Abstract
MORC2 encodes an ATPase that plays a role in chromatin remodeling, DNA repair, and transcriptional regulation. Heterozygous variants in MORC2 have been reported in individuals with autosomal-dominant Charcot-Marie-Tooth disease type 2Z and spinal muscular atrophy, and the onset of symptoms ranges from infancy to the second decade of life. Here, we present a cohort of 20 individuals referred for exome sequencing who harbor pathogenic variants in the ATPase module of MORC2. Individuals presented with a similar phenotype consisting of developmental delay, intellectual disability, growth retardation, microcephaly, and variable craniofacial dysmorphism. Weakness, hyporeflexia, and electrophysiologic abnormalities suggestive of neuropathy were frequently observed but were not the predominant feature. Five of 18 individuals for whom brain imaging was available had lesions reminiscent of those observed in Leigh syndrome, and five of six individuals who had dilated eye exams had retinal pigmentary abnormalities. Functional assays revealed that these MORC2 variants result in hyperactivation of epigenetic silencing by the HUSH complex, supporting their pathogenicity. The described set of morphological, growth, developmental, and neurological findings and medical concerns expands the spectrum of genetic disorders resulting from pathogenic variants in MORC2.
Keywords: MORC2, CMT2Z, microcephaly, developmental delay, intellectual disability, Leigh-like disease
Main Text
Microrchidia CW-type zinc finger protein 2 (MORC2, MIM: 616661) is a member of a family of ATPases fundamental for epigenetic silencing through chromatin modification.1, 2, 3 It has most commonly been associated with autosomal-dominant Charcot-Marie-Tooth (CMT) disease type 2Z (MIM: 616688), a form of axonal neuropathy with progressive weakness, muscle cramps, and sensory impairment presenting in childhood or early adulthood.4, 5, 6, 7 However, some reported individuals presented with hypotonia, generalized muscle weakness, and delayed milestones,4 or occasionally with spinal muscular atrophy, intellectual disability, hearing loss, pyramidal signs, microcephaly, and brain atrophy, in infancy.4,5,8, 9, 10 The association of MORC2 variants with human disease has only recently been noted, and most of the individuals were ascertained through studies of neuropathies. We used a hypothesis-free approach to assess common characteristics in individuals with de novo variants in MORC2 who were referred for exome sequencing at a clinical laboratory.
We identified 15 individuals with de novo variants in MORC2. In addition we included an affected mother-daughter pair harboring a novel variant in MORC2 (subjects 19 and 20) and three individuals heterozygous for recurrent variants for whom parental studies could not be completed (subjects 5, 13, and 16), bringing our total to 20 individuals. Clinical data were obtained from the referring providers or via provider completion of a questionnaire. This study was conducted under GeneDx’s research protocol “Research to Expand the Understanding of Genetic Variants: Clinical and Genetic Correlations,” approved by the Western Institutional Review Board (IRB) (protocol 20171030). All research subjects provided written consent to participate through either GeneDx’s research protocol or as required by their clinical institution. Where applicable, informed written consent was obtained for the use of photographs.
For all subjects except subject 9, we used genomic DNA from the proband and parents (when available) to capture the exonic regions and flanking splice junctions of the genome via the SureSelect Human All Exon V4 (50 Mb), the Clinical Research Exome kit (Agilent Technologies), or the IDT xGen Exome Research Panel v1.0. Massively parallel (NextGen) sequencing was done on an Illumina system with 100 bp or greater paired-end reads. Reads were aligned to human genome build GRCh37/UCSC hg19 and analyzed for sequence variants via a custom-developed analysis tool. Additional sequencing technology and the variant interpretation protocol have been previously described.11 The general assertion criteria for variant classification can be found in Table S1. For subject 9, identified by communication with another testing laboratory (K.W., unpublished data), trio exome sequencing was performed on a Novaseq6500 platform (Illumina) in a CLIA certified laboratory (Gene by Gene) via the Agilent SureSelect Clinical Research Exome Capture Enrichment kit (Agilent Technologies) to capture the protein-coding regions. Mapping of the obtained reads to the reference genome (build GRCh37/hg19), variant calling, annotation, and data analysis were done with the Genoox data analysis platform. Variants were prioritized on the basis of their effect on the protein (missense, nonsense, frameshift, splice-site) and their having a minor allele frequency below 1% in general population databases, such as gnomAD,12 the Greater Middle East Variome, and the Rambam Genetics Institute internal database of over 1,500 Israeli exomes. All variants were confirmed by Sanger sequencing and reported with the NM_001303256.1 transcript of MORC2 (Figure S1).
We identified nine missense variants clustering in the ATPase module of MORC2 (Tables 1 and S1, Figure 1). None of these variants are reported in gnomAD,12 and all were considered to be pathogenic or likely pathogenic in the context of the 2015 American College of Medical Genetics and Genomics (ACMG) standards and guidelines for the interpretation of sequence variants (Table S1).13 To our knowledge, five (55.6%) of the variants were previously unreported. Published individuals for whom clinical information was available were included in Table 1 for comparison.
Table 1.
Detailed Clinical Description of Individuals with Variants in the ATPase module of MORC2
| Affected Individual | Mutation | Inheritance | Sex | Age at Examination |
Growth Parameters (Z Scores)a |
Cognitive and Development |
Facial Dysmorphism | Hearing Loss | Endocrine | Neuromuscular Features | EMG/NCS | Brain MRI | Other Features | Additional Variants Reported | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Height | Weight | HC | Motor Delay | Speech Delay | Intellectual Disability | |||||||||||||
| Subject 1 | Thr24Ile | de novo | female | 4.2 years | −3.41 | −2.95 | −3.72 | + | + | mild | + | − | precocious puberty | high arches, hyperreflexia, spasticity, ataxic and jerking movements of arms and legs, thoracic kyphosis; only walks with a walker | normal | abnormal T2 hyperintensity in right cerebral peduncle, cortical atrophy with ventriculomegaly, abnormal signal in right putamen and diffuse, ill-defined white matter hyperintensity | strabismus | N/A |
| Subject 2 | Glu27Lys | de novo | male | 17 years | −3.66 | −5.6 | −2.05 | + | + | mild | − | − | delayed puberty, growth hormone deficiency, hypothyroidism | high arches, toe walking, spasticity, hyperreflexia | N/A | normal at age 14 years | N/A | N/A |
| Subject 3 | Glu27Lys | de novo | female | 4.8 years | −3.13 | −2.36 | −5.03 | + | + | moderate | + | − | vitamin D deficiency | high arches, hyporeflexia in lower extremities | N/A | severe delay in myelination with T2 hyperintensity in the substantia nigra, mild cerebral atrophy | severe GERD, hyperopia, ptosis, capillary hemangioma | c.81dupG in COX14, heterozygous (LPATH); p.Leu368Arg in DHTKD1, heterozygous (VUS) |
| Subject 4 | Glu27Lys | de novo | male | 26.6 years | −3.28 | −2.91 | −0.94 | + | + | mild | + | SNHL, severe, hearing aid in one ear and a cochlear implant in the other | precocious puberty, mildly increased prolactin | hyporeflexia, reduced range of motion in large joints, knee contractures, crouched and stiff gait | normal | normal at age 12 years | salt and pepper maculopathy, strabismus, oromotor dyspraxia, constipation, autism | m.3397A>G, homoplasmic (VUS) |
| Subject 5 | Glu27Lys | unknown | female | 5.5 years | −2.89 | 0.4 | −4.02 | + | + | severe | − | mixed, moderate-profound, hearing aids | − | hypotonia, weakness, hyperreflexia | N/A | ventriculomegaly, supratentorial and infratentorial volume loss with diffusely abnormal white matter and prominent cavitary encephalomalacia in the putamina and caudate heads | retinal dystrophy on ERG, bilateral ptosis, esotropia, epilepsy, GERD, feeding difficulties, laryngomalacia, sialorrhea, history of acute respiratory failure with illness, neutropenia, lactic acidosis | N/A |
| Subject 6 | Glu27Lys | de novo | female | 1.3 years | −1.58 | −0.93 | −1.35 | + | + | N/A | + | − | − | axial hypotonia, appendicular hypertonia (more pronounced in the lower extremities), hyperreflexia and extensor plantar response | N/A | normal at 10 months | non-specific peripheral retinal deposits | N/A |
| Subject 7 | Ser87Leu | de novo | male | 4.8 years | −2.32 | −2.58 | −0.4 | + | + | severe | + | − | − | hypotonia, decreased muscle bulk, proximal weakness, areflexia, non-ambulatory | sensory motor axonal neuropathy | T2 hyperintensities of the central tegmental tract and superior cerebral peduncles, globus palladi, subthalamic nucleus and substantia nigra | unprovoked episodic deterioration in neurologic symptoms, followed by gradual recovery, frequent respiratory infections | N/A |
| Subject 8 | Ser87Leu | de novo | male | 5 years | −3.34 | −4.08 | −3.46 | + | + | mild-to-moderate | − | − | − | weakness, decreased muscle bulk, mildly elevated creatine kinase, areflexia, wide based and waddling gait, uses a walker and braces for ambulation, mild kyphosis | axonal motor neuropathy | normal at 15 months | proximally placed thumbs, hirsutism | N/A |
| Hyun et al.14 | Ser87Leu | de novo | male | 13 years | N/A | N/A | N/A | + | N/A | N/A | N/A | + | N/A | hypotonia, weakness, areflexia, scoliosis, wheelchair bound | sensory motor neuropathy | normal | N/A | N/A |
| Ser87Leu | de novo | female | 10 years | N/A | N/A | N/A | + | N/A | N/A | + | N/A | N/A | hypotonia, weakness, areflexia, scoliosis, wheelchair bound | sensory motor neuropathy | normal | cataract | N/A | |
| Sevilla et al.4 | Ser87Leu | unknown | female | 1.6 years | N/A | N/A | (<third percentile)b | + | − | − | N/A | N/A | N/A | hypotonia, weakness, areflexia | sensory motor neuropathy | “mild dismaturative features” | N/A | N/A |
| Subject 9 | Ala88Val | de novo | female | 23 years | −6.61 | −3.9 | −9.7 | + | + | severe | + | hearing loss, type unknown | N/A | hammertoes, spasticity, non-ambulatory | N/A | N/A | decreased vision, short and broad toes | N/A |
| Subject 10 | Arg132Cys | de novo | male | 6 years | −2.12 | −0.67 | −2.24 | + | + | mild | + | − | − | mild diffuse hypotonia, hyporeflexia, possible mild muscle weakness, intention tremor of upper extremities, intermittent exotropia, slow gait with trace circumduction at left hip | normal at age 4 years | lesions of the periaqueductal gray matter, thalami, substantia nigra, and geniculocalcarine tract at 17 months; periaqueductal gray matter lesions had resolved by age 4 years, whereas other lesions remained stable | pectus excavatum, fifth finger clinodactyly, broad great toes, cutis marmorata, subtle retinal pigmentary changes at age 7 years | N/A |
| Subject 11 | Arg132Cys | de novo | male | 8.3 years | −3.69 | −3.32 | −2.04 | + | + | mild | + | SNHL, mild-to-moderate | − | hypotonia, weakness, areflexia, hammertoes, pes cavus, intoeing gait | sensory motor polyneuropathy | hypomyelination | N/A | N/A |
| Subject 12 | Arg132Cys | de novo | male | 15 years | −2.5 | −2.82 | −2.12 | + | + | mild (non-verbal) | + | SNHL, moderate, unilateral | precocious puberty | mild hypotonia, patellar hyporeflexia, severe scoliosis | mild axonal neuropathy | normal at 9 years old; previous exams raised concern for possible mild periventricular gliosis | absence seizures, velopharyngeal insufficiency, eosinophilic esophagitis, chronic constipation, history of feeding difficulties | p.Met34Thr in GJB2, heterozygous (PATH); p.Arg603Gln in TCF12, heterozygous, de novo (VUS) |
| Subject 13 | Arg132Cys | not maternal (father not tested) | female | 34 years | −2.76 | 0.1 | −2.64 | − | + | mild | + | SNHL, unilateral hearing aid | hypothyroidism, premature thelarche, PCOS | weakness, hypotonia, normal reflexes, poor balance and coordination, lordosis | N/A | cerebral and cerebellar volume loss, a couple of foci of T2 hyperintensity in cerebral white matter | narrow shoulders, brachydactyly, small hands, clinodactyly, myopia, possible macular degeneration, GERD, gallbladder disease, heterochromia | N/A |
| Hyun et al.14 | Arg132Leu | unknown | male | 30 years | N/A | N/A | N/A | N/A | N/A | N/A | N/A | + | N/A | weakness, high arches, areflexia | sensory motor neuropathy | N/A | N/A | N/A |
| Subject 14 | Arg266Ser | de novo | female | 12 years | −3.3 | −0.96 | −3.5 | + | + | moderate | + | SNHL, mild-to-moderate, bilateral | − | hypotonia, hyperreflexia, mild hemiplegic gait, tremor, dystonia, scoliosis | N/A | cortical dysplasia | fetal finger pads, bilateral sandal gap, clynodactyly of toes 3, 4, and 5 | N/A |
| Subject 15 | Ser388Arg | de novo | female | 9 years | −2.07 | −0.82 | −3.27 | + | + | moderate | + | SNHL, progressive, bilateral, has cochlear implants | precocious puberty | hypotonia, difficulty with tandem gait, trips when running, mild intention tremor | normal at age 9 years | delayed myelination, mild cerebellar volume loss | linear hypopigmentation in extremities, progressive hyperopia, mild retinal changes with normal ERG | p.Glu510Gln in HADHA, heterozygous (PATH) |
| Subject 16 | Ser388Arg | unknown | female | 15 years | −2.07 | −1.23 | −2.05 | + | + | mild | + | SNHL, moderate-to-severe, bilateral | − | severe spasticity (asymmetric), lower extremity hyperreflexia, unsteady, wide based gait, hammertoes and high arches not present on exam at age 12 years but significant at age 15 years | N/A | cerebellar atrophy | resting tremor and dysmetria, history of seizures and staring spells with normal EEG, Wolff-Parkinson-White syndrome, vision impairment | p.Gly145Arg in HCN2, heterozygous (VUS) |
| Subject 17 | Tyr394Cys | de novo | female | 8.6 years | −2.1 | 0.5 | −2.1 | + | + | mild | + | SNHL, bilateral, mild | − | hypotonia, weakness, high arches, intoeing gait, unilateral tremor, dystonic posturing, spasticity | sensory polyneuropathy | multiple foci stable chronic hemosiderin deposition within the supratentorial and infratentorial white matter | Tetralogy of Fallot | N/A |
| Subject 18 | Tyr394Cys | de novo | male | 49 years | −1.2 | 2.36 | 2.02 | + | + | N/A | − | N/A | hypothyroidism | progressive, asymmetric limb weakness (left > right), muscle atrophy, foot drop, areflexia, ataxia, uses walker or wheelchair for ambulation | sensory motor neuropathy | generalized brain atrophy and scattered subcortical and deep white matter microangiopathic changes disproportionate to patient’s age | essential thrombocytosis, grooved tongue, mildly elevated creatine kinase (500–900 U/L) | N/A |
| Ando et al.5 | Tyr394Cys | unknown | male | 29 years | N/A | N/A | N/A | − | − | − | N/A | N/A | N/A | hyporeflexia, gait abnormalities, weakness, distal atrophy | sensory neuropathy | N/A | N/A | N/A |
| Subject 19 | Val413Phe | maternal | female | 5.6 years | −2.29 | −2.35 | 0.09 | + | + | mild | + | − | growth hormone deficiency | hypotonia, weakness, hyporeflexia, ataxia, action tremor | N/A | normal at age 3 years | duplicated collecting system | p.Leu2127Pro in ASXL3, heterozygous, maternal (VUS) |
| Subject 20 | Val413Phe | unknown | female | 29 years | −2.2 | 0.52 | −2.33 | + | + | mild | + | hearing loss, type unknown | growth hormone deficiency | tingling in extremities with onset in 20 s | N/A | N/A | N/A | p.Leu2127Pro in ASXL3, heterozygous (VUS) |
Abbreviations are as follows: EMG, electromyogram; NCS, nerve conduction study; MRI, magnetic resonance imaging; SNHL, sensorineural hearing loss; ERG, electroretinography; GERD, gastresophageal reflux; N/A, not available; LPATH, likely pathogenic; PATH, pathogenic; VUS, variant of uncertain significance.
Reported as standard deviations (SDs) of the raw Z score from the mean based on CDC standards.
Z scores or raw numbers not available in the publication.
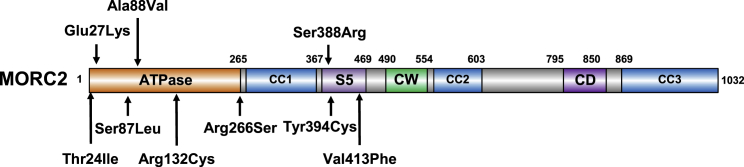
Figure 1.
Neurodevelopmental Variants Cluster in the ATPase Module of MORC2
Schematic representation of the domain structure of MORC2. MORC2 binds ATP through its GHKL-type ATPase module,2,15 which consists of a GHKL-type ATPase domain (residues 1–265), a transducer S5-like domain ([S5], residues 266–494), and an 80 amino acid antiparallel coiled-coil insertion within the transducer-like domain (coiled-coil 1 [CC1], residues 282–361) that projects out of the ATPase module.15 MORC2 also contains two putative chromatin-binding modules, a CW-type zinc finger (CW) and a chromo-like domain (CD), and two additional coiled-coils (CC2 and CC3). All variants described in this paper cluster in the ATPase module of MORC2.
Our cohort includes a diverse group of individuals; 12/20 (60%) were female, and the average age at the time of last examination was 14.2 years (range 1.3–49 years). The primary indication for genetic testing was developmental delay or growth failure, with the exception of the oldest individual (Subject 18) who presented with a diagnosis of sensorimotor neuropathy. The most commonly described features include gross motor delay (19/20, 95%), short stature (height < 2 SDs from the mean for age, 18/20, 90%), intellectual disability (18/20, 90%), and microcephaly (head circumference < 2 SDs from the mean for age, 15/20, 75%) (Tables 1 and 2). Facial dysmorphism was reported in 16/20 (80%) individuals, but the features were subtle and variable. Facial characteristics often consist of a long face with a narrow jaw, deep set eyes, broad nasal tip, thin upper lip, dental crowding, and high palate (Figure 2). Gait abnormalities were reported in 15/16 (94%) individuals; hypotonia (11/16, 69%) and decreased or absent deep tendon reflexes (DTRs; 9/18, 50%) were also commonly noted. Ten individuals had nerve conduction studies (NCSs); four of them reportedly had normal testing, whereas six showed sensorimotor peripheral neuropathy. Hearing loss was reported in 11/19 (58%) and was progressive; two individuals received cochlear implants (subjects 4 and 15) and two more use hearing aids (subjects 5 and 13). Pigmentary retinopathy was reported in 5/6 (83%) individuals who had dilated eye exams. Eighteen individuals had a magnetic resonance imaging (MRI) of the brain, which was abnormal in 12 individuals (66%). Reported changes included Leigh syndrome-like lesions (symmetric abnormalities in the brainstem, basal ganglia, or cerebellum) in five of those 18 individuals. A diagnosis of mitochondrial disease was suspected in seven individuals (subjects 1, 3, 4, 5, 7, 10, and 12).
Table 2.
Summary of Clinical Characteristics of Individuals with Variants in the ATPase Module of MORC2
| This Paper | All Individuals | |||
|---|---|---|---|---|
| Clinical Characteristics | Total | % | Total | % |
| Short staturea | 18/20 | 90 | 18/20 | 90 |
| Microcephalya | 15/20 | 75 | 16/21 | 76 |
| Developmental Delay | ||||
| Motor delay | 19/20 | 95 | 22/23 | 96 |
| Intellectual disability | 18/20 | 90 | 18/22 | 82 |
| Facial dysmorphism | 16/20 | 80 | 17/21 | 81 |
| Hearing Loss | 11/19 | 58 | 12/20 | 60 |
| Pigmentary retinopathy | 5/6 | 83 | 5/6 | 83 |
| Neuromuscular | ||||
| Hypotonia | 11/16 | 69 | 14/19 | 74 |
| Hyporeflexia/areflexia | 9/18 | 50 | 13/22 | 59 |
| Hyperreflexia | 6/18 | 33 | 6/22 | 27 |
| Weakness | 8/15 | 53 | 12/19 | 63 |
| High arches | 7/16 | 44 | 7/16 | 44 |
| Gait abnormalities | 15/16 | 94 | 18/19 | 95 |
| EMG/NCS abnormalities | 6/10 | 60 | 11/15 | 73 |
| Brain MRI | ||||
| Any abnormality | 12/18 | 66 | 12/21 | 57 |
| Leigh-like lesionsb | 5/18 | 28 | 5/21 | 24 |
| White matter abnormalities | 9/18 | 50 | 9/21 | 43 |
Abbreviations are as follows: EMG, electromyogram; NCS, nerve conduction study; MRI, magnetic resonance imaging
2 standard deviations (SDs) below the mean for age by CDC standards.
Based on ClinGen panel Leigh syndrome curation guidelines.
Figure 2.
Facial Characteristics of Individuals Harboring MORC2 Variants
The recurrent variants present in our cohort, c.79G>A (p.Glu27Lys), c.260C>T (p.Ser87Leu), c.394C>T (p.Arg132Cys), and c.1181A>G (p.Tyr394Cys), have been previously reported in the medical literature.4,5,14,16 Clinical findings among individuals harboring the same variant were assessed (Table 1). Five subjects (subjects 2–6) were heterozygous for c.79G>A (p.Glu27Lys). This variant was previously reported de novo in a child with developmental delay by the Deciphering Developmental Disorders Study group,16 but detailed clinical information was not available. Developmental delay, intellectual disability, and microcephaly were reported in four individuals. Subject 6 was referred for evaluation of declining growth parameters, but at the time of her last assessment, her head circumference was in the normal range (1.35 SDs below the mean) and she was too young to have her intellectual abilities assessed. Two of our subjects required hearing aids at age 5 and 10 years, and further progression required a unilateral cochlear implant at age 13 years in one of them. Brain MRI shows abnormalities of the white matter, basal ganglia, and cerebral atrophy in two individuals, while three others were normal at ages 10 months, 12 years, and 14 years. NCSs were normal in one individual but were not performed in the others. In addition, subject 6 has non-specific peripheral retinal deposits at age 1 year, and two other individuals, the youngest at age 5 years, have clear pigmentary retinopathy. Subject 5 also harbors a pathogenic copy number gain of approximately 3 Mb at chromosome 22q11.21, a contiguous gene duplication (MIM: 608363) with a highly variable phenotype ranging from asymptomatic to severe developmental delay, short stature, hearing loss, and hypotonia, which could be contributing to her presentation. Characteristic brain MRI findings and retinal changes have not been described with this chromosomal abnormality.
Two subjects (subjects 7 and 8) harbored the c.260C>T (p.Ser87Leu) variant. Both have muscular weakness, areflexia, and gait impairment; neurophysiologic studies confirmed a sensorimotor neuropathy in each individual. Subject 7 experienced two apparently unprovoked regressions in symptoms, first with worsening hypotonia, weakness, and loss of babbling at age 9 months and again with worsening truncal hypotonia at 33 months; each time was followed by a plateau and slow improvement. He has developmental delay and severe intellectual disability, and his head circumference is normal (Z score = −0.40), but a brain MRI shows brain stem and basal ganglia abnormalities that did not change on repeat imaging at 10 months and 34 months. Subject 8 has decreased muscle bulk and mildly elevated serum creatine kinase activity; exome sequencing also showed a de novo, likely pathogenic variant in MYH7 (MIM: 160760) associated with autosomal-dominant myopathies and cardiomyopathy that could be responsible for those features and contribute to the weakness and areflexia but would not explain the growth abnormalities or intellectual disability. The c.260C>T (p.Ser87Leu) variant in MORC2 has been reported in several children considered to have infantile onset CMT disease type 2Z.4,14,16 Review of the published affected individuals for which clinical information was available revealed that the variant was confirmed de novo in 3/4 individuals, and at least one of them was reported to be microcephalic and one was reported to be dysmorphic (Table 1).4
The c.394C>T (p.Arg132Cys) variant was identified in four subjects (subjects 10–13). Subjects 10–12 have gross motor delay; all have mild intellectual disability. All of them have microcephaly and brain abnormalities on MRIs; periaqueductal gray matter and basal ganglia lesions were reported in one (subject 10), and one individual had findings suggestive of periventricular gliosis that were not present on repeat imaging at age 9 years (subject 12). Three individuals had NCSs: one was normal at age 4 years, whereas the other two showed a sensorimotor neuropathy (ages 4 and 15 years). Subject 12 also harbored a de novo missense variant in TCF12 (MIM: 600480), a gene for which loss-of-function variants are associated with Craniosynostosis 3 (MIM: 615314) and generally normal intelligence, which was not considered a good fit for this child. The c.394C>T (p.Arg132Cys) variant has been described in one individual with developmental delay for whom no additional information was available,16 and substitution of the Arg132 residue for a different amino acid (p.Arg132Leu) has also been reported in an individual who presented in childhood with classic CMT disease type 2Z with progressive weakness and hearing loss.14
We identified the previously unreported c.1164C>G (p.Ser388Arg) variant in two individuals (subjects 15 and 16). Both have developmental delay, intellectual disability, and microcephaly. Subject 15 has mild retinal pigmentary changes, less prominent neuromuscular symptoms, a normal NCS at age 9 years, and the brain MRI shows delayed myelination with mild cerebellar volume loss. Subject 16 has severe, asymmetric spasticity and lower extremity hyperreflexia as well as pes cavus and hammertoes, which were not present on the exam at age 12 years but were prominent at age 15 years. She has not had NCSs; the brain MRI shows cerebellar atrophy. Subject 16 also has a history of seizures and Wolff-Parkinson-White syndrome (MIM: 194200).
The c.1181A>G (p.Tyr394Cys) variant identified in two of our subjects (subjects 17 and 18) has been reported in an adult Japanese individual with a clinical diagnosis of CMT with onset at 6 years of age.5 No cognitive impairment was noted in the published individual, and no segregation data was provided. Our patients, in addition to polyneuropathy symptoms, have developmental delay and brain abnormalities. Subject 17 has facial dysmorphism, short stature (Z score = −2.10), and microcephaly (Z score = −2.10), similar to the other individuals described in this report.
We also identified a mother-daughter pair (subjects 19 and 20) harboring a novel missense variant in MORC2, c.1237G>T (p.Val413Phe). The maternal grandparents were not tested. Subject 19 presented at age 5 years with ataxia and action tremor, growth retardation, and developmental delay. An MRI of the brain was normal at 3 years old. Her mother, subject 20, has short stature, microcephaly, global developmental delay, mild intellectual disability, and adult onset hearing loss. Both were growth hormone deficient; subject 19 had a normal bone age and skeletal survey. Exome sequencing also showed a shared variant of uncertain significance in ASXL3 (MIM: 615115), a gene associated with autosomal-dominant Bainbridge-Ropers syndrome (MIM: 615485), consisting of severe-to-profound developmental delay with growth retardation and facial dysmorphism; this was not considered to be a good phenotypic fit for this family. In addition, pathogenic variants in ASXL3 are usually loss-of-function as opposed to missense such as in this instance, further arguing against pathogenicity.
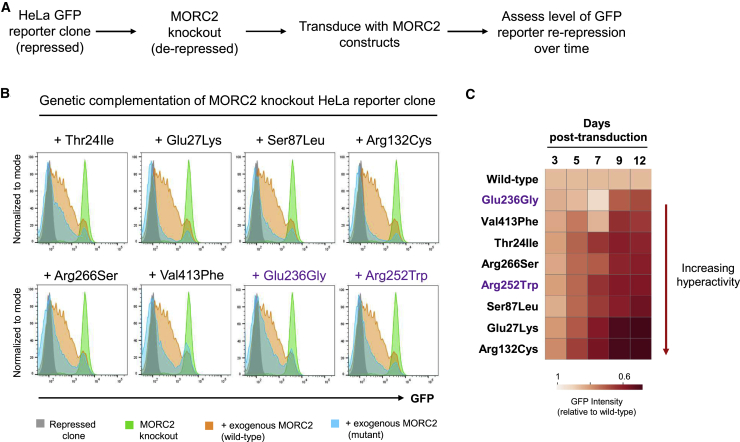
Chromatin remodeling by MORC2 has recently been shown to play a critical role in the process of epigenetic silencing mediated by the Human Silencing Hub (HUSH) complex.2,15 Thus, we performed a genetic complementation experiment to assess the functional consequences of these MORC2 variants in HUSH-mediated silencing (Figure 3A). MORC2-deficient cells harboring a HUSH-sensitive GFP reporter were transduced with lentiviral vectors encoding either wild-type MORC2 or the p.Thr24Ile, p.Glu27Lys, p.Ser87Leu, p.Arg132Cys, p.Arg266Ser, and p.Val413Phe mutants, plus two additional variants previously associated with MORC2-neuropathies (p.Glu236Gly and p.Arg252Trp), and reporter repression was measured over 12 days by flow cytometry (Figure 3A and Figure S2). We found that the p.Thr24Ile, p.Glu27Lys, p.Ser87Leu, p.Arg132Cys, p.Arg266Ser, and p.Val413Phe mutants all hyperactivated HUSH-mediated silencing (Figure 3B); p.Arg132Cys and p.Glu27Lys exhibited the most extreme phenotypes (Figure 3C). These findings mirror the effects observed with the p.Glu236Gly and p.Arg252Trp mutants previously associated with MORC2-neuropathies (Figure 3B)2 and support the notion that these variants in MORC2 are pathogenic. The p.Ala88Val, p.Ser388Arg, and p.Tyr394Cys variants were identified after the functional assays were completed.
Figure 3.
MORC2 Mutations Hyperactivate Epigenetic Silencing by the HUSH Complex
(A) Schematic representation of the complementation experiment in which the ability of MORC2 variants to restore repression of a HUSH-sensitive GFP reporter in MORC2 knockout cells was assessed.
(B and C) MORC2 variants hyperactivate HUSH repression. At day 12 (B), all MORC2 variants exhibit enhanced silencing of the GFP reporter construct (blue histograms) compared to wild-type MORC2 (orange histograms). The full results for the time-course are, which reveal that the c.79G > A (p.Glu27Lys) and c.394C > T (p.Arg132Cys) variants are the most hyperactivating, are shown in (C). The p.Glu236Gly and p.Arg252Trp variants (shown in purple) previously associated with CMT2Z were included as a reference.
Phenotypic heterogeneity, the concept that variation in the same gene can cause more than one disorder, is not uncommon in the era of exome and genome sequencing. In recent years, several genes associated with neuropathies have also been found to be responsible for more severe neurodevelopmental disorders, sometimes representing the extremes of a spectrum17 and, in other cases, two completely different syndromes.18 Constitutional variants in MORC2 were first reported in 2016 in association with a progressive axonal and sensory neuropathy frequently presenting in the first decade of life.8 There have been a few subsequent reports of more severely affected individuals, although with inconsistent features. Indeed, although some of the subjects reported here have characteristics of MORC2-related neuropathies, those features were considered part of a broader presentation, and hence, the subjects were referred for clinical exome sequencing. For this study, we used a genotype-first approach and selected individuals harboring de novo variants in MORC2 in a large dataset. Analysis of their detailed phenotypic information then allowed us to define a new syndrome characterized by global developmental and growth delay with variable facial dysmorphism. Leigh syndrome (or subacute necrotizing encephalomyelopathy, MIM: 256000), characterized by developmental delay and/or regression, movement disorder, neuropathy, and bilateral symmetric abnormalities in the basal ganglia, brainstem, and/or cerebellum on brain MRIs, was considered in several individuals. The presence of hearing loss and pigmentary retinopathy further suggested the diagnosis of a mitochondrial disorder.
MORC2 is involved in epigenetic silencing and expressed in neural tissues predominantly at early stages of development,2,19 providing a potential substrate for neurodevelopmental disability. MORC2 has also been shown to bind ATP citrate lyase, the enzyme that catalyzes the formation of cytosolic acetyl-CoA, a critical building block for multiple biosynthetic pathways,20 providing a potential mechanism for the presence of mitochondrial features in these individuals. Consistent with previous reports, all variants identified in our cohort cluster in the gyrase, Hsp90, histidine kinase, MutL (GHKL)-type ATP binding domain and the transducer-like domain, which together form the ATPase module of MORC2. Similar to other GHKL-type ATPases, ATP binding and hydrolysis by MORC2 functions as a conformational switch.15,21 Upon ATP binding, the ATPase module dimerizes and becomes active, allowing for HUSH complex activity leading to transcriptional repression.15 Assessing the functional effects of different MORC2 variants, Douse et al. showed that, in the setting of normal ATP-dependent dimerization, neuropathic mutations resulting in weaker ATPase activity hyperactivated HUSH-mediated silencing.15 All six of the MORC2 variants that we tested hyperactivated silencing by the HUSH complex in cellular assays, and thus, it is likely that these variants also exert their pathogenic effects by diminishing the ATPase activity of MORC2.
The presence of several recurrent variants allowed us to make some initial genotype-phenotype correlations. Most features appear consistent between individuals sharing the same variants, although the degree of severity varies. In the case of the c.1237G>T (p.Val413Phe) variant, we were able to evaluate the expression of the disorder across two generations; although information was incomplete for the mother, there were enough common findings to consider them similarly affected. All individuals harboring the c.260C>T (p.Ser87Leu) and the c.1181A>G (p.Tyr394Cys) variants have significant neuropathy symptoms, which can explain why they were identified in previous studies.4,5,14 In the case of the c.260C>T (p.Ser87Leu) variant, all published subjects were young and presented with gross motor delay, and at least one of them was also microcephalic.4 One subject heterozygous for the c.1181A>G (p.Tyr394Cys) variant in our cohort has mild facial features, hearing loss, and intellectual disability, and the other has normal growth parameters and no facial dysmorphism; however, they both presented with developmental delay and white matter abnormalities on brain MRIs, neither of which were reported in the published individual.5 On the other hand, individuals harboring the c.79G>A (p.Glu27Lys) and c.394C>T (p.Arg132Cys) variants are more likely to present with developmental delay, growth retardation, brain abnormalities, and inconsistent neuromuscular complaints. Indeed, among our nine patients with these variants, NCSs were reported for two subjects (ages 4 and 26 years) and were not performed in five others, suggesting that neuropathy was not a predominant concern, at least at that time. The lack of neuropathy in these patients might also explain why the c.79G>A (p.Glu27Lys) and c.394C>T (p.Arg132Cys) variants have not been identified in previous studies of MORC2 mutations among cohorts of CMT-affected patients but were found in large cohorts of individuals with neurodevelopmental disorders.16 Furthermore, our functional assays demonstrate that the c.79G>A (p.Glu27Lys) and c.394C>T (p.Arg132Cys) variants exert the most strongly hyperactivating effects on HUSH-mediated silencing (Figure 3B), suggesting that the degree of HUSH hyperactivation in the cellular assays might reflect, at least in part, the severity of the central nervous system phenotype.
Although more work is clearly necessary to delineate the specific mechanisms by which mutations in MORC2 result in the types of multi-organ system involvement described here, we have shown that the MORC2-associated neurodevelopmental disorder is characterized by global developmental delay, short stature, microcephaly, and variable dysmorphic facies, with or without neuropathy. The striking presence of features of Leigh-like syndrome further broadens the spectrum of phenotypes associated with MORC2 variants.
Data and Code Availability
Variant information for all variants described in this manuscript is publicly available through Clinvar under the following accession numbers: p.Thr24Ile (SCV001134972.1), p.Glu27Lys (SCV001134974.1), p.Ser87Leu (SCV000618293.2), p.Ala88Val (SCV000999384.1), p.Arg132Cys (SCV000571490.3), p.Arg266Ser (SCV000573276.4), p.Ser388Arg (SCV001134979.1), p.Tyr394Cys (SCV000589765.2), p.Val413Phe (SCV001134978.1).
Consortia
The members of the Undiagnosed Diseases Network are Maria T. Acosta, Margaret Adam, David R. Adams, Pankaj B. Agrawal, Mercedes E. Alejandro, Justin Alvey, Laura Amendola, Ashley Andrews, Euan A. Ashley, Mahshid S. Azamian, Carlos A. Bacino, Guney Bademci, Eva Baker, Ashok Balasubramanyam, Dustin Baldridge, Jim Bale, Michael Bamshad, Deborah Barbouth, Gabriel F. Batzli, Pinar Bayrak-Toydemir, Anita Beck, Alan H. Beggs, Gill Bejerano, Jimmy Bennet, Beverly Berg-Rood, Raphael Bernier, Jonathan A. Bernstein, Gerard T. Berry, Anna Bican, Stephanie Bivona, Elizabeth Blue, John Bohnsack, Carsten Bonnenmann, Devon Bonner, Lorenzo Botto, Brenna Boyd, Lauren C. Briere, Elly Brokamp, Gabrielle Brown, Elizabeth A. Burke, Lindsay C. Burrage, Manish J. Butte, Peter Byers, William E. Byrd, John Carey, Olveen Carrasquillo, Ta Chen Peter Chang, Sirisak Chanprasert, Hsiao-Tuan Chao, Gary D. Clark, Terra R. Coakley, Laurel A. Cobban, Joy D. Cogan, F. Sessions Cole, Heather A. Colley, Cynthia M. Cooper, Heidi Cope, William J. Craigen, Andrew B. Crouse, Michael Cunningham, Precilla D’Souza, Hongzheng Dai, Surendra Dasari, Mariska Davids, Jyoti G. Dayal, Esteban C. Dell’Angelica, Shweta U. Dhar, Katrina Dipple, Daniel Doherty, Naghmeh Dorrani, Emilie D. Douine, David D. Draper, Laura Duncan, Dawn Earl, David J. Eckstein, Lisa T. Emrick, Christine M. Eng, Cecilia Esteves, Tyra Estwick, Liliana Fernandez, Carlos Ferreira, Elizabeth L. Fieg, Paul G. Fisher, Brent L. Fogel, Irman Forghani, Laure Fresard, William A. Gahl, Ian Glass, Rena A. Godfrey, Katie Golden-Grant, Alica M. Goldman, David B. Goldstein, Alana Grajewski, Catherine A. Groden, Andrea L. Gropman, Irma Gutierrez, Sihoun Hahn, Rizwan Hamid, Neil A. Hanchard, Nichole Hayes, Frances High, Anne Hing, Fuki M. Hisama, Ingrid A. Holm, Jason Hom, Martha Horike-Pyne, Alden Huang, Yong Huang, Rosario Isasi, Fariha Jamal, Gail P. Jarvik, Jeffrey Jarvik, Suman Jayadev, Jean M. Johnston, Lefkothea Karaviti, Emily G. Kelley, Dana Kiley, Isaac S. Kohane, Jennefer N. Kohler, Deborah Krakow, Donna M. Krasnewich, Elijah Kravets, Susan Korrick, Mary Koziura, Joel B. Krier, Seema R. Lalani, Byron Lam, Christina Lam, Brendan C. Lanpher, Ian R. Lanza, C. Christopher Lau, Kimberly LeBlanc, Brendan H. Lee, Hane Lee, Roy Levitt, Richard A. Lewis, Sharyn A. Lincoln, Pengfei Liu, Xue Zhong Liu, Nicola Longo, Sandra K. Loo, Joseph Loscalzo, Richard L. Maas, Ellen F. Macnamara, Calum A. MacRae, Valerie V. Maduro, Marta M. Majcherska, May Christine V. Malicdan, Laura A. Mamounas, Teri A. Manolio, Rong Mao, Kenneth Maravilla, Thomas C. Markello, Ronit Marom, Gabor Marth, Beth A. Martin, Martin G. Martin, Julian A. Martínez-Agosto, Shruti Marwaha, Jacob McCauley, Allyn McConkie-Rosell, Colleen E. McCormack, Alexa T. McCray, Elisabeth McGee, Heather Mefford, J. Lawrence Merritt, Matthew Might, Ghayda Mirzaa, Eva Morava-Kozicz, Paolo M. Moretti, Marie Morimoto, John J. Mulvihill, David R. Murdock, Mariko Nakano-Okuno, Avi Nath, Stan F. Nelson, John H. Newman, Sarah K. Nicholas, Deborah Nickerson, Donna Novacic, Devin Oglesbee, James P. Orengo, Laura Pace, Stephen Pak, J. Carl Pallais, Christina GS. Palmer, Jeanette C. Papp, Neil H. Parker, John A. Phillips III, Jennifer E. Posey, Lorraine Potocki, Barbara N. Pusey, Aaron Quinlan, Wendy Raskind, Archana N. Raja, Genecee Renteria, Chloe M. Reuter, Lynette Rives, Amy K. Robertson, Lance H. Rodan, Jill A. Rosenfeld, Natalie Rosenwasser, Robb K. Rowley, Maura Ruzhnikov, Ralph Sacco, Jacinda B. Sampson, Susan L. Samson, Mario Saporta, C. Ron Scott, Judy Schaechter, Timothy Schedl, Kelly Schoch, Daryl A. Scott, Lisa Shakachite, Prashant Sharma, Vandana Shashi, Jimann Shin, Rebecca Signer, Catherine H. Sillari, Edwin K. Silverman, Janet S. Sinsheimer, Kathy Sisco, Edward C. Smith, Kevin S. Smith, Lilianna Solnica-Krezel, Rebecca C. Spillmann, Joan M. Stoler, Nicholas Stong, Jennifer A. Sullivan, Angela Sun, Shirley Sutton, David A. Sweetser, Virginia Sybert, Holly K. Tabor, Cecelia P. Tamburro, Queenie K.-G. Tan, Mustafa Tekin, Fred Telischi, Willa Thorson, Cynthia J. Tifft, Camilo Toro, Alyssa A. Tran, Brianna M. Tucker, Tiina K. Urv, Matt Velinder, Dave Viskochil, Tiphanie P. Vogel, Colleen E. Wahl, Stephanie Wallace, Nicole M. Walley, Chris A. Walsh, Melissa Walker, Jennifer Wambach, Jijun Wan, Lee-kai Wang, Michael F. Wangler, Patricia A. Ward, Daniel Wegner, Mark Wener, Tara Wenger, Katherine Wesseling Perry, Monte Westerfield, Matthew T. Wheeler, Jordan Whitlock, Lynne A. Wolfe, Jeremy D. Woods, Shinya Yamamoto, John Yang, Guoyun Yu, Diane B. Zastrow, Chunli Zhao, and Stephan Zuchner.
Declaration of Interests
M.J.G.S., C.F., E.T., W.W., and J.J. are employees of GeneDx, Inc. J.S.C. is a consultant to Invitae. A.F. is a consultant to Calico Labs and a paid Data and Safety Monitoring Board (DSMB) member of Bluebirdbio, Inc. and Stealth Therapeutics. M.K.K. is a paid member of the DSMB for Modis and is on the Speakers Bureau for Novartis.
Acknowledgments
I.A.T. is a postdoctoral fellow of the Damon Runyon Cancer Research Foundation. Research reported in this manuscript was supported by the NIH Common Fund through the Office of Strategic Coordination, Office of the NIH Director under award number U01HG007690. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Published: July 20, 2020
Footnotes
Supplemental Data can be found online at https://doi.org/10.1016/j.ajhg.2020.06.013.
Web Resources
Exome Variant Server, NHLBI GO Exome Sequencing Project (ESP), https://evs.gs.washington.edu/EVS/
GeneDx ClinVar, https://www.ncbi.nlm.nih.gov/clinvar/
Greater Middle East Variome, http://igm.ucsd.edu/gme/
OMIM, https://www.omim.org
Supplemental Data
References
- 1.Moissiard G., Cokus S.J., Cary J., Feng S., Billi A.C., Stroud H., Husmann D., Zhan Y., Lajoie B.R., McCord R.P. MORC family ATPases required for heterochromatin condensation and gene silencing. Science. 2012;336:1448–1451. doi: 10.1126/science.1221472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tchasovnikarova I.A., Timms R.T., Douse C.H., Roberts R.C., Dougan G., Kingston R.E., Modis Y., Lehner P.J. Hyperactivation of HUSH complex function by Charcot-Marie-Tooth disease mutation in MORC2. Nat. Genet. 2017;49:1035–1044. doi: 10.1038/ng.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li D.Q., Nair S.S., Ohshiro K., Kumar A., Nair V.S., Pakala S.B., Reddy S.D., Gajula R.P., Eswaran J., Aravind L., Kumar R. MORC2 signaling integrates phosphorylation-dependent, ATPase-coupled chromatin remodeling during the DNA damage response. Cell Rep. 2012;2:1657–1669. doi: 10.1016/j.celrep.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sevilla T., Lupo V., Martínez-Rubio D., Sancho P., Sivera R., Chumillas M.J., García-Romero M., Pascual-Pascual S.I., Muelas N., Dopazo J. Mutations in the MORC2 gene cause axonal Charcot-Marie-Tooth disease. Brain. 2016;139:62–72. doi: 10.1093/brain/awv311. [DOI] [PubMed] [Google Scholar]
- 5.Ando M., Okamoto Y., Yoshimura A., Yuan J.H., Hiramatsu Y., Higuchi Y., Hashiguchi A., Mitsui J., Ishiura H., Fukumura S. Clinical and mutational spectrum of Charcot-Marie-Tooth disease type 2Z caused by MORC2 variants in Japan. Eur. J. Neurol. 2017;24:1274–1282. doi: 10.1111/ene.13360. [DOI] [PubMed] [Google Scholar]
- 6.Laššuthová P., Šafka Brožková D., Krůtová M., Mazanec R., Züchner S., Gonzalez M.A., Seeman P. Severe axonal Charcot-Marie-Tooth disease with proximal weakness caused by de novo mutation in the MORC2 gene. Brain. 2016;139:e26. doi: 10.1093/brain/awv411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semplicini C., Ollagnon-Roman E., Leonard-Louis S., Piguet-Lacroix G., Silvestre M., Latour P., Stojkovic T. High intra-familiar clinical variability in MORC2 mutated CMT2 patients. Brain. 2017;140:e21. doi: 10.1093/brain/awx019. [DOI] [PubMed] [Google Scholar]
- 8.Albulym O.M., Kennerson M.L., Harms M.B., Drew A.P., Siddell A.H., Auer-Grumbach M., Pestronk A., Connolly A., Baloh R.H., Zuchner S. MORC2 mutations cause axonal Charcot-Marie-Tooth disease with pyramidal signs. Ann. Neurol. 2016;79:419–427. doi: 10.1002/ana.24575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanni G., Nardella M., Barresi S., Bellacchio E., Niceta M., Ciolfi A., Pro S., D’Arrigo S., Tartaglia M., Bertini E. De novo p.T362R mutation in MORC2 causes early onset cerebellar ataxia, axonal polyneuropathy and nocturnal hypoventilation. Brain. 2017;140:e34. doi: 10.1093/brain/awx083. [DOI] [PubMed] [Google Scholar]
- 10.Schottmann G., Wagner C., Seifert F., Stenzel W., Schuelke M. MORC2 mutation causes severe spinal muscular atrophy-phenotype, cerebellar atrophy, and diaphragmatic paralysis. Brain. 2016;139:e70. doi: 10.1093/brain/aww252. [DOI] [PubMed] [Google Scholar]
- 11.Retterer K., Juusola J., Cho M.T., Vitazka P., Millan F., Gibellini F., Vertino-Bell A., Smaoui N., Neidich J., Monaghan K.G. Clinical application of whole-exome sequencing across clinical indications. Genet. Med. 2016;18:696–704. doi: 10.1038/gim.2015.148. [DOI] [PubMed] [Google Scholar]
- 12.Lek M., Karczewski K.J., Minikel E.V., Samocha K.E., Banks E., Fennell T., O’Donnell-Luria A.H., Ware J.S., Hill A.J., Cummings B.B., Exome Aggregation Consortium Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E., ACMG Laboratory Quality Assurance Committee Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyun Y.S., Hong Y.B., Choi B.O., Chung K.W. Clinico-genetics in Korean Charcot-Marie-Tooth disease type 2Z with MORC2 mutations. Brain. 2016;139:e40. doi: 10.1093/brain/aww082. [DOI] [PubMed] [Google Scholar]
- 15.Douse C.H., Bloor S., Liu Y., Shamin M., Tchasovnikarova I.A., Timms R.T., Lehner P.J., Modis Y. Neuropathic MORC2 mutations perturb GHKL ATPase dimerization dynamics and epigenetic silencing by multiple structural mechanisms. Nat. Commun. 2018;9:651. doi: 10.1038/s41467-018-03045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deciphering Developmental Disorders Study Prevalence and architecture of de novo mutations in developmental disorders. Nature. 2017;542:433–438. doi: 10.1038/nature21062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shy M.E. Peripheral neuropathies caused by mutations in the myelin protein zero. J. Neurol. Sci. 2006;242:55–66. doi: 10.1016/j.jns.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Verma P., Kumar A., Goswami C. TRPV4-mediated channelopathies. Channels (Austin) 2010;4:319–328. doi: 10.4161/chan.4.4.12905. [DOI] [PubMed] [Google Scholar]
- 19.Sancho P., Bartesaghi L., Miossec O., García-García F., Ramírez-Jiménez L., Siddell A., Åkesson E., Hedlund E., Laššuthová P., Pascual-Pascual S.I. Characterization of molecular mechanisms underlying the axonal Charcot-Marie-Tooth neuropathy caused by MORC2 mutations. Hum. Mol. Genet. 2019;28:1629–1644. doi: 10.1093/hmg/ddz006. [DOI] [PubMed] [Google Scholar]
- 20.Sánchez-Solana B., Li D.Q., Kumar R. Cytosolic functions of MORC2 in lipogenesis and adipogenesis. Biochim. Biophys. Acta. 2014;1843:316–326. doi: 10.1016/j.bbamcr.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearl L.H., Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu. Rev. Biochem. 2006;75:271–294. doi: 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Variant information for all variants described in this manuscript is publicly available through Clinvar under the following accession numbers: p.Thr24Ile (SCV001134972.1), p.Glu27Lys (SCV001134974.1), p.Ser87Leu (SCV000618293.2), p.Ala88Val (SCV000999384.1), p.Arg132Cys (SCV000571490.3), p.Arg266Ser (SCV000573276.4), p.Ser388Arg (SCV001134979.1), p.Tyr394Cys (SCV000589765.2), p.Val413Phe (SCV001134978.1).