45 Establishing a standardized and accurate way to evaluate the skills of a resident would be required if certifying bodies, such as the American Board of Plastic Surgery, ever wanted to develop a Fundamentals of Microsurgery test similar to the standards in General Surgery.
Fig. 1.
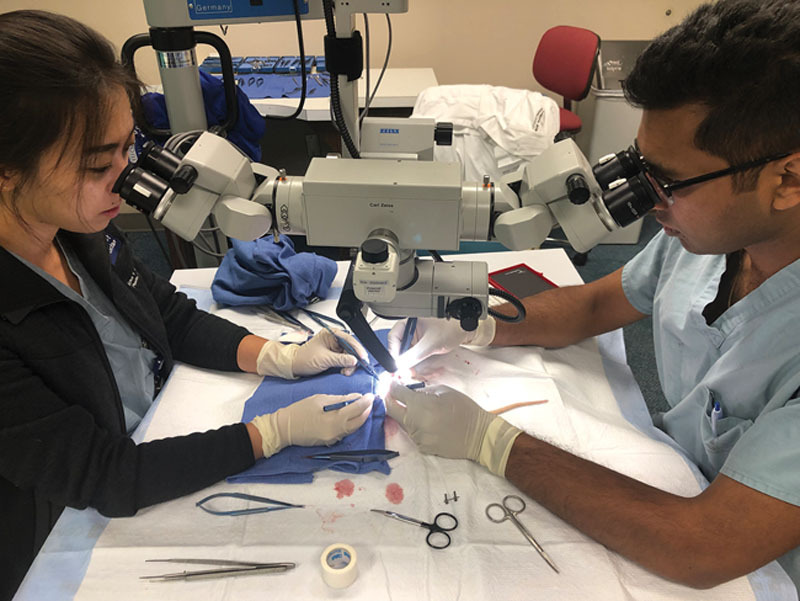
Residents conducting microvascular training using live animal models.
One of the basic simulation models for practicing microsurgical skills, such as vessel anastomosis and nerve coaptation, uses fresh chicken thighs.46 An easily identifiable fat pad is located on the underside of the chicken thigh that includes the femoral artery, vein, and nerve, allowing for both vascular anastomosis and nerve coaptation in the same specimen. While this static model benefits from a lower cost and more basic setup, it is limited by the lack of active blood flow to assess anastomotic patency (Fig. 2). As a result, Zeng et al47 developed a technique for infusing saline mixed with blue food coloring through the vessels of a thicken thigh. This allowed for emulation of bleeding during a case, as well as provides a way to check the patency of an anastomosis.47
Fig. 2.
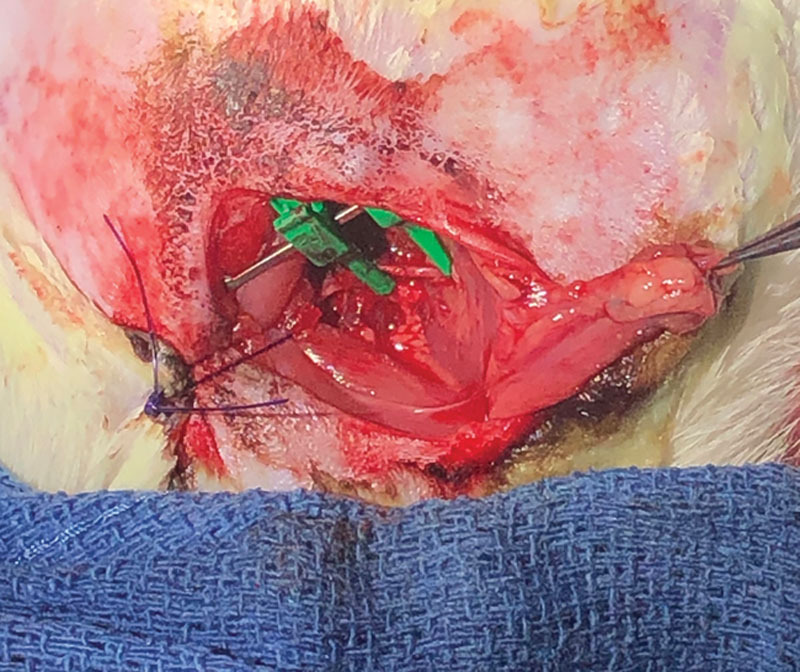
A femoral artery laceration repaired with 11-0 nylon suture in a live rat under anesthesia.
Many institutions use live animal models under general anesthesia to better simulate this part of the operation. For example, live rat models have been frequently used by many training programs, due to their appropriate size of blood vessels, dynamic arterial and venous blood flow, and reasonable cost. Additionally, the model realistically simulates troubleshooting scenarios encountered in the operating room, such as vessel mismatch, low flow states, and poor quality or paucity of recipient vessels. Nevertheless, there are drawbacks to these type of live animal models, including a higher cost, ethical concerns, and associated strict standard operating procedures, as well as their one-time use limit.
Human cadaver models have been shown to be particularly useful in simulating flap elevation and recipient vessel harvest. Cadavers can be additionally injected with latex to better visualize blood vessels and to facilitate dissection. In fact, numerous studies have used human cadavers to better understand perforator anatomy and to develop novel flaps for reconstruction.48–51 In addition, the beneficial use of cadavers for flap elevation and recipient vessel harvest has led to the popularization of various flap reconstruction courses.
High-fidelity synthetic models have been developed as an additional tool to simulation. These include manufactured prosthetic vessels that have a size comparable to that of human vessels, with the added realism of a surrounding adventitial layer.52,53 Though synthetic simulators lack some of the anatomic representation of animal and cadaver models, synthetic models act as tools for basic training without added ethical considerations and high costs. At this time, microsurgical synthetic models are not cheaper or better models than the nonliving and living animal models and therefore are not commonly used.52
A more recent innovation in simulation in plastic surgery is the use of VR to train residents and fellows in microscopic techniques by interfacing synthetic models with instruments connected to computers. Examples of VR in microsurgery include the ANGIO Mentor Symbionix (3D Systems, Littleton, Colo.), AccuTouch Immersion Medical (Industrial Designers Society of America, Herndon, Va.), Procedicus VIST (Mentice, Gothenburg, Sweden), and Simusuite (Medical Simulation Corporation, Denver, Colo.).54 Associated characteristics include haptic feedback, physiological responses, neurological and pharmacological reactions, and metric assessment with modules for carotid, renal, iliac, and coronal procedures.54 The benefits of VR are decreased costs of simulation laboratories compared with animal laboratories. Currently, simulation of anastomotic training is best represented by animal models, while the use of cadavers are the best practice in training the residents on flap elevation.
SIMULATION IN ESTHETIC SURGERY
Some of the greatest potential benefits of simulation for trainees is in the field of esthetic surgery, where trainee participation in cases is perhaps the most limited. This has at least partially been reflected through resident surveys. For example, Zammit et al55 found that residents express the least confidence with rhinoplasty procedures and desire increased availability of simulators to improve these skills. Additionally, one study has demonstrated that residents lack hands-on training in bilateral breast augmentation despite it being the second most common esthetic procedure performed in the United States.55 The authors attributed these findings to the fact that the majority of esthetic training is observation-based as opposed to hands-on. As a result, the demand for simulation in esthetic training is quite high and has been proved to be helpful.56 In a study assessing the quality of simulation training, researchers showed improved surgical performance using simulation compared to video training of Botox administration.57
Current simulators for esthetic surgery consists mainly of cadaveric models. Cadaveric models have been used to help residents learn facial anatomy as well as multiple esthetic surgery procedures. Jung et al58 developed a systematic dissection of a cadaver from medial to lateral, cranial to caudal, and superficial to deep. Residents had significant increases not only in their understanding of anatomy but also in their comfort level in performing esthetic surgery.58
A few prosthetic models have also been developed. One such example is a synthetic breast augmentation model, complete with anatomic landmarks and a submuscular plane that was developed by Kazan et al51 in Montreal, Canada. To overcome the issues with reusability, the authors were able to develop a strip of “skin” that would be placed over the previous incision and Velcro that would be reapplied after performing the breast pocket dissection. The model also included a pneumothorax detector that would identify if the pleural space was violated. In addition, the authors also developed a grading system for trainees on its use, with a future plan to validate its use with a similar scoring system during actual surgery.59 A model for trans-axillary placement of breast implants was developed in China but has not been widely used.60
Cadaveric pig heads have been defined as reliable simulators for various facial procedures and have long been used in dermatology training. Applications in esthetics range from procedures such as Botox injections and laser and chemical peels. For rhinoplasty, there has been a simulation model that uses porcine septal cartilage to train residents in the technique of spreader graft placement. They were able to document improvement using the model over time.61 Human cadaver models have also been proved to be reliable and have validated models for use in rhinoplasty technique training.62
VR in esthetic surgery training is limited, but existing models cover the internal and external intricacies of the aging process, in addition to the more complex details of rhytidectomies, such as malar fat pad manipulation and superficial musculoaponeurotic system manipulation.63 Despite the underdevelopment of simulators in esthetic training, this frontier represents the greatest potential benefit in plastic surgery resident training.
LIMITATIONS
Despite its touted benefits, simulation has been slow to pervade plastic surgery training compared to other surgical subspecialties. Though previous studies have attributed this delay to apprehension on behalf of educators and lack of evidence to support improved intraoperative performance, current studies have accredited the delay to time and energy constraints of both faculty and trainees.4,5 Implementation of simulation into training curriculum requires a large time investment, restricted by the already taxing clinical schedules found within plastic surgery.4
In the era of increased time constraints, paperwork, and responsibility, trainees may not have the time for in-person simulation practice. At institutions that currently have dedicated simulation centers, it has been found that these resources are underutilized, contributing to the issues of cost associated with simulators—an additional limitation of simulation.10 Therefore, programs seeking to incorporate simulation into the educational curriculum must not only carve out time for use in already robust clinical training schedule but also allocate budget for the expenses required, which depend on the quantity and type of simulator(s) desired.
A potential response to both time constraints and increased costs has been proposed by vascular surgeons. Dawson et al64 found that region-wide vascular surgery simulation workshops are associated with lower overall cost compared with single-institution simulation laboratories. These types of workshops were later described in microsurgery training to cut costs and provide training outside the operating room and have potential in other subspecialties in plastic surgery.52 Simulation has been consistently seen as a beneficial tool, but its incorporation into training has yet to be widely manifested in plastic surgery due to associated costs and restrained time for incorporation and use. The nationwide resident “boot camps” that are hosted around the country may be an excellent place to start.65
CONCLUSIONS
Though regularly employed in other surgical specialties, simulation remains underutilized in the field of plastic surgery despite its various applications. Simulation serves as a standardized assessment tool, increases surgical knowledge, and enhances surgical judgment and confidence that can translate directly into the operating room. The various simulation modalities make it a flexible tool for use in all subspecialties of plastic surgery. As the number of validated simulators increases and existing simulation practices become more cost-effective, simulation will increasingly complement plastic surgery training program curricula, promoting the continuation of the standard of excellence in this field in a safe and controlled environment.
Footnotes
Published online 17 July 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Okrainec A, Soper NJ, Swanstrom LL, et al. Trends and results of the first 5 years of Fundamentals of Laparoscopic Surgery (FLS) certification testing. Surg Endosc. 2011;25:1192–1198. [DOI] [PubMed] [Google Scholar]
- 2.Kazan R, Cyr S, Hemmerling TM, et al. The evolution of surgical simulation: the current state and future avenues for plastic surgery education. Plast Reconstr Surg. 2017;139:533e–543e. [DOI] [PubMed] [Google Scholar]
- 3.Gardner AK, Scott DJ, Pedowitz RA, et al. Best practices across surgical specialties relating to simulation-based training. Surgery. 2015;158:1395–1402. [DOI] [PubMed] [Google Scholar]
- 4.Thomson JE, Poudrier G, Stranix JT, et al. Current status of simulation training in plastic surgery residency programs: a review. Arch Plast Surg. 2018;45:395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diaz-Siso JR, Plana NM, Stranix JT, et al. Computer simulation and digital resources for plastic surgery psychomotor education. Plast Reconstr Surg. 2016;138:730e–738e. [DOI] [PubMed] [Google Scholar]
- 6.Kneebone RL, Scott W, Darzi A, et al. Simulation and clinical practice: strengthening the relationship. Med Educ. 2004;38:1095–1102. [DOI] [PubMed] [Google Scholar]
- 7.Kapadia MR, DaRosa DA, MacRae HM, et al. Current assessment and future directions of surgical skills laboratories. J Surg Educ. 2007;64:260–265. [DOI] [PubMed] [Google Scholar]
- 8.Pavlidis I, Zavlin D, Khatri AR, et al. Absence of stressful conditions accelerates dexterous skill acquisition in surgery. Sci Rep. 2019;9:1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plana NM, Rifkin WJ, Kantar RS, et al. A prospective, randomized, blinded trial comparing digital simulation to textbook for cleft surgery education. Plast Reconstr Surg. 2019;143:202–209. [DOI] [PubMed] [Google Scholar]
- 10.Gosman A, Mann K, Reid CM, et al. Implementing assessment methods in plastic surgery. Plast Reconstr Surg. 2016;137:617e–623e. [DOI] [PubMed] [Google Scholar]
- 11.McKinnon VE, Kalun P, McRae MH, et al. A shift on the horizon: a systematic review of assessment tools for plastic surgery trainees. Plast Reconstr Surg. 2018;142:217e–231e. [DOI] [PubMed] [Google Scholar]
- 12.Sachdeva AK, Pellegrini CA, Johnson KA. Support for simulation-based surgical education through American College of Surgeons–accredited education institutes. World J Surg. 2008;32:196–207. [DOI] [PubMed] [Google Scholar]
- 13.Rosen JM, Long SA, McGrath DM, et al. Simulation in plastic surgery training and education: the path forward. Plast Reconstr Surg. 2009;123:729–738; discussion 739. [DOI] [PubMed] [Google Scholar]
- 14.Kempton SJ, Bentz ML. Making master surgeons out of trainees: part I. teaching surgical judgment. Plast Reconstr Surg. 2016;137:1646–1653. [DOI] [PubMed] [Google Scholar]
- 15.Sayadi LR, Naides A, Eng M, et al. The new frontier: a review of augmented reality and virtual reality in plastic surgery. Aesthet Surg J. 2019;39:1007–1016. [DOI] [PubMed] [Google Scholar]
- 16.Seymour NE, Gallagher AG, Roman SA, et al. Virtual reality training improves operating room performance results of a randomized, double-blinded study. Ann Surg. 2002;236:458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitsuno D, Ueda K, Hirota Y, et al. Effective application of mixed reality device hololens: simple manual alignment of surgical field and holograms. Plast Reconstr Surg. 2019;143:647–651. [DOI] [PubMed] [Google Scholar]
- 18.Tepper OM, Rudy HL, Lefkowitz A, et al. Mixed reality with HoloLens: where virtual reality meets augmented reality in the operating room. Plast Reconstr Surg. 2017;140:1066–1070. [DOI] [PubMed] [Google Scholar]
- 19.Davis CR, Rosenfield LK. Looking at plastic surgery through Google Glass: part 1. systematic review of Google Glass evidence and the first plastic surgical procedures. Plast Reconstr Surg. 2015;135:918–928. [DOI] [PubMed] [Google Scholar]
- 20.Greenfield MJ, Luck J, Billingsley ML, et al. Demonstration of the effectiveness of augmented reality telesurgery in complex hand reconstruction in Gaza. Plast Reconstr Surg Glob Open. 2018;6:e1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim Y, Kim H, Kim YO. Virtual reality and augmented reality in plastic surgery: a review. Arch Plast Surg. 2017;44:179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanevsky J, Corban J, Gaster R, et al. Big data and machine learning in plastic surgery: a new frontier in surgical innovation. Plast Reconstr Surg. 2016;137:890e–897e. [DOI] [PubMed] [Google Scholar]
- 23.Malic C, Jivan S, Majumder S. A simple model for hand trauma training. J Hand Surg Eur Vol. 2007;32:578–580. [DOI] [PubMed] [Google Scholar]
- 24.Smith AM, Forder JA, Annapureddy SR, et al. The porcine forelimb as a model for human flexor tendon surgery. J Hand Surg Br. 2005;30:307–309. [DOI] [PubMed] [Google Scholar]
- 25.Wright TC, Widdowson D, Khan M, et al. A cost-effective training tool for flexor tendon repair: pig’s trotters. J Plast Reconstr Aesthet Surg. 2006;59:107–108. [DOI] [PubMed] [Google Scholar]
- 26.Kempton SJ, Salyapongse AN, Israel JS, et al. Surgical education module improves operative proficiency in endoscopic carpal tunnel release: a blinded randomized controlled trial of trainees. J Surg Educ. 2018;75:442–449. [DOI] [PubMed] [Google Scholar]
- 27.Garg R, Fung BKK, Chow SP, et al. A reusable model for teaching and practising tendon repair techniques. J Hand Surg Eur Vol. 2007;32:725–726. [DOI] [PubMed] [Google Scholar]
- 28.Hough M, Southern S. Flexor tendon simulator in patient education. Ann Plast Surg. 2002;49:678. [DOI] [PubMed] [Google Scholar]
- 29.Tare M. Dental rolls: a suitable model for practising tendon repair techniques. J Hand Surg Br. 2004;29:506–507. [DOI] [PubMed] [Google Scholar]
- 30.Bari AS, Woon CY, Pridgen B, et al. Overcoming the learning curve: a curriculum-based model for teaching zone II flexor tendon repairs. Plast Reconstr Surg. 2012;130:381–388. [DOI] [PubMed] [Google Scholar]
- 31.Barrie KA, Wolfe SW, Shean C, et al. A biomechanical comparison of multistrand flexor tendon repairs using an in situ testing model. J Hand Surg Am. 2000;25:499–506. [DOI] [PubMed] [Google Scholar]
- 32.Ingraham JM, Weber RA, III, Weber RA. Utilizing a simulated tendon to teach tendon repair technique. Hand (N Y). 2009;4:150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eberlin KR, Ducic I. Surgical algorithm for neuroma management: a changing treatment paradigm. Plast Reconstr Surg Glob Open. 2018;6:e1952–e1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ilie VG, Ilie VI, Dobreanu C, et al. Training of microsurgical skills on nonliving models. Microsurgery. 2008;28:571–577. [DOI] [PubMed] [Google Scholar]
- 35.Gul BU, Yanilmaz DK, Arslan D, et al. Silicone-based simulation models for peripheral nerve microsurgery. J Plast Reconstr Aesthet Surg. 2019;72:477–483. [DOI] [PubMed] [Google Scholar]
- 36.Cote V, Schwartz M, Arbouin Vargas JF, et al. 3-Dimensional printed haptic simulation model to teach incomplete cleft palate surgery in an international setting. Int J Pediatr Otorhinolaryngol. 2018;113:292–297. [DOI] [PubMed] [Google Scholar]
- 37.Iorio ML, Masden D, Blake CA, et al. Presurgical planning and time efficiency in orthognathic surgery: the use of computer-assisted surgical simulation. Plast Reconstr Surg. 2011;128:179e–181e. [DOI] [PubMed] [Google Scholar]
- 38.Cheng H, Podolsky DJ, Fisher DM, et al. Teaching palatoplasty using a high-fidelity cleft palate simulator. Plast Reconstr Surg. 2018;141:91e–98e. [DOI] [PubMed] [Google Scholar]
- 39.Podolsky DJ, Fisher DM, Wong Riff KW, et al. Assessing technical performance and determining the learning curve in cleft palate surgery using a high-fidelity cleft palate simulator. Plast Reconstr Surg. 2018;141:1485–1500. [DOI] [PubMed] [Google Scholar]
- 40.Smith DM, Oliker A, Carter CR, et al. A virtual reality atlas of craniofacial anatomy. Plast Reconstr Surg. 2007;120:1641–1646. [DOI] [PubMed] [Google Scholar]
- 41.Flores RL, Deluccia N, Grayson BH, et al. Creating a virtual surgical atlas of craniofacial procedures: Part I. Three-dimensional digital models of craniofacial deformities. Plast Reconstr Surg. 2010;126:2084–2092. [DOI] [PubMed] [Google Scholar]
- 42.Flores RL, Deluccia N, Oliker A, et al. Creating a virtual surgical atlas of craniofacial procedures: Part II. Surgical animations. Plast Reconstr Surg. 2010;126:2093–2101. [DOI] [PubMed] [Google Scholar]
- 43.Kantar RS, Alfonso AR, Ramly EP, et al. Knowledge and skills acquisition by plastic surgery residents through digital simulation training. Plast Reconstr Surg. 2019;145:184e–192e. [DOI] [PubMed] [Google Scholar]
- 44.Mueller MA, Pourtaheri N, Evans GRD. Microsurgery training resource variation among US integrated plastic surgery residency programs. J Reconstr Microsurg. 2019;35:176–181. [DOI] [PubMed] [Google Scholar]
- 45.Mcgoldrick RB, Davis CR, Paro J, et al. Motion analysis for microsurgical training: objective measures of dexterity, economy of movement, and ability. Plast Reconstr Surg. 2015;136:231e–240e. [DOI] [PubMed] [Google Scholar]
- 46.Loh CYY, Wang AYL, Tiong VTY, et al. Animal models in plastic and reconstructive surgery simulation-a review. J Surg Res. 2018;221:232–245. [DOI] [PubMed] [Google Scholar]
- 47.Zeng W, Shulzhenko NO, Feldman CC, et al. “Blue-Blood”-infused chicken thigh training model for microsurgery and supermicrosurgery. Plast Reconstr Surg Glob Open. 2018;6:e1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zachara M, Drozdowski P, Wysocki M, et al. Anatomical variability of the anterolateral thigh flap perforators between sexes: a cadaveric study. Eur J Plast Surg. 2013;36:179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tinhofer IE, Meng S, Steinbacher J, et al. The surgical anatomy of the vascularized lateral thoracic artery lymph node flap-A cadaver study. J Surg Oncol. 2017;116:1062–1068. [DOI] [PubMed] [Google Scholar]
- 50.Shin KJ, Lee SH, Koh KS, et al. Anatomical consideration for the safe elevation of the deep circumflex iliac artery in flap surgery. Plast Reconstr Surg. 2018;142:193–201. [DOI] [PubMed] [Google Scholar]
- 51.Tanner C, Johnson T, Majors A, et al. The vascularity and osteogenesis of a vascularized flap for the treatment of scaphoid nonunion: the pedicle volar distal radial periosteal flap. Hand. 2018;14:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh M, Ziolkowski N, Ramachandran S, et al. Development of a five-day basic microsurgery simulation training course: a cost analysis. Arch Plast Surg. 2014;41:213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cooper L, Sindali K, Srinivasan K, et al. Developing a three-layered synthetic microsurgical simulation vessel. J Reconstr Microsurg. 2019;35:15–21. [DOI] [PubMed] [Google Scholar]
- 54.Rudarakanchana N, Van Herzeele I, Desender L, et al. Virtual reality simulation for the optimization of endovascular procedures: current perspectives. Vasc Health Risk Manag. 2015;11:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zammit D, Ponnudurai N, Safran T, et al. Reevaluating the current model of rhinoplasty training and future directions: a role for focused, maneuver-specific simulation. Plast Reconstr Surg. 2019;144:597e–605e. [DOI] [PubMed] [Google Scholar]
- 56.Hashem AM, Waltzman JT, D’Souza GF, et al. Resident and program director perceptions of aesthetic training in plastic surgery residency: an update. Aesthet Surg J. 2017;37:837–846. [DOI] [PubMed] [Google Scholar]
- 57.Mitkov MV, Thomas CS, Cochuyt JJ, et al. Simulation: an effective method of teaching cosmetic botulinum toxin injection technique. Aesthet Surg J. 2018;38:NP207–NP212. [DOI] [PubMed] [Google Scholar]
- 58.Jung JS, Kang DH, Lim NK. Face dissection as the plastic surgery approach: effective method in residency training. J Craniofac Surg. 2019;30:e263–e265. [DOI] [PubMed] [Google Scholar]
- 59.Kazan R, Viezel-Mathieu A, Cyr S, et al. The Montreal Augmentation Mammaplasty Operation (MAMO) simulator: an alternative method to train and assess competence in breast augmentation procedures. Aesthet Surg J. 2018;38:835–849. [DOI] [PubMed] [Google Scholar]
- 60.Wang C, Chen L, Mu D, et al. A low-cost simulator for training in endoscopic-assisted transaxillary dual-plane breast augmentation. Ann Plast Surg. 2017;79:525–528. [DOI] [PubMed] [Google Scholar]
- 61.Oh CJ, Tripathi PB, Gu JT, et al. Development and evaluation of rhinoplasty spreader graft suture simulator for novice surgeons. Laryngoscope. 2019;129:344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coan BS, Neff E, Mukundan S, Jr, et al. Validation of a cadaveric model for comprehensive physiologic and anatomic evaluation of rhinoplastic techniques. Plast Reconstr Surg. 2009;124:2107–2117. [DOI] [PubMed] [Google Scholar]
- 63.Smith DM, Aston SJ, Cutting CB, et al. Applications of virtual reality in aesthetic surgery. Plast Reconstr Surg. 2005;116:898–904; discussion 905. [DOI] [PubMed] [Google Scholar]
- 64.Dawson DL, Lee ES, Hedayati N, et al. Four-year experience with a regional program providing simulation-based endovascular training for vascular surgery fellows. J Surg Educ. 2009;66:330–335. [DOI] [PubMed] [Google Scholar]
- 65.American Society of Plastic Surgeons. ACAPS/ASPS Plastic Surgery Boot Camp. Available at https://www.plasticsurgery.org/for-medical-professionals/education-and-resources/events/resident-boot-camp. Accessed February 21, 2020.


