Abstract
Sperm malformation is a direct factor for male infertility. Multiple morphological abnormalities of the flagella (MMAF), a severe form of asthenoteratozoospermia, are characterized by immotile spermatozoa with malformed and/or absent flagella in the ejaculate. Previous studies indicated genetic heterogeneity in MMAF. To further define genetic factors underlying MMAF, we performed whole-exome sequencing in a cohort of 90 Chinese MMAF-affected men. Two cases (2.2%) were identified as carrying bi-allelic missense DNAH8 variants, variants which were either absent or rare in the control human population and were predicted to be deleterious by multiple bioinformatic tools. Re-analysis of exome data from a second cohort of 167 MMAF-affected men from France, Iran, and North Africa permitted the identification of an additional male carrying a DNAH8 homozygous frameshift variant. DNAH8 encodes a dynein axonemal heavy-chain component that is expressed preferentially in the testis. Hematoxylin-eosin staining and electron microscopy analyses of the spermatozoa from men harboring bi-allelic DNAH8 variants showed a highly aberrant morphology and ultrastructure of the sperm flagella. Immunofluorescence assays performed on the spermatozoa from men harboring bi-allelic DNAH8 variants revealed the absent or markedly reduced staining of DNAH8 and its associated protein DNAH17. Dnah8-knockout male mice also presented typical MMAF phenotypes and sterility. Interestingly, intracytoplasmic sperm injections using the spermatozoa from Dnah8-knockout male mice resulted in good pregnancy outcomes. Collectively, our experimental observations from humans and mice demonstrate that DNAH8 is essential for sperm flagellar formation and that bi-allelic deleterious DNAH8 variants lead to male infertility with MMAF.
Keywords: CRISPR, DNAH8, DNAH17, dynein, exome, flagella, ICSI, infertility, knockout, sperm
Main Text
Human infertility, defined as the inability to achieve a clinical pregnancy despite 12 months of regular and unprotected intercourse, has become a widespread health issue.1 Multiple morphological abnormalities of the flagella (MMAF) are defined by the combination of absent, short, bent, coiled, and/or irregular-caliber flagella.2 Previous genetic studies revealed a series of MMAF-associated genes in cases of primary infertility without primary ciliary dyskinesia (PCD; MIM: 244400) associated symptoms (reviewed by Touré et al.).3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 However, these genetic findings account for approximately 35% to 60% of MMAF cases,11,12 demonstrating the high genetic heterogeneity of this disorder and the necessity for further genetic explorations.
Cilia and flagella are hair-like organelles extending from the cell surface.15,16 Both contain an important core component, termed the axoneme, which is an evolutionarily conserved structure consisting of a highly ordered “9 + 2” arrangement of nine peripheral microtubule doublets and two central microtubules.17 A number of multi-protein complexes (including radial spokes, nexin-dynein regulatory complex, central complex, and dynein arms) constitute the major components of the axoneme.18 Among the complexes, the outer and inner dynein arms (ODAs and IDAs, respectively) play an important role in the beating of cilia and flagella through ATP hydrolysis.19 Previous studies have revealed that mutations in IDA and ODA protein complexes cause several ciliopathies and male infertility. In particular, the deficiency of DNAH1 (MIM: 603332), which encodes an important component of the IDA heavy chain, leads to isolated male infertility with MMAF.2 Importantly, recent studies reported that mutations in DNAH17 (MIM: 610063; encoding a sperm-specific ODA heavy-chain component) also cause isolated male infertility due to asthenoteratozoospermia.13,20 These findings suggest the potential involvement of other components of dynein arms in male infertility and sperm flagellar malformations.
Here, two distinct MMAF cohorts were analyzed. The first cohort comprised 90 Chinese MMAF-affected men enrolled from the First Affiliated Hospital of Anhui Medical University and the Women and Children’s Hospital of Xiamen University in China. The second cohort comprised 167 individuals with MMAF, including 83 men from North Africa (Algeria, Libya, and Tunisia; enrolled at the Clinique des Jasmins in Tunis), 52 men recruited at the Royan Institute (Reproductive Biomedicine Research Center) in Iran, and 32 men recruited in France (mainly at the Reproductive Department of the Cochin Hospital in Paris). The clinical phenotypes of the affected individuals are summarized in the Supplemental Note (see Supplemental Information). Informed consent was obtained from all subjects participating in the study. The study regarding the cohorts was approved by the institutional review boards at all of the participating institutes.
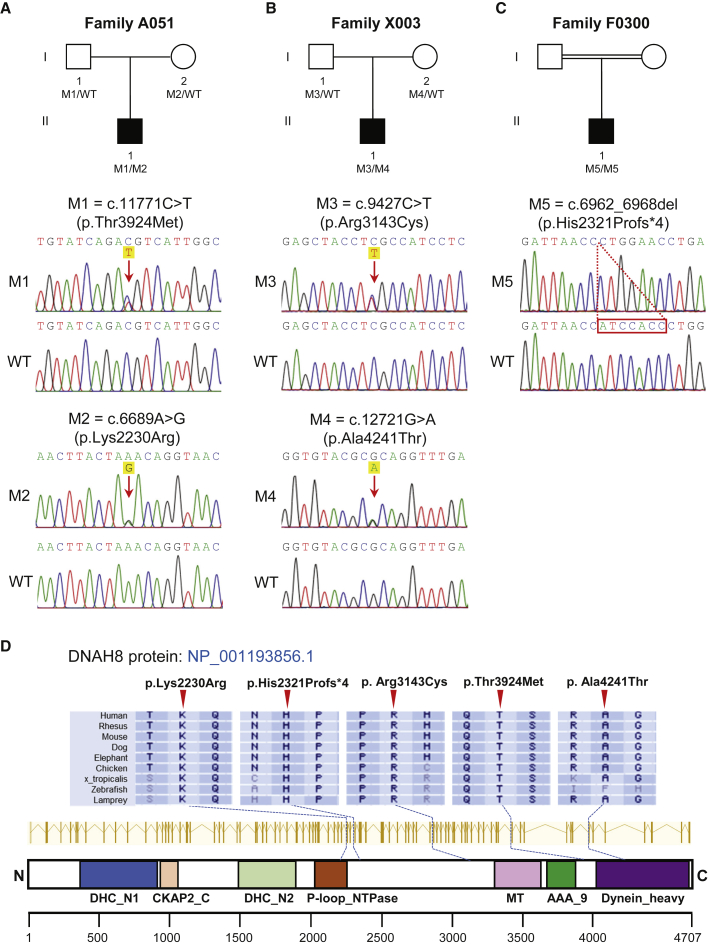
To investigate the unknown genetic factors involved in human MMAF, we performed whole-exome sequencing (WES) analyses in the first cohort of 90 Chinese men with MMAF. After applying stringent bioinformatic analyses according to our previously described protocol,4 we identified two men (2.2%) harboring bi-allelic missense variants in DNAH8 (MIM: 603337; NCBI: NM_001206927.2). The DNAH8-mutated alleles were c.11771C>T (p.Thr3924Met) plus c.6689A>G (p.Lys2230Arg) in subject A051 (II-1 in Figure 1A) and c.9427C>T (p.Arg3143Cys) plus c.12721G>A (p.Ala4241Thr) in subject X003 (II-1 in Figure 1B). Subsequent Sanger sequencing confirmed that these bi-allelic DNAH8 variants were inherited from heterozygous parental carriers (Figures 1A and 1B; Table S1). All of the DNAH8 variants were either absent or rare in the human genome datasets archived in the 1000 Genomes Project and gnomAD databases. These DNAH8 variants were also predicted to be damaging through the use of the PolyPhen-2, SIFT, and MutationTaster tools (Table 1).
Figure 1.
Identification of Bi-allelic DNAH8 Variants in Men with MMAF
(A–C) The pedigrees of three families affected by DNAH8 variants. The NCBI reference sequence number for DNAH8 transcript is NM_001206927.2. Sanger sequencing results are shown below the pedigrees. The variant positions are indicated by red arrows or a dashed box. WT, wild type.
(D) Variant locations and phylogenetic conservation of the mutated residues in DNAH8 protein. The NCBI reference sequence number for DNAH8 protein is NP_001193856.1. Colored squares denote different domains according to the NCBI browser. DHC_N1—dynein heavy chain, N-terminal region 1; CKAP2_C—cytoskeleton-associated protein 2 C terminus; DHC_N2—dynein heavy chain, N-terminal region 2; P-loop_NTPase—P-loop containing nucleoside triphosphate hydrolases; MT—microtubule-binding stalk of dynein motor; AAA_9—ATP-binding dynein motor region D5; Dynein_heavy—dynein heavy chain and region D6 of dynein motor.
Table 1.
Bi-allelic DNAH8 Variants Identified in MMAF-affected Men
| - | Subject A051 | Subject X003 | Subject F0300 | ||
|---|---|---|---|---|---|
| cDNA alteration | c.11771C>T | c.6689A>G | c.9427C>T | c.12721G>A | c.6962_6968del |
| Variant allele | heterozygous | heterozygous | heterozygous | heterozygous | homozygous |
| Protein alteration | p.Thr3924Met | p.Lys2230Arg | p.Arg3143Cys | p.Ala4241Thr | p.His2321Profs∗4 |
| Variant type | missense | missense | missense | missense | frameshift |
| Allele Frequency in Human Population | |||||
| 1000 Genomes | 0 | 0 | 0 | 0 | 0 |
| gnomAD (v3) | 0.006729 | 0 | 0.0004677 | 0.0001187 | 0 |
| Function Prediction | |||||
| SIFT | damaging | damaging | damaging | damaging | NA |
| PolyPhen-2 | damaging | damaging | damaging | damaging | NA |
| MutationTaster | damaging | damaging | damaging | damaging | damaging |
NCBI reference sequence number of DNAH8 is NM_001206927.2.
NA, not applicable.
WES analysis of the 167 MMAF-affected men from the second cohort identified an additional case in an individual of Moroccan ancestry (Figure 1C). This MMAF-affected subject F0300 (II-1 in Figure 1C) harbored a homozygous frameshift variant (c.6962_6968del [p.His2321Profs∗4]) that produced a frameshift and premature stop codon in DNAH8. No DNA was available from family members of subject F0300. His brother was reported to be infertile, although no further investigation could be performed. We note that his parents are consanguineous, thus strongly supporting the likelihood that this DNAH8 frameshift variant was also transmitted under a recessive mode of inheritance.
Importantly, the residues in DNAH8 affected by these aforementioned variants are all highly conserved across species (Figure 1D). Furthermore, no bi-allelic deleterious variants in previously described MMAF- or PCD-associated genes were observed in the three men with bi-allelic DNAH8 variants. These findings further suggest that the infertility phenotypes were likely caused by the identified bi-allelic DNAH8 variants.
DNAH8 contains 92 exons and encodes a predicted 4,707-amino-acid protein (NCBI: NP_001193856.1; UniProt: A0A075B6F3). The DNAH8 protein is preferentially expressed in the human testis, according to the Human Protein Atlas. Our reverse transcription polymerase chain reaction (RT-PCR) assays also indicated that mouse Dnah8 is predominantly expressed in the testis (Figure S1A). Furthermore, the expression of mouse Dnah8 mRNA in the testis began at 14 days after birth, corresponding to the pachytene stage (Figure S1B).
Semen parameters of men harboring bi-allelic DNAH8 variants were analyzed in the source laboratories according to World Health Organization guidelines.21 Sperm motility and progressive motility in the men harboring bi-allelic DNAH8 variants were dramatically lower than the normal reference values (Table 2). Hematoxylin-eosin (H&E) staining and scanning electron microscopy examination were performed to assess sperm morphology. Approximately 70% of the immotile spermatozoa displayed abnormal flagella, including absent, short, and coiled flagella, angulation, and irregular caliber (Figure 2 and Table 2).
Table 2.
Semen Characteristics and Sperm Flagellar Morphology of Men Carrying Bi-allelic DNAH8 Variants
| - | Subject A051 | Subject X003 | Subject F0300 | Reference Values |
|---|---|---|---|---|
| Semen Parameters | ||||
| Semen volume (mL) | 2.2 | 3.2 | 7.1 | >1.5 |
| Sperm concentration (106/mL) | 39.1 | 42.7 | 13.6∗ | >15.0 |
| Total sperm count (106) | 86.0 | 136.7 | 96.6 | >39.0 |
| Motility (%) | 4.0∗ | 2.7∗ | 8.0∗ | >40.0 |
| Progressive motility (%) | 1.3∗ | 1.5∗ | 4.0∗ | >32.0 |
| Sperm Flagellar Morphology | ||||
| Absent flagella (%) | 2.0 | 2.5 | 4.0 | <5.0 |
| Short flagella (%) | 7.1∗ | 9.0∗ | 8.0∗ | <1.0 |
| Coiled flagella (%) | 57.1∗ | 32.0∗ | 33.0∗ | <17.0 |
| Angulation (%) | 0.5 | 6.0 | 19.0∗ | <13.0 |
| Irregular caliber (%) | 1.0 | 26.0∗ | 8.0∗ | <2.0 |
| Normal flagella (%) | 32.3 | 24.5 | 28.0 | >23.0 |
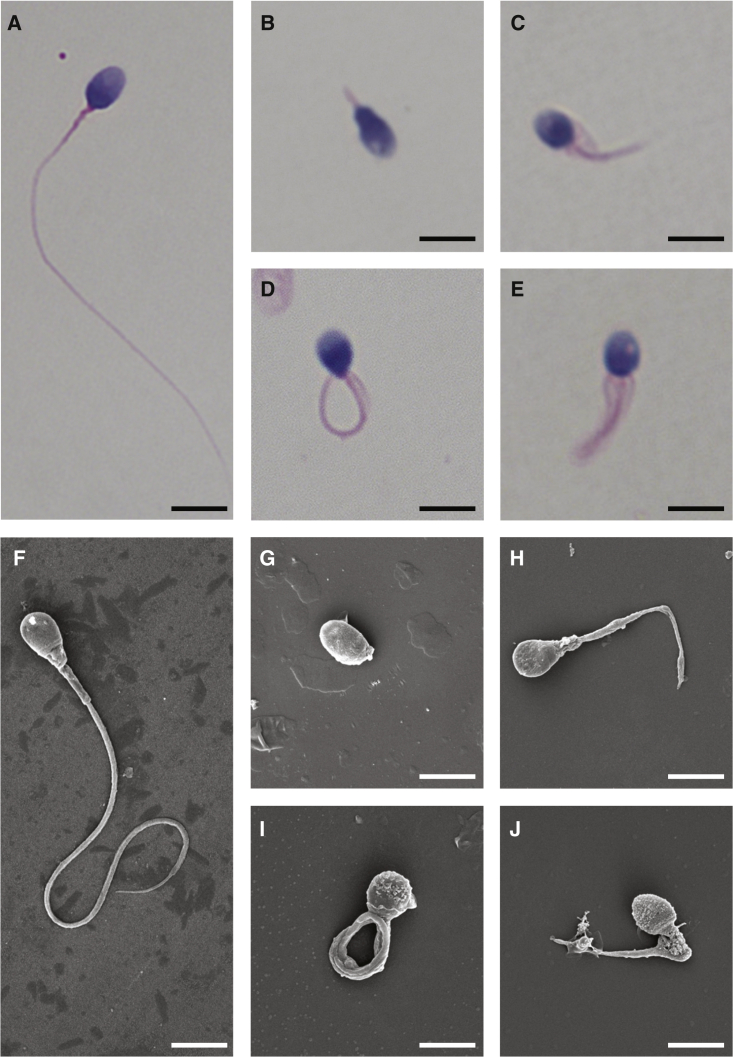
Figure 2.
Morphology of the Spermatozoa from Men Harboring Bi-allelic DNAH8 Variants
(A) Normal morphology of the spermatozoon from a healthy control male evident under light microscopy. Scale bars: 5 μm.
(B–E) Most spermatozoa from men harboring bi-allelic DNAH8 variants presented MMAF phenotypes, including absent (B), short (C), coiled (D), and irregular-caliberflagella (E). The data of subject A051 as an example.
(F) Normal morphology of the spermatozoon from a healthy control male as revealed by scanning electronic microscopy.
(G–J) The MMAF phenotypes, including absent (G), short (H), coiled (I), irregular-caliber flagella (J), were clearly revealed in the spermatozoa from subject A051 harboring bi-allelic DNAH8 variants.
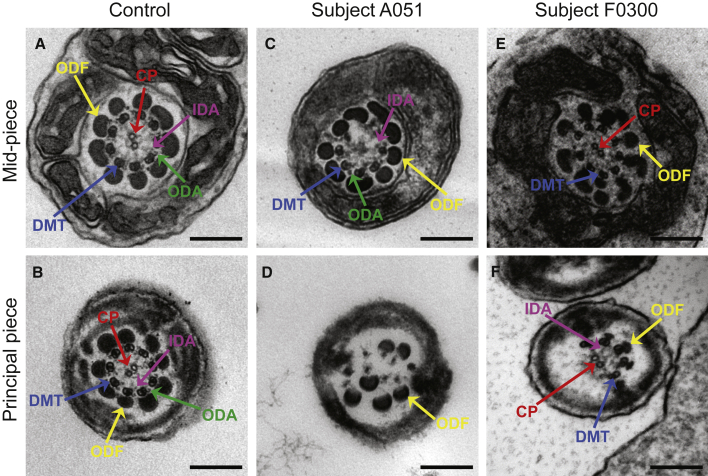
Various ultrastructural defects were revealed by transmission electron microscopy (TEM) in the sperm flagella from men harboring bi-allelic DNAH8 variants. The typical “9 + 2” microtubule structure was observed in the spermatozoa from control men (Figure 3). However, a dramatic disorganization in axonemal or peri-axonemal structures (including disorganized peripheral microtubule doublets and outer dense fibers, missing or disassembled ODAs, and absent central pairs) was detected in the spermatozoa from men harboring bi-allelic DNAH8 variants (Figure 3). Quantification conducted on transverse sections of the sperm flagella indicated higher rates of abnormal flagellar ultrastructure in men harboring bi-allelic DNAH8 variants than those in the normal control (Table S2).
Figure 3.
TEM Analyses of Sperm Cells from Men Harboring Bi-allelic DNAH8 Variants
(A and B) Cross-sections of the mid-piece (A) and principal piece (B) of the sperm flagella in a control individual show the typical ‘‘9 + 2’’ microtubule structure, including nine pairs of peripheral microtubule doublets (DMT; blue arrows), nine outer dense fibers (ODF; yellow arrows), and the central pair of microtubules (CP; red arrows). The outer dynein arms (ODA; green arrows) and inner dynein arms (IDA; pink arrows) are also visible. Scale bars: 200 μm.
(C–F) In the spermatozoa from men harboring bi-allelic DNAH8 variants, various axonemal anomalies can be observed, including the lack of CP (C) or DMT (D). Misarranged (D) or supernumerary ODFs (E) were also observed. ODAs were disassembled or absent in the samples from men carrying bi-allelic DNAH8 variants (C–F).
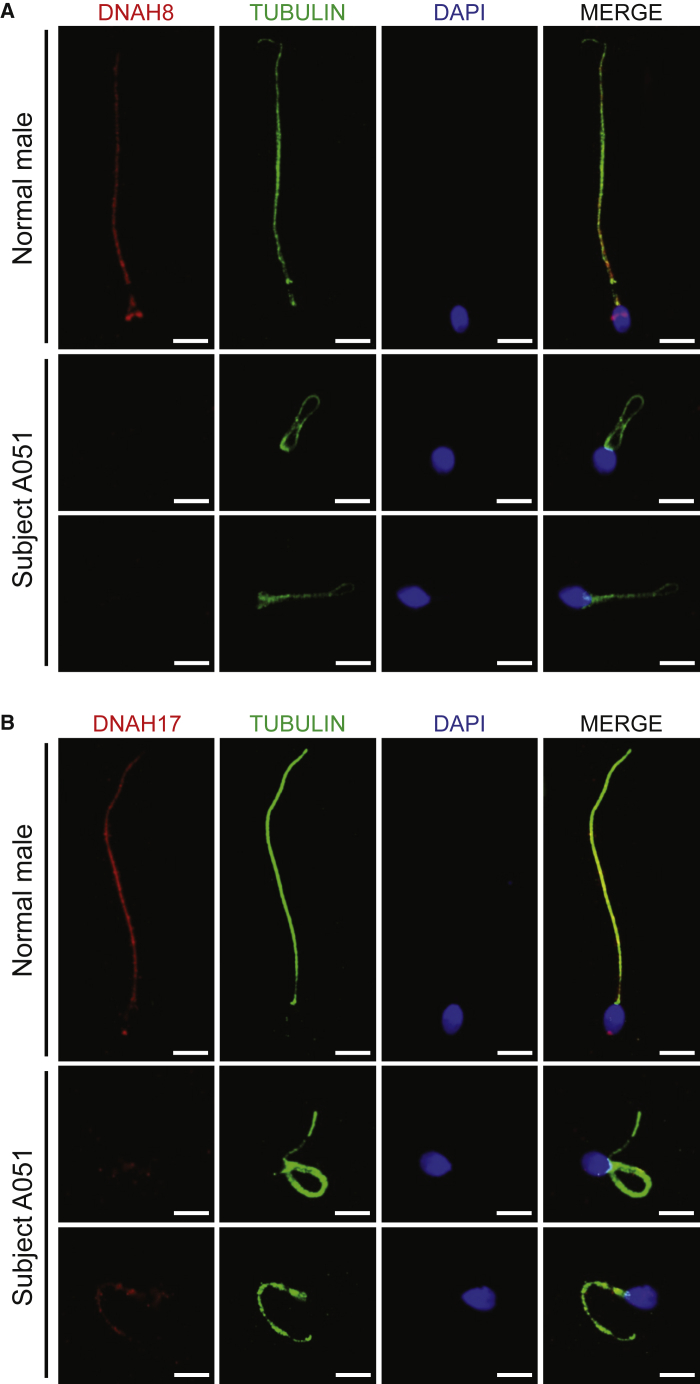
To further investigate the pathogenicity of bi-allelic DNAH8 variants, we analyzed the levels of DNAH8 mRNA and DNAH8 protein using RT-PCR (Table S3) and immunofluorescence assays, respectively. The abundance of DNAH8 mRNA in the sperm from subject A051, who harbored bi-allelic DNAH8 variants, was significantly reduced when compared to the normal control (Figure S2). As for the protein level, in the normal control man, DNAH8 immunostaining was concentrated along the mid-piece and principal piece of the sperm flagella (Figure S3). This observation in humans is consistent with previous evidence in wild-type male mice.22 In contrast, DNAH8 immunostaining was almost absent in the sperm flagella from all three subjects harboring bi-allelic DNAH8 variants, including both missense and frameshift variants (Figure 4A and Figure S3A). We also examined the presence of DNAH17, which is required for accurate localization of DNAH8.13 Notably, the staining of DNAH17 was dramatically reduced in the spermatozoa from men harboring bi-allelic DNAH8 variants (Figure 4B and Figure S3B).
Figure 4.
Localization of DNAH8 and Associated Protein DNAH17 in the Spermatozoa from Men Harboring Bi-allelic DNAH8 Variants
(A) Immunofluorescence staining of the spermatozoa from a normal control male and subjects carrying bi-allelic DNAH8 variants. Anti-DNAH8 (red) and anti-α-tubulin (green) antibodies were used. Spermatozoa were counterstained with 4′,6-diamidino-2-phenylindole as a marker of the cell nucleus. In the fertile control male, DNAH8 immunostaining (red) concentrated along the sperm flagella, but this signal was almost absent from the sperm flagella from subjects harboring bi-allelic DNAH8 variants. The data of subject A051 are provided to illustrate the typical staining observed in the DNAH8-associated cases. Scale bars: 5 μm.
(B) DNAH17 immunostaining is affected in the spermatozoa from men harboring bi-allelic DNAH8 variants. The spermatozoa were stained with anti-DNAH17 (red) and anti-α-tubulin (green) antibodies. DNAH17 staining mainly localized along the spermflagella from a control male, while being evidently reduced in the spermatozoa from subject A051. Scale bars: 5 μm.
DNAH8 is highly conserved among different species during evolution. Consistent with the human data available from the Human Protein Atlas, the murine ortholog Dnah8 is also preferentially expressed in the testis, as per our RT-PCR assays conducted in a set of various mouse tissues (Figure S1, Table S4). To further investigate the role of mouse Dnah8 in sperm flagellar formation, we generated Dnah8-knockout (KO; Dnah8em1/em1) mice through the use of CRISPR-Cas9 technology. Two guide RNAs targeting the regions near the start and stop codons were used to delete the entire coding region of Dnah8 (Figure S4A). Polymerase chain reaction (PCR) and Sanger sequencing were performed to confirm the mutated allele in Dnah8-KO mice (Figures S4B and S4C). We also used an immunoblot assay to investigate the level of DNAH8 protein in the testes of wild-type and Dnah8-KO male mice. As shown in Figure S5, the signal of DNAH8 was absent in the testis from Dnah8-KO male mice. No significant differences were observed in testis weight between Dnah8-KO and heterozygous mutated male mice (Figure S6).
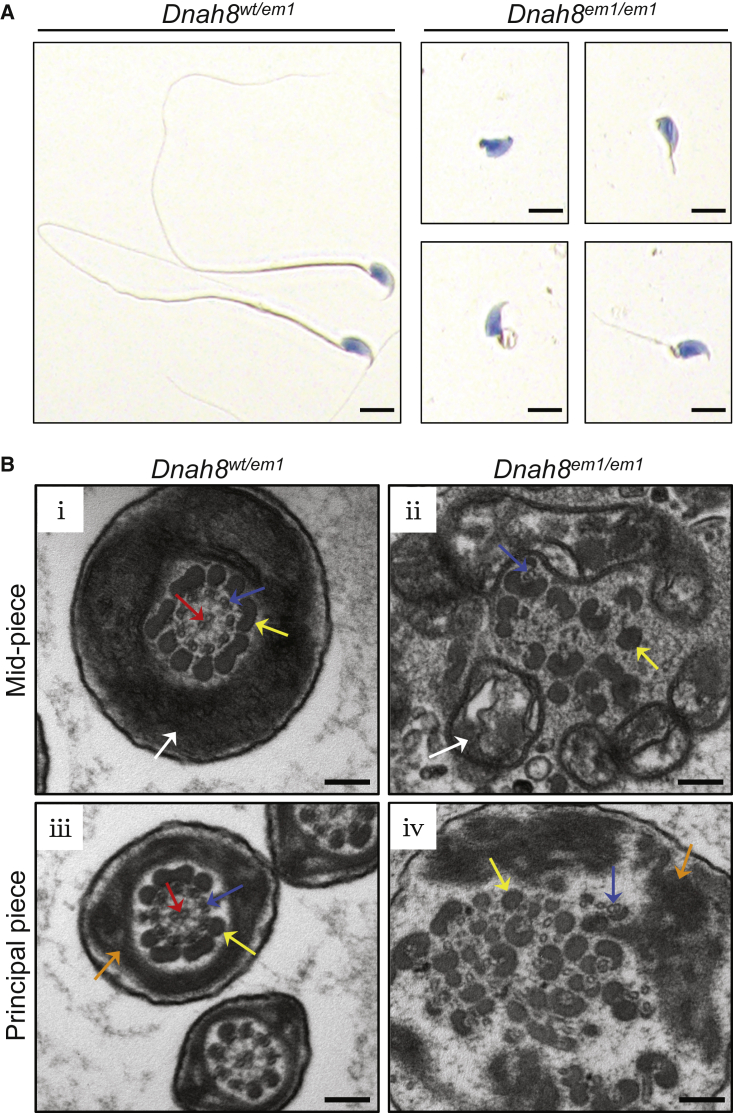
Sperm parameters and morphology of Dnah8-KO male mice were also investigated. As shown in Table 3, Video S1, and Video S2, diminished sperm movement was observed in Dnah8-KO male mice when compared to heterozygous mutated (Dnah8wt/em1) male mice. H&E staining revealed significantly higher rates of abnormal flagella in Dnah8-KO male mice than those in heterozygous mutated male mice (Table 3 and Figure 5A). The sperm flagella of Dnah8-KO male mice also presented with absent, short, coiled, bent, and/or irregular shapes, which recapitulated the clinical phenotypes of MMAF-affected men with bi-allelic DNAH8 variants. Furthermore, TEM analysis of sperm flagella showed disorganized microtubules and outer dense fibers in the spermatozoa from Dnah8-KO male mice (Figure 5B). These experimental observations on Dnah8-KO male mice conclusively demonstrated the crucial role of DNAH8 in sperm flagellar formation.
Table 3.
Sperm Characteristics and Flagellar Morphology of Dnah8-KO Male Mice
| - | Heterozygous Control (Dnah8wt/em1) | KO (Dnah8em1/em1) |
|---|---|---|
| Semen Parameter | ||
| Motility (%) | 91.7 ± 1.5 | 0 ± 0∗∗∗ |
| Sperm Flagellar Morphologya | ||
| Absent flagella (%) | 4.0 ± 2.6 | 18.5 ± 17.8∗ |
| Short flagella (%) | 0.0 ± 0.0 | 25.7 ± 1.2∗∗∗ |
| Coiled flagella (%) | 0.0 ± 0.0 | 25.5 ± 15.6∗∗ |
| Irregular caliber (%) | 0.2 ± 0.3 | 20.5 ± 12.6∗∗∗ |
| Bent flagella (%) | 0.7 ± 0.8 | 9.8 ± 1.6∗∗∗ |
Data represent the mean ± SD of three independent experiments.
p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Figure 5.
Sperm Morphology and Ultrastructure Analyses for Dnah8-KO Male Mice
(A) H&E staining of the spermatozoa obtained from mouse cauda epididymis. The spermatozoa from heterozygous mutated (Dnah8wt/em1) male mice showed normal morphology. In contrast, Dnah8-KO (Dnah8em1/em1) male mice manifested aberrantflagellar morphologies, which were consistent with the clinical phenotypes in the MMAF-affected men. Scale bar: 5 μm.
(B) TEM of cross-sections of the spermatozoa from Dnah8-KO male mice. Cross-sections of the mid-piece (i) and principal piece (iii) of the sperm flagella in heterozygous mutated male mice are shown as controls. (ii) Cross-sections of the mid-piece of the sperm flagella in Dnah8-KO male mice revealed disorganization of mitochondrial sheaths, outer dense fibers, and microtubules. (iv) Cross-sections of the principal piece of the sperm flagella in Dnah8-KO male mice showed disorganization of fibrous sheaths, outer dense fibers, and microtubules. White arrows indicate mitochondrial sheath, orange arrows indicate fibrous sheaths, yellow arrows indicate outer dense fibers, blue arrows indicate peripheral microtubule doublets, and red arrows indicate the central pair of microtubules. Scale bars: 200 nm.
Diminished sperm motility was evident in Dnah8-KO male mice when compared to Dnah8 heterozygous mutated male mice.
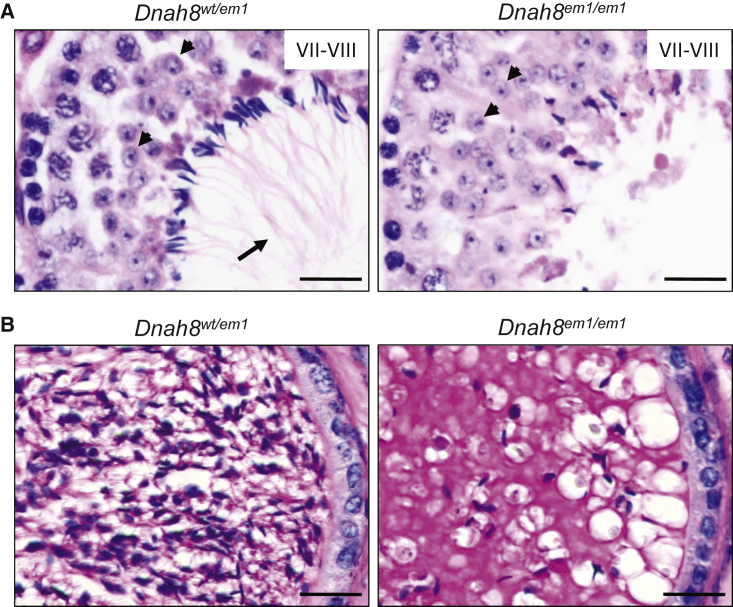
To further investigate the role of DNAH8 in spermatogenesis, we performed H&E staining on the testes of Dnah8-KO and heterozygous mutated male mice (Figure 6A). In stage VII–VIII seminiferous tubules, no elongated tails were observed in the testes from Dnah8-KO male mice, but normal round spermatids were observed, indicating the involvement of DNAH8 in sperm flagellar formation. Periodic acid–Schiff (PAS) staining of the cauda epididymis from Dnah8-KO male mice displayed fewer sperm heads than did those from heterozygous mutated mice (Figure 6B). Collectively, these data suggest that DNAH8 deficiency can result in MMAF and male infertility in both humans and mice.
Figure 6.
Dnah8 is Essential for Normal Spermatogenesis in Mice
(A) The development of sperm flagella was investigated in mouse testis through the use of H&E staining. In stage VII–VIII seminiferous tubules, normal round spermatids (arrowheads) were observed, but elongated tails (arrows) were not observed in the testes from Dnah8-KO (Dnah8em1/em1) male mice. Scale bar: 20 μm.
(B) PAS staining of the cauda epididymis from male mice. Decreased sperm quantity was observed in the epididymis from Dnah8-KO male mice, when compared with that in heterozygous mutated (Dnah8wt/em1) male mice. Scale bar: 20 μm.
To assess the fertility and reproductive behavior of Dnah8-KO male mice, sexually mature Dnah8-KO and heterozygous mutated male mice were individually caged with 8-week-old wild-type B6D2F1 female mice (one male with three females) for 2 months, and plugs were checked every morning. Pups were counted on the day of birth. As shown in Figure S7, normal mounting and copulatory plugs were observed for both groups of Dnah8-KO and heterozygous mutated male mice. However, Dnah8-KO male mice failed to produce any offspring over 2 months of breeding, whereas heterozygous mutated males routinely produced offspring (Figure S7). These experimental observations indicate that DNAH8 is necessary for male fertility in mice.
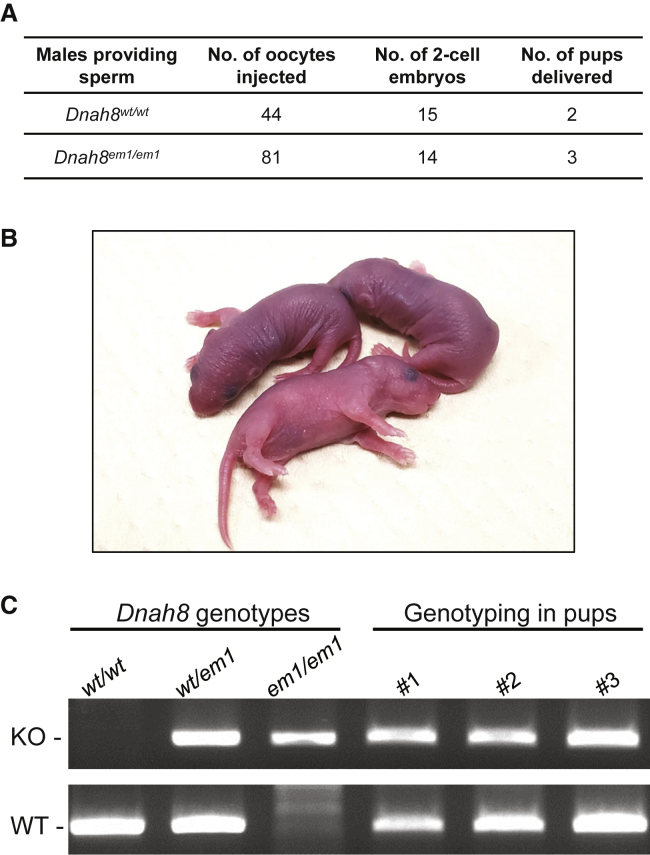
Intracytoplasmic sperm injection (ICSI) has been reported to be efficient for most MMAF-associated asthenoteratozoospermia.23 To examine whether DNAH8-associated male infertility could also be overcome via ICSI, we conducted experiments using the sperm from wild-type and Dnah8-KO male mice. As shown in Figure 7, pups were successfully obtained upon ICSI using the spermatozoa from Dnah8-KO male mice after the transfer of two-cell embryos to pseudopregnant ICR females. Genotyping assays confirmed that all these pups were heterozygous Dnah8-mutated carriers, as anticipated. Our findings indicated that Dnah8-associated KO male infertility in mice could be overcome by ICSI. Consistent with these experimental observations, the second ICSI attempt performed using the sperm from subject F0300 (who harbored a homozygous DNAH8 frameshift variant) was successful and resulted in a live birth. Overall, our data strongly suggest that ICSI could serve as a promising treatment for infertile men harboring bi-allelic DNAH8 variants.
Figure 7.
Pups Obtained upon ICSI using the Spermatozoa from Dnah8-KO Male Mice
(A) Development of ICSI embryos. Fourteen (17.3%) of the 81 oocytes injected with the spermatozoa from Dnah8-KO (Dnah8em1/em1) male mice developed to the two-cell stage, and three pups were obtained after embryo transfer. ICSI data from the use of sperm from wild-type male mice are shown as controls.
(B) The pups obtained from ICSI using the spermatozoa from Dnah8-KO male mice.
(C) Genotyping of these pups. All of the pups carried the heterozygous Dnah8-mutated allele. KO denotes the mutated allele and WT denotes the wild-type allele.
As highly conserved beating organelles, motile cilia and flagella are required for cell motility and signaling.24 The axonemal ultrastructure, which is shared by cilia and flagella, consists of a circle of nine peripheral microtubule doublets arranged around a central pair of microtubules.17 Distinct multi-protein dynein complexes, which are attached regularly to peripheral microtubule doublets, contain molecular motors that can generate microtubule sliding and thus regulate the movement of motile cilia and flagella.19
The mammalian ODA is a complex structure that is attached to the peripheral microtubules via a docking complex. It is responsible for ciliary and flagellar beating.25, 26, 27 Previous studies reported two types of human ODAs. In airway epithelial cells, type 1 ODAs (e.g., DNAH11) reside in the proximal part of the cilium, whereas type 2 ODAs (e.g., DNAH9) reside in the distal part of the cilium.28,29 The deficiency of ODA-associated proteins results in PCD and/or asthenoteratozoospermia. For example, disruptions in DNAH5 and DNAH11 are associated with PCD.28,30 Furthermore, mutations in DNAH9 (MIM: 603330), DNAI1 (MIM: 604366), and DNAI2 (MIM: 605483) induce PCD and male infertility.18,31,32 These previous observations indicate the important roles of ODA-associated proteins in ciliary and flagellar morphology and motility.
Here, our genetic analyses using WES on two distinct cohorts with MMAF (in total, 257 cases) identified three (1.2%) unrelated men carrying bi-allelic variants in DNAH8, which encodes an ODA heavy chain component of the axoneme that is preferentially expressed in the testis. These DNAH8 variants are either rare or absent in human populations, but they were enriched in the MMAF-affected cases of different ancestries, indicating that DNAH8 deficiency could be another important cause of MMAF across human populations.
Further phenotypic analysis revealed that men harboring bi-allelic DNAH8 variants displayed typical MMAF phenotypes, including reduced sperm motility, missing microtubule doublets, and disassembled or absent ODAs. Intriguingly, ODAs were still present in small amounts on observable doublets in the sperm flagella from subject A051 (who harbored bi-allelic DNAH8 missense variants), whereas ODAs were absent from those of subject F0300 (who harbored a homozygous frameshift variant of DNAH8). This phenomenon may be due to a possible phenotype continuum depending on the severity of variants in MMAF-associated genes, as previously shown for CFAP70, DNAH1, and DNAH17.2,13,20,33
Functional experiments further revealed the pathogenicity of bi-allelic DNAH8 variants. The level of DNAH8 mRNA in the spermatozoa from subject A051 harboring bi-allelic DNAH8 missense variants was significantly reduced (Figure S2). This may be due to the recognition of specific sets of mutated mRNAs by RNA-binding proteins, which serve as adaptors and control decay rates by recruitment of RNA-degrading enzymes.34 The reduced mRNA abundance in low-motility sperm was also previously observed for other members (such as DNAH1 and DNAH7) of the axonemal dynein family.35
The remaining mutated mRNA of DNAH8 may produce a small amount of mis-folded DNAH8 protein. However, the immunostaining of DNAH8 was almost absent in the sperm flagella from men harboring bi-allelic DNAH8 variants. This near absence of DNAH8 protein may result from specific degradation of unfolded proteins by the “unfolded protein response” described in previous studies.36,37
A previous study described the absence of DNAH8 protein in the spermatozoa from men harboring bi-allelic DNAH17 variants.13 The present investigations on the amount and localization of DNAH17 revealed a dramatic reduction of DNAH17 in the sperm cells from men harboring bi-allelic DNAH8 variants. These observations confirm the interaction between DNAH8 and DNAH17 during spermatogenesis. Several previous studies also reported that DNAH8 and DNAH17 were categorically detectable in human sperm proteome analyses.38,39 DNAH17 is required for flagellar biogenesis and stabilization of microtubule doublets 4–7.20 Our TEM analysis of sperm also indicated a destabilization of microtubule doublets 4–7 in the principal piece of spermatozoa from men harboring bi-allelic DNAH8 variants. This characteristic defect shared by DNAH8-associated and DNAH17-associated cases further suggests that DNAH8 and DNAH17 proteins are dependent on each other during flagellar assembly and spermatogenesis.
Presently, Dnah8-KO male mice displayed MMAF phenotypes, including diminished sperm motility and abnormal flagella. Furthermore, H&E and PAS staining analyses revealed abnormal spermatogenesis in the testis and reduced sperm counts in the cauda epididymis from Dnah8-KO male mice. Previous studies showed that ODAs are preassembled in the cell body and transported as a holo-complex into the cilium, where they dock to the axonemal microtubules via intraflagellar transport (IFT).40, 41, 42, 43 These observations further indicate the important role of ODAs in cell differentiation. Similar results were also found in the spermatozoa with defects in IDA-associated proteins. For example, the spermatozoa from men with DNAH1 mutations showed severely disarranged axonemal structures with loss of IDAs.2 All these observations indicate that mutations in dynein proteins not only induce the lack of ODAs and IDAs, but also affect assembly of the axoneme. Therefore, structural abnormalities in the sperm axoneme from men harboring bi-allelic DNAH8 variants and Dnah8-KO male mice are likely caused by the flagellar assembly defects during spermatogenesis. Notably, the decreased sperm counts in Dnah8-KO male mice were not observed in men harboring bi-allelic DNAH8 variants, and this may potentially reflect evolutionarily divergent protein regulatory networks of DNAH8 between human and mouse.
As an assisted reproductive technology, ICSI has been regarded as an effective way to help infertile couples achieve a successful pregnancy. Previous studies have suggested that MMAF-affected men harboring DNAH1 variants could acquire good prognoses following ICSI, with 70.8% overall fertilization, 50.0% pregnancy, and 37.5% delivery rates.44 In contrast, ICSI has been reported to fail for the MMAF-affected men carrying CEP135 (MIM: 611423) or DNAH17 variants.13,45 In this study, ICSI experiments were performed using the sperm from Dnah8-KO male mice. Although the rate of two-cell embryos in the Dnah8-KO group was lower than that of the wild-type control group, we obtained healthy pups, indicating a successful ICSI treatment in the Dnah8-KO mouse model. Furthermore, ICSI was also successful for human subject F0300 and resulted in a positive pregnancy outcome and a live birth. Therefore, our findings indicate that ICSI can be recommended for DNAH8-associated asthenoteratozoospermia.
In conclusion, we identified DNAH8 as an asthenoteratozoospermia-associated gene in both humans and mice. The observed effects of DNAH8 deficiency on DNAH17 localization in the sperm flagella further suggested that dynein-arms-associated proteins may affect spermatogenesis in a collaborative manner. Furthermore, DNAH8-associated MMAF and male infertility could be treated through the use of ICSI. Our findings will be informative for genetic and reproductive counseling of infertile men with asthenoteratozoospermia.
Declaration of Interests
The authors declare no competing interests.
Data and Code Availability
The NCBI reference sequence number for DNAH8 transcript is NM_001206927.2. The NCBI reference sequence number for DNAH8 protein is NP_001193856.1.
Acknowledgments
We would like to thank the families for participating and supporting this study. We also thank the Center of Cryo-electron Microscopy at Zhejiang University, the transmission electron microscopy core facility of the Institut Cochin (INSERM U1016, Paris), and Yonggang Lu at Osaka University for technical support. This work was supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT)/Japan Society for the Promotion of Science (JSPS) (KAKENHI grants JP17H04987 to H.M. and JP19H05750 to M.I.), the National Natural Science Foundation of China (31625015, 31521003, 81901541, 81971441, and 81871200), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (P01HD087157 and R01HD088412 to M.I.), the Bill and Melinda Gates Foundation (INV-001902 to M.I.), the Institut National de la Santé et de la Recherche Médicale (Inserm), the Centre National de la Recherche Scientifique (CNRS), the Université de Paris and the French National Research Agency (MASFLAGELLA ANR-14-CE15-0002 and FLAGEL-OME ANR18-CE17-0014 to P.F.R. and A.T.), Shanghai Medical Center of Key Programs for Female Reproductive Diseases (2017ZZ01016), and Shanghai Municipal Science and Technology Major Project (2017SHZDZX01).
Published: July 2, 2020
Footnotes
Supplemental Data can be found online at https://doi.org/10.1016/j.ajhg.2020.06.004.
Contributor Information
Feng Zhang, Email: zhangfeng@fudan.edu.cn.
Masahito Ikawa, Email: ikawa@biken.osaka-u.ac.jp.
Web Resources
1000 Genomes Project, https://www.internationalgenome.org
HUGO Gene Nomenclature Committee, https://www.genenames.org
Human Protein Atlas, https://www.proteinatlas.org
National Center for Biotechnology Information (NCBI), https://www.ncbi.nlm.nih.gov/
Online Mendelian Inheritance in Man, https://www.omim.org
PolyPhen-2, http://genetics.bwh.harvard.edu/pph2/
UniProt, https://www.uniprot.org
Supplemental Data
References
- 1.Hosseini B., Nourmohamadi M., Hajipour S., Taghizadeh M., Asemi Z., Keshavarz S.A., Jafarnejad S. The Effect of Omega-3 Fatty Acids, EPA, and/or DHA on Male Infertility: A Systematic Review and Meta-analysis. J. Diet. Suppl. 2019;16:245–256. doi: 10.1080/19390211.2018.1431753. [DOI] [PubMed] [Google Scholar]
- 2.Ben Khelifa M., Coutton C., Zouari R., Karaouzène T., Rendu J., Bidart M., Yassine S., Pierre V., Delaroche J., Hennebicq S. Mutations in DNAH1, which encodes an inner arm heavy chain dynein, lead to male infertility from multiple morphological abnormalities of the sperm flagella. Am. J. Hum. Genet. 2014;94:95–104. doi: 10.1016/j.ajhg.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baccetti B., Collodel G., Estenoz M., Manca D., Moretti E., Piomboni P. Gene deletions in an infertile man with sperm fibrous sheath dysplasia. Hum. Reprod. 2005;20:2790–2794. doi: 10.1093/humrep/dei126. [DOI] [PubMed] [Google Scholar]
- 4.Tang S., Wang X., Li W., Yang X., Li Z., Liu W., Li C., Zhu Z., Wang L., Wang J. Biallelic Mutations in CFAP43 and CFAP44 Cause Male Infertility with Multiple Morphological Abnormalities of the Sperm Flagella. Am. J. Hum. Genet. 2017;100:854–864. doi: 10.1016/j.ajhg.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong F.N., Amiri-Yekta A., Martinez G., Saut A., Tek J., Stouvenel L., Lorès P., Karaouzène T., Thierry-Mieg N., Satre V. Absence of CFAP69 Causes Male Infertility due to Multiple Morphological Abnormalities of the Flagella in Human and Mouse. Am. J. Hum. Genet. 2018;102:636–648. doi: 10.1016/j.ajhg.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kherraf Z.E., Amiri-Yekta A., Dacheux D., Karaouzène T., Coutton C., Christou-Kent M., Martinez G., Landrein N., Le Tanno P., Fourati Ben Mustapha S. A Homozygous Ancestral SVA-Insertion-Mediated Deletion in WDR66 Induces Multiple Morphological Abnormalities of the Sperm Flagellum and Male Infertility. Am. J. Hum. Genet. 2018;103:400–412. doi: 10.1016/j.ajhg.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auguste Y., Delague V., Desvignes J.P., Longepied G., Gnisci A., Besnier P., Levy N., Beroud C., Megarbane A., Metzler-Guillemain C., Mitchell M.J. Loss of Calmodulin- and Radial-Spoke-Associated Complex Protein CFAP251 Leads to Immotile Spermatozoa Lacking Mitochondria and Infertility in Men. Am. J. Hum. Genet. 2018;103:413–420. doi: 10.1016/j.ajhg.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coutton C., Martinez G., Kherraf Z.E., Amiri-Yekta A., Boguenet M., Saut A., He X., Zhang F., Cristou-Kent M., Escoffier J. Bi-allelic Mutations in ARMC2 Lead to Severe Astheno-Teratozoospermia Due to Sperm Flagellum Malformations in Humans and Mice. Am. J. Hum. Genet. 2019;104:331–340. doi: 10.1016/j.ajhg.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu W., He X., Yang S., Zouari R., Wang J., Wu H., Kherraf Z.E., Liu C., Coutton C., Zhao R. Bi-allelic Mutations in TTC21A Induce Asthenoteratospermia in Humans and Mice. Am. J. Hum. Genet. 2019;104:738–748. doi: 10.1016/j.ajhg.2019.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coutton C., Vargas A.S., Amiri-Yekta A., Kherraf Z.E., Ben Mustapha S.F., Le Tanno P., Wambergue-Legrand C., Karaouzène T., Martinez G., Crouzy S. Mutations in CFAP43 and CFAP44 cause male infertility and flagellum defects in Trypanosoma and human. Nat. Commun. 2018;9:686. doi: 10.1038/s41467-017-02792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu C., He X., Liu W., Yang S., Wang L., Li W., Wu H., Tang S., Ni X., Wang J. Bi-allelic Mutations in TTC29 Cause Male Subfertility with Asthenoteratospermia in Humans and Mice. Am. J. Hum. Genet. 2019;105:1168–1181. doi: 10.1016/j.ajhg.2019.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorès P., Dacheux D., Kherraf Z.E., Nsota Mbango J.F., Coutton C., Stouvenel L., Ialy-Radio C., Amiri-Yekta A., Whitfield M., Schmitt A. Mutations in TTC29, Encoding an Evolutionarily Conserved Axonemal Protein, Result in Asthenozoospermia and Male Infertility. Am. J. Hum. Genet. 2019;105:1148–1167. doi: 10.1016/j.ajhg.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitfield M., Thomas L., Bequignon E., Schmitt A., Stouvenel L., Montantin G., Tissier S., Duquesnoy P., Copin B., Chantot S. Mutations in DNAH17, Encoding a Sperm-Specific Axonemal Outer Dynein Arm Heavy Chain, Cause Isolated Male Infertility Due to Asthenozoospermia. Am. J. Hum. Genet. 2019;105:198–212. doi: 10.1016/j.ajhg.2019.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Touré A., Martinez G., Kherraf Z.E., Cazin C., Beurois J., Arnoult C., Ray P.F., Coutton C. The genetic architecture of morphological abnormalities of the sperm tail. Hum. Genet. 2020 doi: 10.1007/s00439-00020-02113-x. [DOI] [PubMed] [Google Scholar]
- 15.Reiter J.F., Leroux M.R. Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 2017;18:533–547. doi: 10.1038/nrm.2017.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zariwala M.A., Knowles M.R., Omran H. Genetic defects in ciliary structure and function. Annu. Rev. Physiol. 2007;69:423–450. doi: 10.1146/annurev.physiol.69.040705.141301. [DOI] [PubMed] [Google Scholar]
- 17.Ishikawa T. Axoneme Structure from Motile Cilia. Cold Spring Harb. Perspect. Biol. 2017;9:a028076. doi: 10.1101/cshperspect.a028076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loges N.T., Olbrich H., Fenske L., Mussaffi H., Horvath J., Fliegauf M., Kuhl H., Baktai G., Peterffy E., Chodhari R. DNAI2 mutations cause primary ciliary dyskinesia with defects in the outer dynein arm. Am. J. Hum. Genet. 2008;83:547–558. doi: 10.1016/j.ajhg.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibañez-Tallon I., Heintz N., Omran H. To beat or not to beat: roles of cilia in development and disease. Hum. Mol. Genet. 2003;12:R27–R35. doi: 10.1093/hmg/ddg061. [DOI] [PubMed] [Google Scholar]
- 20.Zhang B., Ma H., Khan T., Ma A., Li T., Zhang H., Gao J., Zhou J., Li Y., Yu C. A DNAH17 missense variant causes flagella destabilization and asthenozoospermia. J. Exp. Med. 2020;217:e20182365. doi: 10.1084/jem.20182365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y., Yang J., Jia Y., Xiong C., Meng T., Guan H., Xia W., Ding M., Yuchi M. Variability in the morphologic assessment of human sperm: use of the strict criteria recommended by the World Health Organization in 2010. Fertil. Steril. 2014;101:945–949. doi: 10.1016/j.fertnstert.2013.12.047. [DOI] [PubMed] [Google Scholar]
- 22.Samant S.A., Ogunkua O.O., Hui L., Lu J., Han Y., Orth J.M., Pilder S.H. The mouse t complex distorter/sterility candidate, Dnahc8, expresses a gamma-type axonemal dynein heavy chain isoform confined to the principal piece of the sperm tail. Dev. Biol. 2005;285:57–69. doi: 10.1016/j.ydbio.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Chemes H.E., Alvarez Sedo C. Tales of the tail and sperm head aches: changing concepts on the prognostic significance of sperm pathologies affecting the head, neck and tail. Asian J. Androl. 2012;14:14–23. doi: 10.1038/aja.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viswanadha R., Sale W.S., Porter M.E. Ciliary Motility: Regulation of Axonemal Dynein Motors. Cold Spring Harb. Perspect. Biol. 2017;9:a018325. doi: 10.1101/cshperspect.a018325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loges N.T., Antony D., Maver A., Deardorff M.A., Güleç E.Y., Gezdirici A., Nöthe-Menchen T., Höben I.M., Jelten L., Frank D. Recessive DNAH9 Loss-of-Function Mutations Cause Laterality Defects and Subtle Respiratory Ciliary-Beating Defects. Am. J. Hum. Genet. 2018;103:995–1008. doi: 10.1016/j.ajhg.2018.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King S.M. Axonemal Dynein Arms. Cold Spring Harb. Perspect. Biol. 2016;8:a028100. doi: 10.1101/cshperspect.a028100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pazour G.J., Agrin N., Walker B.L., Witman G.B. Identification of predicted human outer dynein arm genes: candidates for primary ciliary dyskinesia genes. J. Med. Genet. 2006;43:62–73. doi: 10.1136/jmg.2005.033001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fliegauf M., Olbrich H., Horvath J., Wildhaber J.H., Zariwala M.A., Kennedy M., Knowles M.R., Omran H. Mislocalization of DNAH5 and DNAH9 in respiratory cells from patients with primary ciliary dyskinesia. Am. J. Respir. Crit. Care Med. 2005;171:1343–1349. doi: 10.1164/rccm.200411-1583OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dougherty G.W., Loges N.T., Klinkenbusch J.A., Olbrich H., Pennekamp P., Menchen T., Raidt J., Wallmeier J., Werner C., Westermann C. DNAH11 Localization in the Proximal Region of Respiratory Cilia Defines Distinct Outer Dynein Arm Complexes. Am. J. Respir. Cell Mol. Biol. 2016;55:213–224. doi: 10.1165/rcmb.2015-0353OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu S., Chen W., Zhan Y., Li S., Ma X., Ma D., Sheng W., Huang G. DNAH11 variants and its association with congenital heart disease and heterotaxy syndrome. Sci. Rep. 2019;9:6683. doi: 10.1038/s41598-019-43109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guichard C., Harricane M.C., Lafitte J.J., Godard P., Zaegel M., Tack V., Lalau G., Bouvagnet P. Axonemal dynein intermediate-chain gene (DNAI1) mutations result in situs inversus and primary ciliary dyskinesia (Kartagener syndrome) Am. J. Hum. Genet. 2001;68:1030–1035. doi: 10.1086/319511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fassad M.R., Shoemark A., Legendre M., Hirst R.A., Koll F., le Borgne P., Louis B., Daudvohra F., Patel M.P., Thomas L. Mutations in Outer Dynein Arm Heavy Chain DNAH9 Cause Motile Cilia Defects and Situs Inversus. Am. J. Hum. Genet. 2018;103:984–994. doi: 10.1016/j.ajhg.2018.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beurois J., Martinez G., Cazin C., Kherraf Z.E., Amiri-Yekta A., Thierry-Mieg N., Bidart M., Petre G., Satre V., Brouillet S. CFAP70 mutations lead to male infertility due to severe astheno-teratozoospermia. A case report. Hum. Reprod. 2019;34:2071–2079. doi: 10.1093/humrep/dez166. [DOI] [PubMed] [Google Scholar]
- 34.Stoecklin G., Mühlemann O. RNA decay mechanisms: specificity through diversity. Biochim. Biophys. Acta. 2013;1829:487–490. doi: 10.1016/j.bbagrm.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Caballero-Campo P., Lira-Albarrán S., Barrera D., Borja-Cacho E., Godoy-Morales H.S., Rangel-Escareño C., Larrea F., Chirinos M. Gene transcription profiling of astheno- and normo-zoospermic sperm subpopulations. Asian J. Androl. 2020;22 doi: 10.4103/aja.aja_4143_4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rutkowski D.T., Hegde R.S. Regulation of basal cellular physiology by the homeostatic unfolded protein response. J. Cell Biol. 2010;189:783–794. doi: 10.1083/jcb.201003138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang S., Kaufman R.J. The impact of the unfolded protein response on human disease. J. Cell Biol. 2012;197:857–867. doi: 10.1083/jcb.201110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang G., Guo Y., Zhou T., Shi X., Yu J., Yang Y., Wu Y., Wang J., Liu M., Chen X. In-depth proteomic analysis of the human sperm reveals complex protein compositions. J. Proteomics. 2013;79:114–122. doi: 10.1016/j.jprot.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Amaral A., Castillo J., Estanyol J.M., Ballescà J.L., Ramalho-Santos J., Oliva R. Human sperm tail proteome suggests new endogenous metabolic pathways. Mol. Cell. Proteomics. 2013;12:330–342. doi: 10.1074/mcp.M112.020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fowkes M.E., Mitchell D.R. The role of preassembled cytoplasmic complexes in assembly of flagellar dynein subunits. Mol. Biol. Cell. 1998;9:2337–2347. doi: 10.1091/mbc.9.9.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dean A.B., Mitchell D.R. Chlamydomonas ODA10 is a conserved axonemal protein that plays a unique role in outer dynein arm assembly. Mol. Biol. Cell. 2013;24:3689–3696. doi: 10.1091/mbc.E13-06-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dean A.B., Mitchell D.R. Late steps in cytoplasmic maturation of assembly-competent axonemal outer arm dynein in Chlamydomonas require interaction of ODA5 and ODA10 in a complex. Mol. Biol. Cell. 2015;26:3596–3605. doi: 10.1091/mbc.E15-05-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desai P.B., Freshour J.R., Mitchell D.R. Chlamydomonas axonemal dynein assembly locus ODA8 encodes a conserved flagellar protein needed for cytoplasmic maturation of outer dynein arm complexes. Cytoskeleton (Hoboken) 2015;72:16–28. doi: 10.1002/cm.21206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wambergue C., Zouari R., Fourati Ben Mustapha S., Martinez G., Devillard F., Hennebicq S., Satre V., Brouillet S., Halouani L., Marrakchi O. Patients with multiple morphological abnormalities of the sperm flagella due to DNAH1 mutations have a good prognosis following intracytoplasmic sperm injection. Hum. Reprod. 2016;31:1164–1172. doi: 10.1093/humrep/dew083. [DOI] [PubMed] [Google Scholar]
- 45.Sha Y.W., Xu X., Mei L.B., Li P., Su Z.Y., He X.Q., Li L. A homozygous CEP135 mutation is associated with multiple morphological abnormalities of the sperm flagella (MMAF) Gene. 2017;633:48–53. doi: 10.1016/j.gene.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 46.Cooper T.G., Noonan E., von Eckardstein S., Auger J., Baker H.W., Behre H.M., Haugen T.B., Kruger T., Wang C., Mbizvo M.T., Vogelsong K.M. World Health Organization reference values for human semen characteristics. Hum. Reprod. Update. 2010;16:231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 47.Auger J., Jouannet P., Eustache F. Another look at human sperm morphology. Hum. Reprod. 2016;31:10–23. doi: 10.1093/humrep/dev251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Diminished sperm motility was evident in Dnah8-KO male mice when compared to Dnah8 heterozygous mutated male mice.
Data Availability Statement
The NCBI reference sequence number for DNAH8 transcript is NM_001206927.2. The NCBI reference sequence number for DNAH8 protein is NP_001193856.1.