Highlights
-
•
The objective of the study was to measure the benefits of supplemental telehealth via mobile apps videotelephony for home-dwelling older adults with cognitive impairment and their caregivers during social distancing compared with telehealth by phone calls only over a period of 4 weeks.
-
•
Supplemental video-conferencing telehealth was associated with 1) resilience against cognitive deterioration and improved quality of life in persons with cognitive impairment, and 2) enhanced well-being and functioning in the spousal caregivers. By contrast, notable deterioration was apparent in both parties who received telehealth by telephone alone.
-
•
To maximize impact for older adults with neurocognitive disorders and their caregivers, video conference should be investigated as the modus operandi of telehealth not only under the unusual circumstances of COVID-19 but also beyond the context of pandemic-related social distancing.
Key Words: Caregiver, COVID-19 pandemic, dementia, telehealth
Abstract
Objectives
Social distancing under the COVID-19 pandemic has restricted access to community services for older adults with neurocognitive disorder (NCD) and their caregivers. Telehealth is a viable alternative to face-to-face service delivery. Telephone calls alone, however, may be insufficient. Here, we evaluated whether supplementary telehealth via video-conferencing platforms could bring additional benefits to care-recipient with NCD and their spousal caregivers at home.
Participants
Sixty older adults NCD-and-caregiver dyads were recruited through an activity center.
Design, Intervention
The impact of additional services delivered to both care-recipient and caregiver through video conference (n = 30) was compared with telehealth targeted at caregivers by telephone only (n = 30), over 4 weeks in a pretest–post-test design. Interviews and questionnaires were conducted at baseline and study's end.
Measurements, Results
Supplementary telemedicine had averted the deterioration in the Montreal Cognitive Assessment evident in the telephone-only group (ηp2 = 0.50). It also reversed the falling trend in quality of life observed in the telephone only group (QoL-AD, ηp2 = 0.23). Varying degrees of improvements in physical and mental health (Short-Form 36 v2), perceived burden (Zarit Burden Interview Scale) and self-efficacy (Revised Caregiving Self-Efficacy Scale) were observed among caregivers in the video-conferencing group, which were absent in the telephone-only group (ηp2 = 0.23–0.51).
Conclusion
Telemedicine by video conference was associated with improved resilience and wellbeing to both people with NCD and their caregivers at home. The benefits were visible already after 4 weeks and unmatched by telephone alone. Video conference as the modus operandi of telehmedicine beyond the context of pandemic-related social distancing should be considered.
INTRODUCTION
Social distancing measures have been adopted worldwide to contain the spread of transmission of the COVID-19. Regardless of the success of such measures, social distancing invariably imposes limitations and constrains on diverse daily living activities.1 The older adults, and especially those with cognitive impairments under home care, are particularly vulnerable to the disruption caused by social distancing. Besides their known sensitivity to loneliness,2 severe disruptions of the normal routine,3 including access to social supports at the community level are expected. The lack of such community services is particularly damaging to the older adults because they rely on them to maintain interpersonal links and to make relevant life adjustments to the environment.4 Hence, social distancing could markedly compromise the quality of life and long-term health of community-dwelling older adults as well as their caregivers.5 Telehealth has become an obvious and cost-effective alternative route of service delivery to this vulnerable group, which is compatible with the social distancing measures during the ongoing COVID-19 pandemic. While the effectiveness of telehealth outside pandemic periods has been investigated,6, 7, 8, 9 how best to utilize telehealth during social distancing requires timely examination among diverse communities.
In its simplest form, supportive telehealth for older adults with neurocognitive disorder (NCD) cared at home may be conducted via regular phone calls whereby consultation and health information updates are made available to the caregivers.10 It is expected that supportive telehealth can be readily enhanced by utilizing commonly accessible mobile communication platforms that permit video conference, such as Zoom, WhatsApp and Facetime. Such video-conferencing platforms should facilitate engagement with the older adults with NCD as well as their caregivers by allowing more direct and immersive interaction with the health service providers.11 , 12 This mode of communication engages not only verbal, but also non-verbal communication and facial expression, which better approximates natural human face-to-face interaction.13 , 14 Hence, video conference compared with conventional phone calls alone may boost social interaction in home-dwelling older adults with NCD when the opportunity for face-to-face engagement is curtailed by social distancing.
We hypothesized that telehealth via video conference could minimize the possible negative impact of social distancing measures made necessary by the COVID-19 pandemic. Here, we tested the extent to which telehealth delivered via video-conferencing platforms in addition to routine telephone calls would yield notable benefits to older adults with NCD (65–80 years old) in home care and their spousal caregivers over a period of social distancing. Care-recipient/caregiver dyads were allocated to either a control group with telehealth targeted at caregivers by telephone only or the intervention group receiving additional services delivered to both care-recipient and caregiver through video conference using mobile devices. Over a period of 4 weeks, we monitored the changes in general cognitive functions, behavioral and psychological symptoms of dementia and quality of life in the NCD subjects, and the caregivers’ health status, perceived burden, and self-efficacy.
METHODS
Participants
Community-dwelling people with cognitive impairment and their spousal caregivers were recruited by convenient sampling through an activity day center for older adults. All participants had been visiting the center since early 2019. The present study began in March 2020 and was completed by mid-May 2020. Social distancing began officially in Hong Kong on 17th February 2020, but the public had already begun modifying its social behavior weeks ahead. We approached people who were between 65 and 80 with a diagnosis of NCD according to DSM-5 and were cared at home with their spouse as the primary caregiver. We contacted the caregivers and sought informed consents from them and the care-recipients to participate in the study. NCD subjects with major physical disabilities, such as strokes, were excluded. Following informed consents, the recruited dyads were allocated alternately into the two groups: interventionversus control groups, until 30 dyads were collected for each group. Group allocation was thus not randomized. The study was conducted according to the Declaration of Helsinki with prior approval by the Hong Kong Polytechnic University's Research Human Subjects Ethics Committee.
Telehealth
Both intervention and control groups received a weekly care service via telephone covering topics and information relevant to older adults’ well-being of community living, focusing on healthy aging, psychosocial needs, and physical well-being (adopted the Report on Hong Kong Healthy Ageing Executive Summary, www.elderlycommission.gov.hk/en/library/Ex-sum.htm#3) (see Supplemental Digital Content S1). The caregivers in the control group received weekly telephone calls that lasted 30 minutes. The intervention group received, in addition, weekly health services delivered through video communication apps, namely, Zoom, WhatsApp, or FaceTime. The applications had been preinstalled on all caregivers’ mobile devices at the time of enrolment. The NCD care-recipients were always present during video conference, and the healthcare provider was able to communicate directly to them. Each video-conferencing session was conducted on a separate day which also lasted 30 minutes. Outcome measures were obtained before and after the 4-week period by interviews and questionnaires conducted under blind conditions.
Primary Outcome Measures
Neurocognitive functioning, behavioral and psychological problems, and quality of life were assessed in the care-recipient with NCD by validated Chinese versions of Montreal Cognitive Assessment (MoCA), the Revised Memory and Behavior Problem Checklist (RMBPC), and the Quality of Life in Alzheimer's Disease (QoL-AD) assessment, respectively. MoCA was administered to the care-recipients.15 The frequency measures of specified behavioral problems (over the previous week) of the RMBPC were obtained from the caregiver.16 QoL-AD assessment was performed with the caregivers.17 The validated Chinese version of three other instruments were employed to characterize the caregivers. The Short Form 36 version 2 (SF-36v2) provided separate components for physical and mental health status.18 The Zarit Burden Interview Scale (ZBI) indexed caregiver burden based on feelings of over-sacrifice, perceived care-recipient's dependence, negative emotions during care, feelings of inadequacy, and uncertainty about the care-recipient's future.19 Finally, the Revised Caregiving Self-Efficacy Scale (RCSES) evaluated responding to problematic behavior, obtaining support, managing the household, regulating moods related to caregiving, and gathering healthcare information.20 , 21
Statistical Analysis
Demographic data were compared by one-way ANOVA or χ² test for group differences. Outcome measures were initially subjected to separate ANOVAs with a 2 × 2 × 2 mixed design, with between-subjects factors: Group (intervention versus control groups) and Gender, and a within-subject factor: Time (pretest baseline versus post-test). The effect of group over time never depended on gender (see Supplemental Digital Content S2), and therefore the factor Gender was dropped in the final analysis to increase power. Additional 2 × 2 (Group × Time) ANCOVAs were performed with the inclusion of age and years of education of both the care-recipients and caregivers as covariates. A significant Group by Time interaction in the 2 × 2 (Group × Time) ANOVA and ANCOVA would indicate that change over time of a given outcome measure differed significantly between groups. Significant interactions were investigated further by ANOVA restricted to a group and a given time point, and ANCOVA of post-test scores using pretest scores as covariate were conducted. Effect sizes of significant effects from ANOVA/ANCOVA are reported in η p 2. Ad hoc correlative analysis and linear regression were performed to examine the strength of the association between overall concomitant changes observed in care-recipients and caregivers. All analyses were conducted using SPSS-IBM v23. Type-I error rate was set at p <0.05.
RESULTS
Demographics
As summarized in Table 1 , the sex-ratio and average age of care-recipients or the caregivers, and caregivers’ education were comparable between groups. The years of education could only be unambiguously determined in 24 and 26 care-recipients in the control and intervention groups, respectively. One-way ANOVA indicated a significant group difference [F (1,48) = 4.70, p <0.05, η p 2 = 0.09] with care-recipients in the intervention group had almost one additional year of education (∼13% more) than their counterparts in the control group. The two groups did not differ significantly in terms of number of chronic diseases in the caregivers, the hours of support provided by all carers (spouse and other family members/relatives) per day, and the major source of income (see statistics in Table 1).
TABLE 1.
Sociodemographic Characteristics
| Control (n = 30) |
Intervention (n = 30) |
Group Difference | |
|---|---|---|---|
| Age (years) | |||
| Care-recipients with NCD | 72.73 ± 0.84 (64–80 yr) | 72.87 ± 0.84 (65–80 yr) | F(1,58) = 0.01, p = 0.91 |
| Caregivers | 71.83 ± 0.80 (66–82 yr) | 72.43 ± 0.80 (66–82 yr) | F(1,58) = 0.28, p = 0.60 |
| Years of education | |||
| Care-recipients with NCDa | 7.04 ± 0.31 (5–9 yr) | 7.96 ± 0.29 (5–11 yr) |
F(1,48) = 4.70, p = 0.035, ηp2 = 0.09 |
| Caregivers | 8.23 ± 0.25 (6–11 yr) | 7.90 ± 0.25 (6–11 yr) | F(1,58) = 0.88, p = 0.35 |
| Female: male ratio | |||
| Care-recipients with NCD | 12:18 | 13:17 | χ2 = 0.07, df = 1, p = 0.79 |
| Caregivers | 18:12 | 17:13 | |
| Number of Chronic diseases reported in caregiversb | |||
| 0 | 0 | 1 | χ2 = 1.15, df = 2, p = 0.56 |
| 1–2 | 22 | 25 | |
| >3 | 5 | 4 | |
| Hours of support by family (including primary caregivers) per day | |||
| 4–8 hr | 21 | 15 | χ2 = 2.50, df = 1, p = 0.11 |
| >8 hr | 9 | 15 | |
| Major source of financial incomec | |||
| Social security | 8 | 6 | χ2 = 0.38, df = 2, p = 0.83 |
| Family/relatives | 9 | 10 | |
| Own saving | 11 | 12 | |
Summary of demographic data of the 30 dyads in the control group and the 30 dyads in the intervention group. Values refer to group means ± standard error (SE), and the range in years are given in parenthesis. The last four variables are frequency counts, classified by groups and categories. There were some data missing in some measures. One-way ANOVA was used to assess group difference in age and years of education in care-recipients with NCD and caregivers. χ2 goodness-of-fit test was used to evaluate frequency counts between group on sex ratio, presence of chronic diseases in caregivers, level of support by family per day (more or less than 8 hours per day), and the major source of income.
Years of education in recipient with NCD were calculated based on 24 and 26 dyads in the control and intervention groups, respectively.
Total hours of care provided by all family members (including the primary caregivers) were classified for 27 and 30 dyads in the control and intervention groups, respectively. Due to the low counts in some cells, the Fisher-Freeman-Halton Exact Test was also performed, which confirmed the lack of statistical significance based on the reported χ2 test of independence.
The major source of financial income could only be reliably determined in 28 dyads of each group.
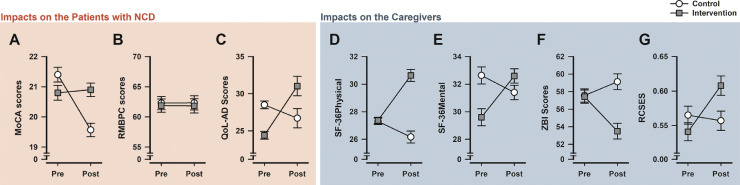
Impacts on Care-Recipients With NCD
The intervention with supplementary telehealth delivered via video-conferencing apps was associated with to a resilience against a fall in general cognitive functioning in the control group over the study period (Fig. 1 A). The MoCA scores in the intervention group remained largely stable, and thus by the end of 4 weeks their MoCA scores were superior to controls. The falling trend in quality of life observed in the control group over the study period was absent in the intervention group (Fig. 1C). Despite the baseline (pretest) difference in favor of the control group, the intervention group enjoyed a higher quality of life by the end of the study period. Finally, the two groups never differed in terms of behavioral and psychological problems, which hardly changed across time (Fig. 1B).
FIGURE 1.
Comparison of all primary measures obtained in the care-recipients with NCD [A–C] and in their caregivers [D–G]at baseline and at study's end 4-week later, denoted as "Pre" and "Post", respectively, in the abscissa of each individual plot. * denotes group difference at p <0.05 based on one-way ANOVA of pretest or post-test scores. # denotes group difference at p <0.05 based on ANCOVA of post-test scores with pretest scores as covariate. § denotes a significant time effect based on one-way repeated measures ANOVA restricted to either group. All values refer to group means ± standard error (SE, estimated from the error variance in the 2 × 2 ANOVA).
The above impressions were confirmed by separate two-way (Group × Time) ANOVAs. The analyses of both MoCA and QoL-AD scores yielded a highly significant interaction [MoCA: F (1,58) = 57.18, p <0.001, η p 2 = 0.50; QoL-AD: F (1,58) = 17.17, p <0.001, η p 2 = 0.23]. The ANOVA also yielded a significant Time effect [MoCA: F (1,58) = 45.97, p <0.001, η p 2 = 0.44; QoL-AD: F (1,58) = 5.64, p <0.05, η p 2 = 0.09] but the Group effect was far from significance [MoCA: F (1,58) = 1.45, p = 0.23; QOL-AD: F (1,58) = 0.02, p = 0.90]. The critical interaction terms remained statistically significant with comparable effect sizes when the age and years of education of both care-recipients and caregivers were covariates in supplementary ANCOVAs. By contrast, the ANOVA of RMBPC scores did not yield any significant effects [all F’s <1].
One-way ANOVAs confirmed that MoCA and QoL-AD scores were significantly higher in the intervention group at study's end [MoCA: F (1,58) = 17.97, p <0.001, η p 2 = 0.24; QoL-AD: F (1,58) = 5.54, p <0.05, η p 2 = 0.09]. When pretest scores were controlled as covariate by ANCOVA, the effect size of the group difference in post-test MoCA was substantially elevated [F (1,57) = 55.23, p <0.001, η p 2 = 0.49]. However, controlling baseline difference by ANCOVA had rendered the post-test group difference in QoL-AD no longer significant [F (1,57) = 2.44, p = 0.12, η p 2 = 0.04].
To gauge the clinical significance, we classified our subjects based on their MoCA into major (≤18), mild (=19 to 21) and pre-NCD (≥22) levels (see Table 2 ).22 The classification based on the post-treatment MoCA scores significantly depended on grouping [χ2 = 9.09, df = 2, p <0.05], while such dependency was absent at baseline [χ2 = 3.35, df = 2, p = 0.19]. According to this set of criteria, a high proportion (16 of 17) of care-recipients with “pre-NCD” MoCA scores in the control group had attained the MoCA criterion for mild NCD in the study period. By contrast, no such shift of clinical status was seen in the intervention group.
TABLE 2.
Classification of Care-Recipients Based on their MoCA Into Major (≤18), mild (=19–21) and Pre-NCD (≥22) Levels
| Baseline |
At Study's End |
|||||
|---|---|---|---|---|---|---|
| Classification | Major | Mild | Pre-NCD | Major | Mild | Pre-NCD |
| by MoCA | ≤18 | 19–21 | 22–24 | ≤18 | 19–21 | 22–24 |
| Intervention | 2 | 18 | 10 | 1 | 19 | 10 |
| Control | 1 | 11 | 17 | 2 | 27 | 1 |
Classification of the MoCA scores obtained at baseline and study's end of the care-recipients into “major”, “mild” and “pre-NCD” range. According to these cut-offs, substantial deterioration was evident in the control group with a substantial proportion of care-recipients with “pre-NCD” levels of MoCA at baseline had shifted to the “mild” category. Such a shift was absent in care-recipients in the intervention group.
Impacts on Caregivers
A deteriorating trend in all outcome measures for the caregivers was discernible in the control group over the study period. This was detected as a pre-to-post fall in the SF-36v2 mental and physical well-being component scores, in self-efficacy (RCSES), and a rise in perceived burden (Fig. 1D–G). Regardless of the magnitude of these deteriorations seen in the control group, an opposite trend was evident in the intervention group, suggesting that supplementary video conference was associated with a general positive impact on the caregivers. This interpretation is supported by the emergence of the critical Group × Time interaction in the ANOVAs of all four measures. The effect size of the interaction was the largest in the physical and mental components of the SF-36v2 questionnaire [F (1,58) = 60.30, p <0.001, η p 2 = 0.51 and F (1,58) = 49.13, p < 0.001, η p 2 = 0.46, respectively], followed by the ZBI scale of perceived burden [F (1,58) = 19.04, p <0.001, η p 2 = 0.25], and the RCSES self-efficacy score [F (1,58) = 17.30, p <0.001, η p 2 = 0.23]. Again, these interaction terms all remained statistically significant when the age and education of both care-recipients and caregivers were covariates in ANCOVAs.
The SF-36v2 physical well-being component and ZBI scores of perceived burden were closely matched between groups at baseline, which subsequently diverged at the study's end (Fig. 1D,1F). Consistent with these graphical impressions, ANOVA of post-test scores yielded a significant group difference in the SF-36v2 physical well-being component [F(1,58)=53.72, p <0.001, η p 2=0.48], ZBI scores [F(1,58) = 19.94, p <0.001, η p 2=0.26]. Their respective ANCOVAs (with pretest scores as covariate) yielded significant group effects [all p's<0.001] with marginally larger effect sizes [η p 2= 0.52 and 0.28, respectively]. Although the two groups were not as closely matched in RCSES scores at baseline, the ANCOVA of post-test RCSES scores (with pretest scores as covariate) also yielded a significant group effect [p <0.001, η p 2 = 0.23] and an effect size comparable with the interaction term in the original ANOVA. Baseline difference was most pronounced in the mental component of SF-36v2 (Fig. 1E). When the post-test scores were analyzed by ANCOVA (with pretest scores as covariate), the effect size was revised downward [η p 2 = 0.35] relative to the interaction term in the original ANOVA, whilst the main group effect remained statistically significant.
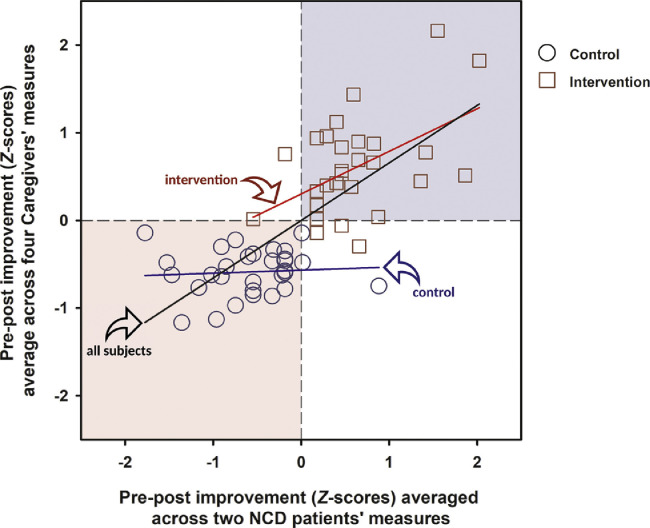
Correlative Improvement Between Care-Recipients and Caregivers
To examine strength of association between the improvement between care-recipients and caregivers on the relevant outcome measures above, we performed correlative analysis between two composite variables. To index the improvement in care-recipients across different measures, the pre–post changes in MoCA and QoL-AD scores were z-transformed and then averaged. To index the improvement in caregivers, all four relevant measures (physical components of SF-36v2, mental components of SF-36v2, ZIB, and RCSES) were likewise combined, except that the pre–post changes in ZIB scores were reversed in sign to reflect that a reduction in perceived burden corresponds to a benefit. As depicted in Figure 2 , a strong positive association [R 2 = 0.53] was evident across all 60 dyads [Pearson's r = +0.73, df = 58, p <0.001]. However, the control and intervention groups largely segregated into two diagonally opposite quadrants of the scatter plot. An association of moderate effect size was detected in the intervention group [r = +0.50, df = 28, p = 0.005, R 2 = 0.25] but not in the control group [r = +0.07, df = 28, p = 0.70, R 2 = 0.005]. The sole presence of an association in the intervention group may suggest that the supplementary video conference facilitated positive synergistic interaction between care-recipients and caregivers of a dyad.
FIGURE 2.
Scatter plot of pre–post changes observed in the care-recipients with NCD and their spousal caregivers based on relevant outcome measures that yielded statistical evidence for a group effect across time. Pre–post changes were normalized with respect to the mean and standard deviation of all subjects (N = 60), and then averaged to provide the summative indices for improvement (i.e., positive changes) in care-recipients and their caregivers, as represented by the abscissa and ordinate axes, respectively. Three regression lines, indicated by the arrows, are fitted to all or a subset of the data. The black regression line through the origin is fitted to all 60 dyads [ANOVA of the linear regression was highly significant at F(1,58) = 65.25, p <0.001; R2 = 0.53, b = 0.66 ± 0.08]. The red regression line is fitted to the dyads in the intervention group (N = 30) [ANOVA of this linear regression was significant at F(1,28) = 9.21, p = 0.005; R2 = 0.25, b = 0.49 ± 0.16], whereas the blue regression line is fitted to dyads in the control group (N = 30), of which no significant association in pre–post changes between partners of the dyads was found [ANOVA of this linear regression was far from statistical significance F(1,28) = 0.15, p = 0.70; R2 = 0.005, b = 0.04 ± 0.09]. The light blue and pink backgrounds show the location of quadrants I and III in the Cartesian plane, where most of the intervention and control groups lay, respectively.
DISCUSSION
Supplementary telehealth delivered via video-conferencing apps implemented through mobile devices over 4 weeks – when social distancing due to the COVID-19 pandemic was in place – was associated with positive effects for community-dwelling older adults with neurocognitive impairment and their caregivers in comparison with conventional telehealth conducted by phone conversation alone. We also see that the overall benefits to the older adults with NCD and their caregivers were well correlated, especially in the intervention group receiving telehealth through video conference (Fig. 2). Our findings suggest that the use of video conference in telehealth should be further explored especially in time of unfavorable social circumstances that limit social interaction and connectedness in this vulnerable group.
A positive impact was observed in all measures except for problem behavior indexed by the total frequency score in the 24-item RMBPC. Notably, the RMBPC frequency scores remained very stable over time. This is in keeping with suggestion that depressive symptoms, anxiety, and apathy are at least moderately stable over time.23 However, we cannot exclude the possibility that longer period of social distancing or isolation may worsen problem behavior. Alternatively, the stability may suggest a ceiling effect. Here, the total frequency scores of RMBPC were well above 60. It is well above the 33–34 incidences of problem behavior reported per week in a previous study of people (47–90 years old) with moderate to severe Alzheimer's disease in Taiwan.16 Hence, one may suspect that the on-going social distancing had already elevated our baseline RMBPC scores, thus limiting the scope of further exacerbation of problem behavior.
On the other hand, we saw a reduction in cognitive functioning in the NCD subjects in the control group, and the additional delivery of health content via video conference was associated with a resilience against this reduction. Telehealth via video conference did not improve performance in the MoCA. Instead, it was maintained at a stable level over the study period. At post-treatment, the MoCA scores of the control group had fallen to a level below that of the intervention group. Indeed, the fall of 1.83 in the MoCA observed in the control group was substantial considering that it had occurred within only 4 weeks. Over the same period, the intervention group showed a marginal reduction of 0.1 in the MoCA. When the MoCA scores were translated into clinical classifications according to standard criteria,22 we saw a notable proportion of care-recipients with “pre-NCD” MoCA scores in the control group attaining the MoCA criterion for mild NCD by study's end, whereas no such shift was apparent in the intervention group. It is therefore reasonable to suspect that the atypical, rapid fall in MoCA scores in our control group might stem from the barriers to social stimulation and interaction imposed by social distancing. However, we lack any direct measures of social activities to test this hypothesis. Given that the social distancing measures in Hong Kong24 were less strict and less stringently applied compared with communities in North America and Europe, one may speculate that the negative impact on health and care of people with NCD would be more severe in these regions.3 , 10 , 11 A direct comparison between communities with varying degrees of social measures is therefore warranted.
While the impact of telehealth via video conference on the care-recipients’ cognitive functioning appeared best described as resilience, notable improvement in quality of life (indexed by QoL-AD) over time was demonstrated in the intervention group. It contrasted sharply with the deteriorating trend in the control group over the same period, such that care-recipients in the intervention group enjoyed better quality of life by the study's end despite them being inferior to controls at baseline (Fig. 1C). This was paralleled by multiple improvements from the caregivers’ perspective as demonstrated by all four measures of caregiver functioning.
Improvement in both physical and mental status of the caregivers was supported by the SF-36v2 health survey, which was accompanied by a reduction in perceived burden indexed by ZBI and increase in self-efficacy indexed by RMBPC (Fig. 1D–G). Hence, the benefits attributable to the supplementary video-conferencing intervention clearly went beyond resilience. Interpretation of physical health and perceived burden is straightforward because the two groups were closely matched at baseline. Although a baseline difference was apparent in SF-36v2 mental health component and self-efficacy scores, the direction was such that the intervention group was initially lower than the control group in both measures and yet the group difference was reversed by the end of the study. This should not detract our observations that the use of video conference in telehealth was associated with multiple improvements in caregivers’ well-being and functioning.
The correlation between the concomitant betterment observed in care-recipients and caregivers – largely driven by the intervention group (Fig. 2), was not surprising since synergistic interaction between care-recipients and caregivers is well known in many chronic conditions under home care.25 What may be surprising however is the lack of such a correlation within the control group, where the general well-being of both care-recipients and carers deteriorated over time. Within the intervention group, the correlative analysis provided an estimate of 25% for the shared variance between the betterment observed in care-recipients and caregivers in this period. The regression line further suggests that one standard deviation (SD) unit of improvement observed in the care-recipients was associated with 0.49 SD unit in the caregivers, of their respective composite indexes.
Conclusion
Compared with telephone conversation alone, video conference could capture important social elements intrinsic to face-to-face interaction.14 We suggest that this could be critically beneficial for people with NCD and their caregivers at home. Social distancing likely had exacerbated the impact of social isolation resulting from mobility limitation in this group of older adults. Indeed, the feeling of loneliess is known to be associated with lower engagment in face-to-face social interaction as well as lower use of mobile communcation devices.13 , 14 Video-conferencing was apt in meeting such needs by this vulnerable group under the unusual social conditions caused by the ongoing pandemic. In addition, older adults reportedly find the experience of video-conferencing more user-centered and interesting.26 The telehealth came across as more engaging, and the caregivers as well as the care-recipients likely had paid more attention to the content, whereas telephone call alone was perceived as passive. In addition, our subjects were positive toward learning to improve their operation of the mobile apps, which is a potential determinant on the effective content delivery and benefits of telehealth in older adults.27
Limitations
First, we may not exclude the possibility that the superior benefits of video-conference supported telehealth stemmed primarily from the increased time or frequency of contacts as such. A head-to-head comparison between video conferences and phone calls with matching contact time is necessary. Second, the switch from phone calls to video conference likely had affected the content, style, and manner of the delivery by the health care providers, and these should have been recorded and subjected to analysis to isolate potential mediator variables. Third, randomization of group allocation would strengthen the impacts of our present findings. Fourth, the duration of the study only permitted examination of short-term impact associated with supplemental video conference to conventional telephone only delivery of health information for caregivers and care-recipients with NCD. Within the 4-week period, only four video-conferencing sessions were conducted. Although a strong impact was clearly identified, it is essential to examine whether further benefits may be possible with extended periods of supplementary video conference, and whether any such benefits would be sustainable when supplementary video conferences ceased. Fifth, the generalizability of our findings remains to be fully evaluated across communities differed in the manners and severity by which social distancing is enforced. Such comparative investigations between different geographical areas and socioeconomic segments would be instructive.
AUTHOR CONTRIBUTION
Dr Frank Lai coordinated the whole study and developed the research idea. He executed the research plan and monitored the progress. Dr Frank Lai and Professor Benjamin K. Yee both had substantially contributed to the conception and design of the work, analysis and interpretation of research data, and preparation of the manuscript. Ms. Elaine Yan and Mr. Daniel Chan had conducted the literature review and data collection, including the arrangement for interventions for participants. Ms. Kathy Yu and Ms. Wing Tsui assisted in the literature search and served as the blinded assessors in the study. They had helped in the earlier drafts of the manuscript and assisted in subsequent revision of the text, contributing significantly to the paper’s intellectual content. All authors have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgments
Disclosure
All authors report no declarations of interest. There is no disclosure reporting any conflicts of interest, financial or otherwise, related to the submitted manuscript.
This study was supported by Innovation and Technology Fund for Better Living awarded by the Innovation and Technology Bureau of the Hong Kong Special Administrative Region Government (ITB/FBL/2004/19/P).
Footnotes
Ethics Statement: Approval was given by The Hong Kong Polytechnic University Human Research Ethics Committee and the study was conducted according to the Declaration of Helsinki.
Supplementary materials associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jagp.2020.07.019.
Appendix. SUPPLEMENTARY MATERIALS
References
- 1.D'Onofrio G, Sancarlo D, Ricciardi F. Information and communication technologies for the activities of daily living in older patients with dementia: a systematic review. J Alzheimers Dis. 2017;57:927–935. doi: 10.3233/JAD-161145. [DOI] [PubMed] [Google Scholar]
- 2.Berg-Weger M, Morley JE: Loneliness and social isolation in older adults during the Covid-19 pandemic: implications for gerontological social work. J Nutr Health Aging 2020; 24:456–458; 10.1007/s12603-020-1366-8 [DOI] [PMC free article] [PubMed]
- 3.Jawaid A. Protecting older adults during social distancing. Science. 2020;368:145. doi: 10.1126/science.abb7885. [DOI] [PubMed] [Google Scholar]
- 4.Fricke J, Unsworth C. Time use and importance of instrumental activities of daily living. Aust Occup Ther J. 2001;48:118–131. doi: 10.1046/j.0045-0766.2001.00246.x. [DOI] [Google Scholar]
- 5.Palmer AD, Carder PC, White DL. The impact of communication impairments on the social relationships of older adults: pathways to psychological well-being. J Speech Lang Hear Res. 2019;62:1–21. doi: 10.1044/2018_JSLHR-S-17-0495. [DOI] [PubMed] [Google Scholar]
- 6.Banbury A, Nancarrow S, Dart J. Adding value to remote monitoring: co-design of a health literacy intervention for older people with chronic disease delivered by telehealth - the telehealth literacy project. Patient Educ Couns. 2020;103:597–606. doi: 10.1016/j.pec.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Bernocchi P, Giordano A, Pintavalle G. Feasibility and clinical efficacy of a multidisciplinary home-telehealth program to prevent falls in older adults: a randomized controlled trial. J Am Med Dir Assoc. 2019;20:340–346. doi: 10.1016/j.jamda.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Pimentel CB, Gately M, Barczi SR. GRECC connect: geriatrics telehealth to empower health care providers and improve management of older veterans in rural communities. Fed Pract. 2019;36:464–470. [PMC free article] [PubMed] [Google Scholar]
- 9.VanRavenstein K, Davis BH. When more than exercise is needed to increase chances of aging in place: qualitative analysis of a telehealth physical activity program to improve mobility in low-income older adults. JMIR Aging. 2018;1:e11955. doi: 10.2196/11955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zubatsky M, Berg-Weger M, Morley J. Using telehealth groups to combat loneliness in older adults through COVID-19. J Am Geriatr Soc. 2020;68:1678–1679. doi: 10.1111/jgs.16553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Middleton A, Simpson KN, Bettger JP. COVID-19 pandemic and beyond: considerations and costs of telehealth exercise programs for older adults with functional impairments living at home-lessons learned from a pilot case study. Phys Ther. 2020;100:1278–1288. doi: 10.1093/ptj/pzaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodman-Casanova JM, Dura-Perez E, Guzman-Parra J. Telehealth home support during COVID-19 confinement for community-dwelling older adults with mild cognitive impairment or mild dementia: survey study. J Med Internet Res. 2020;22:e19434. doi: 10.2196/19434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan Y. Ablex Publishing Corporation, Stamford, CT; 2000. Politeness in Chinese Face-To-Face Interaction. [Google Scholar]
- 14.Jin B, Park N. In-person contact begets calling and texting: interpersonal motives for cell phone use, face-to-face interaction, and loneliness. Cyberpsychol Behav Soc Netw. 2010;13:611–618. doi: 10.1089/cyber.2009.0314. [DOI] [PubMed] [Google Scholar]
- 15.Wong A, Xiong YY, Kwan PW. The validity, reliability and clinical utility of the Hong Kong Montreal Cognitive Assessment (HK-MoCA) in patients with cerebral small vessel disease. Dement Geriatr Cogn Disord. 2009;28:81–87. doi: 10.1159/000232589. [DOI] [PubMed] [Google Scholar]
- 16.Fuh JL, Liu CY, Wang SJ. Revised memory and behavior problems checklist in Taiwanese patients with Alzheimer's disease. Int Psychogeriatr. 1999;11:181–189. doi: 10.1017/s1041610299005736. [DOI] [PubMed] [Google Scholar]
- 17.Chan IW, Chu LW, Lee PW. Effects of cognitive function and depressive mood on the quality of life in Chinese Alzheimer's disease patients in Hong Kong. Geriatr Gerontol Int. 2011;11:69–76. doi: 10.1111/j.1447-0594.2010.00643.x. [DOI] [PubMed] [Google Scholar]
- 18.Dong A, Chen S, Zhu L. The reliability and validity of Chinese version of SF36 v2 in aging patients with chronic heart failure. Aging Clin Exp Res. 2017;29:685–693. doi: 10.1007/s40520-016-0614-6. [DOI] [PubMed] [Google Scholar]
- 19.Ko KT, Yip PK, Liu SI, Huang CR. Chinese version of the Zarit caregiver Burden Interview: a validation study. Am J Geriatr Psychiatry. 2008;16:513–518. doi: 10.1097/JGP.0b013e318167ae5b. [DOI] [PubMed] [Google Scholar]
- 20.Steffen AM, Gallagher-Thompson D. Validating the revised scale for caregiving self-efficacy: a cross-national review. Gerontologist. 2019;59:e325–e342. doi: 10.1093/geront/gny004. [DOI] [PubMed] [Google Scholar]
- 21.Steffen AM, McKibbin C, Zeiss AM. The revised scale for caregiving self-efficacy: reliability and validity studies. J Gerontol B Psychol Sci Soc Sci. 2002;57:P74–P86. doi: 10.1093/geronb/57.1.p74. [DOI] [PubMed] [Google Scholar]
- 22.Yeung PY, Wong LL, Chan CC. A validation study of the Hong Kong version of Montreal Cognitive Assessment (HK-MoCA) in Chinese older adults in Hong Kong. Hong Kong Med J. 2014;20:504–510. doi: 10.12809/hkmj144219. [DOI] [PubMed] [Google Scholar]
- 23.van der Linde R, Dening T, Stephan B. Longitudinal course of behavioural and psychological symptoms of dementia: systematic review. Br J Psychiatry. 2016;209:366–377. doi: 10.1192/bjp.bp.114.148403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The Centre for Health Protection (2020) Guidelines for Centre-based Services for the Prevention of Coronavirus disease (COVID-19) (Interim).www.chp.gov.hk/files/pdf/guideline_for_centre_based_services_eng.pdf
- 25.Jeyathevan G, Cameron JI, Craven BC. Re-building relationships after a spinal cord injury: experiences of family caregivers and care recipients. BMC Neurol. 2019;19:117. doi: 10.1186/s12883-019-1347-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Väyrynen S, Röning J, Alakärppä I. User-centered development of video telephony for servicing mainly older users: review and evaluation of an approach applied for 10 years. Human Technol. 2006;20:8–37. doi: 10.17011/ht/urn.2006157. [DOI] [Google Scholar]
- 27.Arnaert A, Klooster J, Chow V. Attitudes toward videotelephones: an exploratory study of older adults with depression. J Gerontol Nurs. 2007;33:5–13. doi: 10.3928/00989134-20070901-02. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.