Abstract
Abstract
The current outbreak of the highly transmittable and life-threatening severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has evolved rapidly and posed a global health emergency. Many clinical trials are now being conducted to test possible therapies. To assist this, virtual screening via molecular docking was performed on several FDA-approved drugs, previously used in epidemics, and the top ten compounds were selected. These ten well-characterized drugs, previously used to treat malaria and Ebola infections, were screened based on their interactions with the SARS-CoV-2 ACE2 receptor and 3C-like protease. Compared to the other nine medicines, brincidofovir, an ether lipid ester analog of cidofovir with potent antiviral activity, showed the highest docking scores and binding interactions. Therefore, brincidofovir is worth further investigations and clinical trials as a possible therapeutic agent for the COVID-19 disease caused by the novel SARS-CoV-2.
Graphic abstract
Electronic supplementary material
The online version of this article (10.1007/s13721-020-00263-6) contains supplementary material, which is available to authorized users.
Keywords: Novel coronavirus, SARS-CoV-2, Molecular docking, ACE2 receptor, 3C-like protease, Brincidofovir, COVID-19
Introduction
Humankind has previously witnessed the outbreak of many life-threatening pathogens including Ebola, Zika, the Middle East respiratory syndrome (MERS) coronavirus, severe acute respiratory syndrome (SARS) coronavirus and, nowadays, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Memish et al. 2013; Organization; Sikka et al. 2016; Stawicki et al. 2016; Su et al. 2016; Xiong et al. 2020). The novel coronavirus has initially spread from China and propagated rapidly throughout the globe and has received worldwide attention due to its alarming levels of transmission and aggressive behavior in causing acute respiratory disease. The virus was then officially declared pandemic as a public health emergency of international concern by the World Health Organization (WHO). Researchers throughout the globe are working around the clock to develop potential vaccines and drugs to fight the new coronavirus, SARS-CoV-2, the causative agent of the COVID-19 disease. However, developing a new drug or vaccine usually takes a long time as it should be intensively tested and confirmed to be safe through clinical trials before they can be approved for human use (Control and Prevention 2014; GSK files for approval of world’s first malaria vaccine 2014). Therefore, repurposing FDA-approved drugs seems to be a quicker way to treat patients who otherwise have no option. The SARS-CoV-2 is a single-stranded positive-sense RNA virus that relies on its spike (S) protein to attach and enter the target cells (Chiang 2020; Hoffmann et al. 2020; Wu et al. 2020). The virus S protein binds to the host cell angiotensin-converting enzyme 2 (ACE2) receptor, allowing the virus particles to enter the cells (Chiang 2020; Hoffmann et al. 2020; Wan et al. 2020). Thus, blocking the ACE2 receptor reveals a potential therapeutic target for drug discovery to prevent SARS-CoV-2 transmissibility. Besides, two coronavirus proteases, designated 3-chymotrypsin-like protease (3CLpro) and a papain-like protease (PLpro), were previously considered vital targets to combat the SARS and MERS coronavirus epidemics (Wu et al. 2020). These two proteases were shown to be highly conserved with the novel SARS-CoV-2, especially in the functional regions (Wu et al. 2020). Viruses use their proteases to break down their viral peptides into functional units essential for replication and packaging inside the host cells, and thus considered antiviral drug targets. Molecular docking is a popular bioinformatic modeling tool broadly used in structure-based drug design (Kitchen et al. 2004; Mubarak et al. 2020; Naik 2020; Naik and Pardasani 2017, 2018; Naik and Zu 2020). It is an efficient way to predict the type of interaction, binding affinity and the appropriate target-binding sites between the drug and corresponding receptor using, for instance, scoring functions (Kitchen et al. 2004; Lengauer and Rarey 1996). Elucidating the binding behavior plays an important role in the rational design of drugs as well as to explicate fundamental biochemical processes (Kitchen et al. 2004; Lengauer and Rarey 1996). In this study, molecular docking was performed on dozens of FDA-approved drugs and the top ten hits, previously used in the treatment of malarial, fungal/bacterial and Ebola infections and FDA-approved/fast-tracked for human treatment, were selected. For the selected drugs used in this study, the MOE modeling program was used to predict the binding sites and their docking score.
Materials and methods
Molecular docking method
Software and machinery used
All docking studies were performed and characterized by the MOE "Molecular Operating Environment" program. Drug preparation was compiled through ChemDraw, 3D structures were constructed using Chem 3D ultra 12.0 software (Molecular Modeling and Analysis; Cambridge Soft Corporation), then they were energetically minimized using MOPAC and saved as MDL MolFile (*.mol). All calculations were carried out on an Intel(R) Core(TM)i7, 3.8 GHz-based machine running MS Windows 10 as the operating system(Mubarak et al. 2020; Naik 2020; Naik and Pardasani 2017, 2018; Naik and Zu 2020).
SARS-CoV-2 protease and receptor structure
Generation of the protein structures and the crystal structure of the new COVID-19 protease (PDB code = 1Q2W) and ACE2 receptor (PDB code = 6M0J) were retrieved from the Protein Data Bank (http://www.rcsb.org/pdb/welcome.do) (Bonanno et al. 2003). All bound waters, ligands and cofactors were removed from the proteins and then we added hydrogen atoms for optimization.
Molecular docking procedure
The docking protocol was done against the SARS-CoV-2 ACE2 receptor (PDB code = 6M0J), the SARS-CoV-2 3CL protease (PDB code = 1Q2W) and its four active sites. The active sites were isolated and used as dummy atoms. The parameters and charges were assigned with the MMFF94x force field. After alpha-site spheres were generated using the site finder module of MOE, the structural model of the compounds were docked on the surface of the interior of the receptor using the DOCK module of MOE (Abdel-Rhman et al. 2019). The Dock scoring in MOE software was done through the London dG scoring function and has been upgraded by two unrelated refinement methods. Auto rotatable bonds were allowed, and the best five binding poses were directed for analysis to achieve the best score. We used the database browsers to compare the docking poses to the ligand in the co-crystallized structure and to obtain RMSD of the docking pose. To rank the binding affinity of the synthesized compounds to the protein molecule, the binding free energy and hydrogen bonds between the compounds and amino acid in the receptor were calculated (Abdellattif et al. 2018; Ketan et al. (2020). Evaluation of the hydrogen bonds was done by measuring the hydrogen bond length, which does not exceed 3.5 A°. Also, the RMSD of the drug position compared to the docking pose was used in the ranking. RMSD, as well as the mode of interaction of the native ligand within the structure of the receptor, was used as a standard docked model (Abdel-Rhman et al. 2019; Abdellattif et al. 2018; Althagafi et al. 2019; Mashat et al. 2019; Ketan et al. 2020).
Results and discussions
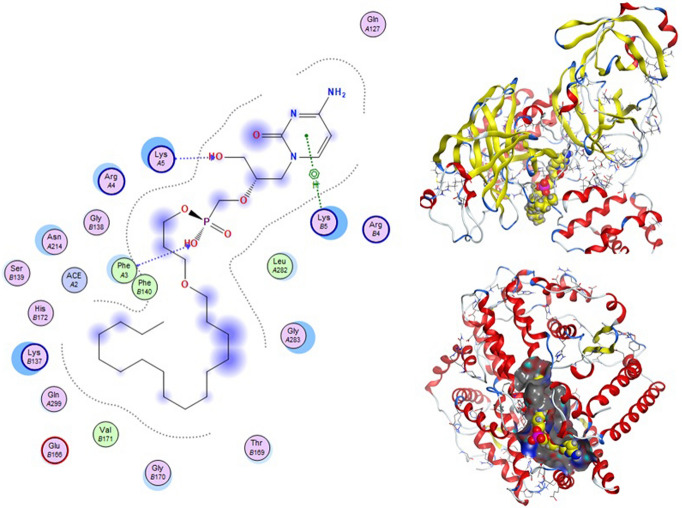
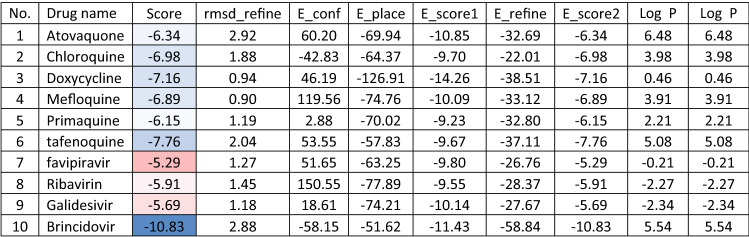
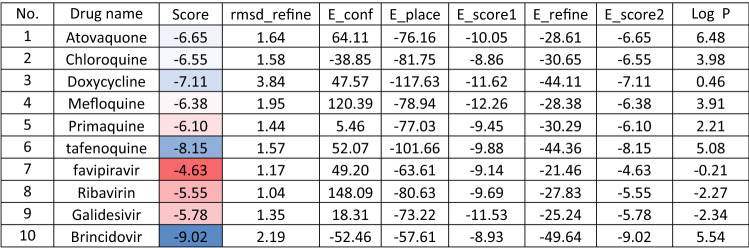
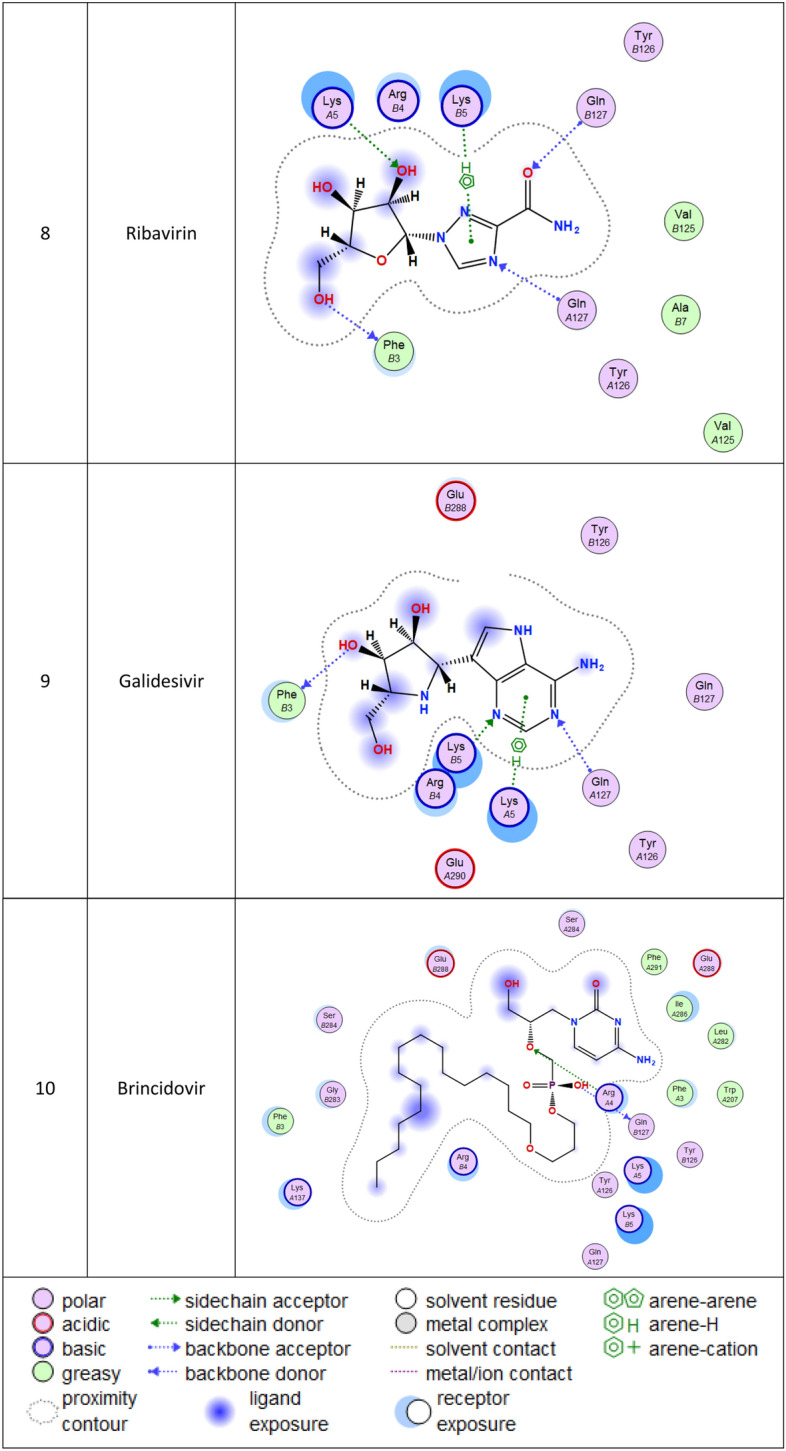
Molecular docking and other computer-related methods are efficient tools broadly used to understand the molecular aspects of protein–ligand interactions during drug discovery against many of previous emerging and fatal diseases including SARS coronavirus (Kitchen et al. 2004; Lengauer and Rarey 1996). In this study, virtual screening of several FDA-approved/fast-tracked drugs was performed against the SARS-CoV-2 ACE2 host receptor (PDB code = 6M0J), the SARS-CoV-2 3CL protease (PDB code = 1Q2W) and its four active sites, to find the most predicated drug–ligand interactions. The presented parameters include the docking scores, ligand binding efficiency and hydrogen bonding interactions. The top ten ranked compounds were selected and are presented in Tables 1, 2, 3, 4, 5 and 6 and Figs. 1, 2, 3, 4 and 5. These ten drugs include four antivirals (favipiravir, ribavirin, brincidofovir, and galidesivir), four antimalarial (chloroquine, mefloquine, primaquine, and tafenoquine) and two antimicrobial agents (doxycycline and atovaquone). Whether we docked against the ACE2 receptor (PDB code = 6M0J), the SARS-CoV-2 3CL protease (PDB code = 1Q2W) or the four main active sites within the SARS-CoV-2 3CL protease, the docking scores of)brincidofovir or BCV) were shown to be the top hit (ranked #1) compared to the other nine drugs. The docking scores for the BCV were − 10.83, − 8.30 and − 9.02 toward the SARS-CoV-2 3CL protease active site 1 (PDB code = 1Q2W), the SARS-CoV-2 3CL whole protease (PDB code = 1Q2W) (Tables 1 and 2 and Figs. 1, 2) and the ACE2 receptor (PDB code = 6M0J) (Tables 3 and 4 and Figs. 3 and 4), respectively. The antimalarial drug tafenoquine comes second in the rank where it scored − 8.15 and − 7.76 with the AC2 receptor and the SARS-CoV-2 3CL protease active site 1, respectively (Tables 2 and 4).
Table 1.
Docking score and energy of the malaria and Ebola drugs and 1Q2W of COVID-19 with site 1 of COVID-19 protease (PDB code = 1Q2W)
Table 2.
Interaction between malaria and Ebola drugs and 1Q2W of COVID-19 with site 1 of COVID-19 protease (PDB code = 1Q2W)
| Drug name | Ligand | Receptor | Interaction | Distance | E (kcal/mol) |
|---|---|---|---|---|---|
| Atovaquone | 6-ring | CD PRO 122 (B) | pi-H | 4.11 | − 0.5 |
| Chloroquine | O 5 | NZ LYS 5 (A) | H-acceptor | 3.34 | − 0.9 |
| 6-ring | CB LYS 137 (A) | pi-H | 4.16 | − 0.6 | |
| 6-ring | CA GLY 2 (B) | pi-H | 3.49 | − 0.5 | |
| Doxycycline | N 6 | N GLN 127 (A) | H-acceptor | 3.27 | − 3.2 |
| Mefloquine | O 41 | OG1 THR 285 (A) | H-donor | 3.09 | − 0.9 |
| Primaquine | F 1 | N GLN 127 (B) | H-acceptor | 3.05 | − 0.6 |
| 6-ring | CG LYS 5 (B) | pi-H | 3.72 | − 0.8 | |
| Tafenoquine | N 27 | NH1 ARG 4 (B) | H-acceptor | 3.58 | − 1.6 |
| 6-ring | CD LYS 5 (A) | pi-H | 4.49 | − 0.7 | |
| Favipiravir | N 13 | O LYS 5 (A) | H-donor | 3.16 | − 1.6 |
| N 9 | N GLN 127 (B) | H-acceptor | 3.32 | − 2.3 | |
| Ribavirin | O 1 | O PHE 3 (B) | H-donor | 2.98 | − 0.8 |
| O 15 | NZ LYS 5 (A) | H-acceptor | 3.26 | − 1.2 | |
| O 26 | N GLN 127 (B) | H-acceptor | 3.14 | − 3.2 | |
| N 27 | N GLN 127 (A) | H-acceptor | 3.32 | − 2.1 | |
| 5-ring | CB LYS 5 (B) | pi-H | 3.99 | − 0.7 | |
| Galidesivir | O 33 | O PHE 3 (B) | H-donor | 3.00 | − 1.2 |
| N 9 | NH1 ARG 4 (B) | H-acceptor | 3.25 | − 4.0 | |
| N 12 | N GLN 127 (A) | H-acceptor | 3.59 | − 1.0 | |
| 6-ring | CD LYS 5 (A) | pi-H | 4.39 | − 0.7 | |
| Brincidovir | O 63 | O GLN 127 (B) | H-donor | 3.02 | − 2.9 |
| O 68 | NH1 ARG 4 (A) | H-acceptor | 2.95 | − 2.4 |
Table 3.
Docking score and energy of the malaria and Ebola drugs with ACE-2 receptor (PDB code = 6M0J)
Table 4.
Interaction between malaria and Ebola drugs with ACE-2 receptor (PDB code = 6M0J)
| Drug | Ligand | Receptor | Interaction | Distance | E (kcal/mol) |
|---|---|---|---|---|---|
| Atovaquone | 6-ring | CA VAL 209 (A) | pi-H | 3.90 | − 1.0 |
| Chloroquine | N 17 | O GLU 208 (A) | H-donor | 3.16 | − 0.6 |
| CL 1 | NZ LYS 94 (A) | H-acceptor | 3.45 | − 0.9 | |
| 6-ring | CA VAL 209 (A) | pi-H | 4.40 | − 0.5 | |
| 6-ring | CG1 VAL 209 (A) | pi-H | 4.14 | − 0.6 | |
| 6-ring | N ASN 210 (A) | pi-H | 3.62 | − 0.6 | |
| Doxycycline | O 24 | OE1 GLU 208 (A) | H-donor | 3.01 | − 1.8 |
| 6-ring | CG2 VAL 209 (A) | pi-H | 4.28 | − 0.7 | |
| Mefloquine | N 29 | O ASN 210 (A) | H-donor | 2.91 | − 0.7 |
| N 29 | N ASN 210 (A) | H-acceptor | 3.33 | − 0.5 | |
| 6-ring | CB GLU 208 (A) | pi-H | 4.42 | − 0.5 | |
| 6-ring | CG2 VAL 209 (A) | pi-H | 4.46 | − 0.6 | |
| Primaquine | 6-ring | CG1 VAL 209 (A) | pi-H | 4.24 | − 0.7 |
| 6-ring | CG1 VAL 209 (A) | pi-H | 4.52 | − 0.7 | |
| Tafenoquine | No measured interaction | ||||
| Favipiravir | O 12 | NZ LYS 94 (A) | H-acceptor | 3.12 | − 3.5 |
| Ribavirin | O 15 | NZ LYS 562 (A) | H-acceptor | 3.03 | − 3.6 |
| Galidesivir | N 14 | O ASN 210 (A) | H-donor | 3.05 | − 1.0 |
| O 29 | CE LYS 562 (A) | H-acceptor | 3.16 | − 0.7 | |
| 5-ring | CA VAL 209 (A) | pi-H | 3.79 | − 2.1 | |
| 6-ring | CA VAL 209 (A) | pi-H | 4.40 | − 0.5 | |
| 5-ring | N ASN 210 (A) | pi-H | 4.25 | − 2.7 | |
| 6-ring | ND2 ASN 210 (A) | pi-H | 4.58 | − 1.3 | |
| Brincidovir | O 63 | OE2 GLU 208 (A) | H-donor | 2.79 | − 6.4 |
| O 74 | NE2 GLN 98 (A) | H-acceptor | 3.01 | − 1.2 | |
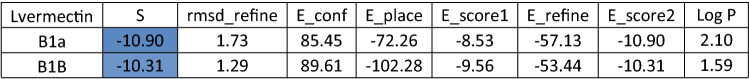
Table 5.
Docking score and energy of ivermectin drug and 1Q2W of COVID-19 with site 1 of COVID-19 protease (PDB code = 1Q2W)
Table 6.
Docking score and energy of ivermectin drug with ACE-2 receptor (PDB code = 6M0J)
Fig. 1.
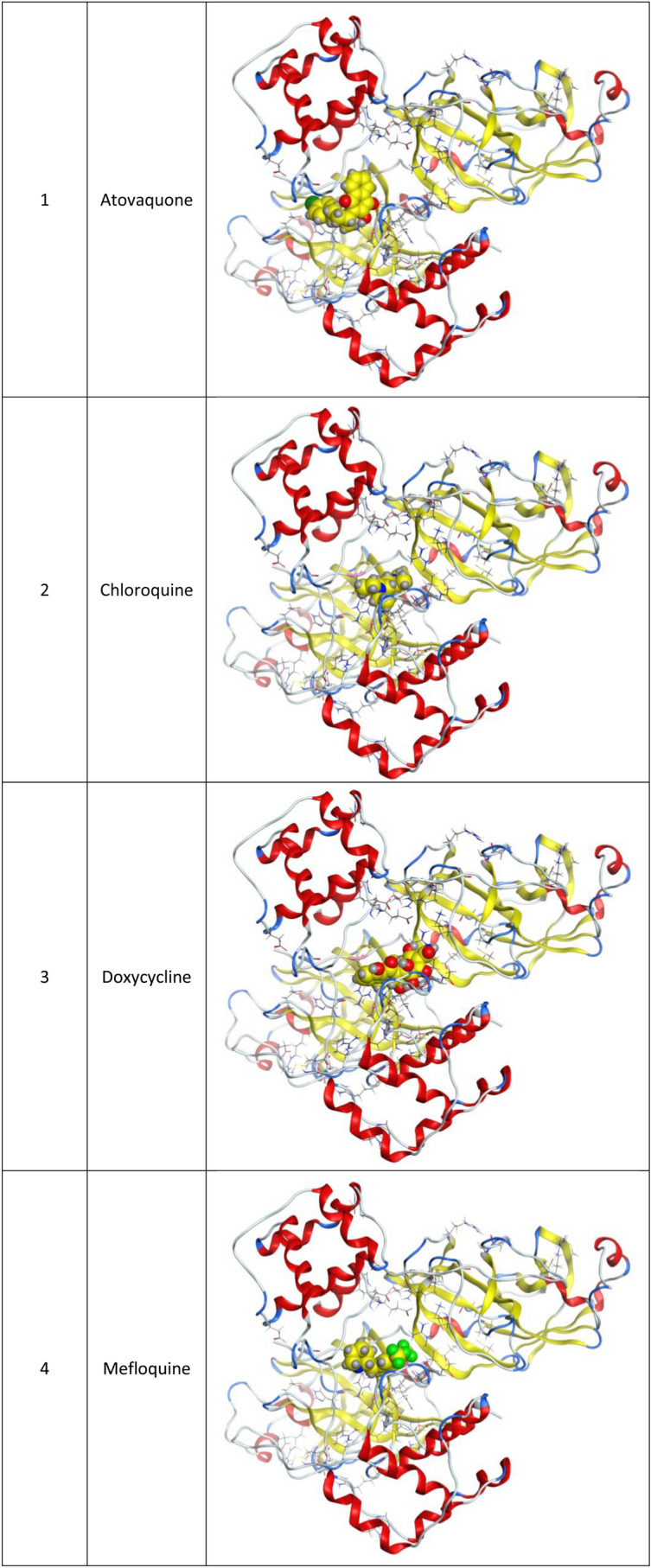
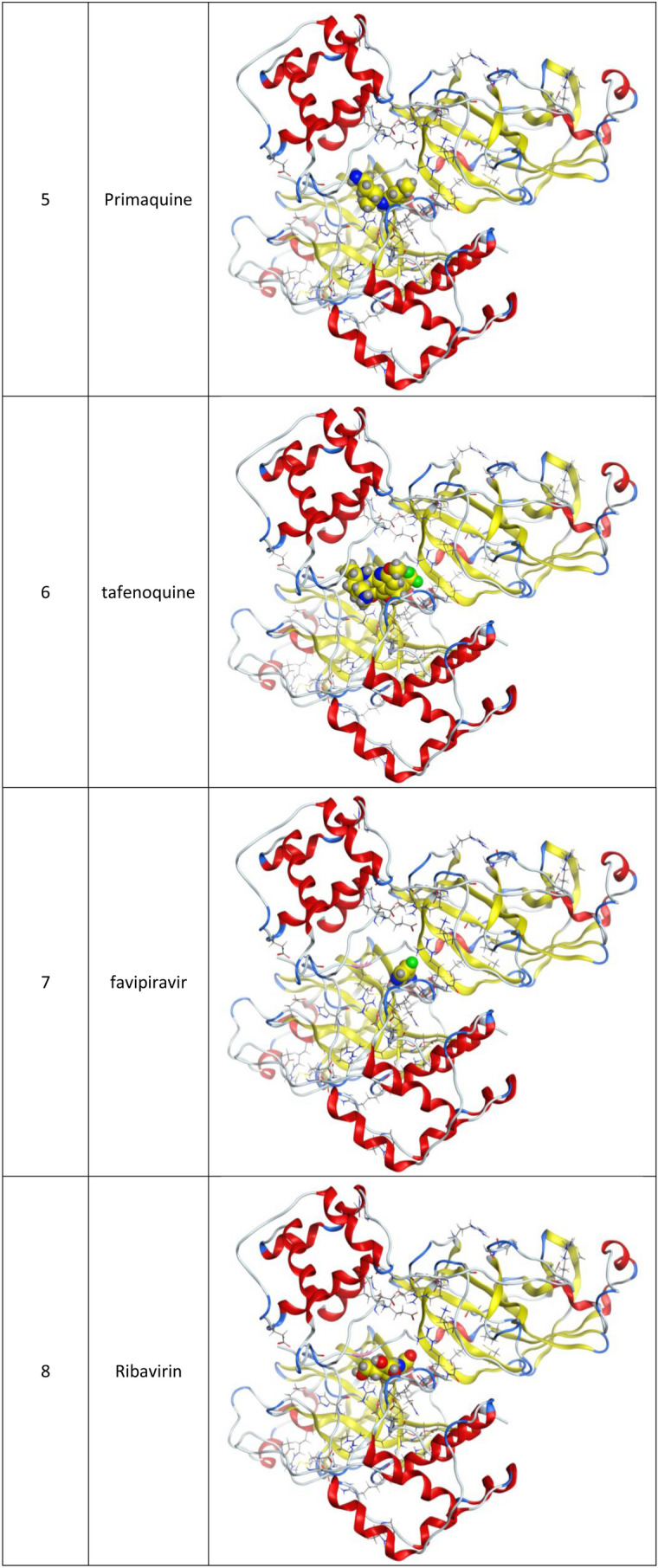
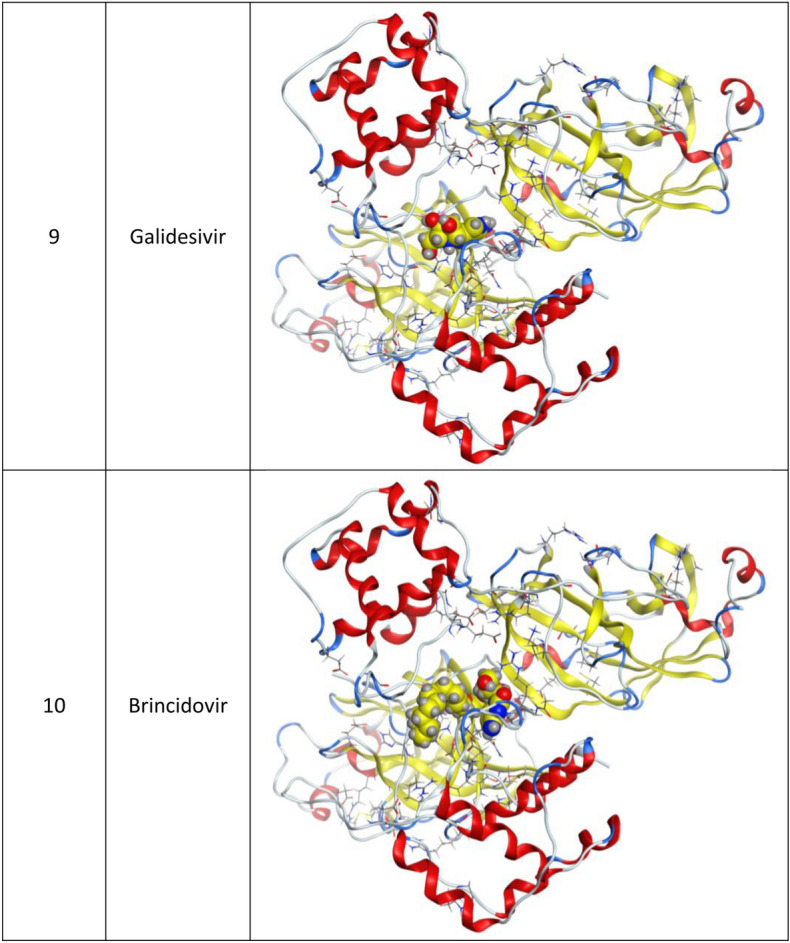
3d Docking of malaria and Ebola drugs and 1Q2W of COVID-19 with site 1 of COVID-19 protease (PDB code = 1Q2W)
Fig. 2.
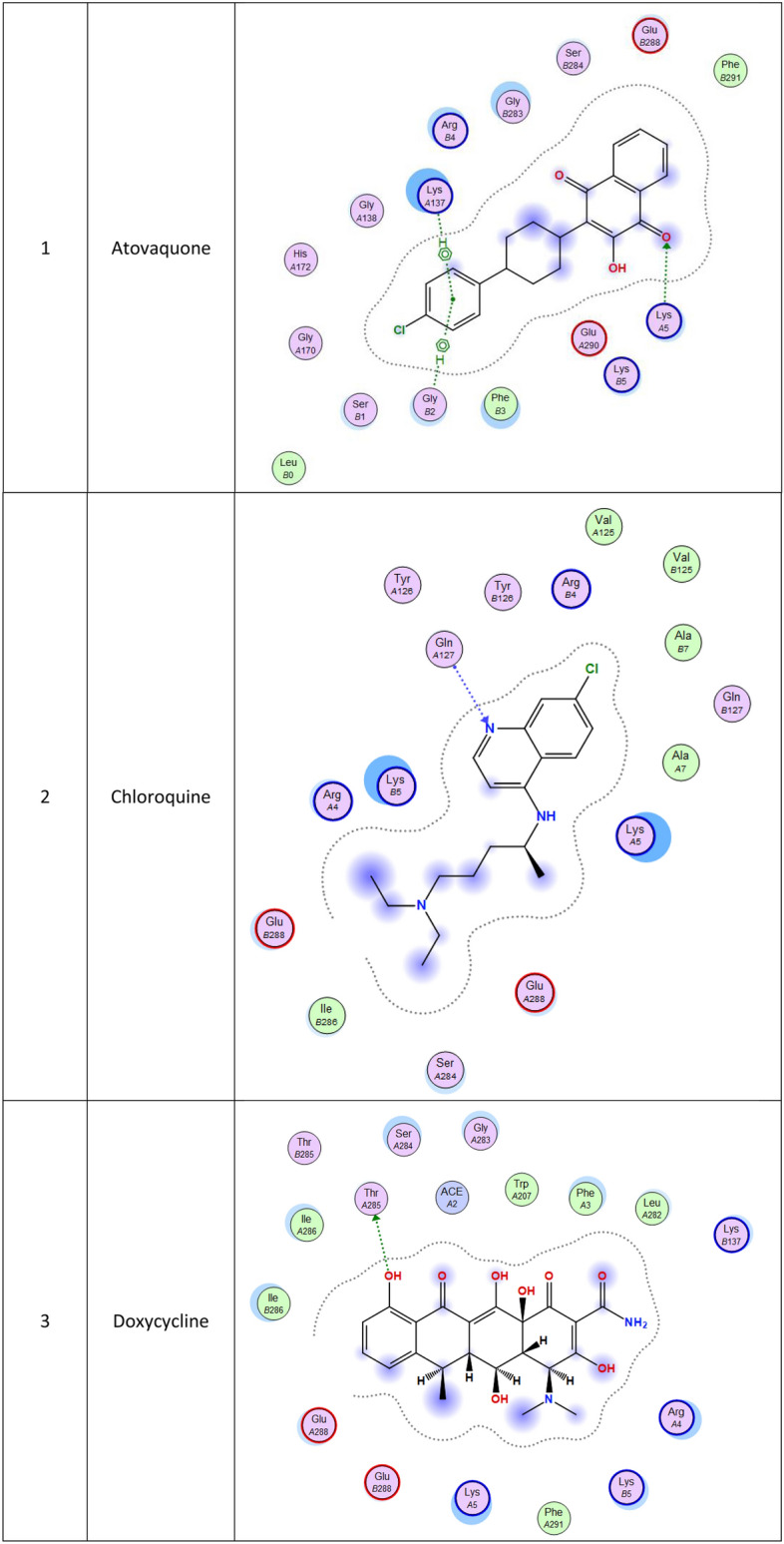
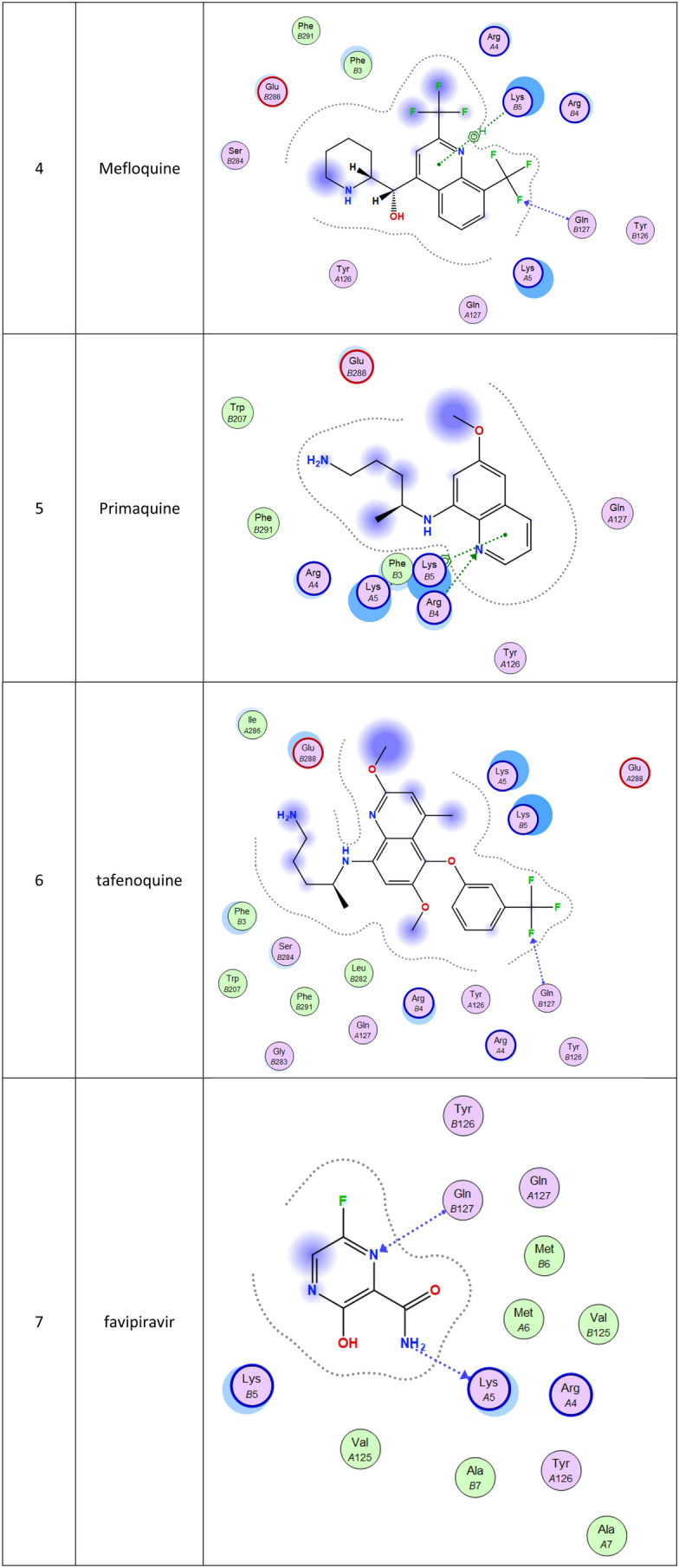
2d Docking of malaria and Ebola drugs and 1Q2W of COVID-19 with fixing the active site 1 of of COVID-19 protease (PDB code = 1Q2W)
Fig. 3.
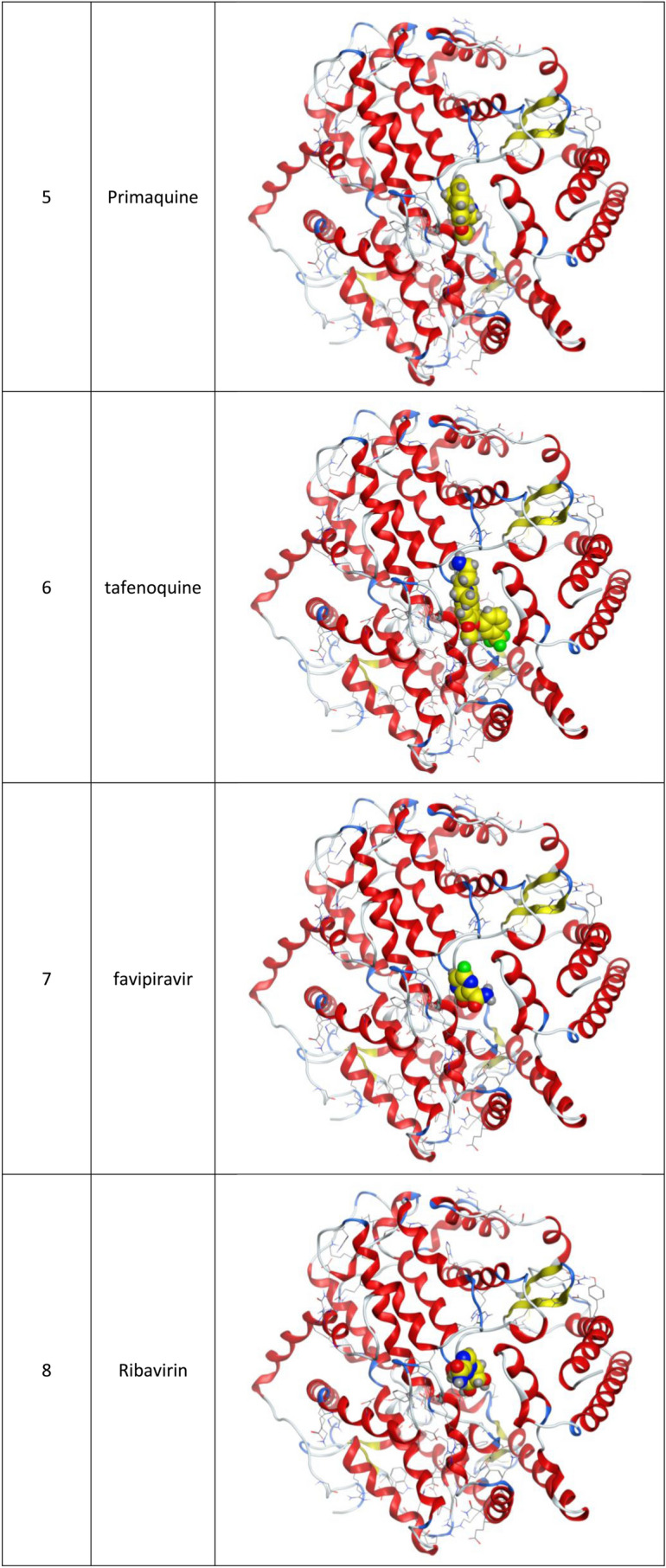
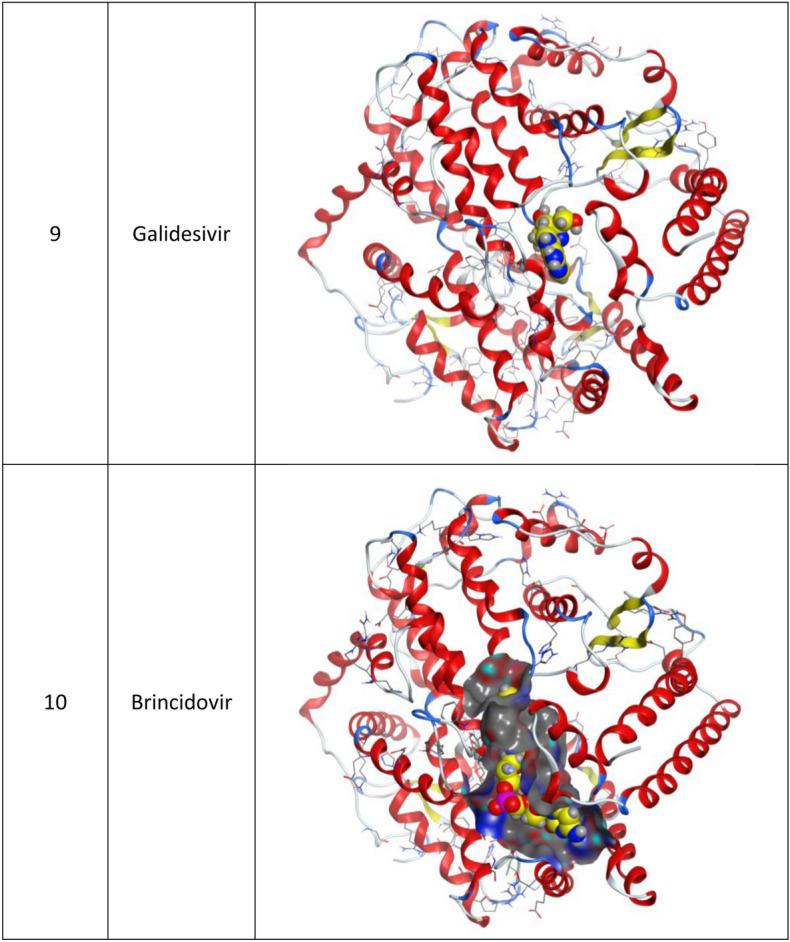
3d Docking of malaria and Ebola drugs with ACE-2 receptor (PDB code = 6M0J)
Fig. 4.
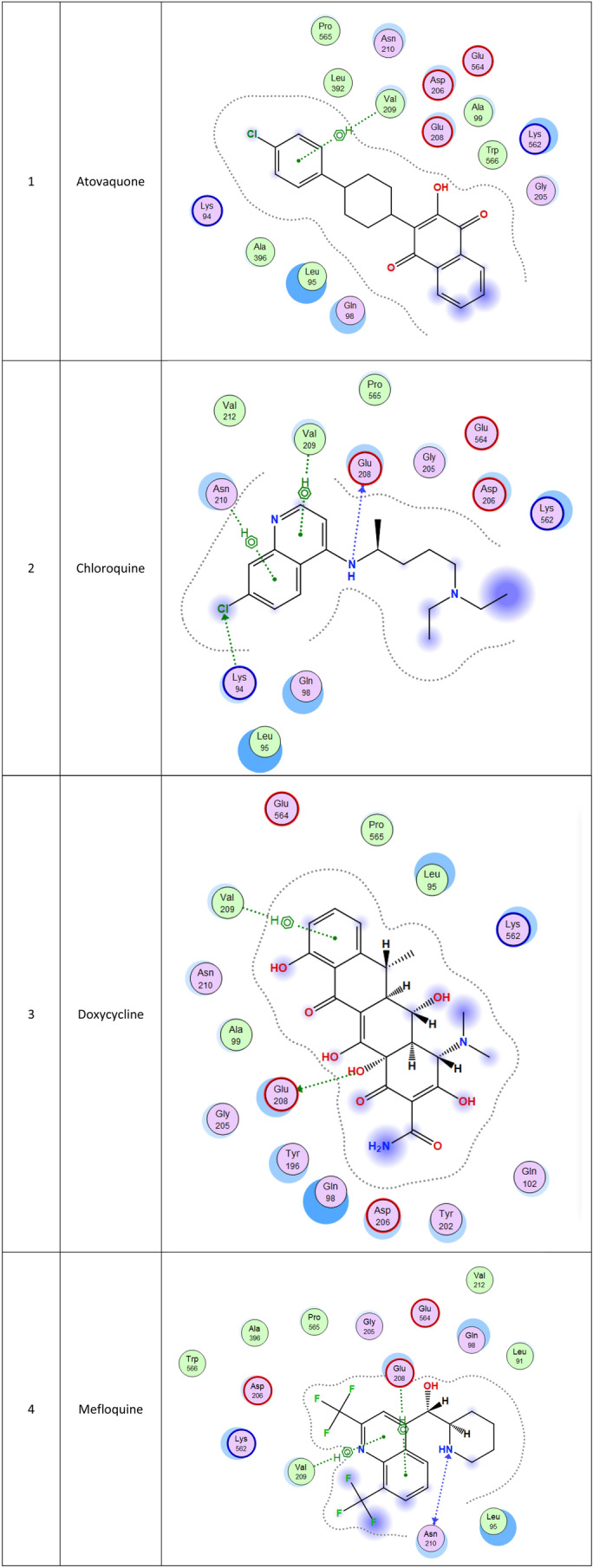
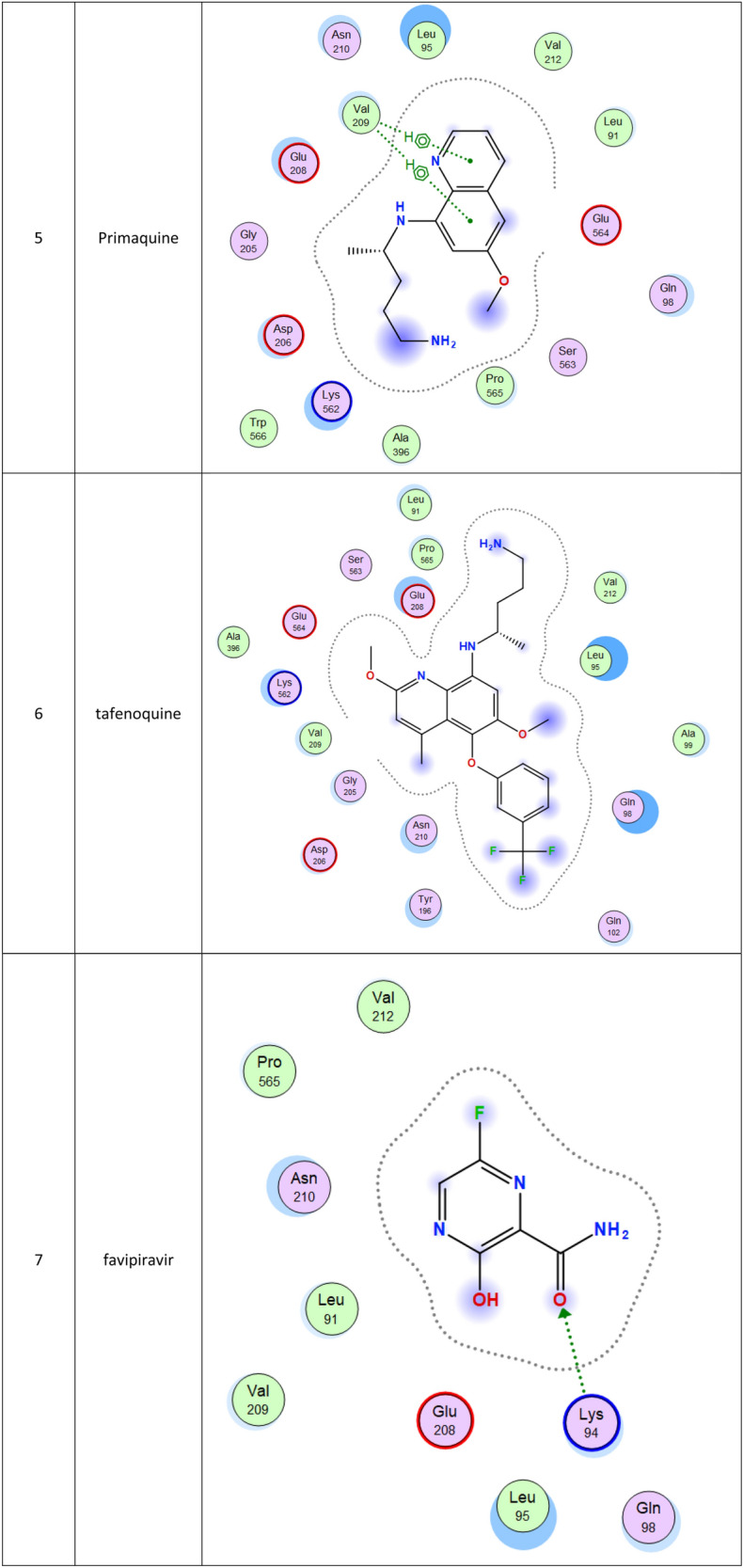
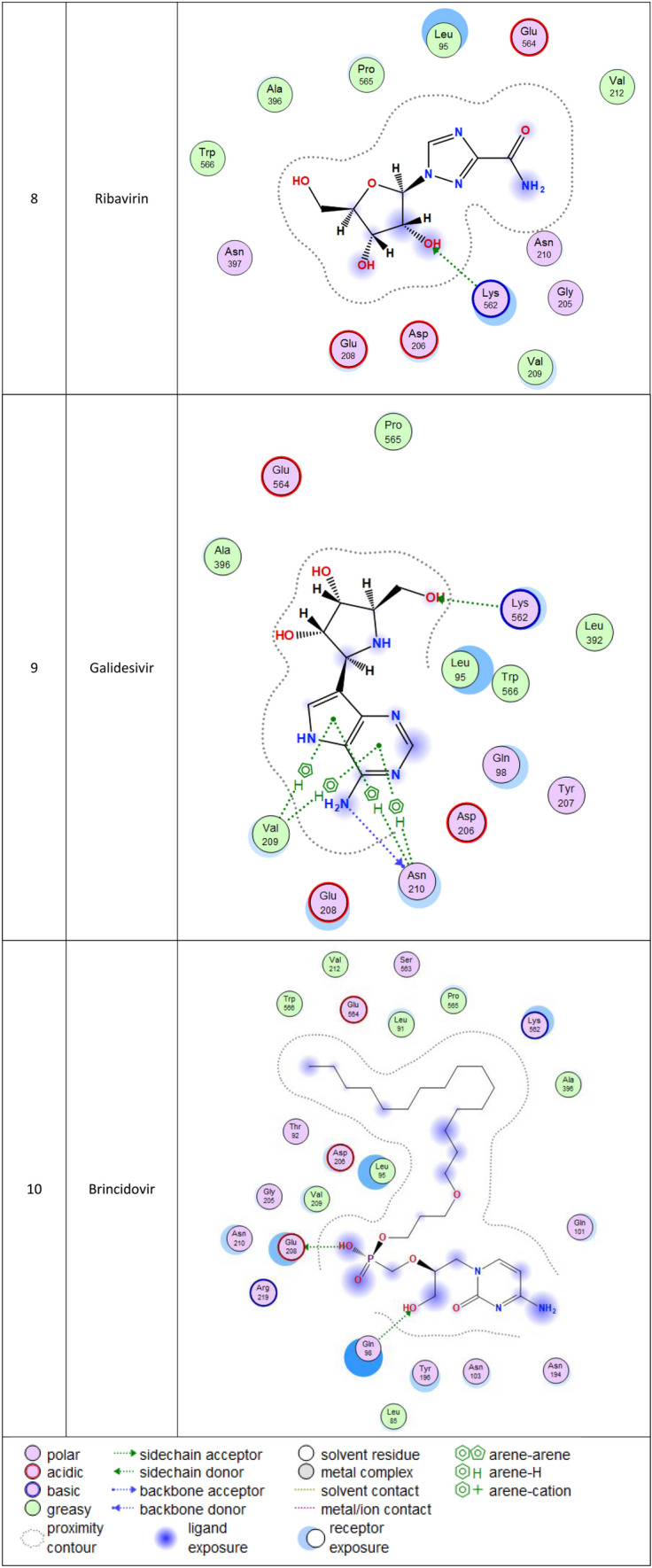
2d Docking of malaria and Ebola drugs with ACE-2 RECEPTOR (PDB CODE = 6M0J)
Fig. 5.
Chemical structure of ivermectin
Brincidofovir (BCV) is an orally bioavailable, long-acting nucleotide analog broad-spectrum antiviral developed by Chimerix Inc. of Durham, North Carolina, USA, for the treatment of double-stranded DNA (dsDNA) viruses (Lanier et al. 2010). BCV is less toxic with an enhanced cellular penetration prodrug of cidofovir, wherein the cidofovir acyclic nucleoside monophosphate is conjugated through its phosphonate group to a lipid, 3-(hexadecyloxy)-1-propanol (Florescu and Keck 2014). Being linked to a lipid particle, the compound ensures better and higher intracellular releases of cidofovir and lower plasma concentrations of the active drug, effectively increasing its antiviral activity. When intracellular, the released free cidofovir from the BCV is phosphorylated to its active metabolite cidofovir diphosphate, which, due to its structural similarity to the deoxycytidine triphosphate (dCTP) nucleotides, gets incorporated into the growing viral DNA strands (Parker et al. 2008a, b). Once incorporated, it prevents further DNA polymerization and disrupts DNA replication of viruses. The drug received FDA Fast Track Designation and has been evaluated in healthy individuals in Phase I studies and in hematopoietic cell transplant recipients and other immunocompromised patients in Phase II/III clinical trials and revealed to be well tolerated and highly efficacious against adenoviruses, BK virus, herpes simplex viruses, and smallpox, but eventually somehow failed for cytomegalovirus (Chittick et al. 2017; Hoiberg and Rapaka 1991; Schepers). Preliminary in vitro tests have also shown the drug potential for the treatment of Ebola virus disease, despite that Ebola is an RNA virus, albeit trials were eventually discontinued (Dunning et al. 2016). Being acted on the Ebola RNA virus so, it is encouraging to act as well on the novel RNA SARS-CoV-2. In addition to its intracellular therapeutic strategy of arresting viral replication and packaging, our study shows here that it also interferes efficiently with the SARS-CoV-2 ACE2 receptor, revealing a different therapeutic mode of action through potentially blocking or inhibiting the virus entry to the host cell, thereby slowing the progression of the infection.
The second top-ranked drug is tafenoquine which is an orally active 8-aminoquinoline, a long-acting analog of primaquine, antimalarial medicine developed by GlaxoSmithKline and 60 Degrees Pharmaceuticals (Baird 2018; Frampton 2018). The drug was FDA approved for the radical cure of Plasmodium vivax (P. vivax) malaria and the prophylaxis of malaria in 2018. The drug is active against pre-erythrocytic, erythrocytic forms and the gametocytes of Plasmodium species that include P. falciparum and P. vivax (Baird 2018; Frampton 2018). Clinical trials for this drug may be also recommended. Chloroquine, which is an antimalaria and immunosuppressive drug, has been recently shown to improve the outcomes in patients with the novel coronavirus pneumonia, which made the FDA issue an Emergency Use Authorization to be tested as a treatment for COVID-19, ranked at the fourth position in this study (Gao et al. 2020).
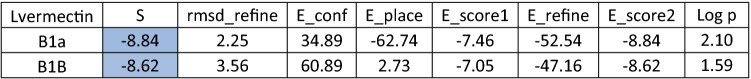
Lastly, while we were working in this research, an Australian study showed that ivermectin, an antiparasitic drug, was effective against COVID-19 disease, although further clinical trials are underway to confirm this effectiveness (Caly et al. 2020). We decided to do some investigations using molecular docking to check the binding interaction between ivermectin and the SARS-CoV-2 protease and receptor. We got comparable data to the antiviral Brincidofovir where the docking scores were -10.31 and -8.84 with the SARS-CoV-2 protease and ACE2 receptor (Tables 5 and 6), respectively. But overall, brincidofovir is better recommended because for its high lipophilicity “5.54”, whereas for ivermectin it is “2.01”.
In conclusion, molecular modeling tools were used to screen for potential anti-SARS-CoV-2 therapeutic agents. After a virtual screening against SARS-CoV-2 protease and ACE2 receptor, a set of antivirals, antimalarials, and antimicrobials drugs showed a potent binding interaction, wherein biocidofovir was the top hit. Therefore, repurposing of biocidofovir against COVID-19 disease is suggested for clinical trials.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (DOCX 27413 kb)
Compliance with ethical standards
Conflict of interest
The authors declare no competing financial interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdellattif MH, Hussien MA, Alzahrani E. New approaches of 4-aryl-2-hydrazinothiazole derivatives synthesis, molecular docking, and biological evaluations. Int J Pharm Sci Res. 2018;9:5060–5078. [Google Scholar]
- Abdel-Rhman MH, Hussien MA, Mahmoud HM, Hosny NM. Synthesis, characterization, molecular docking and cytotoxicity studies on N-benzyl-2-isonicotinoylhydrazine-1-carbothioamide and its metal complexes. J Mol Struct. 2019;1196:417–428. doi: 10.1016/j.molstruc.2019.06.092. [DOI] [Google Scholar]
- Althagafi I, El-Metwaly NM, Farghaly T. Characterization of new Pt(IV)—thiazole complexes: analytical, spectral, molecular modeling and molecular docking studies and applications in two opposing pathways. Appl Organomet Chem. 2019;33:e5099. doi: 10.1002/aoc.5099. [DOI] [Google Scholar]
- Baird JK. Tafenoquine for travelers’ malaria: evidence, rationale and recommendations. J Travel Med. 2018;25:tay110. doi: 10.1093/jtm/tay110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanno JB, et al. X-ray crystal structure of the SARS coronavirus main protease. Worldw Protein Data Bank. 2003 doi: 10.2210/pdb1q2w/pdb. [DOI] [Google Scholar]
- Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C-M. Faculty opinions recommendation of SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Fac Opin Ltd. 2020 doi: 10.3410/f.737494462.793573238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittick G, Morrison M, Brundage T, Nichols WG. Short-term clinical safety profile of brincidofovir: a favorable benefit–risk proposition in the treatment of smallpox. Antivir Res. 2017;143:269–277. doi: 10.1016/j.antiviral.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Control CfD, Prevention (2014) Vaccine testing and the approval process. Webpage. Updated: May
- Dunning J, et al. Experimental treatment of Ebola virus disease with brincidofovir. PLOS ONE. 2016;11:e0162199. doi: 10.1371/journal.pone.0162199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florescu DF, Keck MA. Development of CMX001 (Brincidofovir) for the treatment of serious diseases or conditions caused by dsDNA viruses. Expert Rev Anti-infect Ther. 2014;12:1171–1178. doi: 10.1586/14787210.2014.948847. [DOI] [PubMed] [Google Scholar]
- Frampton JE. Tafenoquine: first global approval. Drugs. 2018;78:1517–1523. doi: 10.1007/s40265-018-0979-2. [DOI] [PubMed] [Google Scholar]
- Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. BioSci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- GSK files for approval of world’s first malaria vaccine (2014) Pharm J. 10.1211/pj.2014.20065965
- Hoffmann M et al. (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor Cell [DOI] [PMC free article] [PubMed]
- Hoiberg CP, Rapaka RS. FDA regulatory requirements for investigational new drugs and new drug applications: an update. Am Psychol Assoc (APA) 1991 doi: 10.1037/e496242006-019. [DOI] [PubMed] [Google Scholar]
- Ketan ZA, Naser AW, AL-Shmgani HS (2020) Synthesis, design, docking and biological activity of new benzo hydrazide derivatives (2020) Int J Pharm Res. 10.31838/ijpr/2020.12.03.070
- Kitchen DB, Decornez H, Furr JR, Bajorath J. Docking and scoring in virtual screening for drug discovery: methods and applications. Nat Rev Drug Discov. 2004;3:935–949. doi: 10.1038/nrd1549. [DOI] [PubMed] [Google Scholar]
- Lanier R, et al. Development of CMX001 for the treatment of poxvirus infections. Viruses. 2010;2:2740–2762. doi: 10.3390/v2122740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengauer T, Rarey M. Computational methods for biomolecular docking. Curr Opin Struct Biol. 1996;6:402–406. doi: 10.1016/s0959-440x(96)80061-3. [DOI] [PubMed] [Google Scholar]
- Mashat KH, Babgi BA, Hussien MA, Arshad MN, Abdellattif MH. Synthesis, structures, DNA-binding and anticancer activities of some copper (I)-phosphine complexes. Polyhedron. 2019;158:164–172. doi: 10.1016/j.poly.2018.10.062. [DOI] [Google Scholar]
- Memish ZA, et al. Middle East respiratory syndrome coronavirus in bats. Saudi Arabia Emerg Infect Dis. 2013;19:1819. doi: 10.3201/eid1911.131172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mubarak S, Khanday MA, Lone AUH. Mathematical analysis based on eigenvalue approach to study liver metastasis disease with applied drug therapy. Netw Model Anal Health Inf Bioinf. 2020 doi: 10.1007/s13721-020-00231-0. [DOI] [Google Scholar]
- Naik PA. Modeling the mechanics of calcium regulation in T lymphocyte: a finite element method approach. Int J Biomath. 2020 doi: 10.1142/s1793524520500382. [DOI] [Google Scholar]
- Naik PA, Pardasani KR. Three-dimensional finite element model to study calcium distribution in oocytes. Netw Model Anal Health Inf Bioinf. 2017 doi: 10.1007/s13721-017-0158-5. [DOI] [Google Scholar]
- Naik PA, Pardasani KR. 2D finite-element analysis of calcium distribution in oocytes. Netw Model Anal Health Inf Bioinf. 2018 doi: 10.1007/s13721-018-0172-2. [DOI] [Google Scholar]
- Naik PA, Zu J. Modeling and simulation of spatial-temporal calcium distribution in T lymphocyte cell by using a reaction-diffusion equation. J Bioinf Comput Biol. 2020;18:2050013. doi: 10.1142/s0219720020500134. [DOI] [PubMed] [Google Scholar]
- Organization WH Ebola virus disease. Fact sheet N 103. Updated September 2014. Fecha de consulta: 18 de octubre de 2014
- Parker S, Oberle C, Touchette E, Buller RM. Efficacy of therapeutic intervention with an oral ether lipid analogue of cidofovir (CMX001) in a lethal mousepox model. Antivir Res. 2008;78:A61–A62. doi: 10.1016/j.antiviral.2008.01.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker S, et al. Efficacy of therapeutic intervention with an oral ether–lipid analogue of cidofovir (CMX001) in a lethal mousepox model. Antiviral Res. 2008;77:39–49. doi: 10.1016/j.antiviral.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers J. Chimerix announces emergency investigational new drug applications for brincidofovir authorized by FDA for patients with Ebola virus disease
- Sikka V, et al. The emergence of Zika virus as a global health security threat: a review and a consensus statement of the INDUSEM Joint Working Group (JWG) J Global Infect Dis. 2016;8:3. doi: 10.4103/0974-777X.176140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020 doi: 10.1128/jvi.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharmaceutica Sinica B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Wang Y, Chen F, Zhu M. Spatial statistics and influencing factors of the novel coronavirus pneumonia 2019 epidemic in Hubei Province. China: Res Square; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (DOCX 27413 kb)