Abstract
Background
Approximately half of the 2.3 million people with multiple sclerosis (PwMS) will fall in any three-month period. Currently clinicians rely on self-report measures or simple functional assessments, administered at discrete time points, to assess fall risk. Wearable inertial sensors are a promising technology for increasing the sensitivity of clinical assessments to accurately predict fall risk, but current accelerometer-based approaches are limited.
Research question
Will metrics derived from wearable accelerometers during a 30-second chair stand test (30CST) correlate with clinical measures of disease severity, balance confidence and fatigue in PwMS, and can these metrics be used to accurately discriminate fallers from non-fallers?
Methods
Thirty-eight PwMS (21 fallers) completed self-report outcome measures then performed the 30CST while triaxial acceleration data were collected from inertial sensors adhered to the thigh and chest. Accelerometer metrics were derived for the sit-to-stand and stand-to-sit transitions and relationships with clinical metrics were assessed. Finally, the metrics were used to develop a logistic regression model to classify fall status.
Results
Accelerometer-derived metrics were significantly associated with multiple clinical metrics that capture disease severity, balance confidence and fatigue. Performance of a logistic regression for classifying fall status was enhanced by including accelerometer features (accuracy 74%, AUC 0.78) compared to the standard of care (accuracy 68%, AUC 0.74) or patient reported outcomes (accuracy 71%, AUC 0.75).
Significance
Accelerometer derived metrics were associated with clinically relevant measures of disease severity, fatigue and balance confidence during a balance challenging task. Inertial sensors could feasibly be utilized to enhance the accuracy of functional assessments to identify fall risk in PwMS. Simplicity of these accelerometer-based metrics could facilitate deployment for community-based monitoring.
Keywords: wearable, accelerometer, chair stand test, multiple sclerosis, falls
Introduction
Multiple sclerosis is a degenerative neurological disease characterized by inflammation, demyelination and axonal loss of the central nervous system that leads to sensorimotor impairments affecting gait and balance. While disability severity (as measured by the Expanded Disability Status Scale) is weighted toward gait impairment in the middle to end range of the scale, even those with low disability levels experience sensorimotor and balance deficits [1]. Approximately two-thirds of PwMS report mobility problems [2]. As a result of these deficits, falls are common with approximately half of the 2.3 million PwMS experiencing a fall in any three-month period [3]. Although contributions to balance impairment and fall risk are multi-factorial, clinicians are limited by the equipment and time available to conduct complex assessments. Thus, they often rely on self-report or standardized functional assessments to screen for fall risk and direct intervention [4–6]. In particular, quick assessments requiring minimal equipment, such as repeated chair stand tests, the Timed 25 Foot Walk (T25W), or the Timed Up and Go (TUG), lend themselves readily to outpatient and community-based settings. While these tests inherently require postural control to maintain balance during transitions or gait and could theoretically provide deeper insight into specific contributions to balance deficits, clinicians are limited to interpreting results based on gross measures of performance such as completion time or number of repetitions.
The repeated sit-to-stand (STS) paradigm in particular has been shown to provide insight into functional strength [7], balance deficits and fall risk for multiple patient populations, (e.g., see including PwMS [8,9]). The STS is a functionally demanding task [10] that elicits muscular fatigue when performed repeatedly [11]. The 30-second chair stand test (30CST)[7] requires the participant to perform repeated STS repetitions for 30-seconds, and the number of repetitions performed serves as the primary outcome measure [12]. The 30CST has been well studied in geriatric populations to establish healthy normative data for community dwellers [13] and to approximate lower extremity strength [12,14]; however, it has not been used to classify fallers nor to characterize balance impairment in PwMS. While lower limb strength plays an important role in generating the propulsive forces required for completing a STS transition, the STS also requires precise coordination of muscle activation patterns of the lower extremity and erector spinae musculature to stabilize the body center of mass as it transitions from forward to upward motion [15]. Thus, successful completion of a STS transition depends on the interplay of lower limb strength and postural control mechanisms [9,16]. Given that fatigue and balance problems are two of the most commonly reported symptoms in PwMS [17,18], this type of assessment seems particularly suited to characterizing balance deficits and fall risk in PwMS.
The utility of wearable sensors has been established for biomechanical and gait analyses and longer-term activity monitoring in research settings for PwMS (see reviews [19,20]). They are portable and inexpensive, and thus have the potential to provide clinicians with detailed performance data. Clinical translation could be enhanced by initial efforts focused on translating research findings into simple metrics that augment assessments and can be used to inform clinical decision making. Establishing correlation between validated clinical tools and accelerometer-based metrics may facilitate clinical adoption. Previous studies have characterized the STS transition using wearable sensors and derived metrics capable of discriminating between groups; however, these studies relied on the gyroscope signal [21–23] which limits long-term deployment to the home because of high power requirements [24]. Additionally, previous studies have not explored the development of a statistical model to identify fall risk based on 30CST performance in PwMS. Thus, the purpose of this study was to derive accelerometer-based metrics from a minimal number of sensors to characterize STS performance in PwMS during the 30CST. Metrics were used to explore (1) if there was agreement with clinical outcome measures of disease severity, balance confidence, and fatigue, (2) if they could be utilized to detect differences between fallers and nonfallers, and (3) if they provided additional information to better inform identification of fall status when compared to manually counted repetitions from the 30CST.
Methods
Thirty-eight PwMS (mean age 50.6 ± 12.1 (Table 1), 21 fallers, inclusion: no major health conditions other than MS, no acute exacerbations within the previous 3-months, ambulatory without assistive devices) were recruited from the neurology department at the University of Vermont Medical Center. The experimental protocol was approved by the University of Vermont IRB and all participants provided informed consent. A neurologist administered the expanded disability status scale (EDSS) to quantify disease severity, and participants were classified as fallers if they had sustained at least one fall in the previous 6-months. Participants completed clinical outcome measures including the Modified Fatigue Impact Scale (MFIS)[25], and the Activities-Specific Balance Confidence scale (ABC)[26]. These were selected because they have previously been related to fall risk [27,28].
Table 1.
Characteristics and results of clinical and accelerometer derived metrics for study participants
| Participant Characteristic / Metric | Cohort (Mean ± SD) | Fallers (Mean ± SD) | Non-Fallers (Mean ± SD) | P | d |
|---|---|---|---|---|---|
| Age (yrs) | 50.6 ± 12.1 | 55.3 ± 8.6 | 44.8 ± 13.5 | 0.006 | 0.87 |
| CLINICAL METRICS | |||||
| EDSS | 2.9 ± 1.3 | 3.4 ± 1.2 | 2.3 ± 1.2 | 0.006 | 0.88 |
| MFIS | 35.6 ± 17.6 | 42.3 ± 14.3 | 27.8 ± 18.2 | 0.01 | 0.82 |
| ABC | 81.9 ± 18.0 | 73.7 ± 19.4 | 91.5 ± 9.2 | 0.001 | 1.00 |
| 30CST (#) | 11.6 ± 3.1 | 10.4 ± 2.7 | 13.1 ± 3.0 | 0.003 | 0.88 |
| T25W (s) | 5.8 ± 1.4 | 6.2 ± 1.5 | 5.2 ± 1.0 | 0.024 | 0.74 |
| TUG (s) | 9.2 ± 2.5 | 10.0 ± 2.3 | 8.3 ± 2.6 | 0.006 | 0.67 |
| ACCELEROMETER DERIVED METRICS | |||||
| Mean si-st time (s) | 1.35 ± 0.33 | 1.49 ± 0.34 | 1.18 ± 0.23 | 0.005 | 0.93 |
| Mean st-si time (s) | 1.38 ± 0.36 | 1.52 ± 0.38 | 1.19 ± 0.24 | 0.004 | 0.91 |
| CV st-si time (s) | 0.06 ± 0.05 | 0.08 ± 0.06 | 0.05 ± 0.02 | 0.001 | 0.63 |
| Mean peak CC si-st (1) accel (G) | 0.17 ± 0.10 | 0.13 ± 0.08 | 0.21 ± 0.10 | 0.007 | 0.85 |
| Mean peak CC st-si (1) accel (G) | 0.11 ± 0.06 | 0.10 ± 0.06 | 0.13 ± 0.06 | 0.035 | 0.68 |
| Mean peak AP st-si (1) accel (G) | 0.18 ± 0.11 | 0.15 ± 0.08 | 0.22 ± 0.13 | 0.037 | 0.67 |
P-values are based on Independent sample T-test (or Wilcoxon Rank Sum Tests when the test for normality was violated) and effect sizes were evaluated using Cohen’s d. Clinical measures include Expanded Disability Status Scale (EDSS), Modified Fatigue Impact Scale (MFIS), Activity Balance Confidence (ABC), 30-Second Chair Stand Test (30CST), Timed 25 Foot Walk (T25W) and the Timed Up and Go (TUG).
Triaxial accelerometer data (sample rate 250 Hz, ±16G) were recorded from inertial sensors (Biostamp, MC10, Inc., Lexington, MA) adhered directly to the skin on the anterior right thigh and chest, which comprised a subset of sensors deployed as part of a larger study. The participants performed a series of functional assessments including one trial each of the 30CST, T25W, and TUG. The 30CST was performed using a standard height (17-inch) chair, and participants were instructed to complete as many sit-to-stand transitions as possible with arms crossed over their chest, as quickly and safely as they felt comfortable. Each participant also performed a 30-second static standing trial with instructions to maintain a tall posture with their feet facing forward.
A fully automated Matlab algorithm (Mathworks, Natick MA) was used to process the raw accelerometer data and derive the metrics of interest. Raw accelerometer data were transformed into anatomically-relevant reference frames using data from the static standing trial and methods adapted from [28]. This was performed by projecting accelerometer data onto anatomical axes using the direction of gravity and the known sensor locations on the anterior aspect of the chest and thigh to resolve the accelerometer signal in a reference frame with axes approximately aligned with the cranial-caudal (CC), anterior-posterior (AP) and medial-lateral (ML) directions (Figure 1a). This convention was maintained throughout the analysis, but it should be noted that while the CC component was aligned with gravity during the standing calibration, the CC component is fixed in the thigh and will thus represent an AP-directed acceleration at the onset of the sit-to-stand transition (i.e. while the thigh is horizontal). Sit-to-stand (si-st) and stand-to-sit (st-si) transition phases (see Figure 1) were determined by locating the stand (solid gray line in Figure 1b) and sit (solid black line in Figure 1b) events during the task. The stand and sit events were identified as the maximum and minimum values, respectively, in the CC component of the thigh accelerometer signal that had been low pass filtered using a 3rd order Butterworth IIR filter with a cutoff frequency equal to the dominant frequency observed during the 30CST (Figure 1b). This filtering approach isolates accelerometer signal content related to the changes in body segment orientation characteristic of the STS task. Sit and stand events were used to compute sit-stand duration (si-st time) and the stand-sit duration (st-si time) for each transition. Chest accelerometer data were low-pass filtered in the same way to isolate the gravitational acceleration of the CC and AP components of the trunk and trunk flexion angles were approximated throughout the task using a method adapted from [29,30]. Briefly, flexion was computed as the arctangent of the ratio of the AP and CC components of the gravitational acceleration.
Figure 1.
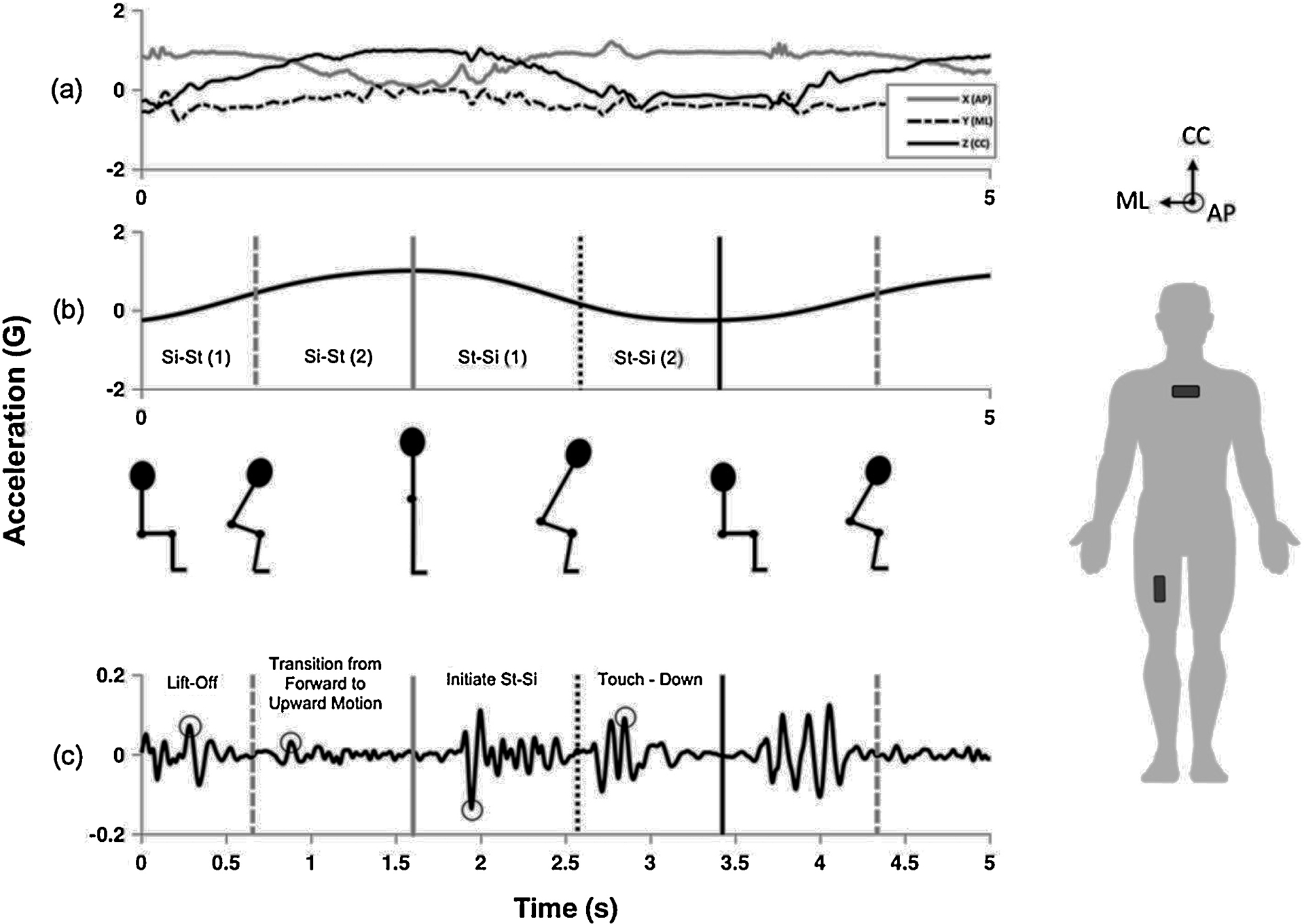
Overview of the algorithm for identifying and extracting accelerometer metrics from a single thigh sensor during a portion of the 30CST. Raw tri-axial accelerometer data (a) was transformed into the anatomically-relevant reference frame (right) using the direction of gravity to align the z-axis (CC component). The CC component of the signal was low pass filtered (b) to identify sit (solid black line), stand (solid gray line), and inflection points (dotted lines) to isolate four phases during the sit-stand-sit transition. Raw accelerometer data were then bandpass filtered (c) and used to extract peaks (indicated by circles) in the identified regions of interest.
The si-st and st-si phases were further delineated (four phases total, Figure 1b) to reflect the change from AP to CC directed movement mid-transition [15], and to isolate the initiation of the si-st transition (lift-off phase, Figure 1c) and the portion of the st-si phase immediately preceding the sit phase during eccentric control, including the final forward lean [31] to touch down (touch-down phase, Figure 1c). As illustrated in Figure 1, these events were located for each transition by identifying the inflection points, computed by taking the maximum and minimum of the derivative of the low pass filtered CC accelerometer signal, which corresponded to the sit-to-stand (gray dashed line) and stand-to-sit (black dotted line) events, respectively. Peak thigh accelerations were extracted in the four phases (absolute maximum) from raw accelerometer signals bandpass filtered using a 3rd-order Butterworth IIR filter with cutoff frequencies of 5 and 20Hz. These cutoffs were selected to limit signal content to a physiologically relevant range while also removing the effects of changes in sensor orientation.
Metrics for characterizing task performance included the number of repetitions (automatically computed from thigh accelerometer data) and the mean and coefficient of variation (CV) of each accelerometer-derived measure extracted from each STS repetition completed during the 30CST by each participant described above. These measures were the si-st time and the st-si time (4 features), peak CC, AP and ML acceleration of the thigh during the four phases (24 features), and peak trunk flexion during the si-st and st-si phases (4 features). Matlab (Mathworks, Natick MA) source code for extracting these metrics is available from https://github.com/M-SenseResearchGroup/30CST.
Independent sample t-tests and Wilcoxon Rank Sum Tests (for non-normal variables determined via Kolmogorov-Smirnov test) were used to evaluate differences between fallers and non-fallers for all clinical outcome measures and accelerometer-derived metrics.
Supervised machine learning was used to train logistic regression models for classifying participant fall status based on accelerometer-derived metrics. Model performance was established using leave-one-subject-out cross validation (LOSO-CV). In this approach data from all but one subject was partitioned into a training set, features that best discriminated between fallers and non-fallers were selected via Davies-Bouldin feature selection, and a model was trained to predict the fall status of the held-out subject. This process was repeated until fall status had been predicted for each subject. To provide context, the performance of the accelerometer-based model was compared to models trained to classify fall status based on clinical features (ABC score, number of 30CST repetitions, TUG time and T25W time). Model performance was primarily assessed by considering the area under the receiver operating characteristic curve (AUC), but model accuracy, sensitivity, and specificity for a posterior probability threshold of 0.5 were also computed.
Finally, Spearman correlations were used to evaluate the relationship between accelerometer derived metrics and clinical measures of disease severity (EDSS), balance confidence (ABC) and fatigue (MFIS). Correlation strength was interpreted as per [32]. Effect size was evaluated for all metrics demonstrating statistically significant differences using the Cohen’s d statistic (d). All statistical analyses were performed in SPSS (SPSS Inc., Chicago, IL).
Results
Fallers and non-fallers demonstrated statistically significant differences in age (p=0.006, d=0.0.87), EDSS (0.006, d=0.88), ABC (p=0.001, d=1.00) and MFIS (p=0.01, d=0.82) (Table 1). We also found a statistically significant difference between fallers and non-fallers for 30CST (p=0.003, d=0.88), T25W (p = 0.024, d=0.74) and TUG (p = 0.006, d=0.67) performance. As for the acceleration derived metrics, we detected differences between the groups for mean si-st time (p=0.005, d=0.93), mean st-si time (p=0.004, d=0.91), CV st-si time (p=0.001, d= 0.63), mean peak CC thigh acceleration during the si-st transition (p=0.007, d=0.85), mean peak CC thigh acceleration during the st-si transition (p=0.035, d=0.68) and mean peak AP thigh acceleration during the st-si transition (p=0.037, d=0.67) (Table 1).
Model performance for a logistic regression model based only on clinical outcome measures was highest for the ABC as the sole model feature (Table 2, Model 1) with an AUC of 0.75 and accuracy of 71%. The functional assessment gross performance measure that best classified falls status was the 30CST number of repetitions, which outperformed both the TUG and T25W (Table 2, Models 2–4). Of the 32 accelerometer derived metrics, three metrics were chosen within each iteration of the LOSO-CV to include in the accelerometer feature only driven model. The accelerometer features that optimized model performance were a combination of acceleration and temporal features with information from both si-st and st-si transition phases (si-st time, st-si time, and mean peak CC si-st (1) accel) and yielded model accuracy of 74% with an AUC of 0.77 (Table 2, Model 5) . When all possible clinical outcome measures and accelerometer metrics were combined, the features selected within the LOSO-CV were the ABC and the same three accelerometer features, resulting in a slight improvement in AUC, but not accuracy (Table 2, Model 6). Model features selected by LOSO-CV were chosen for ≥90% of the LOSO iterations.
Table 2.
Classification performance for logistic regression models comparing model features from clinical outcome measures and accelerometer-derived metrics. Model features were chosen for inclusion based on a leave-one-subject-out cross-validation. All parameters chosen by the leave-one-out cross validation were chosen for at least 90% of the test observations.
| Model | AUC | Accuracy | Sensitity | Specificity | Parameters |
|---|---|---|---|---|---|
| 1 | 0.75 | 0.71 | 0.71 | 0.71 | ABC |
| 2 | 0.74 | 0.68 | 0.81 | 0.53 | 30CST performance (# Reps) |
| 3 | 0.65 | 0.66 | 0.62 | 0.71 | TUG performance (s) |
| 4 | 0.63 | 0.62 | 0.56 | 0.59 | T25W performance (s) |
| 5 | 0.77 | 0.74 | 0.81 | 0.65 | Mean si-st time, mean st-si time, mean peak CC si-st (1) accel |
| 6 | 0.78 | 0.71 | 0.71 | 0.71 | ABC, mean si-st time, mean st-si time, mean peak CC si-st (1) accel |
Multiple accelerometer derived metrics from a single thigh sensor moderately correlated to all three clinical outcome measures: EDSS, ABC and MFIS (Table 3), including number of repetitions performed and all metrics selected for model inclusion through LOSO cross-validation (mean si-st time, mean st-ti time, and mean peak CC si-st (1) accel). All computed metrics demonstrating statistically significant correlations are presented in Table 3.
Table 3.
Statistically significant correlations (r) and the associated p-values (p) between accelerometer derived metrics and clinical measures (non-significant correlations not reported). All metrics were derived from the thigh sensor except for mean st-si trunk flexion, which was derived from the chest sensor.
| Metric | EDSS | MFIS | ABC | |||
|---|---|---|---|---|---|---|
| P | r | P | r | P | r | |
| 30CST Repetitions | p < 0.001 | −0.56 | 0.005 | −0.45 | p < 0.001 | 0.54 |
| SIT-TO-STAND METRICS | ||||||
| Mean si-st time (s) | p < 0.001 | 0.56 | 0.006 | 0.44 | p < 0.001 | −0.54 |
| Mean peak CC si-st (1) accel (G) | 0.002 | −0.50 | p < 0.001 | −0.62 | p < 0.001 | 0.58 |
| Mean peak ML si-st (1) accel (G) | p < 0.001 | −0.58 | 0.001 | −0.52 | p < 0.001 | 0.55 |
| Mean peak ML si-st (2) accel (G) | 0.024 | −0.36 | 0.019 | −0.39 | 0.008 | 0.42 |
| Mean peak CC si-st (2) accel (G) | 0.035 | −0.34 | p < 0.001 | −0.56 | 0.007 | 0.43 |
| STAND-TO-SIT METRICS | ||||||
| Mean st-si time (s) | 0.001 | 0.53 | 0.005 | 0.45 | 0.001 | −0.51 |
| CV st-si time (s) | 0.026 | 0.36 | 0.014 | 0.40 | 0.01 | −0.41 |
| Mean peak CC st-si (1) accel (G) | 0.001 | −0.51 | 0.026 | −0.37 | 0.013 | 0.40 |
| Mean peak ML st-si (1) accel (G) | 0.009 | −0.42 | 0.029 | −0.36 | 0.018 | 0.38 |
| Mean peak AP st-si (1) accel (G) | 0.001 | −0.52 | 0.001 | −0.52 | 0.004 | 0.45 |
Expanded Disability Status Scale (EDSS), Modified Fatigue Impact Scale (MFIS), Activity Balance Confidence (ABC), 30-second chair stand test (30CST), sit-to-stand transition (si-st), stand-to-sit transition (st-si), caudal-cranial (CC), anterior-posterior (AP), medial-lateral (ML), acceleration (accel)
Discussion
Wearable sensors are emerging as a new tool for quantifying biomechanics, especially as they relate to balance and mobility dysfunction in PwMS [19,20,33,34]. Their use to augment functional assessments like the 30CST can provide clinicians with deeper insight into performance [21,22,35]. However, to enable long term deployment in the community setting, there is a need for simple and interpretable metrics that enhance the sensitivity of common clinical tools to detect elevated fall risk and inform intervention strategies. Herein, we characterize the 30CST using a minimal number of wearable sensors and explore associations with balance confidence, fatigue and disease severity in a sample of PwMS. We show that biomechanically-relevant metrics extracted from data recorded by a single wearable accelerometer are correlated with clinically-meaningful measures. Performance of a logistic regression model to classify fallers with only accelerometer-derived metrics was comparable to models with features consisting only of clinical outcome measures and functional assessment gross performance measures (see Table 2, Model 5 AUC, Accuracy and Sensitivity compared to Models 1–4).
We demonstrated that the 30CST outperforms (Table 2, Model 2) other functional assessments (Table 2, Models 3 and 4) in this cohort, suggesting that the 30CST might lend itself to discriminating between fallers and non-fallers in PwMS by exacerbating balance deficits with muscular fatigue, thus impacting performance. Interestingly, the ABC outperforms all functional outcome measures in terms of AUC and accuracy (Table 2, Model 1) for classifying fallers, which is consistent with previous studies [36]; however, patient reported outcome measures may not accurately quantify changes over time due to interventions [37], thus limiting their utility for assessing the effectiveness of interventions to improve balance and decrease fall risk in research studies and in the clinic. Taken together, these findings suggest the 30CST may be especially useful for monitoring balance impairment and fall risk in PwMS.
Generally, greater impairment in all domains (fatigue, balance, disease severity) was reflected in decreased acceleration signatures in all components (AP, CC and ML), and increased time to complete the transitions (see Table 3), suggesting participants adopt a slower [38], more cautious strategy as they fatigue and/or confidence in their balance is diminished. Increased CC acceleration at the thigh, which was anteriorly directed at the onset of the si-st transition, might reflect the importance of knee extensor strength for a successful sit-to-stand transition, which is consistent in other studies of PwMS and total knee arthroplasty [22,39].
The results presented herein demonstrate that a combination of temporal and acceleration-derived features at specific phases of the sit-to-stand and stand-to-sit transitions compare favorably to the current outcome measure of the 30CST for classifying fall status (see Table 2). One key difference in our study was the derivation of features solely based on the accelerometer signal, obviating the need for gyroscopes, which expands the applicability to community and home monitoring. Additionally, these data suggest that a single thigh-worn sensor would be sufficient for analyzing the 30CST. This creates exciting possibilities for clinicians who may want to monitor the effectiveness of an intervention or understand how fluctuating symptoms directly lead to changes in balance and motor control. While counting the number of repetitions is quick and requires minimal equipment, we have shown that including accelerometer features at specific transition phases of interest improves the model’s ability to discriminate fallers from non-fallers (Table 2). Practically, a single accelerometer could be made readily available for quick measurements in clinical settings, or the model could be deployed as a mobile application that leverages the accelerometer inherent to every smart phone. Alternatively, given that adults perform an average of 60 sit-to-stands transitions per day [40], accelerometer metrics could be used to monitor single sit-to-stand repetitions during daily life to inform acute fall risk.
While our study included a relatively small sample size, it was comparable to similar studies that derived metrics from thigh and chest sensors to classify fall status in the elderly [41] and PwMS [22]. Witchel et al [22] analyzed 819 features from 40 participants (17 PwMS) using the gyroscope signal and compared results from PwMS to healthy participants. Our feature set was limited to metrics that could be related to physiological factors associated with performance, and in the future might be informative for directing intervention. Despite our relatively small feature set and generally lower cohort EDSS score, our accelerometer-derived features from a single sensor were discriminatory for classifying falls, which further supports our clinical goal of identifying people at risk for falling and deploying interventions pre-emptively. These promising results point toward the need for future validation of this model using motion capture in a larger sample including greater disability to better understand how it will generalize to new subjects. Future studies will include accelerometer data from unsupervised daily life and longitudinal follow up data to understand if our metrics are sensitive to changes over time and predictive of future falls for PwMS.
Conclusions
We describe a new method for automatically analyzing the performance of the 30CST using data from wearable sensors. Simple accelerometer-based metrics were moderately correlated with clinically validated measures and were better able to differentiate fallers from non-fallers than the standard outcome measure of the 30CST. These results motivate future research investigating the use of this technology for quantifying fall risk in PwMS.
Highlights.
Open-source, automated analysis of 30-second chair stand test for PwMS
Wearable sensor metrics correlate with EDSS, balance confidence and fatigue
Wearable sensor metrics differ significantly between fallers and non-fallers
Fall status classification enhanced by inclusion of wearable sensor metrics
Acknowledgements:
We gratefully acknowledge UVM Medical Center and UVM College of Engineering and Mathematical Sciences for funding to support this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Solomon, AJ: reports consulting for EMD Serono, Biogen, and Alexion. Research funding from Biogen. McGinnis, RS: reports stock ownership for MC10, Inc. and Impellia, Inc., consulting for HX Innovations Inc. and University of Washington, research funding from MC10, Inc. and Epicore Biosystems, Inc.
References
- [1].Martin CL, Phillips BA, Kilpatrick TJ, Butzkueven H, Tubridy N, Mcdonald E, Galea MP, Gait and balance impairment in early multiple sclerosis in the absence of clinical disability, Mult. Scler. J 12 (2006) 620–628. [DOI] [PubMed] [Google Scholar]
- [2].Zwibel HL, Contribution of impaired mobility and general symptoms to the burden of multiple sclerosis, Adv. Ther 26 (2009) 1043–1057. [DOI] [PubMed] [Google Scholar]
- [3].Coote S, Hogan N, Franklin S, Falls in people with multiple sclerosis who use a walking aid: prevalence, factors, and effect of strength and balance interventions, Arch. Phys. Med. Rehabil 94 (2013) 616–621. [DOI] [PubMed] [Google Scholar]
- [4].Phelan EA, Mahoney JE, Voit JC, Stevens JA, Assessment and management of fall risk in primary care settings, Med. Clin 99 (2015) 281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Algorithm for Fall Risk Screening, Assessment, and Intervention, (n.d.) https://www.cdc.gov/steadi/pdf/STEADI-Algorithm-508.pdf (accessed September 9, 2019).
- [6].Fabre JM, Ellis R, Kosma M, Wood RH, Falls Risk Factors and a Compendium of Falls Risk Screening Instruments, J. Geriatr. Phys. Ther 33 (2010) 184 10.1097/JPT.0b013e3181ff2a24. [DOI] [PubMed] [Google Scholar]
- [7].Jones CJ, Rikli RE, Beam WC, A 30-s chair-stand test as a measure of lower body strength in community-residing older adults, Res. Q. Exerc. Sport 70 (1999) 113–119. [DOI] [PubMed] [Google Scholar]
- [8].Nilsagård Y, Andreasson M, Carling A, Vesterlin H, Examining the validity and sensitivity to change of the 5 and 10 sit- to- stand tests in people with multiple sclerosis, Physiother. Res. Int 22 (2017) e1681. [DOI] [PubMed] [Google Scholar]
- [9].Møller AB, Bibby BM, Skjerbӕk AG, Jensen E, Sørensen H, Stenager E, Dalgas U, Validity and variability of the 5-repetition sit-to-stand test in patients with multiple sclerosis, Disabil. Rehabil 34 (2012) 2251–2258. [DOI] [PubMed] [Google Scholar]
- [10].Kralj A, Jaeger RJ, Munih M, Analysis of standing up and sitting down in humans: Definitions and normative data presentation, J. Biomech 23 (1990) 1123–1138. 10.1016/0021-9290(90)90005-N. [DOI] [PubMed] [Google Scholar]
- [11].Roldán-Jiménez C, Bennett P, Cuesta-Vargas AI, Muscular Activity and Fatigue in Lower-Limb and Trunk Muscles during Different Sit-To-Stand Tests, PLOS ONE. 10 (2015) e0141675 10.1371/journal.pone.0141675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].McCarthy EK, Horvat MA, Holtsberg PA, Wisenbaker JM, Repeated chair stands as a measure of lower limb strength in sexagenarian women, J. Gerontol. A. Biol. Sci. Med. Sci 59 (2004) 1207–1212. [DOI] [PubMed] [Google Scholar]
- [13].Rikli RE, Jones CJ, Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years, The Gerontologist. 53 (2013) 255–267. [DOI] [PubMed] [Google Scholar]
- [14].Jones CJ, Rikli RE, Beam WC, A 30-s Chair-Stand Test as a Measure of Lower Body Strength in Community-Residing Older Adults, Res. Q. Exerc. Sport 70 (1999) 113–119. 10.1080/02701367.1999.10608028. [DOI] [PubMed] [Google Scholar]
- [15].Millington PJ, Myklebust BM, Shambes GM, Biomechanical analysis of the sit-to-stand motion in elderly persons, Arch. Phys. Med. Rehabil 73 (1992) 609–617. 10.5555/uri:pii:000399939290124F. [DOI] [PubMed] [Google Scholar]
- [16].Lord SR, Murray SM, Chapman K, Munro B, Tiedemann A, Sit-to-Stand Performance Depends on Sensation, Speed, Balance, and Psychological Status in Addition to Strength in Older People, J. Gerontol. Ser. A 57 (2002) M539–M543. 10.1093/gerona/57.8.M539. [DOI] [PubMed] [Google Scholar]
- [17].Freal JE, Kraft GH, Coryell JK, Symptomatic fatigue in multiple sclerosis., Arch. Phys. Med. Rehabil 65 (1984) 135–138. [PubMed] [Google Scholar]
- [18].Bakshi R, Fatigue associated with multiple sclerosis: diagnosis, impact and management, Mult. Scler. J 9 (2003) 219–227. [DOI] [PubMed] [Google Scholar]
- [19].Frechette ML, Meyer BM, Tulipani LJ, Gurchiek RD, McGinnis RS, Sosnoff JJ, Next Steps in Wearable Technology and Community Ambulation in Multiple Sclerosis, Curr. Neurol. Neurosci. Rep 19 (2019) 80 10.1007/s11910-019-0997-9. [DOI] [PubMed] [Google Scholar]
- [20].Sun R, McGinnis R, Sosnoff JJ, Novel technology for mobility and balance tracking in patients with multiple sclerosis: a systematic review, Expert Rev. Neurother 18 (2018) 887–898. [DOI] [PubMed] [Google Scholar]
- [21].Millor Muruzábal N, Lecumberri Villamediana P, Gómez Fernández M, Martínez Ramírez A, Izquierdo Redín M, An evaluation of the 30-s chair stand test in older adults: frailty detection based on kinematic parameters from a single inertial unit, J. NeuroEngineering Rehabil 2013 10 86. (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Witchel HJ, Oberndorfer C, Needham R, Healy A, Westling CE, Guppy JH, Bush J, Barth J, Herberz C, Roggen D, Thigh-derived inertial sensor metrics to assess the sit-to-stand and stand-to-sit transitions in the timed up and go (TUG) task for quantifying mobility impairment in multiple sclerosis, Front. Neurol 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Van Lummel RC, Ainsworth E, Lindemann U, Zijlstra W, Chiari L, Van Campen P, Hausdorff JM, Automated approach for quantifying the repeated sit-to-stand using one body fixed sensor in young and older adults, Gait Posture. 38 (2013) 153–156. 10.1016/j.gaitpost.2012.10.008. [DOI] [PubMed] [Google Scholar]
- [24].Liu Q, Williamson J, Li K, Mohrman W, Lv Q, Dick RP, Shang L, Gazelle: Energy-Efficient Wearable Analysis for Running, IEEE Trans. Mob. Comput 16 (2017) 2531–2544. 10.1109/TMC.2016.2623304. [DOI] [Google Scholar]
- [25].Modified Fatigue Impact Scale (MFIS), Natl. Mult. Scler. Soc. (n.d.) http://www.nationalmssociety.org/For-Professionals/Researchers/Resources-for-Researchers/Clinical-Study-Measures/Modified-Fatigue-Impact-Scale-(MFIS) (accessed October 28, 2019).
- [26].Powell LE, Myers AM, The Activities-specific Balance Confidence (ABC) Scale, J. Gerontol. Ser. A 50A (1995) M28–M34. 10.1093/gerona/50A.1.M28. [DOI] [PubMed] [Google Scholar]
- [27].Dibble LE, Lopez-Lennon C, Lake W, Hoffmeister C, Gappmaier E, Utility of Disease-Specific Measures and Clinical Balance Tests in Prediction of Falls in Persons With Multiple Sclerosis:, J. Neurol. Phys. Ther 37 (2013) 99–104. 10.1097/NPT.0b013e3182a18460. [DOI] [PubMed] [Google Scholar]
- [28].Tajali S, Shaterzadeh-Yazdi M-J, Negahban H, van Dieën JH, Mehravar M, Majdinasab N, Saki-Malehi A, Mofateh R, Predicting falls among patients with multiple sclerosis: Comparison of patient-reported outcomes and performance-based measures of lower extremity functions, Mult. Scler. Relat. Disord 17 (2017) 69–74. [DOI] [PubMed] [Google Scholar]
- [29].McGinnis RS, Patel S, Silva I, Mahadevan N, DiCristofaro S, Jortberg E, Ceruolo M, Aranyosi AJ, Skin mounted accelerometer system for measuring knee range of motion, in: 2016 38th Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. EMBC, 2016: pp. 5298–5302. 10.1109/EMBC.2016.7591923. [DOI] [PubMed] [Google Scholar]
- [30].Chapman RM, Moschetti WE, Van Citters DW, Stance and swing phase knee flexion recover at different rates following total knee arthroplasty: An inertial measurement unit study, J. Biomech 84 (2019) 129–137. 10.1016/j.jbiomech.2018.12.027. [DOI] [PubMed] [Google Scholar]
- [31].Kerr KM, White JA, Barr DA, Mollan RAB, Analysis of the sit-stand-sit movement cycle in normal subjects, Clin. Biomech 12 (1997) 236–245. [DOI] [PubMed] [Google Scholar]
- [32].Taylor R, Interpretation of the Correlation Coefficient: A Basic Review, J. Diagn. Med. Sonogr 6 (1990) 35–39. 10.1177/875647939000600106. [DOI] [Google Scholar]
- [33].Moon Y, McGinnis RS, Seagers K, Motl RW, Sheth N, Wright JA, Ghaffari R, Sosnoff JJ, Monitoring gait in multiple sclerosis with novel wearable motion sensors, PLOS ONE. 12 (2017) e0171346 10.1371/journal.pone.0171346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sun R, Moon Y, McGinnis RS, Seagers K, Motl RW, Sheth N, Wright JA, Ghaffari R, Patel S, Sosnoff JJ, Assessment of Postural Sway in Individuals with Multiple Sclerosis Using a Novel Wearable Inertial Sensor, Digit. Biomark 2 (2018) 1–10. 10.1159/000485958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Greene BR, Healy M, Rutledge S, Caulfield B, Tubridy N, Quantitative assessment of multiple sclerosis using inertial sensors and the TUG test, in: 2014 36th Annu. Int. Conf. IEEE Eng. Med. Biol. Soc, 2014: pp. 2977–2980. 10.1109/EMBC.2014.6944248. [DOI] [PubMed] [Google Scholar]
- [36].Cattaneo D, Regola A, Meotti M, Validity of six balance disorders scales in persons with multiple sclerosis, Disabil. Rehabil 28 (2006) 789–795. 10.1080/09638280500404289. [DOI] [PubMed] [Google Scholar]
- [37].Nilsagård YE, von Koch LK, Nilsson M, Forsberg AS, Balance Exercise Program Reduced Falls in People With Multiple Sclerosis: A Single-Group, Pretest-Posttest Trial, Arch. Phys. Med. Rehabil 95 (2014) 2428–2434. 10.1016/j.apmr.2014.06.016. [DOI] [PubMed] [Google Scholar]
- [38].Bowser B, O’rourke S, Brown CN, White L, Simpson KJ, Sit-to-stand biomechanics of individuals with multiple sclerosis, Clin. Biomech 30 (2015) 788–794. [DOI] [PubMed] [Google Scholar]
- [39].Boonstra MC, Schwering PJA, De Waal Malefijt MC, Verdonschot N, Sit-to-Stand Movement as a Performance-Based Measure for Patients With Total Knee Arthroplasty, Phys. Ther 90 (2010) 149–156. 10.2522/ptj.20090119. [DOI] [PubMed] [Google Scholar]
- [40].Dall PM, Kerr A, Frequency of the sit to stand task: An observational study of free-living adults, Appl. Ergon 41 (2010) 58–61. 10.1016/j.apergo.2009.04.005. [DOI] [PubMed] [Google Scholar]
- [41].Doheny EP, Walsh C, Foran T, Greene BR, Fan CW, Cunningham C, Kenny RA, Falls classification using tri-axial accelerometers during the five-times-sit-to-stand test, Gait Posture. 38 (2013) 1021–1025. 10.1016/j.gaitpost.2013.05.013. [DOI] [PubMed] [Google Scholar]


