Abstract
Introduction
The novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has emerged early December 2019 and was recently confirmed by the World Health Organization (WHO) to be a public health emergency of international concern. Earlier reports have shown coagulopathy in patients with severe coronavirus disease 2019 (Covid-19).
Main symptoms and important clinical findings
We present four critically ill Covid-19 patients, who were admitted to our hospital. They were treated with supportive care, oral chloroquine, and standard 2500 or 5000 International Units (IU) of dalteparine subcutaneously once daily. Two patients died during the course of their stay as a consequence of severe large vessel arterial thromboembolism. The other two patients survived but symptoms of paralysis and aphasia persisted after cerebral ischemia due to large vessel arterial thromboembolism. Patients showed no signs of overt disseminated intravascular coagulation (DIC) in their laboratory analysis.
Conclusion
This case series suggest that even in absence of overt DIC, arterial thromboembolic complications occur in critically ill patients with Covid-19. Further studies are needed to determine which parameters are useful in monitoring coagulopathy and which dose of anti-thrombotic therapy in Covid-19 patients is adequate, even when overt DIC is not present.
Keywords: Thrombosis, COVID-19, Disseminated intravascular coagulation, Anti-coagulant therapy, Coagulopathy, Imaging
Highlights
-
•
There is a higher risk of coagulopathy in critically ill Covid-19 patients
-
•
Coagulopathy in Covid-19 patients can increase the risk of arterial thromboembolism despite absence of overt DIC.
-
•
Further studies are needed to determine which parameters are useful in monitoring coagulopathy
-
•
More research is needed to determine adequate anti-thrombotic therapy in Covid-19 patients, even in absence of overt DIC.
1. Introduction
The novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) emerged in Wuhan, the capital city of the Hubei province in China at the end of 2019 [1,2]. Infection with SARS-CoV-2 results in coronavirus disease 2019 (Covid-19). In the United States alone over 2,000,000 patients were already diagnosed with Covid-19, and the World Health Organization has declared Covid-19 a public health emergency of international concern [3,4].
Recent reports and studies suggest that Covid-19 can cause coagulopathy in severe cases, which has a negative prognostic factor for survival. The majority of non-survivors with Covid-19 show signs of disseminated intravascular coagulation (DIC) [[5], [6], [7]]. Recent studies mainly focus on pulmonary thromboembolism and small arterial vessel disease. [[8], [9], [10], [11]] Only small patient series are available on systemic arterial thromboembolic disease of the larger vessels. [[12], [13], [14]]
We present four cases of Covid-19 patients, who presented with large vessel arterial thromboembolic disease, without showing signs of overt DIC. These cases highlight the importance of recognizing signs of arterial thromboembolism in critically ill Covid-19 patients. It also emphasizes the importance of monitoring the presence of hypercoagulopathy, which may have implications for adjustment of anti-coagulant treatment regimens in these critically ill patients.
2. Patients
2.1. Patient 1
A 58-year-old male was admitted to our hospital on March 27, 2020. He had a history of psoriasis and psoriatic arthritis for which he used methotrexate from 2011 until 2016. He presented to the ER with cough, fever and malaise which had started 12 days earlier. His chest radiograph showed signs of peripheral consolidations, and real-time reverse-transcriptase–polymerase-chain-reaction (rRT-PCR) proved positive for SARS-CoV-2. A summary is provided in Table 1 . He received daily subcutaneous injections of 2500 IU of dalteparin (body weight 65 kg). Treatment with chloroquine was started orally (start dose 600 mg, followed by 300 mg twice daily).
Table 1.
Characteristics and laboratory analysis of patients.
| Characteristics | Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|---|
| Age | 58 | 81 | 58 | 48 | |
| Sex | Male | Male | Female | Male | |
| Medical History | Psoriasis and psoriatic arthritis | MGUS | Hypertension, gout | None | |
| Symptoms at onset | Cough, fever and malaise | Back pain, malaise, and fever | Cough, fever, malaise | Cough, fever, malaise | |
| Thoracic imaging | CT: Extensive bilateral ground glass opacity and consolidations | Radiograph: extensive bilateral consolidations | Radiograph: extensive bilateral consolidations | Radiograph: extensive bilateral consolidations | |
| Medication on admission | Dalteparin 2500 s.c. Second generation cephalosporin i.v. Chloroquine oral |
Dalteparin 2500 s.c. Second generation cephalosporin i.v. Chloroquine oral |
Dalteparin 2500 s.c. Second generation cephalosporin i.v. |
Dalteparin 5000 s.c. Haloperidol |
|
| Oxygen saturation (%) during admission, mean (range) | 94 (91–98) Nnasal cannula, 4 L O2) |
96 (95–98) Nasal cannula, tube after admission ICU |
95 (93–98) Nasal cannula (4 L O2) |
97 (92–99) Tube during admission to ICU, nasal cannula on ward |
|
| MAP (mmHg), mean (range) | 93 (87–101) | 85 (76–129 | 85 (74–101) | 81 (63–107) | |
| Diagnosis of arterial thromboembolism | Bilateral occlusion of carotid arteries, middle cerebral arteries and proximal anterior cerebral arteries | Extensive bilateral ischemia in territory of middle and posterior cerebral artery and superior cerebellar arteries | Occlusion of the left middle cerebral artery | Bilateral ischemia in territory of middle and posterior cerebral arteries. | |
| SOFA-score at diagnosis of arterial thromboembolism | 3 | 8 | 3 | 2 | |
| Department at time of diagnosis of arterial thromboembolism | Normal ward | ICU | Normal ward | Normal ward | |
| Vasopression/inotropy during admission | No | Yes, 2 days before arterial thromboembolism, noradrenalin | No | Yes, last administration 6 days before arterial thromboembolism, noradrenalin | |
| Outcome | Deceased | Deceased | Alive, right sided paralysis and global aphasia | Alive, minimal left sided paralysis of the arm and leg, severe aphasia | |
| Parameter | Reference | ||||
| Hemoglobin | 8.5–11.0 mmol/L | 7.4 | 6.6 | 7.2 | 6.2 |
| Leukocyte count | 4.0–10.0 × 109/L | 12.6 (+) | 12.8 (+) | 8.9 | 12.7 |
| Lymphocyte count | 1.0–3.5 × 109/L | 0.5 (−) | 0.34 (−) | 1.2 | 2.2 |
| Thrombocyte count | 150–400 × 109/L | 570 (+) | 170 | 320 | 830 |
| CRP | <6.0 mg/L | 210 (+) | 84 (+) | 49 | 57 |
| Troponin-T | <30 ng/L | 11 | 25 | 7 | – |
| Lactate | <1.3 mmol/L | 8.1 (+) | 1.2 | – | 1.1 |
| LD | <250 U/L | 781 (+) | 699 (+) | 176 | 354 (+) |
| Total Bilirubin | < 17 μmol/L | 9.3 | 9.4 | 4.6 | 5.8 |
| PT | 12.0–14.5 s | – | 15.3 (+) | – | 19.4 (+) |
| D-dimer | <0.8 mg/L | 2.2 (+) | >4 (+) | >4 (+) | >20 (+) |
| DIC-score (ISTH) | Not available | 4 | Not available | 4 | |
On March 31, 2020, he was found unresponsive, with extensor posturing, and gasping breath. Under suspicion of stroke or intracranial haemorrhage, an unenhanced CT-scan of the brain was performed which showed no signs of ischemia or haemorrhage. CT-angiography of the aortic arch, carotids and circle of Willis (Fig. 1 ) showed bilateral occlusion of the carotid arteries, the middle cerebral arteries, and proximal anterior cerebral arteries. Laboratory analysis showed markedly elevated D-dimer values (Table 1). Due to severe neurologic decline and the extensiveness of thrombosis with no possibilities for further treatment, palliative sedation was started and the patient died several hours later.
Fig. 1.
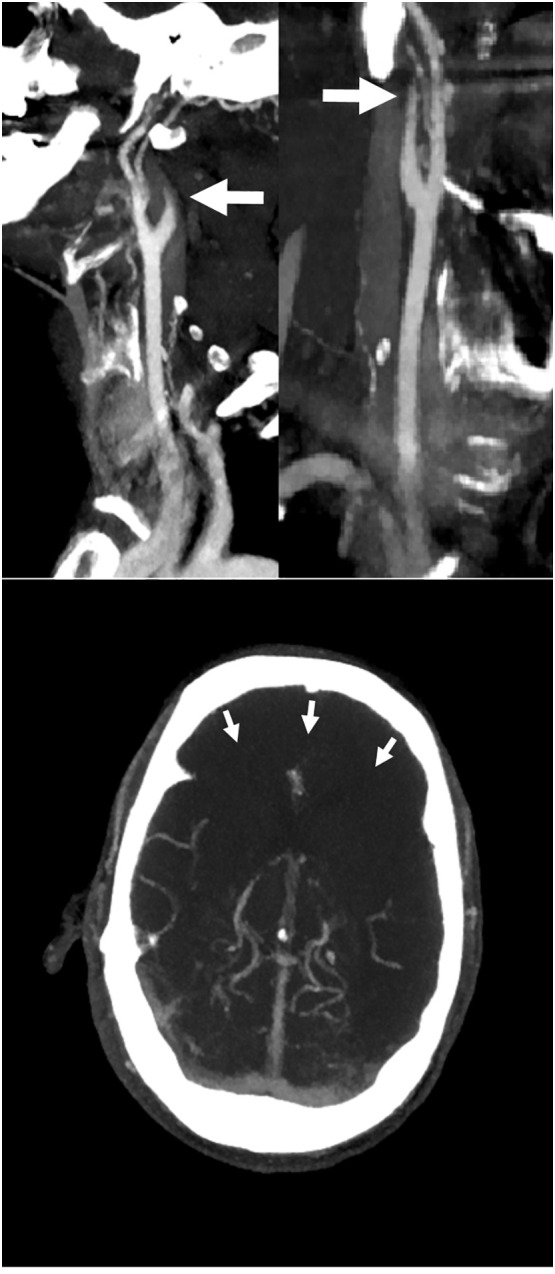
CTA of the carotid arteries. On the upper left panel, maximum intensity projection (MIP) of the left carotid bifurcation is shown, on the upper right panel a MIP of the right carotid bifurcation. White arrows indicate decreased contrast opacification in the internal carotid arteries, the actual thrombus could not be visualized.
In the lower panel an axial MIP of the circle of Willis. The arrows indicate near complete absence of vascular enhancement in the vascular territories of the anterior and part of the medial cerebral arteries due to diffuse arterial thrombosis.
2.2. Patient 2
An 81-year-old male was admitted to our hospital on March 22, 2020. He had a medical history of stable IgG kappa monoclonal gammopathy of unknown significance (MGUS). He presented with back pain, malaise, and fever for seven days. A summary is provided in Table 1. A chest radiograph showed extensive bilateral consolidations, and rRT-PCR proved positive for SARS-CoV-2.
He received daily subcutaneous injections of 2500 IU of dalteparin (body weight 75 kg). Treatment with chloroquine was started orally (start dose 600 mg, followed by 300 mg twice daily).
His condition deteriorated and 5 days after admission he was transferred to the ICU. He was intubated and ventilated due to respiratory insufficiency. Laboratory analysis is shown in Table 1. When a wake-up call was carried out by stopping all sedatives, the patient remained unresponsive and showed an abnormal breathing pattern on mechanical ventilation.
An unenhanced CT-scan of the brain was performed (Fig. 2 ), which showed extensive bilateral ischemia with haemorrhagic transformation and oedema in the vascular territory of both middle cerebral arteries, the posterior cerebral artery and superior cerebellar arteries on both sides The patient died the same day after discontinuation of treatment.
Fig. 2.
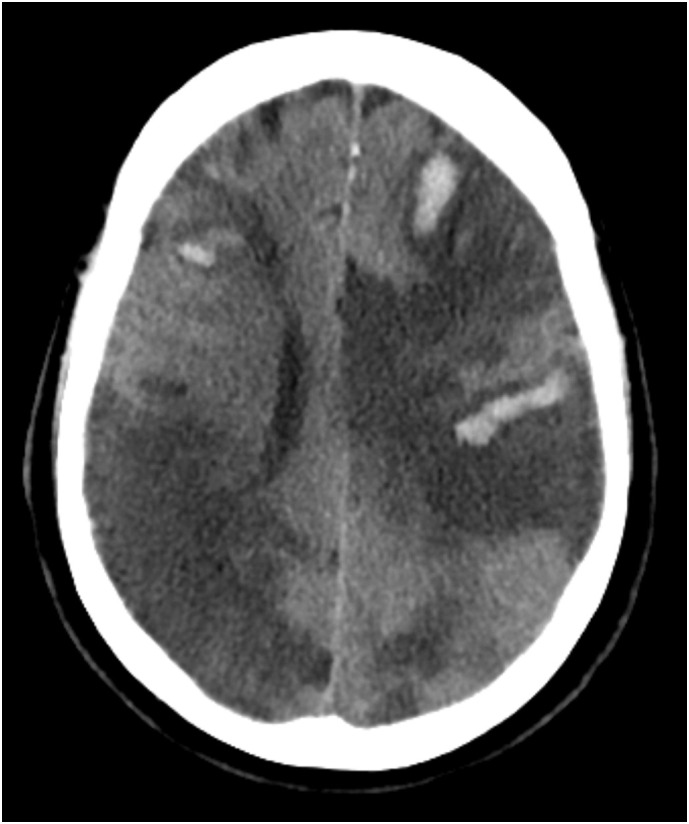
Unenhanced CT-scan of the brain showing bilateral, diffuse ischemia in multiple vascular territories with haemorrhagic transformation of ischemic areas.
2.3. Similar patients
In the period of March 2020 until April 2020, two other Covid-19 patients at our institution presented with cerebral ischemia.
Patient 3 was a 58-year old female. She was transferred from another institution with sudden apathy and right-sided paralysis. She had a medical history of hypertension and gout. CT-angiography showed occlusion of the left middle cerebral artery, and a successful intra-arterial mechanic thrombectomy was performed. She recovered from her Covid-19. She still suffers from right sided paralysis at the time of writing, as well as a global aphasia, however some improvements were seen during admission.
Patient 4 was a 48-year old male without relevant medical history who presented with minimal left-sided paralysis of the arm and leg, brisk reflexes on the left side and severe aphasia in the course of his Covid-19. An unenhanced CT showed bilateral ischemia in vascular territories of the middle and posterior cerebral arteries. There were no options for intra-arterial treatment in this patient. The patient has largely recovered from his Covid-19 and was discharged recently. At the time of discharge, he still suffered from aphasia, but had largely recovered from his minimal left sided paralysis.
Patient characteristics and laboratory analyses are shown in Table 1.
3. Discussion
In this case series, we report four patients with severe Covid-19, who developed severe thromboembolism of cerebral arteries. Of importance is that these patients had no medical history of clinically significant atherosclerotic disease, cardiovascular disease, hypertension, stroke or coagulopathy. These cases highlight the importance of adequate monitoring of coagulopathy in critically ill Covid-19 patients and vigilance for early signs of arterial thromboembolic disease even in patients without overt cardiovascular risk profiles.
DIC is a well-established cause of coagulopathy in sepsis and septic shock. In DIC, interleukin-6 (IL-6) and tumor necrosis factor (TNF) play a central role in sepsis mediated coagulopathy and fibrin deposits are caused by tissue-factor dependent thrombin generation. At the same time, physiologic anticoagulant mechanisms (antithrombin, tissue factor pathway inhibitor (TFPI) and the protein C system) function inappropriately. Furthermore, fibrinolysis is inactivated by high plasma concentrations of plasminogen activator-inhibitor-1 (PAI-1) [15]. As a consequence, DIC leads to thrombotic occlusion of small and middle large arteries, but at the same time increases the risk of severe bleeding, because of consumption of platelets and coagulation factors (i.e. consumption coagulopathy). According to the International Society on Thrombosis and Haemostasis (ISTH) criteria for DIC, low platelet counts, increased prothrombin time (PT), decreased fibrinogen and high d-dimer levels dictate the risk of overt DIC [16].
Recent reports have been published on coagulopathy in critically ill Covid-19 patients, as well as large series which focused on pulmonary thromboembolism [5,7,9,11]. In the majority of cases elevated levels of D-dimer and prolonged PT are found in non-survivors compared to survivors, with the majority of non-survivors showing signs of overt DIC. Coagulopathy is suggested to be a poor prognostic indicator of survival in cases of Covid-19 [5]. In this case series we found markedly elevated D-dimer levels in all patients as well, however there was no accompanying thrombocytopenia. Reports also show an increased risk of thrombosis in patients with a history of cardiovascular disease and stroke, as well as hypertension and diabetes. Surprisingly, our patients had no such medical history [14,17,18].
It has to be mentioned, that in the early months after SARS-CoV-2 emerged, chloroquine and hydroxychloroquine have been widely used in the treatment of Covid-19 due to their in vitro Covid-19 inhibiting properties. Also, at the time the patients in this case series were admitted to our hospital, treatment with hydroxychloroquine was standard practice. In a more recent report, treatment with hydroxychloroquine has not been proven beneficial in terms of intubation rate or survival. [19] Nonetheless, procoagulant effects of hydroxychlorquine have not been described. Rather, anti-coagulant effects have been shown in the treatment of systemic lupus erythematosus (SLE). [20].
Although DIC-scores could not reliably be calculated according to ISTH criteria in some of our patients because of missing values, available laboratory results were not suggestive of overt DIC. Especially, normal or elevated thrombocyte counts as found in all patients argue against presence of overt DIC [16]. Our findings suggest that in absence of overt DIC, arterial thromboembolic complications can still occur in critically ill patients with Covid-19.
Adequate thromboembolic prophylaxis is important in treating Covid-19 patients and might be associated with decreased mortality in patients [6]. Patients currently receive standard prophylactic doses of low molecular weight heparin (LMWH) on admission in our hospital. Up until now, no clear benefit of higher doses of LMWH has been shown for septic patients with clinically relevant coagulopathy, including patients with DIC [21,22]. Specific features of critically ill Covid-19 patients (e.g. severe hypoxemia, hyper inflammatory status and ACE2 receptor mediated endothelial cell activation) might influence coagulopathy in different ways than expected based on current knowledge.
With emerging data on coagulopathy in critically ill Covid-19 patients, higher doses of LMWH might be indicated to prevent both venous and arterial thromboembolism. [12,14,23] Partly because of these cases of cerebral artery thromboembolism we changed our regimen, and currently use dalteparin 5000 IU twice-daily for patients above 80 kg, and 5000 IU once-daily for patients below 80 kg on admission to our ICU. On indication, this regimen might need to be extended to Covid-19 patients admitted at nursing wards, supported by the fact that patients 1 and 2 presented with arterial thromboembolism before they were admitted to the ICU. Further studies are needed to determine adequate treatment regimens in Covid-19 patients with coagulopathy without overt signs of DIC. Also, the extensiveness of arterial thromboembolism related to Covid-19 should be evaluated in larger studies, in order to estimate the prevalence of this serious complication. Most likely, prevalence is underestimated as coagulopathy and thrombosis might have been unrevealed in deceased Covid-19 patients.
There are some limitations in this case series. PT-values are not routinely tested at our institution, especially in patients admitted to the nursing wards. At the time of diagnosis of stroke, limited knowledge was available on large vessel arterial thrombosis in Covid-19 patients. Therefore, routine blood sample analyses of Covid-19 patients only included a subset of coagulation parameters. This impeded calculation of DIC-scores according to ISTH criteria, and also impeded analysis of trends in coagulation parameters. Second, we did not perform contrast-enhanced CT in patient 2. However, due to diffuse ischemia in multiple vascular territories, it was concluded in consensus (by radiologists, neurologist and intensivist) that this resulted from diffuse large vessel arterial thromboembolism.
We report the clinical course of four patients infected with SARS-CoV-2, who presented with arterial thromboembolic events during their admission to our hospital, despite a medical history which was negative for diabetes, hypertension, cardiovascular disease, atrial fibrillation and stroke. Key aspects include the risk of hypercoagulopathy in critically ill Covid-19 patients, which result in an increased risk for large arterial vessel thromboembolism. Further studies are needed to determine the prevalence of arterial thrombo-embolism in Covid-19 and to determine which parameters are useful in monitoring coagulopathy in different stages of their illness. Adjustment of anticoagulation management regimens needs to be considered in order to prevent Covid-19 patients from developing not only venous, but also severe arterial thrombotic complications.
Financial support
None.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coronavirus COVID-19 global cases by the center for systems science and engineering (CSSE) at Johns Hopkins University (JHU) 2020. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6
- 4.Organization WH Coronavirus disease (COVID-2019) situation reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
- 5.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020 doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han H., Yang L., Liu R., Liu F., Wu K.L., Li J. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020 doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 8.Xie Y., Wang X., Yang P., Zhang S. COVID-19 complicated by acute pulmonary embolism. Radiol Cardiothorac Imag. 2020;2(2) doi: 10.1148/ryct.2020200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y., Cao W., Xiao M., Li Y.J., Yang Y., Zhao J. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia. Zhonghua Xue Ye Xue Za Zhi. 2020;41(0):E006. doi: 10.3760/cma.j.issn.0253-2727.2020.0006. [DOI] [PubMed] [Google Scholar]
- 10.Oudkerk M., Buller H.R., Kuijpers D., van Es N., Oudkerk S.F., McLoud T.C. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: report of the National Institute for public health of the Netherlands. Radiology. 2020 doi: 10.1148/radiol.2020201629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382(17) doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kashi M., Jacquin A., Dakhil B., Zaimi R., Mahe E., Tella E. Severe arterial thrombosis associated with Covid-19 infection. Thromb Res. 2020;192:75–77. doi: 10.1016/j.thromres.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levi M., van der Poll T., ten Cate H., van Deventer S.J. The cytokine-mediated imbalance between coagulant and anticoagulant mechanisms in sepsis and endotoxaemia. Eur J Clin Invest. 1997;27(1):3–9. doi: 10.1046/j.1365-2362.1997.570614.x. [DOI] [PubMed] [Google Scholar]
- 16.Toh C.H., Hoots W.K., SSCoDICot I.S.T.H. The scoring system of the scientific and standardisation committee on disseminated intravascular coagulation of the international society on thrombosis and haemostasis: a 5-year overview. J Thromb Haemost. 2007;5(3):604–606. doi: 10.1111/j.1538-7836.2007.02313.x. [DOI] [PubMed] [Google Scholar]
- 17.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020 doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020 doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G. Observational study of Hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruiz-Irastorza G., Ramos-Casals M., Brito-Zeron P., Khamashta M.A. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis. 2010;69(1):20–28. doi: 10.1136/ard.2008.101766. [DOI] [PubMed] [Google Scholar]
- 21.Umemura Y., Yamakawa K., Ogura H., Yuhara H., Fujimi S. Efficacy and safety of anticoagulant therapy in three specific populations with sepsis: a meta-analysis of randomized controlled trials. J Thromb Haemost. 2016;14(3):518–530. doi: 10.1111/jth.13230. [DOI] [PubMed] [Google Scholar]
- 22.Zarychanski R., Abou-Setta A.M., Kanji S., Turgeon A.F., Kumar A., Houston D.S. The efficacy and safety of heparin in patients with sepsis: a systematic review and metaanalysis. Crit Care Med. 2015;43(3):511–518. doi: 10.1097/CCM.0000000000000763. [DOI] [PubMed] [Google Scholar]
- 23.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]


